Abstract
Objectives
Although uterine cancer is the fourth most common cause for cancer death in women worldwide, the molecular underpinnings of tumor progression remain poorly understood. The High Mobility Group A1 (HMGA1) gene is overexpressed in aggressive cancers and high levels portend adverse outcomes in diverse tumors. We previously reported that Hmga1 transgenic mice develop uterine tumors with complete penetrance. Because HMGA1 drives tumor progression by inducing matrix metalloproteinase (MMP) and other genes involved in invasion, we explored the HMGA1-MMP-2 pathway in uterine cancer.
Methods
To investigate MMP-2 in uterine tumors driven by HMGA1, we used a genetic approach with mouse models. Next, we assessed HMGA1 and MMP-2 expression in primary human uterine tumors, including low-grade carcinomas (endometrial endometrioid) and more aggressive tumors (endometrial serous carcinomas, uterine carcinosarcomas/malignant mesodermal mixed tumors).
Results
Here, we report for the first time that uterine tumor growth is impaired in Hmga1a transgenic mice crossed on to an Mmp-2 deficient background. In human tumors, we discovered that HMGA1 is highest in aggressive carcinosarcomas and serous carcinomas, with lower levels in the more indolent endometrioid carcinomas. Moreover, HMGA1 and MMP-2 were positively correlated, but only in a subset of carcinosarcomas. HMGA1 also occupies the MMP-2 promoter in human carcinosarcoma cells.
Conclusions
Together, our studies define a novel HMGA1-MMP-2 pathway involved in a subset of human carcinosarcomas and tumor progression in murine models. Our work also suggests that targeting HMGA1 could be effective adjuvant therapy for more aggressive uterine cancers and provides compelling data for further preclinical studies.
Keywords: HMGA1, High Mobility Group A1, chromatin remodeling proteins, MMP-2, uterine cancer, tumor progression
Graphical abstract
1. Introduction
Uterine cancer is the most frequently diagnosed gynecologic malignancy in the United States and the fourth most common cancer in American women. In addition, it is the sixth most common cancer in women worldwide [1-3]. Moreover, the incidence of uterine cancer has been increasing over the last two decades, possibly due to increasing obesity rates, and the death rate for uterine corpus cancer has been rising since 2000 [2-3]. The five year survival rate for women with distant metastases is less than 20% reflecting the limited treatment options available [1-3]. Thus, further study has the potential to significantly benefit women's health. Carcinomas are the most frequent type of uterine cancers, with endometrioid carcinomas being the most common subtype. The majority of endometrioid carcinomas are low-grade, with indolent behavior in most cases [1-2]. They are generally treated with surgery alone and have favorable outcomes. In contrast, uterine serous carcinomas are less common and are, by definition, high-grade, with generally aggressive behavior even when present at lower stages. Uterine sarcomas are relatively uncommon, comprising only ∼5% of all uterine cancers. They occur in older women, behave aggressively, and are often associated with poor outcomes. Carcinosarcomas are high-grade uterine cancers with both malignant epithelial (carcinomatous) and mesenchymal (sarcomatous) components. The 5-year survival for carcinosarcomas is 24-50% for all stages [3]. Adenosarcomas are even less common, but have significantly better survival rates than carcinosarcomas [3]. Despite the high overall prevalence of uterine cancers, the molecular events that lead to the distinct subtypes are poorly understood.
The high mobility group A1 (HMGA1) gene is overexpressed in diverse, refractory tumors [4-31], including uterine carcinomas and sarcomas [4-5]. The HMGA1 gene encodes the HMGA1a and HMGA1b chromatin remodeling proteins, which function in modulating gene expression [6, 30-31]. HMGA1 proteins are members of the HMGA family of AT-hook DNA binding proteins that consists of HMGA1a, HMGA1b, and HMGA2 [30-32]. HMGA1 is enriched in aggressive cancers and embryonic stem cells [4-31; 33-34]. In a previously published pilot study of 19 primary tumors, we found that HMGA1 is overexpressed in high-grade uterine cancers, but not in normal uterine tissue, benign tumors, or most low-grade neoplasms of the uterus [4]. We also discovered that Hmga1a transgenic mice develop aggressive lymphoid tumors and uterine sarcomas by 9 months of age with complete penetrance [4, 10]. Together, these findings highlight a central role for HMGA1 in diverse high-grade tumors.
Matrix metalloproteases (MMPs) are a family of over 20 zinc-dependent proteinases important to the homeostasis of the extracellular matrix [35-40]. They were originally characterized based on their ability to degrade the extracellular matrix and basement membrane, which facilitates tumor cell invasion, migration, intravasation into the circulation, extravasation out of the bloodstream, and ultimately metastasis. In some tumors, MMP activity correlates with cellular invasiveness and metastatic potential [35-36; 40]. More recently, MMPs were shown to exert other important biologic effects relevant to cancer including the processing of critical proteins involved in angiogenesis, apoptosis, chemotaxis, cell migration, and cell proliferation [35-39]. Surprisingly, tumor suppressor functions have also been identified for MMP family members [37, 40]. We previously found that HMGA1 up-regulates expression of MMP-2 in lung cancer cells, but only in poorly differentiated tumors, indicating that this pathway could drive tumor progression and anaplasia in a subset of poorly differentiated, stem-like lung cancers [23]. HMGA1 also up-regulates expression of MMP-2 in prostate cancer [11]. In addition, HMGA1 induces MMP-9 in pancreatic cancer [35] and MMP-13 in breast cancer tumor models [30], suggesting that the HMGA1-MMP axis is important in diverse human cancers.
