Abstract
Annual infusions of zoledronic acid (5 mg) significantly reduced the risk of vertebral, hip, and nonvertebral fractures in a study of postmenopausal women with osteoporosis and significantly reduced clinical fractures and all‐cause mortality in another study of women and men who had recently undergone surgical repair of hip fracture. In this analysis, we examined whether timing of the first infusion of zoledronic acid study drug after hip fracture repair influenced the antifracture efficacy and mortality benefit observed in the study. A total of 2127 patients (1065 on active treatment and 1062 on placebo; mean age, 75 yr; 76% women and 24% men) were administered zoledronic acid or placebo within 90 days after surgical repair of an osteoporotic hip fracture and annually thereafter, with a median follow‐up time of 1.9 yr. Median time to first dose after the incident hip fracture surgery was ∼6 wk. Posthoc analyses were performed by dividing the study population into 2‐wk intervals (calculated from time of first infusion in relation to surgical repair) to examine effects on BMD, fracture, and mortality. Analysis by 2‐wk intervals showed a significant total hip BMD response and a consistent reduction of overall clinical fractures and mortality in patients receiving the first dose 2‐wk or later after surgical repair. Clinical fracture subgroups (vertebral, nonvertebral, and hip) were also reduced, albeit with more variation and 95% CIs crossing 1 at most time points. We concluded that administration of zoledronic acid to patients suffering a low‐trauma hip fracture 2 wk or later after surgical repair increases hip BMD, induces significant reductions in the risk of subsequent clinical vertebral, nonvertebral, and hip fractures, and reduces mortality.
Keywords: hip fracture, bisphosphonate, osteoporosis, infusion, zoledronic acid
INTRODUCTION
Zoledronic acid is a potent bisphosphonate that can be administered intravenously once yearly to suppress bone turnover markers and increase BMD. A large, randomized, double‐blind clinical trial in 7736 women with postmenopausal osteoporosis showed that an annual 5‐mg dose of intravenous zoledronic acid for 3 yr significantly reduced the risk of vertebral, hip, and nonvertebral fractures. (1) A second trial (HORIZON‐Recurrent Fracture trial [RFT]) in 2127 men and women dosed within 90 days after surgical repair, and annually thereafter for up to 3 yr, showed that zoledronic acid significantly reduced all clinical fractures, as well as subgroups thereof (clinical vertebral and nonvertebral fractures). Moreover, a significant reduction of all‐cause mortality in patients treated with zoledronic acid was observed. (2)
Previous animal studies have suggested that zoledronic acid is preferentially deposited at an acute fracture site, thereby potentially leaving less drug available to the rest of the skeleton. (3) We conducted a posthoc analysis of the HORIZON‐RFT data to examine whether the timing of first infusion had any relationship to fracture and mortality benefit.
MATERIALS AND METHODS
Study design
The Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) Recurrent Fracture Trial was an international, multicenter, randomized, double‐blind, placebo‐controlled trial involving women and men with recent low‐trauma hip fracture. (2) Patients were randomly assigned to receive either zoledronic acid 5 mg or placebo by intravenous infusion over 15 min. More than 99% of all patients received either a loading dose of vitamin D3 or D2 (50,000–100,000 IU, PO or IM) 14 days before first study drug infusion and daily oral calcium (1000–1500 mg) and vitamin D (400–800 IU) supplements during the study period. Patients were infused within 90 days after the surgical repair of a hip fracture and every 12 mo thereafter for up to 3 yr or until 211 patients had experienced a new clinical fracture. Concomitant therapy with nasal calcitonin, selective estrogen receptor modulators, hormone replacement, tibolone, and external hip protectors was allowed at the discretion of the investigator and primary physician. Current use of other bisphosphonates, strontium ranelate, anabolic steroids, and sodium fluoride were not allowed. Previous bisphosphonate use was allowed, provided suitable washout periods were met. Minimal previous therapy with PTH was allowed for a maximum of 1 wk if a prespecified washout of >6 mo had occurred. Patients were monitored with quarterly telephone interviews and yearly clinic visits. All study procedures were approved by the local institutional review board at each site.
