Abstract
High mobility group AT-hook 2 (HMGA2) protein is composed of three AT-hook domains. HMGA2 expresses at high levels in both embryonic stem cells and cancer cells, where it interacts with and stabilizes replication forks (RFs), resulting in elevated cell proliferation rates. In this study, we demonstrated that HMGA2 knockdown reduces cell proliferation. To understand the features required for interaction between HMGA2 and RFs, we studied the solution structure of HMGA2, free and in complex with RFs, using an integrated host of biophysical techniques. Circular dichroism and NMR experiments confirmed the disordered state of unbound HMGA2. Dynamic light scattering and sedimentation velocity experiments demonstrated that HMGA2 and RF are monodisperse in solution, and form an equimolar complex. Small-angle x-ray scattering studies revealed that HMGA2 binds in a side-by-side orientation to RF where 3 AT-hooks act as a clamp to wrap around a distorted RF. Thus, our data provide insights into how HMGA2 interacts with stalled RFs and the function of the process.
Introduction
The high-mobility group (HMG) family of proteins consists of HMGA (HMG-I/Y), HMGB (HMG-1), and HMGN (HMG-14/17) superfamilies. They play significant roles in a number of biological activities, including chromatin remodeling and DNA repair pathways (1, 2). The HMGA group of proteins have preferential binding to the minor groove of AT-rich regions in B-form DNA as well as many bent or distorted DNA structures (3). HMGA proteins are highly expressed in self-renewing human embryonic stem cells (ESCs) but upon differentiation, the levels of HMGA drop to undetectable levels due to a reduction in Lin28b transcription (4), which leads to suppression of HMGA production by let-7 microRNA (5).
In mammals, HMGA is composed of two functional members, HMGA1 (also known as HMGI-Y) and HMGA2 (also known as HMGI-C), which are encoded by separate genes (1, 6). HMGA1 has three experimentally observed splice variants (HMGA1a, HMGA1b, and HMGA1c) (7), whereas only one major transcript has been observed for HMGA2 (HMGA2a) (8). In vivo, HMGA1 and HMGA2 regulate transcription of many of the same genes; however, they also assume distinct roles. For example, HMGA1 is involved in cardiomyocyte cell growth (9) and regulation of glucose and insulin pathways (10), whereas HMGA2 aids in controlling skeletal size (11) and is a critical component for normal spermatogenesis (2). The high expression of HMGA2 observed in ESCs is necessary for their proper growth and development (11). Unfortunately, if HMGA2 expresses at high levels in adult somatic cells, the effects can be detrimental. This occurs when let-7 microRNA gets degraded in somatic cells, relieving the inhibition of HMGA2 expression. The presence of HMGA2 in the nucleus leads to high cell proliferation and the formation of tumors (5). Thus, HMGA2 has become a useful biomarker for various cancers (12, 13, 14), for pathological subtyping of testicular germ cell tumors (15), and for predicting poor prognosis in breast cancer (16).
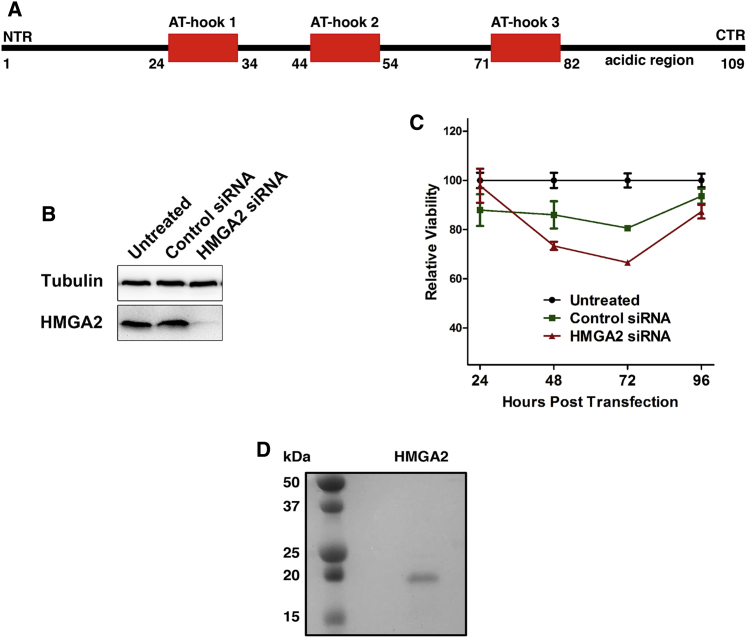
HMGA2a (referred to as HMGA2, 11.8 kDa; Fig. 1 A) contains three independent AT-hook DNA binding motifs (Pro-Arg-Gly-Arg-Pro), which are flanked by positively charged Lys/Arg residues (17). HMGA2 also contains a high number of prolines (∼15% of total sequence) and is highly basic in nature. The AT-hook binding motif is conserved in HMGAs throughout evolution, from bacteria to humans, and is also found in many transcription factor proteins involved in chromatin remodeling complexes (17, 18). AT-hooks are DNA binding motifs that insert into the minor groove of DNA. It is speculated that the proline residues are necessary to maintain the rigidity of this domain, whereas the arginine residues in the Arg-Gly-Arg motif are interacting with the bases of the DNA (18). These three AT-hooks are responsible for DNA binding in transcriptional regulation (19), base excision repair (20), and the stabilization of stalled replication forks (RFs) (21).