Here, we discovered that the Hmga1-Mmp-2 pathway is important in uterine tumorigenesis using a genetic approach in mice. In primary human tumor samples, we also found that HMGA1 is up-regulated in most tumors, with highest levels in the more aggressive, high-grade carcinosarcomas and serous tumors. We also found that the HMGA1-MMP-2 pathway was up-regulated, in a subset of carcinosarcomas. These findings indicate that HMGA1 could serve as a therapeutic target in aggressive uterine carcinomas and sarcomas. Although further studies are needed, our findings also suggest that the HMGA1-MMP-1 pathway may be a rational target for cancer therapy in a subset of carcinosarcomas, thus highlighting the role for personalized therapy in these aggressive tumors.
2. Materials and methods
2.1 Primary tumor samples, RNA preparation and quantification, and quantitative RT-PCR
A total of 76 primary uterine tumor samples (29 endometrioid carcinomas, 30 carcinosarcomas and 17 serous carcinomas) were obtained from de-identified patient samples. Sufficient RNA was generated from 24 endometrioid carcinomas, 23 carcinosarcomas, and 14 serous carcinomas using Trizol as we previously described [4-5]. From the RNA, cDNA was prepared, and HMGA1a and MMP-2 mRNA levels were assessed by quantitative RT-PCR (qRT-PCR) as we previously described [4-5].
To expand our sample size of primary uterine tumors, we also compared gene expression in the Cancer Genome Atlas (TCGA) database from uterine corpus and endometrial carcinoma (UCEC), which included 546 primary uterine tumors and 35 normal uterine tissue controls for expression of HMGA1 and MMP-2. TCGA UCEC expression data, version 2015_11_01, was downloaded using the Broad Institute's firehose application (https://gdac.broadinstitute.org/). Clinical data, including histology, was downloaded from the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/).
2.2 Generation of Hmga1a transgenic–Mmp-2 null mice
The Hmga1a mice have been previously reported [4, 10]. Briefly, the murine Hmga1a cDNA is driven by the H-2K promoter and immunoglobulin μ enhancer, which is expressed in MHC Class 1 expressing cells, including B and T cells [10], uterine tissue [4], and intestinal epithelium [17]. All mice (100%) develop lymphoid malignancy by 5-10 months and die from their disease by 8-13 months [10]. The female mice also develop uterine sarcomas with complete (100%) penetrance [4]. The Mmp-2 null mice (a kind gift from Dr. Lynn Matrisian) were also previously described [37]. To generate mice that are both transgenic for Hmga1a and null for Mmp-2, we mated Hmga1a males with Mmp-2-/- mice. Hmga1a males were used because the females are infertile [4]. First, we generated mice that are transgenic for Hmga1a and heterozygous for the Mmp-2 alleles (Mmp-2+/-). The Hmga1a transgenic-Mmp-2+/- males were then crossed again with Mmp-2-/- mice to generate mice that are transgenic for Hmga1a and homozygous null for Mmp-2 (designated Hmga1a-Mmp-2-/-). We generated groups of mice (n=5-8/group; Table 1) from each genotype for comparison, including: Hmga1a-Mmp-2-/- (n=7), Hmga1a-Mmp-2+/- (n=7), Hmga1a-Mmp-2+/+ (n=7), nontransgenic-Mmp-2-/- (n=5), nontransgenic-Mmp-2+/- (n=8), nontransgenic-Mmp-2+/+ (n=6). All mice were maintained on a C57Bl6 background and the genotype was determined by PCR. The presence of the Hmga1a transgene was assessed using the primers 5′-TAAGCTTGATGTCATGAGCGAG and 5′-CCTGAGACTTCCACACTGATG, which produced a 565 base pair PCR fragment specific to the transgene. As a PCR control, primers specific to mouse beta-2 microglobulin (T5′-ACCCACCGGAGAATGGGAAG and 5′-TCTTGGGCTCGGCCATACTG) were used, which produced a 237 base pair fragment. The Mmp-2 genotype was assessed as previously described.
Table 1. Relative uterine weights/uterine tumor burdens in Hmga1 transgenic - Mmp-2-/- mice and controls.
| Genotype | Number of mice/genotype | Body weight (grams) | Uterine weight (grams) | Relative uterine weight |
|---|---|---|---|---|
| Hmga1-Mmp-2-/- | 7 | 25.08 +/- 2.50 | 0.89 +/- 0.40 | 3.58 +/- 1.62 |
| Hmga1-Mmp-2+/- | 7 | 26.41 +/- 2.66 | 1.11+/- 0.73 | 4.25 +/- 2.93 |
| Hmga1-Mmp-2+/+ | 7 | 26.57 +/- 2.27 | 2.11 +/- 1.27 | 7.78 +/- 4.45 |
| Nontransgenic Mmp-2-/- | 5 | 25.75+/- 2.38 | 0.15 +/- 0.02 | 0.58 +/- 0.11 |
| Nontransgenic Mmp-2+/- | 8 | 23.91+/- 2.39 | 0.10 +/- 0.02 | 0.42 +/- 0.10 |
| Nontransgenic Mmp-2+/+ | 6 | 23.70+/- 1.78 | 0.13 +/- 0.03 | 0.55 +/- 0.13 |
2.3 Histopathological analysis of uterine tumors
Tissues were fixed and analyzed as we described [4, 10]. Uterine weights were obtained and relative tumor sizes were estimated by dividing the uterine weight by the total body weight as we previously described [4, 10]. Spleen weights and relative lymphoid tumor sizes were also obtained as previously described [22].