Patients
Men and women ≥50 yr of age were eligible for inclusion within 90 days after surgical repair of a low‐trauma hip fracture. Details of the study methodology have been published previously by Lyles et al. (2)
Endpoints
The primary endpoint was time to first new clinical fracture of the axial or appendicular skeleton, excluding facial and digital fractures and fractures in pathological bone. Secondary endpoints included the change in BMD in the nonfractured hip measured annually with DXA and time to clinical vertebral, nonvertebral, and hip fractures.
Measurements and outcomes assessment
Lateral chest and lumbar spine X‐rays were obtained for most patients at baseline. At each study visit, patients were asked whether they had (1) sustained a new nonvertebral fracture or (2) had new or worsening back pain and spine radiographs obtained in routine clinical care. Affirmative responses to either question triggered sites to submit documentation to the central blinded adjudication committee at Duke University Medical Center. A nonvertebral fracture was confirmed when a radiograph, radiograph report, or medical record documented a new fracture. A possible vertebral fracture required review of both the baseline and recent radiographs using a semiquantitative technique. (4)
DXA measurements of the contralateral hip were performed at baseline and annually thereafter. Sites were requested to use the same machine for each patient over time, and local quality assurance was monitored. BMD and T‐scores were adjusted to correct for site and brand variations.
Statistical analysis
This trial was event driven, requiring patients with 211 clinical fractures to have 90% power. A two‐sided 5% level significance test with two interim analyses using O'Brien‐Fleming boundaries (5) was used to detect a 35% reduction in clinical fracture rate with zoledronic acid compared with placebo.
A predefined analysis of BMD response to the first dose of zoledronic acid occurring within or later than 6 wk was performed. The percent change in BMD in subjects dosed earlier or later than 6 wk was compared using t‐tests. ANOVA models were used to correct for imbalances in baseline risk factors.
In further posthoc analyses, subjects were divided into infusion time groups by the week of their infusion after hip fracture repair, with 2‐wk time intervals used to allow sufficient subjects for analysis in each group. Summary statistics for background and demographic variables were calculated by treatment group. Randomization comparability between treatment groups was evaluated for these background and demographic variables and compared by χ2 test for discrete variables and t‐tests for the continuous variables. Hazard ratios (HRs) for the fracture and mortality variables were calculated for each infusion time group using survival analysis, and tests for trend were completed.
RESULTS
A total of 2127 patients were randomized to zoledronic acid 5 mg (n = 1065) or placebo (n = 1062). The median follow‐up time was 1.9 yr. Compared with placebo, the zoledronic acid group reduced the risk of clinical fractures by 35% (8.6% versus 13.9%, p = 0.001). Total hip and femoral neck BMD of the nonfractured hip was significantly increased at each time point (12, 24, and 36 mo). This study also showed a 28% reduction in overall mortality in the zoledronic acid–treated group.
Analyses of changes in BMD and fractures within and after 6 wk
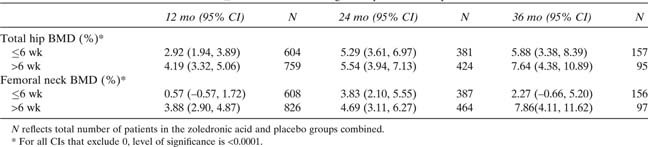
A preplanned analysis of BMD response in relationship to the timing of first dose (within or later than 6 wk after surgical repair of the hip fracture) was performed. Of the randomized patients, 46.12% were dosed within 6 wk and 53.83% after. For one patient, the timing of dosing was unknown (Table 1). Patients dosed later than 6 wk after hip fracture exhibited a greater increase in total hip and femoral neck BMD at month 12 compared with patients dosed earlier than 6 wk (Table 2). At 12 mo, total hip BMD increases over placebo were 2.92% in the ≤6 wk group versus 4.19% in the >6 wk group (both p < 0.0001 versus placebo; Table 2). For femoral neck BMD at 12 mo, smaller significant increases relative to placebo in the ≤6 wk group were also observed (0.57% versus 3.88%, respectively; Table 2). At 24 mo, the increases in hip and femoral neck BMD were similar in both subgroups. At 36 mo, there were fewer patients available for analysis, but the BMD increases in both subgroups were similar.