Figure 1.
(A) Given here is a schematic of the amino acid sequence of human HMGA2 highlighting the AT-hook domains. (B) Given here is a Western blot of HMGA2 knockdown at 48 h post transfection using an anti-Tubulin (control) and anti-HMGA2 antibody. (C) The effect of HMGA2 knockdown on cell viability was monitored for HEK293T cells at 24-h intervals over 96 h. A significant loss in viability was observed upon transfection with the HMGA2 siRNA. Data points are shown as an average of eight measurements with error bars corresponding to the SD. (D) Shown here is the Coomassie-stained SDS-PAGE of purified HMGA2. To see this figure in color, go online.
During replication, a fraction of RFs is arrested as they translocate along the parental DNA. For the RF to resume replication, adult cells are equipped with a RF protection complex that recognizes ssDNA and attempts to stabilize the RF (22). The RFs that are unable to be stabilized lead to the collapse of the DNA and eventual cell death. In ESCs and cancer cells, the overexpression of HMGA2 provides additional support to the fork protection complex and is one of the reasons for the observed increase in cell proliferation (21).
In this study, we have confirmed a positive role for HMGA2 in regulating cell proliferation. Furthermore, we characterized HMGA2 and a DNA RF individually and in complex using biophysical methods. Our results confirm that human HMGA2 exists as a disordered monomer in solution (in contrast to the dimeric nature of mouse HMGA2 (23)); however, upon interaction with a RF, it forms an equimolar complex by binding in a side-by-side orientation. Our study suggests that it is necessary for HMGA2 to be in a disordered state such that it has the flexibility to stabilize a stalled RF by binding to the necessary AT-rich sites. HMGA2 acts as a clamp by taking advantage of the rigidity of the double-stranded region to stabilize the strands being newly synthesized.
Materials and Methods
Protein expression and purification
A pET-28a (+) vector containing Escherichia coli-optimized codons (24) for the expression of full-length human HMGA2 (residues 1–109, Uniprot: P52926) with a thrombin cleavable N-terminal hexa-histidine tag was transformed into E. coli BL21 cells. Cells were inoculated into 1.6 L Luria Broth containing 50 μg/mL kanamycin and grown at 37°C until the cell density reached an OD600 of 0.6. Cells were then induced with 1 mM isopropyl β-D-1-thiogalactopyranoside and protein was expressed for 3 h before harvesting the cells by centrifugation at 6500 rpm for 20 min. The cell pellet was frozen at −80°C until further processing. Purification and removal of the N-terminal hexa-histidine tag was performed as previously described (25).
Formation of RF DNA with and without HMGA2
Polyacrylamide gel electrophoresis (PAGE)-purified oligonucleotides oSG309 (5′-TTTTTTATAATGCCAACTTAGTATAAAAAAGCTGAACGAGAAACGTAAAA-3′), oSG606 (5′-TTTTACGTTTCTCGTTCAGCTTTTTTATACTAACTTGAGCGAAACGGGAA-3′), and oSG607 (5′-TTCCCGTTTCGCTCAAGGTTGGCATTATAAAAAA-3′) (Alpha DNA, Montreal, QC) were annealed as described previously (21) to form the RF. Assembled RF was separated from free oligonucleotides by means of size exclusion chromatography (SEC) using a HiLoad Superdex 75 column (GE Healthcare, Fairfield, CT) in 10 mM HEPES, pH 7.5, 154 mM NaCl buffer. To purify the DNA:protein complex using SEC, equal volumes of RF (2 μM) and HMGA2 (3 μM) were mixed and incubated for 30 min at room temperature. The complex was then concentrated and loaded onto a Superdex 200 column (GE Healthcare) in 10 mM HEPES, pH 7.5, 154 mM NaCl buffer to remove any unbound species.