2.4 Chromatin immunoprecipitation and silencing HMGA1
Chromatin immunoprecipitation experiments were performed in MES-SA uterine carcinosarcoma cells as previously described [4, 12, 19] using MES-SA cells (human uterine carcinosarcoma cells, ATCC). Proteins cross-linked to chromatin were immunoprecipitated with the following antibodies: HMGA1 [12], histone H3 (as positive control), or IgG (as a negative control). The MMP-2 promoter region with the consensus HMGA1 DNA-binding site was amplified from the immunoprecipitated protein-DNA complexes using the forward and reverse primers 5′- GGGGAAAAGAGGTGGAGAAA -3′ and 5′- CGCCTGAGGAAGTCTGGAT -3′, respectively [12].
2.5 Statistical analysis
Statistical significance was assessed by the Student's t-test and Mann-Whitney test. To determine if HMGA1 and MMP-2 expression correlate, both Pearson's and Spearman's correlations were calculated. P<0.05 was considered significant.
For analysis of primary tumor and normal tissue gene expression data from TCGA, the association between gene expression and histological type (endometrioid, serous or carcinosarcomas) was assessed by linear regression. Because two different RNAseq platforms, IlluminaHiSeq and IlluminaGa were used for the UCEC dataset, platform was included as a covariate in the analysis to account for possible batch effects.
3. Results
3.1 Deficiency of Mmp-2 interferes with uterine tumorigenesis in Hmga1 transgenics
To define the role of Mmp-2 in the development of tumorigeneis in the Hmga1a transgenic model, we crossed the Hmga1a transgenics with mice null for Mmp-2 (Mmp-2-/-), to generate mice with each of the following genotypes: Hmga1a-Mmp-2-/- (n=7), Hmga1a-Mmp-2+/- (n=7), Hmga1a-Mmp-2+/+ (n=7), and nontransgenic Mmp-2-/- (n=6), Mmp-2+/- (n=8), Mmp-2+/+ (n=5) (Table 1). The total uterine weight (grams; p = 0.045), which reflects tumor burden [4], and relative uterine weight (uterine weight/body weight; p = 0.049), which reflects relative tumor burden [4], were significantly decreased in the Hmga1a mice that were null for Mmp-2 (Fig. 1A-C). The total and relative uterine weights were also decreased in the Hmga1a-Mmp-2+/- mice, although this did not reach statistical significance in the relatively small sample sizes. Histopathologically, the tumors with Mmp-2 deficiency were similar in appearance to those with wildtype Mmp-2 (Fig. 1D). There was no difference in mean body weights in all groups and there were no significant differences in the uterine weightss in the nontransgenic Mmp-2-/-, Mmp-2+/-, Mmp-2+/+ mice (Table 1). Of note, absolute spleen weights and relative spleen weights, which reflect the lymphoid tumor burdens [10], showed a trend towards decreasing size in Hmga1a transgenics that were deficient for Mmp-2, although this did not reach statistical significance in the small samples sizes studied (Supplementary Fig. 1; Supplementary Table 1).
FIGURE 1. Deficiency of Mmp-2 interferes with uterine tumorigenesis in Hmga1 transgenics.
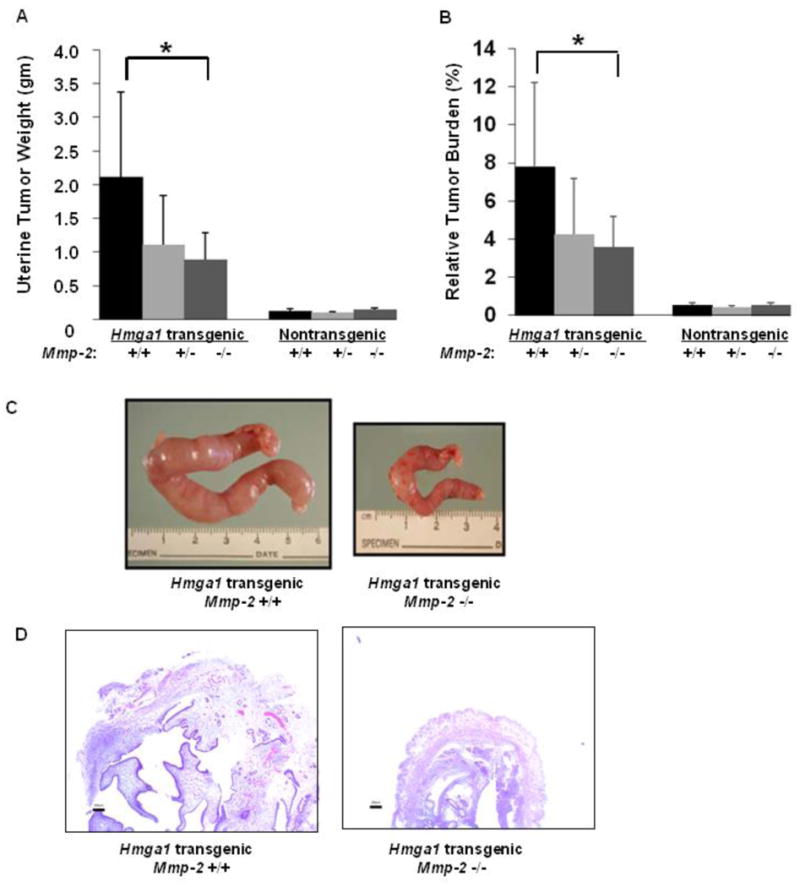
A. Mean uterine tumor weights (grams) in Hmga1 transgenic mice with Mmp-2 that was wildtype (+/+; n=7), heterozygous (+/-; n=7), or null (-/-; n=7) as compared to wildtype (nontransgenic) mice with Mmp-2 that was wildtype (+/+; n=6), heterozygous (+/-; n=8), or null (-/-; n=5). * denotes P = 0.045. B. Relative tumor burden in Hmga1 transgenic or wildtype mice with Mmp-2 that was wildtype (+/+), heterozygous deficiency (+/-) or null (-/-). * denotes P = 0.049. The number of mice/group is indicated above.