Table Table 1.
Distribution of Timing of Dosing of First Dose of Zoledronic Acid (ZOL) and Placebo After Surgical Repair of Hip Fracture
|
|
Table Table 2.
Least‐Squares Mean Differences in the Percentage Change in Total Hip and Femoral Neck BMD Over Placebo in Patients Dosed ≤6 vs. >6 wk After Surgical Hip Fracture Repair
|
|
A posthoc analysis was performed to evaluate the relationship of timing of infusion to the incidence of clinical fractures. Clinical fractures were reduced by 33% (p < 0.05) in patients first dosed ≤6 wk after hip fracture compared with 37% (p < 0.05) in patients dosed >6 wk. Similar benefits in clinical fracture risk reduction were found regardless of the timing of the first infusion.
Analysis of changes in BMD, fracture, and mortality at 2‐wk time intervals
To further evaluate any relationship between timing of first dose and changes in BMD, antifracture efficacy, and mortality in more detail, an additional posthoc analysis based on infusion time broken into 2‐wk intervals was performed. The proportion of patients dosed at each 2‐wk interval between 0 and 12 wk after fracture repair are shown in Table 1. Only 5% of patients were dosed within 2 wk after fracture repair. The numbers of patients dosed in the other time intervals were similar, ranging between 12% and 16%, except in the 4‐ to 6‐wk interval, where 24% of all patients were dosed.
Timing of first infusion and changes in BMD
At 12 mo, total hip BMD showed significant increases at all time points except in the small subgroup that was dosed ≤2 wk (Fig. 1A). In the ≤2 wk group, the increases were of similar magnitude compared with those seen at later time points, but the 95% CIs were wide. At 24 mo, when patients had received two infusions, all infusion time groups achieved significant BMD increases (Fig. 1B).
Figure Figure 1.

(A) Least‐squares (LS) mean between‐treatment differences (zoledronic acid vs. placebo) in the percentage change in total hip BMD at 12 mo by timing of first study drug infusion after hip fracture repair. (B) LS mean between‐treatment differences (zoledronic acid vs. placebo) in the percentage change in total hip BMD at 24 mo by timing of first study drug infusion after hip fracture repair. (C) Hazard ratios (HRs) for reductions in clinical fractures by timing of first study drug infusion.† (D) HR for reductions in nonvertebral fractures by timing of first study drug infusion.† (E) HR for reductions in hip fractures by timing of first study drug infusion.† (F) HR for reductions in clinical vertebral fractures by timing of first study drug infusion.† (G) HR for reductions in death by timing of first study drug infusion.† *p < 0.05; **p < 0.01; ***p < 0.001. †Significance determined based on log‐rank test.
Timing of first infusion and changes in antifracture efficacy
To further analyze the relationship between timing of first dose and antifracture efficacy, time to fracture endpoints were also re‐evaluated based on 2‐wk intervals (Figs. 1C–1F). The magnitude of the hazard reduction for all clinical fractures, the primary endpoint, was already maximal after 2 wk. However, because of the small sample size in the 2‐wk subgroups, the confidence limits all crossed 1, with the exception of the 4‐ to 6‐wk subgroup (the largest one). Similarly, there was no consistent variation of antifracture efficacy by time of infusion for nonvertebral fractures, hip fractures, or clinical vertebral fractures (Figs. 1C–1F), although the 95% CIs were wide. The CIs were consistently wider for all fractures in the ≤2‐wk group with the lower limit crossing 1. Except for the small ≤2 wk subgroup, the data suggest a consistent reduction in clinical fractures regardless of timing of infusion.