Knockdown of HMGA2
The HEK293T cell line was a gift from Dr. Thomas Klonisch. Cells were grown in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum as described previously (26). For siRNA transfections, cells were reverse-transfected with Lipofectamine RNAiMAX and 1.5 pmoles siRNA (Life Technologies, Waltham, MA) according to the manufacturer’s protocol. Transfection of a nontargeting control siRNA and siRNA-targeting HMGA2 (5′-GGUGAGCCCUCUCCCUAAGATT-3′) were performed simultaneously. Cell viability was monitored by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay according to a previously described protocol (27). Cell lysis and Western blot analysis were performed as described previously (25) using an anti-HMGA2 antibody (Cat. No. 5269; Cell Signaling Technology, Danvers, MA) and an anti-Tubulin antibody (Cat. No. T6074; Sigma-Aldrich, St. Louis, MO) for detection.
Coomassie SDS-PAGE
HMGA2 protein was mixed at a 1:1 ratio with reducing Lämmli buffer and incubated at 95°C for 5 min. Samples were then loaded onto a 16% SDS-PAGE gel containing 6 M urea. The gel was run at 5 W for 2.5 h and then stained with Coomassie Brilliant Blue.
DNA gel shift assays
DNA-protein interactions were studied by incubating a constant amount of RF (200 nM) with increasing amounts of HMGA2 (0–3 μM) in 10 mM HEPES, pH 7.5, 154 mM NaCl for 20 min. The binding reactions were resolved by electrophoresis on a 10% Tris-borate ethylenediaminetetraacetic acid native gel. DNA was stained using SYBR Gold fluorescent nucleic acid dye (Invitrogen, Carlsbad, CA) and visualized on a FluorChem Q imaging system (Cell Biosciences, Santa Clara, CA) (25). The gel-shift was performed in triplicate after densitometry analysis to determine the fraction of bound RF. Binding affinity was calculated using a modified Hill equation:
| (1) |
where Bmax represents the fraction of bound DNA at the upper limits of the titration, X is the protein concentration, and h is the Hill coefficient (28).
Hydrodynamic characterization
The homogeneity of HMGA2, RF, and their complex was studied by dynamic light scattering (DLS) and sedimentation velocity (SV) methods in 10 mM HEPES, pH 7.5, 154 mM NaCl buffer. SEC-purified samples were subjected to 0.1 μm filtration before equilibrating at 20°C for 5 min for DLS analysis. The hydrodynamic radius (Rh) distributions were obtained using a Nano-S Zetasizer (Malvern, Toronto, ON, Canada) at concentrations between 1 and 10 mg/mL as described previously (29, 30, 31). SV experiments were performed as previously described (30, 32) using a ProteomeLab XL-I analytical ultracentrifuge and an An50Ti 8-cell rotor (Beckman Coulter, Mississauga, ON, Canada). Standard 12 mm double-sector cells were filled with 400 μL of sample and 420 μL of buffer. A total of 120 absorbance and interference radial scans were performed at a speed of 45,000 rpm (163,296 × g) at every 11.5 min interval with a concentration range of 0.2–2 mg/mL. Data were analyzed using the SEDFIT program to determine the sedimentation coefficients of each macromolecule (s20,b) (33, 34). The sedimentation boundaries are determined from numerical solutions of the Lamm equation:
| (2) |
and the data is then fit to a sum of the Lamm equations using least-squares to determine the concentration of each species in the sample. The sedimentation coefficients were then corrected to standard solvent conditions using the buffer viscosity and density calculated from SEDNTERP (s20,w) (35). Plotting the s20,w values for multiple concentrations provided the Svedberg constant at infinite dilution (s020,w), which, combined with the Rh, allows calculation of the molecular mass (31, 36):
| (3) |
Spectropolarimetry