C. Gross necropsy shows a significant decrease in uterine tumor size in the Hmga1 transgenic mice that are null for Mmp-2 as compared to Hmga1 transgenics that are wildtype for Mmp-2. D. Histopathologic findings in tumors from Hmga1a transgenics wildtype or deficient for Mmp-2
3.2 HMGA1 is overexpressed in primary high grade, aggressive uterine tumors
Our previous study showed that HMGA1 is overexpressed in uterine carcinosarcomas and leiomyosarcomas [4]. To compare HMGA1 expression in uterine carcinosarcomas to that of pure endometrial carcinomas, we assessed mRNA expression in primary human tumors, including: 1) low-grade endometrioid carcinomas, 2) serous carcinomas, and, 3) carcinosarcomas by qRT-PCR. We discovered that HMGA1 levels are significantly higher in the carcinosarcomas and serous carcinomas as compared to the endometrioid carcinomas (Fig. 2A). Interestingly, HMGA1 expression was significantly higher in the carcinosarcomas as compared to the endometrioid carcinomas (P=0.0021) and in the serous carcinomas as compared to the endometrioid carcinomas (P=0.0004). The levels of HMGA1 in the serous carcinoma and carcinosarcoma samples were similar (Fig. 2A).
FIGURE 2. HMGA1is overexpressed in more aggressive uterine tumors and correlates positively with MMP-2expression in carcinosarcomas.
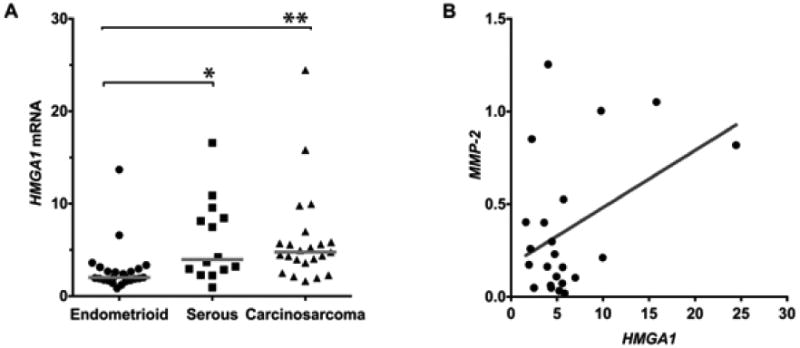
A. HMGA1 mRNA expression is highest in the high grade serous and carcinosarcoma tumors as compared to endometrioid tumors. * denotes P = 0.002 and ** denotes P = 0.004.
B. HMGA1 and MMP-2 expression correlate positively in the carcinosarcomas (r = 0.42, P<0.05).
3.3 HMGA1 expression correlates with MMP-2 expression in primary carcinosarcomas
Next, we sought to determine whether expression levels of HMGA1a and MMP-2 are correlated in human uterine tumors. To this end, we measured HMGA1a and MMP-2 expression in the primary uterine tumors, including the endometrioid carcinomas, serous carcinomas, and carcinosarcomas. There was no correlation between HMGA1a and MMP-2 mRNA expression in the pure carcinomas. There was, however, a significant, positive correlation between HMGA1a and MMP-2 mRNA in the carcinosarcomas (Fig. 2B; r = 0.42, P<0.05). Taken together, these results suggest that HMGA1 could drive tumor progression in uterine carcinosarcomas by up-regulating MMP-2 to promote metastatic disease.
3.4 HMGA1 binds directly to the MMP-2 promoter in a human uterine carcinosarcoma cells
Because we found that HMGA1a expression is positively correlated with MMP-2 in carcinosarcomas, we sought to determine if HMGA1 binds directly to the MMP-2 promoter in carcinosarcoma cells to up-regulate its expression. We therefore performed chromatin immunoprecipitation experiments in the MES-SA uterine carcinosarcoma cell line. We found that HMGA1 binds to the MMP-2 promoter in a highly conserved region near the transcription start sites (Fig. 3A).
FIGURE 3. HMGA1 binds directly to the MMP-2 promoter to regulate MMP-2 expression.
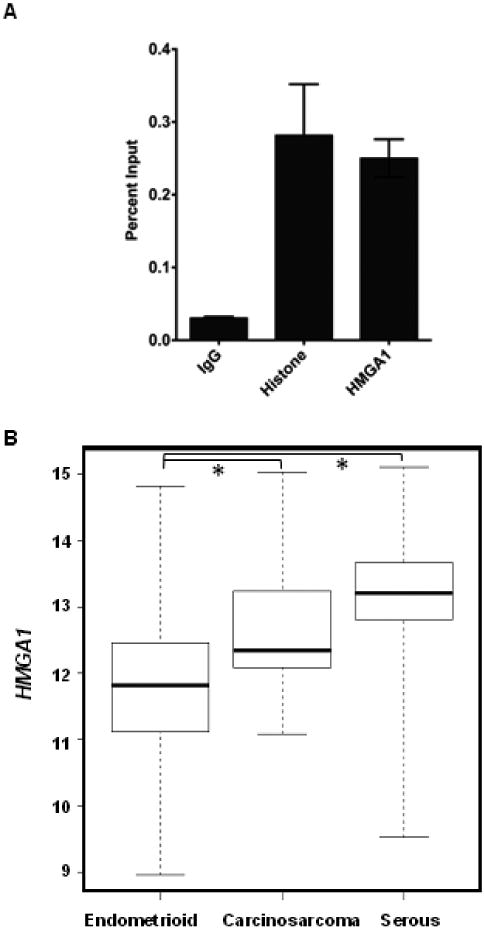
A. Chromatin immunoprecipiation was performed with sheared chromatin form human MES-SA carcinosarcoma cells after cross-linking proteins bound to DNA with formaldehyde. Quantity of immunoprecipitated DNA with the following antibodies (all from Upstate, except for the HMGA1 antibody, which is from Abcam): HMGA1, Histone H3 (positive control), or rabbit IgG (negative control); bars represent relative enrichment as the mean percent of total input DNA +/- standard deviations as assessed by quantitative RT-PCR performed in triplicate. The primers used to amplify the HMGA1 binding site in the MMP-2 promoter were previously described [23].