Timing of first infusion and changes in mortality after hip fracture
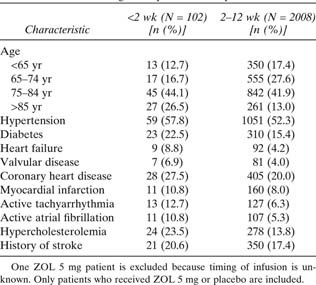
For time to all‐cause mortality (Fig. 1G), the ≤2 wk group showed no reduction in the hazard of death, whereas other infusion time periods did, although the 95% CIs crossed 1 for all but one time point. The apparent trend toward greater reduction in mortality when the first infusion was given at longer intervals after fracture repair was not significant (p = 0.31) A review of the baseline characteristics for the patients in the ≤2 wk group compared with the rest of the patient population was performed. Patients dosed <2 wk were older and exhibited a higher baseline prevalence of hypertension, coronary artery disease, diabetes, atrial fibrillation, and stroke (Table 3). These patients were also more likely to have come from an institutional setting before hospitalization for hip fracture and return to an institution after fracture compared with the subgroups dosed after the first 2 wk.
Table Table 3.
Baseline Characteristics in Patients Dosed <2 vs. ≥2 wk After Surgical Hip Fracture Repair
|
|
Antifracture efficacy and mortality reduction after exclusion of the ≤2‐wk group
To study the impact of patients dosed within 2 wk on the overall results, we analyzed overall antifracture efficacy and reduction of mortality after exclusion of the ≤2‐wk group (Table 4). When time points >2 wk after fracture repair were used for statistical testing only, all time to fracture endpoints, as well as time to all‐cause mortality, showed significant and generally larger relative risk reductions (Table 4). Clinical fractures were reduced by 41% (p = 0.0002), nonvertebral fractures by 44% (p = 0.0077), clinical vertebral fractures by 53% (p = 0.0084), and hip fractures by 48% (p = 0.0305). There was a significant 30% reduction in mortality (p = 0.0095). Thus, exclusion of the ≤2 wk group resulted in preservation of significant antifracture efficacy for all fracture subgroups and the significant reduction in mortality.
Table Table 4.
HRs for Clinical Fractures for Zoledronic Acid–Treated Subjects After Exclusion of Patients Dosed Within 2 wk
|
|
DISCUSSION
Patients with hip fracture represent an important population to target for secondary fracture prevention. The study on which this posthoc analysis was based (2) previously showed a significant 35% reduction in clinical fractures and a 28% reduction in all‐cause mortality in patients administered zoledronic acid up to 90 days after surgical repair of hip fracture. However, the administration of an intravenous bisphosphonate in patients with ongoing fracture healing could potentially affect the dynamics of BMD response, antifracture efficacy, and mortality benefit, depending on when the dose was administered. We found a suggestion of reduced drug efficacy in subjects dosed within 2 wk of their fracture repair, with all subsequent infusion time groups achieving a consistent relative benefit with respect to fracture reduction and mortality. In a rodent study, Amanat et al. (3) administered 14C‐zoledronic acid at 0, 1, and 2 wk after femoral diaphyseal fracture. Zoledronic acid administered during fracture healing (weeks 1 and 2) was concentrated at the fracture site and reduced bisphosphonate exposure to the rest of the skeleton, as reflected in lower incorporation of radioactivity at the contralateral nonfractured leg. Our patients underwent osteosynthesis or implant surgery, and from bone scintigraphy studies, it is well known that bisphosphonates accumulate at sites of surgery and osteosynthesis. (6) Thus, in theory, our patients were facing a situation similar to the one reported by Amanat et al., with potential accumulation of drug in the fracture callus and at sites of osseointegration. However, further analysis by 2‐wk intervals showed that total hip BMD at 12 mo was significantly increased in all patients dosed after 2 wk. No trend toward better BMD response with increasing time between hip fracture repair and first infusion was seen (Figs. 1A and 1B). This suggests that no major trapping of bisphosphonate took place at the fracture site. It is possible that the marginally reduced effect in patients dosed within the first 2 wk could be caused by such a phenomenon, but the absence of a general trend over time speaks against this mechanism. Moreover, as outlined above, the increased frailty and number of comorbidities associated with this group might render them less susceptible to pharmaceutical intervention.