Far-UV circular dichroism (CD) spectra were recorded on a J-810 Spectropolarimeter (Jasco, Easton, PA) that was calibrated using 2.583 mM (1S)-(+)-camphor-10-sulfonic acid monohydrate (Alfa Aesar, Ward Hill, MA). The protein and RF spectra were measured at concentrations of 0.25 and 0.21 mg/mL, respectively, in 10 mM sodium phosphate, pH 7.5, 154 mM NaF buffer at 20.0°C. The RF/HMGA2 complex was measured in the same phosphate buffer at 0.17 mg/mL DNA concentration. Data were collected using an integration time of 8 s/nm in a 1 mm cell. The spectra were normalized by the light path length (L) and the number of peptide bonds (in the case of protein) or nucleotides (in the case of RF or RF/HMGA2 complex) per unit volume as described previously (30, 32):
| (4) |
Chemical shift mapping of amide backbone
HMGA2 was expressed from E. coli cells grown in M9 minimal media with 15NH4Cl as the sole nitrogen source to label the nitrogens and purified as described above. Purified HMGA2 was concentrated to 500–700 μM with and without the presence of unlabeled RF in NMR buffer (10 mM HEPES, pH 7.5, 154 mM NaF, 10% D2O, 75 μM 2,2-dimethyl-2-silapentane-5-sulfonate). The 15N-heteronuclear single quantum coherence (HSQC) spectra (37) were collected with 1024 × 128 complex points at 25°C on an INOVA 600 MHz spectrometer (Varian, Cary, NC). Sixteen scans were performed for each sample with a 1H sweep of 16.0 ppm and a 15N sweep of 32.0 ppm. Data were zero-filled, multiplied with an apodization function, Fourier-transformed, and base-line corrected using the software NMRPipe (38) before being visualized using the software SPARKY (39).
Small-angle x-ray scattering
Small-angle x-ray scattering (SAXS) data for 1) SEC-purified HMGA2 were collected at 6, 7, 8 ,and 10 mg/mL; 2) RF at 2, 3, 4, and 5 mg/mL; and 3) complex at 2, 3, and 4 mg/mL in 10 mM HEPES, pH 7.5, 154 mM NaCl buffer using a 3-pinhole camera (S-MAX3000; Rigaku Americas, The Woodlands, TX) equipped with a MicroMax+002 Microfocus sealed tube (Cu Kα radiation at 1.54 Å; Rigaku) and a Confocal Max-Flux optics system operating at 40 W (Rigaku). Scattering data for all three species were recorded using a 200 mm multiwire 2D detector and processed as previously described (30, 40, 41). Briefly, the buffer scattering data was subtracted from the sample scattering data using the program PRIMUS (42) followed by merging of buffer-subtracted data for all concentrations of each sample and the complex. The data were further processed using the program GNOM (43) to obtain Rg and Dmax. The ab initio structures for RF were calculated using the program DAMMIF (44) whereas those for the complex were calculated using the softwares DAMMIN and MONSA (45). The quality of the low-resolution structures was verified by the goodness-of-fit parameter (χ-value) for each model of RF and complex. The ab initio models for RF and the complex were then rotated and averaged using the program DAMAVER (46).
Calculation of hydrodynamic parameters from ab initio models
The hydrodynamic properties of RF and complex were calculated for each model using the program HYDROPRO (47) as described previously (30, 40, 41). The density (1.005 g/mL) and viscosity (1.025 Poise) of buffer was calculated using SEDNTERP (35). The molecular weight (Mw) and partial specific volume for HMGA2 were calculated based on amino acid sequence using SEDNTERP (35) whereas NucProt Calculator (48) was used for RF. The partial specific volume of complex was calculated using Eq. 1 from Dzananovic et al. (41).
Results
HMGA2 plays a key role in cell proliferation
To investigate the effect that HMGA2 has on cell proliferation, the expression of HMGA2 was knocked down using siRNA transfection against the mRNA of HMGA2 and confirmed using a Western blot analysis (Fig. 1 B). At 48 and 72 h post transfection, a significant reduction in viability (p < 0.05) was observed when compared to the HEK293T cells transfected with a control siRNA. However, after 96 h, the viability of both transfected cell lines was found to be within the error of the untreated cells (Fig. 1 C).