B. HMGA1 is highly overexpressed in the more aggressive primary tumors with adverse outcomes, including serous and carcinosarcomas, as compared to endometrioid tumors. Gene expression data was retrieved from TCGA database. (Specific datapoints are shown in Supplementary Fig. 2).
3.5 HMGA1 and MMP-2 expression in uterine tumors from TCGA
To expand our study sample size of primary tumors, we investigated independent gene expression analyses from TCGA (Fig. 3B). Similar to our results from primary tumor samples, we found that HMGA1 is significantly overexpressed in all uterine tumors compared to normal uterine tissue (Supplementary Fig. 2; P<0.00001). In addition, HMGA1 was highest in the aggressive serous carcinomas (P<0.00001) and carcinosarcomas (P<0.00001) as compared to the more indolent endometrioid tumors. In contrast to our analysis of 30 primary carcinosarcomas, however, we did not find a correlation between HMGA1 and MMP-2 in this larger dataset of carcinosarcomas, nor was there a correlation in normal uterine tissue, endometrioid or seous tumors (Supplementary Fig. 3). The basis for this difference is unclear, but suggests that the HMGA1-MMP-2 pathway may only be up-regulated in a subset of carcinosarcomas. Taken together, these data are consistent with the model whereby increasing expression of HMGA1 drives both tumor initiation and tumor progression in uterine tumors, and higher levels reprogram cancer cells to more aggressive phenotype. The HMGA1-MMP-2 pathway is activated in a subset of carcinocarcomas, which could contribute to driving tumor progression (Fig. 4).
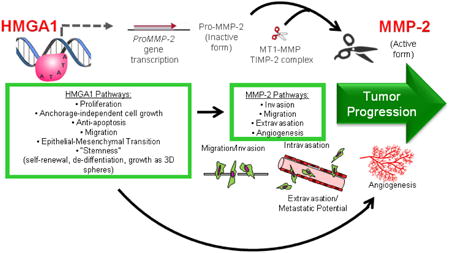
FIGURE 4. Model for the HMGA1-MMP-2 pathway in uterine carcinosarcomas.
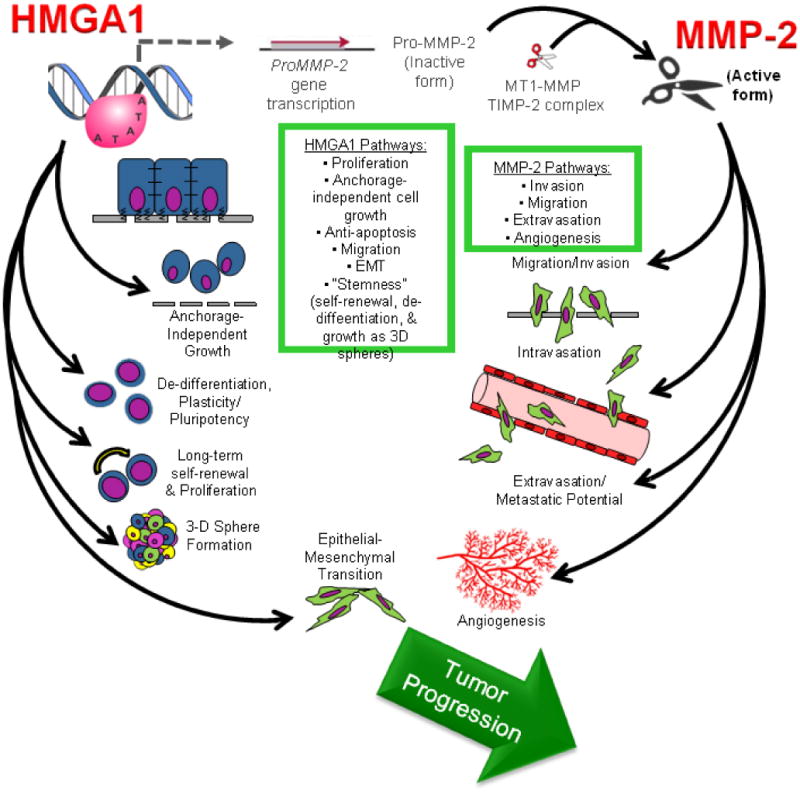
HMGA1 induces multiple transcriptional networks which activate tumor initiation, tumor progression, and stem cell pathways, including induction of the MMP-2 gene in a subset of high-grade carcinosarcomas. The inactive, pro-MMP-2 protein is generated and subsequently cleaved to an active form by a complex that includes a membrane-type MMP (MT-MMP) and tissue inhibitor of metalloproteinase (TIMP-2, itself a protease). The active MMP-2 drives tumor cell mobility, invasion, migration and angiogenesis and the HMGA1-MMP-2 pathway could then collectively culminate in tumor progression.