The primary endpoint, all clinical fractures, exhibited maximal reduction in patients dosed at 2–4 wk, and no clear trend over time was demonstrable. Moreover, reductions in clinical vertebral, nonvertebral, and hip fractures did not show any significant time‐dependent trends over the 90‐day dosing period of the study. This further speaks against significant entrapment of bisphosphonate at the fracture site. Only patients dosed ≤2 wk after surgical repair showed absence of clinical fracture reduction compared with the rest of the group. We hypothesize that this discrepancy may be caused by differences in baseline risk factor characteristics of this group compared with patients dosed at later time points.
Several studies have shown a very high peri‐ and postoperative mortality in hip fracture patients, which amounts to 9% within the first 30 days and 19% within 120 days. 7 , 8 Davidson et al. (9) reported 26% mortality in the year after hip fracture in one hospital in New Zealand; others have found mortality rates in the 15–25% range, 10 , 11 with the greatest risk in the first 6 mo. Clinical fractures (including clinical vertebral fractures) have been shown to be associated with a substantial increase in mortality, even in relatively healthy older women. (12) In support of this concept, mortality in this study population was ∼3‐fold higher than in the zoledronic acid Pivotal Fracture Trial in postmenopausal women with osteoporosis. (13) In the post–hip fracture population of our study, patients dosed within 2 wk showed a much higher variance for virtually every endpoint studied, including mortality. Moreover, analysis of baseline characteristics of this group showed that they had more comorbidities than patients dosed later after surgical repair (Table 3). The increased frailty of this group may explain the much higher response variability and lack of treatment response in this group. Sensitivity analysis also supported this notion. As shown in Table 4, all fracture subtypes, as well as mortality, were significantly reduced in patients dosed 2–12 wk after hip fracture repair.
In conclusion, infusions of zoledronic acid in patients with a recent hip fracture led to an increase in total hip BMD as early as 2 wk after surgical repair. Patients receiving zoledronic acid within 2 wk after surgical repair were frailer, had more comorbidities, and did not show a significant reduction in fractures or mortality. Patients dosed 2–12 wk after hip fracture, however, showed significant reduction in clinical vertebral fractures, nonvertebral fractures, and hip fractures, as well as all‐cause mortality.
Acknowledgements
This research was funded by Novartis Pharma, Basel, Switzerland.
Dr. Eriksen has been an employee of and owns stock in Novartis. Dr. Lyles receives research grants from Novartis, the Alliance for Better Bone Health (Sanofi‐Aventis and Procter & Gamble), and Amgen, consults for Novartis, Procter & Gamble, Merck, Amgen, GTx, GlaxoSmithKline, Eli Lilly, and Bone Medical, and holds patents. Dr. Colón‐Emeric consults for Novartis and receives research grants from Novartis and the Alliance for Better Bone Health. Dr. Pieper receives research grants from Novartis. Dr. Magaziner receives research grants from Novartis and Merck, consults for Amgen, Merck, Aventis, and GTx, and speaks for Merck and Pfizer. Dr. Adachi receives research grants from Amgen, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Procter & Gamble, and Roche and consults for Amgen, AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Procter & Gamble, Roche, Sanofi‐Aventis, and Servier. Dr. Hyldstrup receives research grants from Eli Lilly, Novartis, Pfizer, Nycomed, Roche, and GlaxoSmithKline, consults