HMGA2, RF, and the complex can be purified to homogeneity
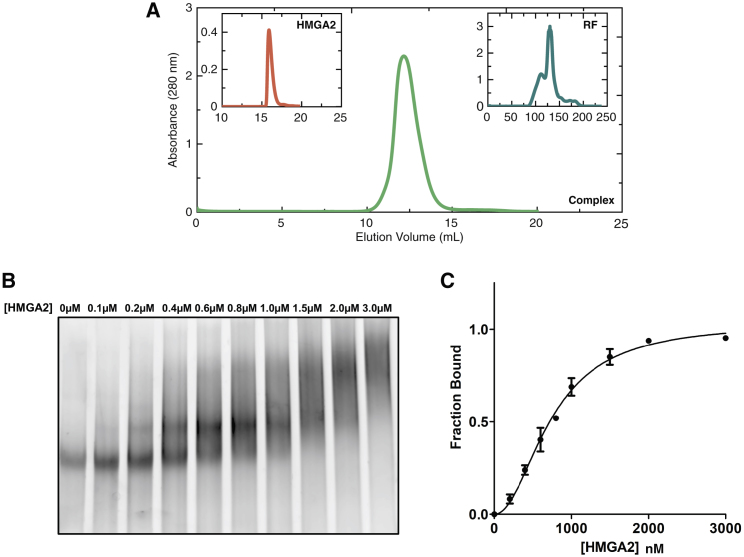
Codon-optimized (24) HMGA2 was recombinantly expressed in E. coli and affinity-purified by immobilized metal ion affinity chromatography using a hexa-histidine tag. The tag was subsequently removed by thrombin cleavage. As a secondary purification step, HMGA2 was subjected to SEC. The presence of a single band on Coomassie SDS-PAGE (Fig. 1 D) and the single peak at ∼15.9 mL (Fig. 2 A) confirmed the homogeneity of HMGA2.
Figure 2.
(A) Shown here are elution profiles of HMGA2 (coral), RF (teal), and complex (sea green) from the Superdex 75 10/300 GL, HiLoad 16/600 Superdex 75, and Superdex 75 10/300 GL SEC columns, respectively, indicating purity of both individual components as well as the complex. (B) Given here is EMSA indicating slower migration of RF (200 nM) upon interaction with HMGA2 (0–3 μM). (C) Shown here is the binding profile of HMGA2 with RF fit to a modified Hill equation. Data points are determined using densitometry and displayed as the average of three independent measurements (740 ± 50 nM). To see this figure in color, go online.
The RF was assembled by annealing three oligonucleotides (oSG309, oSG606, and oSG607; see Materials and Methods for details) followed by purification on a HiLoad SEC column that yielded two distinctive peaks at ∼120 and ∼130 mL (Fig. 2 A). The second, more prominent peak, was collected to use for subsequent experiments.
Initial studies using electrophoretic mobility shift assays (EMSAs) suggested that recombinantly produced HMGA2 binds to the SEC-purified RF (Fig. 2 A), as we observed a shift in the RF gel position to higher molecular weight with increasing concentration of HMGA2 (Fig. 2 B). Through densitometry analysis of the gel-shifts, we were able to determine the fraction of bound HMGA2. Furthermore, with this data and using a modified Hill equation (see Materials and Methods), we determined that HMGA2 binds to RF DNA with Kd = 740 ± 50 nM and a Hill coefficient of 2.0 ± 0.2, which suggests cooperative binding of the AT-hooks (Fig. 2 C). Based on this, we prepared the complex by incubating RF with a small excess of HMGA2 (to ensure that all DNA was in a bound state) before purifying it by SEC. The complex eluted as a single peak at ∼12.5 mL with no unbound HMGA2 (Fig. 2 A). The purified complex was used for subsequent biophysical studies.