Discussion
Cancers of the uterine corpus are among the most common gynecologic malignancies in developed countries, accounting for about 6% of female cancers in the US [1-4]. This past year, approximately 46,000 new cases were diagnosed with over 8,000 deaths in the US alone and the incidence is rising. Endometrial endometrioid carcinomas are the most common uterine cancers, comprising over 80% of uterine cancers. They arise from the endometrium, usually in the setting of endometrial hyperplasia, and are often cured by surgery alone as they tend to be indolent. Serous carcinomas also arise from the endometrium, but primarily occur in post-menopausal women with atrophic endometrium, and generally behave more aggressively. In contrast, carcinosarcomas are mixed tumors with both malignant epithelial and mesenchymal components. Like serous tumors, they tend to be high-grade or poorly differentiated and behave aggressively. The molecular underpinnings that give rise to more aggressive uterine cancers are poorly understood, particularly in the carcinosarcomas. Here, we focused on HMGA1 because it encodes a chromatin remodeling protein that correlates with poor differentiation status and adverse clinical outcomes in diverse tumors [6,20]. Emerging evidence also indicates that HMGA1 drives tumor progression by co-opting pathways involved in stem cells, an epithelial-mesenchymal transition, and metastatic progression [6, 17, 27]. In addition, prior studies suggest that MMPs cooperate with HMGA1 during tumor progression [11, 23, 36].
MMPs are key proteolytic enzymes that contribute to the ability of tumors to invade surrounding tissues and intravasate into the vascular system, thus setting up early events required for metastatic progression [35-39]. MMPs are also required for angiogenesis, which likewise involves degradation of the vascular basement membrane and extracellular matrix followed by migration of vascular endothelial precursors into the surrounding tissue. During the proteolysis of the basement membrane, MMPs release endogenous extracellular matrix and angiogenic factors (VEGF, TGF-beta) that are sequestered in the basement membrane [35-39]. MMPS are held in check by tissue inhibitors or metalloproteases (TIMPs) and studies suggest that nonmetastatic tumors secrete higher levels of TIMPS and thereby prevent tumor invasion and angiogenesis. The discovery of MMPs as essential factors in tumor invasion and metastatic progression led to the development of pharmacologic inhibitors, although early clinical trials were uniformly disappointing. Limitations of the early clinical trials included lack of MMP specificity, inconsistent pharmacokinetics in cancer patients, and drug intolerance [35]. In addition, most patients treated in the early trials had advanced disease, which could limit the efficacy of therapy with MMP inhibitors. A subset of MMPs has tumor-suppressor effects, and inhibitors could potentially enhance tumor progression [37, 40]. Recent work also indicates that MMPs regulate innate immunity and host defense [39]. Thus, the current challenge in the field will be to elucidate the specific roles of MMPs in tumor progression and host defense so that more effective inhibitors can be developed.
In this study, we investigated the HMGA1-MMP-2 pathway because HMGA1 had been shown to directly activate MMP-2 expression in experimental models of prostate cancer [11] and poorly differentiated lung cancer [23]. In fact, prior studies in other tumors provide additional evidence that MMP and HMGA1 proteins cooperate to drive neoplastic transformation in diverse tumor types. For example, in the breast cancer cells (MCF-7), MMP-13 was identified as a gene induced in cells engineered to express high levels of HMGA1a or HMGA1b [30]. Studies in prostate cancer cells showed that HMGA1 increases levels of the precursor protein to MMP-2, MT-MMP-2 [11]. HMGA1 also induces MMP-9 expression and promotes tumor invasion through the PI3K/Akt pathway in pancreatic cancer cells [36]. Work from our group uncovered the link between MMP-2 and HMGA1 in poorly differentiated lung cancers [23]. More recently, HMGA1 gene and protein levels were found to correlate significantly with MMP-9 gene and protein expression as well as other markers of invasion and angiogenesis of malignant gliomas [24]. In fact, the 5′ promoter regions of many MMP genes include consensus DNA binding sites for HMGA1 [11, 23-24, and Resar unpublished data].
Here, we found that MMP-2 deficiency impaired the growth of the uterine tumors that were induced by HMGA1 in a mouse model, although there was no consistent effect on the lymphoid tumors. This finding suggests that uterine tumors induced by HMGA1 in this model are dependent on MMP-2 for growth at the stage in which we measured tumor size, in contrast to the lymphoid tumors, which may depend on other HMGA1 pathways [10, 12, 21-22]. In primary human carcinosarcomas, we also found a positive correlation between HMGA1 and MMP-2, but only in a subset of these tumors. This relationship was not observed in primary tumors from the TCGA database, although the basis for this difference could not be discerned from this study. It is possible that carcinosarcomas rely on transient activation of this pathway for early invasion, after which selective pressures from the tumor microenvironment no longer require functional MMP-2. Alternatively, there may be molecularly distinct subtypes of carcinosarcomas, including those in which the HMGA1-MMP-2 pathway contributes to tumor progression. Finally, different methodologies to assess MMP-2 could rely on differential sensitivities of detection. It has become clear that HMGA1 orchestrates many tumor progression and stem cells pathways, which appear to be cell-type and context specific. Further studies will be needed to determine under which contexts the HMGA1-MMP-2 pathway functions in uterine carcinosarcomas.
In conclusion, we also found that HMGA1 is highly overexpressed in primary high-grade, poorly differentiated tumors, including aggressive serous carcinomas and carcinosarcomas, as compared to the more differentiated and indolent endometrioid tumors or normal uterine tissue. We also found that HMGA1 and MMP-2 expression are positively correlated in a subset of primary human carcinosarcomas. Moreover, we report for the first time that HMGA1 binds directly to the MMP-2 promoter in poorly differentiated, cultured human uterine carcinosarcoma cells. Finally, we show that growth of uterine sarcomas is impaired in Hmga1 transgenics that are deficient in Mmp-2, providing additional evidence that targeting this pathway could be beneficial in the treatment of a subset of uterine cancers. Our results further highlight the role of HMGA1 in driving tumorigenesis in poorly differentiated, aggressive human uterine tumors and suggest that HMGA1 could be a rational therapeutic target. Further studies with a focus on personalized medicine will be needed to define subsets of patients who may respond to therapy to interrupt the HMGA1-MMP-2 pathway.