for Novartis, Eli Lilly, and Nycomed, and speaks for Merck, Eli Lilly, Nycomed, Novartis, Novo Nordisk, and Servier. Dr. Recknor receives research grants from Procter & Gamble, consults for Procter& Gamble, Roche, and Eli Lilly, and speaks for Procter & Gamble, Eli Lilly, Roche, GlaxoSmithKline, Merck, and Aventis. Dr. Nordsletten receives research grants from Biomet, consults for Novartis and DePuy, and speaks for Wyeth. Ms. Lavecchia is an employee of and owns stock in Novartis. Dr. Hu is an employee of Novartis. Dr. Boonen receives research grants from Amgen, Eli Lilly, Novartis, Pfizer, Procter & Gamble, Sanofi‐Aventis, and Roche–GlaxoSmithKline and consults or speaks for Amgen, Eli Lilly, Merck, Novartis, Procter & Gamble, Sanofi‐Aventis, and Servier. Dr. Mesenbrink is an employee of and owns stock, restricted stock, exercisable options, and tradable options in Novartis
Published online on February 16, 2009
REFERENCES
- 1. Black DM, Delmas P, Eastell R, Reid IR, Boonen S, Cauley J, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Jensen TR, Krasnow J, Hue T, Sellmeyer DE, Eriksen EF, Cummings SR 2007. Once‐yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 2. Lyles KW, Colón‐Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S 2007. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357: 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amanat N, McDonald M, Godfrey C, Bilston L, Little D 2007. Optimal timing of a single dose of zoledronic acid to increase strength in rat fracture repair. J Bone Miner Res 22: 867–876. [DOI] [PubMed] [Google Scholar]
- 4. Genant HK, Nevitt MC, Black DM, Palemo L, Jergas M, Cummings SR 1993. Assessment of prevalent vertebral fractures combining visual, semiquantitative and morphometric analyses. J Bone Miner Res 8: S338. [DOI] [PubMed] [Google Scholar]
- 5. O'Brien P, Fleming T 1997. A multiple testing procedure for clinical trials. Biometrics 35: 556. [PubMed] [Google Scholar]
- 6. Voggenreiter G, Klaes W, Assenmacher S, Schmitneuerburg KP 1995. Massive intercalary bone allografts in the treatment of primary and secondary bone‐tumors: A report on 21 cases. Arch Orthop Trauma Surg 114: 308–318. [DOI] [PubMed] [Google Scholar]
- 7. Moran CG, Wenn RT, Sikand M, Taylor AM 2005. Early mortality after hip fracture: Is delay before surgery important ? J Bone Joint Surg Am 87: 483–489. [DOI] [PubMed] [Google Scholar]
- 8. Robbins JA, Biggs ML, Cauley J 2006. Adjusted mortality after hip fracture: From the Cardiovascular Health Study. J Am Geriatr Soc 54: 1885–1891. [DOI] [PubMed] [Google Scholar]
- 9. Davidson CW, Merrilees MJ, Wilkinson TJ, McKie JS, Gilchrist NL 2001. Hip fracture mortality and morbidity—can we do better ? N Z Med J 114: 329–332. [PubMed] [Google Scholar]
- 10. Boonen S, Autier P, Barette M, Vanderschueren D, Lips P, Haentjens P 2004. Functional outcome and quality of life following hip fracture in elderly women: A prospective controlled study. Osteoporos Int 15: 87–94. [DOI] [PubMed] [Google Scholar]
- 11. Ray NF, Chan JK, Thamer M, Melton LJ 1997. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: Report from the National Osteoporosis Foundation. J Bone Miner Res 12: 24–35. [DOI] [PubMed] [Google Scholar]
- 12. Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D 2000. Risk of mortality following clinical fractures. Osteoporos Int 11: 556–561. [DOI] [PubMed] [Google Scholar]
- 13. Black DM, Boonen S, Cauley J, Delmas P, Eastell R, Reid I, Rosario‐Jansen T, Caminis J, Zhang J, Hu H, Cummings S 2006. Effect of once‐yearly infusion of zoledronic acid 5 mg on spine and hip fracture reduction in postmenopausal women with osteoporosis: The HORIZON Pivotal Fracture Trial. J Bone Miner Res 21: S16. [Google Scholar]