HMGA2 and RF are monomeric in solution and form an equimolar complex
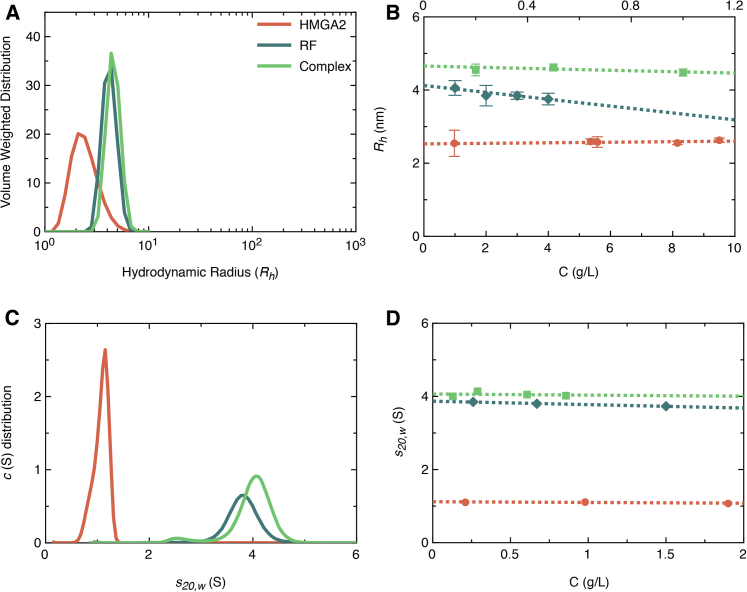
DLS analysis of HMGA2, RF, and complex at multiple concentrations suggest that they are highly monodispersed (Fig. 3 A). Determination of hydrodynamic radii (Rh) for each species at multiple concentrations demonstrated the concentration independence of HMGA2 (2.58 ± 0.25 nm), RF (4.12 ± 0.24 nm), and complex (4.65 ± 0.15 nm) (Fig. 3 B; Table 1). SV analysis using analytical ultracentrifugation (AUC) confirmed that HMGA2, RF, and their complex is monodispersed in solution (Fig. 3 C). Measurements taken at concentrations between 0.1 and 2 mg/mL displayed concentration independence, affirming homogeneity of the components and a strong interaction of HMGA2 to RF in the complex. Sedimentation coefficients corrected to standard solvent conditions (pure water at 20°C, s20,w) were extrapolated from individual concentrations to infinite dilution yielding a Svedberg constant (s020,w) of 1.12 ± 0.12 S (HMGA2), 3.86 ± 0.12 S (RF), and 4.06 ± 0.11 S (complex). The modest increase in Rh and s020,w values for RF upon incubation with HMGA2 indicated the formation of a protein:DNA complex.
Figure 3.
(A) Given here is the hydrodynamic radius (Rh) distribution and (C) sedimentation coefficient (s20,w) distribution of HMGA2 (coral), RF (cyan), and complex (sea green) at 1 mg/mL, suggesting monodisperse samples. (B) The Rh for HMGA2 and RF is plotted at multiple concentrations (1–10 mg/mL, bottom x axis) and for the complex between 0.2 and 1 mg/mL (top x axis). The vertical error bars indicate the SD from five measurements. (D) The Svedberg coefficient in water (s20,w) was plotted at multiple concentrations (0.2–2 mg/mL) for each sample with vertical error bars indicative of the SE from multiple scans. This plot presents the concentration independence of all samples under the experimental conditions. To see this figure in color, go online.
Table 1.
Summary of Hydrodynamic Parameters
| Parameters | HMGA2 |
RF |
HMGA2-RF Complex |
|||
|---|---|---|---|---|---|---|
| Experimental Analysis | Experimental Analysis | HYDROPRO DAMMIN | Experimental Analysis | HYDROPRO DAMMIN | HYDROPRO MONSA | |
| Rh (nm)a | 2.58 ± 0.25 | 4.12 ± 0.24 | 4.06 ± 0.05 | 4.65 ± 0.15 | 4.57 ± 0.04 | 4.37 ± 0.10 |
| Rg (nm)b | 2.45 ± 0.06 | 4.54 ± 0.14 | 4.51 ± 0.12 | 4.85 ± 0.06 | 4.88 ± 0.07 | 4.77 ± 0.01 |
| Dmax (nm)b | 8.0 | 15.4 | 15.7 ± 0.27 | 15.0 | 15.3 ± 0.12 | 15.0 ± 0.20 |
| s020,w (S)c | 1.12 ± 0.12 | 3.86 ± 0.12 | 4.03 ± 0.05 | 4.06 ± 0.11 | 4.11 ± 0.05 | 4.31 ± 0.10 |
| Mwd | 11.70 | 41.40 | — | 53.10 | — | — |
| Mwe | 11.80 | 43.03 | — | 55.47 | — | — |
| Chif | — | 1.2h | — | 1.0h | — | — |
| 1.2, 1.2, 1i | ||||||
| NSDg | — | 0.73 ± 0.08h | — | 0.83 ± 0.03h | — | — |
| 0.87 ± 0.06i | ||||||
Experimentally measured using DLS with errors obtained from linear regression analysis.
Experimentally measured using GNOM analysis from SAXS data.
Experimentally measured using AUC.
Calculated from amino acid/nucleotide sequence.
Calculated from Rh and s020,w.
Goodness-of-fit parameter suggesting agreement between raw data and data back-calculated from the ab initio model.
Normalized spatial discrepancy indicating agreement between individual models.
Data from DAMMIN.
Data from MONSA.