Supplementary Material
Highlights.
Deficiency of Mmp-2 impairs uterine tumorigenesis in Hmga1 transgenic mice
HMGA1 is overexpressed in aggressive human uterine carcinosarcomas and serous carcinomas
HMGA1 and MMP-2 are positively correlated in a subset of human carcinosarcomas
HMGA1 occupies the MMP-2 promoter in human carcinosarcoma cells
Targeting the HMGA1 pathways could disrupt progression of aggressive uterine tumors
Acknowledgments
The authors thank the generous support of the NIH, Prevent Cancer Foundation, the Maryland Stem Cell Research Fund. Grant support: R01 CA092339, R21 CA118343, R03 CA139331 (L.M.S. Resar), CA149550, P01 CA134292 (D. L. Huso), Prevent Cancer Foundation (J. Hillion), Maryland Stem Cell Research Fund (J. Hillion, L. Xian, S. N. Shah, and L. Resar)
Grant Support: R01 CA092339, R21 CA118343, R03 CA139331 (L.M.S. Resar), CA149550, P01 CA134292 (D. L. Huso), Prevent Cancer Foundation (J. Hillion), Alex's Lemonade Stand Foundation (J. Hillion, L.M.S. Resar) and Maryland Stem Cell Research Fund (L.M.S. Resar, L. Xian, J. Hillion).
Abbreviations
- HMGA1a
high mobility group A1 gene
- MMP-2
matrix metalloproteinase-2
- RT-PCR
real time polymerase chain reaction
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-based Report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2015. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Ross S, Coebergh JWW, Comber H, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. European Journal of Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Tesfaye A, Di Cello F, Hillion J, Ronnett BM, Elbahloul O, Ashfaq R R, et al. The high-mobility group A1 gene up-regulates cyclooxygenase 2 expression in uterine tumorigenesis. Cancer Res. 20007;67:3998–4004. doi: 10.1158/0008-5472.CAN-05-1684. [DOI] [PubMed] [Google Scholar]
- 5.Di Cello F, Hillion J, Kowalski J, Ronnett BM, Aderinto A, Huso DL, et al. Cyclooxygenase inhibitors block uterine tumorigenesis in HMGA1a transgenic mice and human xenografts. Mol Cancer Ther. 2008;7:2090–5. doi: 10.1158/1535-7163.MCT-07-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resar LMS. The high mobility group A1 gene: transforming inflammatory signals into cancer? Cancer Res. 2010;70:436–9. doi: 10.1158/0008-5472.CAN-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood LJ, Maher JF, Bunton TE, Resar LM. The oncogenic properties of the HMG-I gene family. Cancer Res. 2000;60:4256–61. [PubMed] [Google Scholar]
- 8.Wood LJ, Mukherjee M, Dolde CE, Xu Y, Maher JF, Bunton TE, et al. HMG-I/Y, A new c-Myc target gene and potential oncogene. Mol Cell Biol. 2000;20:5490–502. doi: 10.1128/mcb.20.15.5490-5502.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedulla ML, Treff NR, Resar LMS, Reeves R. Sequence and analysis of the murine Hmgiy (Hmga1) gene locus. Gene. 2001;271:51–8. doi: 10.1016/s0378-1119(01)00500-5. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Sumter TF, Bhattacharya R, Tesfaye A, Fuchs EJ, Wood LJ, et al. The HMG-I oncogene causes highly penetrant, aggressive lymphoid malignancy in transgenic mice and is overexpressed in human leukemia. Cancer Res. 2004;64:3371–5. doi: 10.1158/0008-5472.CAN-04-0044. [DOI] [PubMed] [Google Scholar]
- 11.Takaha N, Resar LM, Vindivich D, Coffey DS. High mobility group protein HMGI(Y) enhances tumor cell growth, invasion, and matrix metalloproteinase-2 expression in prostate cancer cells. Prostate. 2004;60:160–167. doi: 10.1002/pros.20049. [DOI] [PubMed] [Google Scholar]
- 12.Hillion J, Dhara S, Sumter TF, Mukherjee M, Di Cello F, Belton A, et al. The high-mobility group A1a/signal transducer and activator of transcription-3 axis: an achilles heel for hematopoietic malignancies? Cancer Res. 2008;68:10121–7. doi: 10.1158/0008-5472.CAN-08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Cello F, Hillion J, Hristov A, Wood LJ, Mukherjee M, Schuldenfrei A, et al. HMGA2 participates in transformation in human lung cancer. Mol Cancer Res. 2008;6:743–50. doi: 10.1158/1541-7786.MCR-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hristov AC, Cope L, Di Cello F, Reyes MD, Singh M, Hillion JA, et al. HMGA1 correlates with advanced tumor grade and decreased survival in pancreatic ductal adenocarcinoma. Mod Pathol. 2010;23:98–104. doi: 10.1038/modpathol.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hristov AC, Cope L, Reyes MD, Singh M, Iacobuzio-Donahue C, Maitra A, et al. HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:43–9. doi: 10.1038/modpathol.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson DM, Joseph B, Hillion J, Segal J, Karp JE, Resar LMS. Flavopiridol induces BCL-2 expression and represses oncogenic transcription factors in leukemic blasts from adults with refractory acute myeloid leukemia. Leuk Lymphoma. 