We used the Svedberg equation (Eq. 3), that combines the Rh (DLS) and s020,w (SV) to calculate the Mw for each species in solution (31, 36). We found that the Mw of HMGA2 (11.80) and RF (43.03) agreed with their sequence-based Mw (11.70 and 41.40, respectively), implying that both species are monomeric in solution. Furthermore, the calculated Mw of 55.47 for HMGA2-RF complex established that the molecules interact in a 1:1 stoichiometry (Table 1).
HMGA2 is disordered in solution
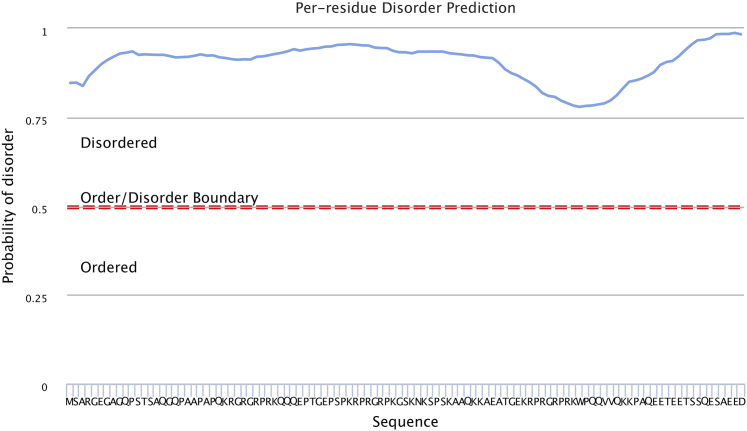
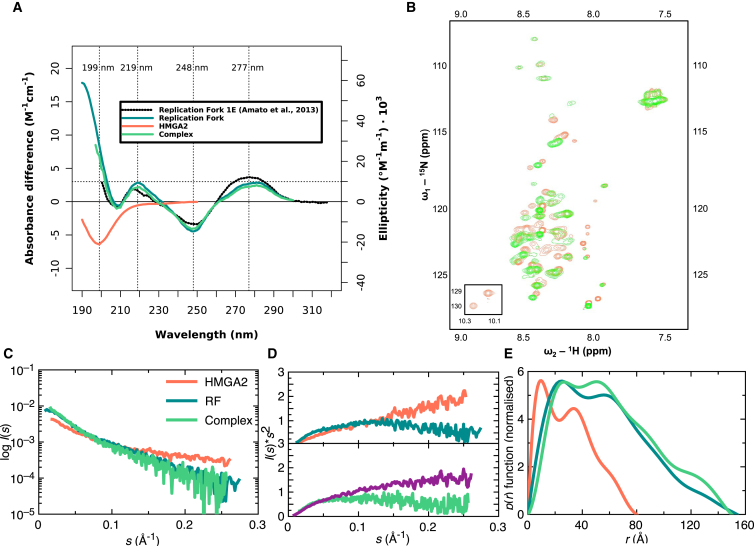
Based on the amino acid sequence of HMGA2, bioinformatics tools (PrDOS (49), PSIPRED (50, 51), and RONN (52)) predicted a disordered secondary structure (Fig. 4). To experimentally validate the disordered nature of HMGA2, we employed three separate techniques: CD spectropolarimetry, NMR HSQC spectroscopy, and SAXS. Far-UV CD spectropolarimetry of HMGA2 produced a spectrum with a minimum at 199 nm followed by a flat line from 210 to 250 nm (Fig. 5 A). This spectrum appeared identical to that of a disordered protein (53). To investigate the secondary structure elements by NMR spectroscopy, HMGA2 was labeled with 15N and an HSQC spectrum was acquired. The HSQC spectrum of HMGA2 (Fig. 5 B) displayed a very narrow chemical shift range in the proton dimension that is highly characteristic of a disordered protein. In particular, the 1H resonances fall between 7.9 and 8.6 ppm, whereas the range normally observed for folded proteins is from 6.5 to 10.0 ppm, or even wider. Finally, SAXS studies of HMGA2 provided information on its solution behavior at multiple concentrations. Scattering curves for each concentration were buffer-subtracted and merged to generate the SAXS plots (Fig. 5, C–E). In general, the Kratky analysis of SAXS data for proteins provided qualitative information on the overall shape of the biomolecule of interest (54). The ascending linear curve (coral) observed for HMGA2 in the Porod-Debye Plateau region was indicative of a disordered protein (54) (Fig. 5 D), which agrees with results from the CD and NMR analyses.