2011;52:1999–2006. doi: 10.3109/10428194.2011.591012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belton A, Gabrovsky A, Bae YK, Reeves R, Iacobuzio-Donahue C, Huso DL, et al. HMGA1 induces intestinal polyposis in transgenic mice and drives tumor progression and stem cell properties in colon cancer cells. PLoS One. 2012;7:e30034. doi: 10.1371/journal.pone.0030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah SN, Kerr C, Cope L, Zambidis E, Liu C, Hillion J, et al. HMGA1 reprograms somatic cells into pluripotent stem cells by inducing stem cell transcriptional networks. PLoS One. 2012;7:e48533. doi: 10.1371/journal.pone.0048533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillion J, Smail SS, Di Cello F, Belton A, Shah SN, Huso T, et al. The HMGA1-COX-2 axis: a key molecular pathway and potential target in pancreatic adenocarcinoma. Pancreatology. 2012;12:372–9. doi: 10.1016/j.pan.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SN, Resar LM. High mobility group A1 and cancer: potential biomarker and therapeutic target. Histol Histopathol. 2012;27:567–79. doi: 10.14670/HH-27.567. [DOI] [PubMed] [Google Scholar]
- 21.Schuldenfrei A, Belton A, Kowalski J, Talbot CC, Jr, Di Cello F, et al. HMGA1 drives stem cell, inflammatory pathway, and cell cycle progression genes during lymphoid tumorigenesis. BMC Genomics. 2011;12:549. doi: 10.1186/1471-2164-12-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Cello F, Dhara S, Hristov AC, Kowalski J, Elbahloul O, Hillion J, et al. Inactivation of the Cdkn2a locus cooperates with HMGA1 to drive T-cell leukemogenesis. Leuk Lymphoma. 2013;54(8):1762–8. doi: 10.3109/10428194.2013.764422. [DOI] [PubMed] [Google Scholar]
- 23.Hillion J, Wood LJ, Mukherjee M, Bhattacharya R, Di Cello F, Kowalski J, et al. Upregulation of MMP-2 by HMGA1 promotes transformation in undifferentiated, large-cell lung cancer. Mol Cancer Res. 2009;7:1803–12. doi: 10.1158/1541-7786.MCR-08-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang B, Fan H, Zhang IY, Liu B, Feng B, Meng L, et al. HMGA1 expression in human gliomas and its correlation with tumor proliferation, invasion and angiogenesis. J Neurooncol. 2012;106:543–9. doi: 10.1007/s11060-011-0710-6. [DOI] [PubMed] [Google Scholar]
- 25.Williams MD, Reeves R, Resar LS, Hill HH., Jr Metabolomics of colorectal cancer: past and current analytical platforms. Anal Bioanal Chem. 2013;405:5013–30. doi: 10.1007/s00216-013-6777-5. [DOI] [PubMed] [Google Scholar]
- 26.Roy S, Di Cello F, Kowalski J, Hristov AC, Tsai HL, Bhojwani D, et al. HMGA1 overexpression correlates with relapse in childhood B-lineage acute lymphoblastic leukemia. Leuk Lymphoma. 2013;54:2565–7. doi: 10.3109/10428194.2013.782610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah SN, Cope L, Poh W, Belton A, Roy S, Talbot C, et al. HMGA1: A master regulator of tumor progression in triple negative breast cancer. PLoS ONE. 2013;8:e63419. doi: 10.1371/journal.pone.0063419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams MD, Zhang X, Park JJ, Siems WF, Gang DR, Resar LM, et al. Characterizing metabolic changes in human colorectal cancer. Anal Bioanal Chem. 2015;16:4581–95. doi: 10.1007/s00216-015-8662-x. [DOI] [PubMed] [Google Scholar]
- 29.Williams MD, Zhang X, Belton AS, Xian L, Huso T, Park JJ, et al. HMGA1 drives metabolomics reprogramming of intenstinal epithelium druing hyperproliferation, polyposis and colorectal carcinogenesis. J Proteome Res. 2015;14:1420–31. doi: 10.1021/pr501084s. [DOI] [PubMed] [Google Scholar]
- 30.Reeves R, Edberg DD, Li Y. Architectural transcription factor HMGI(Y) promoters tumor progression and mesenchymal transition of human epithelial cells. Mol Cell Biol. 2001;21:575–94. doi: 10.1128/MCB.21.2.575-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fusco A, Fedele M. Roles of HMGA proteins in cancer. Nat Rev Cancer. 2007;7:899–910. doi: 10.1038/nrc2271. [DOI] [PubMed] [Google Scholar]
- 32.Resar LM, Brodsky RA. “Let”-ing go with clonal expansion? Blood. 2011;117:5788–90. doi: 10.1182/blood-2011-04-346668. [DOI] [PubMed] [Google Scholar]
- 33.Chiappetta G, Avantaggiato V, Visconti R, Fedele M, Battista S, Trapasso F, et al. High level expression of the HMGI (Y) gene during embryonic development. Oncogene. 1996;13:2439–46. [PubMed] [Google Scholar]
- 34.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zucker S, Cao J. Selective matrix metalloproteinase (MMP) inhibitors in cancer therapy: ready for prime time? Cancer Biol Ther. 2009;8:2371–3. doi: 10.4161/cbt.8.24.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liau SS, Jazag A, Whang EE. HMGA1 is a determinant of cellular invasiveness and in vivo metastatic potential in pancreatic adenocarcinoma. Cancer Res. 2006;66:11613–22. doi: 10.1158/0008-5472.CAN-06-1460. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–8. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 38.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–92. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 39.Chung AS, Waldeck H, Schmidt DR, Kao WJ. Monocyte inflammatory and matrix remodeling response modulated by grafted ECM-derived ligand concentration. J Biomed Mater Res A. 2009;91:742–52. doi: 10.1002/jbm.a.32259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palavalli LH, Prickett TD, Wunderlich JR, Wei X, Burrell AS, Porter-Gill P, et al. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet. 2009;41:518–20. doi: 10.1038/ng.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