Figure 4.
The entire sequence of HMGA2 is predicted to be disordered according to the program RONN (52). To see this figure in color, go online.
Figure 5.
(A) Given here is the far-UV CD spectra of HMGA2, RF, and complex displayed as an average of three measurements indicating disordered HMGA2 and confirmation of RF formation. A reference RF (56) (dotted black) spectrum was overlaid onto the experimental RF (teal) for comparison. (B) Shown here is the 15N HSQC spectral overlay of free HMGA2 (coral) with HMGA2 in complex with RF (green), suggesting a conformational change of HMGA2 upon interaction with RF. (C) Given here is merged SAXS data of HMGA2 (coral), RF (teal), and complex (sea green) obtained from multiple concentrations. The corresponding (D) Kratky plot suggests that HMGA2 (coral) is a disordered protein and that RF (teal) is structured (upper panel). Theoretical Kratky analysis of the complex (purple) is distinctly different from the experimental analysis (sea green), suggesting that upon interaction with RF, HMGA2 undergoes a conformational change (lower panel). (E) Given here is the P(r) function of HMGA2, RF, and complex (same color scheme as C and D) outlining the multidomain nature of the molecules and their Dmax. To see this figure in color, go online.
CD spectropolarimetry confirms assembly of RF
To validate the assembly of RF by annealing of three DNA oligonucleotides, far-UV CD analysis was performed (Fig. 5 A). The CD spectrum of the RF had extrema at positions that correlated with that of B-DNA; two distinct maxima at 219 nm and near 280 nm and a minimum at 248 nm (55) (Fig. 5 A). A properly assembled RF can be distinguished from B-DNA by a depressed peak magnitude at 219 and 248 nm. We have overlaid a spectrum of RF from this study with a spectrum of RF previously published by Amato et al. (56) for comparison. Our peak heights correspond to those of their RF, indicating that the RF from this study assembled properly.
HMGA2 retains flexibility when in complex with RF
CD spectropolarimetry, NMR, and SAXS studies confirmed that HMGA2 is disordered in solution. Furthermore, SEC, DLS, and SV experiments have demonstrated that the individual species, as well as the complex between them, are highly pure. Our next goal was to study changes in secondary structure of HMGA2 upon interaction with RF. We first analyzed the complex using CD spectropolarimetry, which is very sensitive to changes in the secondary structure. The CD signal of nucleic acids originates from the glycosidic bond angles. The spectrum below 230 nm contains contributions from DNA and protein, whereas above 230 nm the protein signal is zero. We have therefore ignored the protein contribution when we normalize the spectra, so the spectrum of the complex can be directly compared to the spectrum of the RF in the range below 230 nm. The CD spectra of our RF in the presence and absence of HMGA2 are identical within experimental uncertainty, indicating that the RF maintains its conformation when the protein is bound.
Next, we studied the complex through HSQC NMR using 15N-labeled HMGA2 bound to unlabeled RF. The HSQC spectrum of bound HMGA2 (green) is superimposed on the spectrum of unbound HMGA2 (coral) in Fig. 5 B. A 70–75% decrease in peak intensity was observed upon complex formation. It was evident that HMGA2 is in a bound state with the addition of the RF because it resulted in slower tumbling. Interestingly, the chemical shift range in the complex is nearly identical to that observed for the free protein, suggesting that HMGA2 is disordered even when bound to the RF. One noticeable difference between the two spectra was the disappearance of the tryptophan (W83) side-chain peak in the bound form (bottom-left inset to Fig. 5 B). This infers that W83 is near or part of a binding site in the interaction with RF. Finally, Kratky analysis of SAXS data for the complex displayed a curved shape (sea green) that approaches the baseline at high s, which is indicative of a macromolecule with only partial flexibility. This is opposed to an ascending linear curve in the Porod-Debye plateau region for unbound HMGA2 (coral) that suggests a fully disordered protein (54) (Fig. 5 D).
HMGA2 interacts with RF in a side-by-side manner
SAXS data for HMGA2, RF, and the complex were collected at multiple concentrations, buffer-subtracted, and merged to study their size and shape in solution (Fig. 5, C and E). As mentioned, the Kratky analysis for HMGA2 suggests that it is a disordered protein (Fig. 5 D), therefore we only studied the radius of gyration (Rg = 2.45 ± 0.06 nm) and the maximum particle dimension (Dmax = 8 nm) using the pair-distance distribution function (P(r); Fig. 5 E, coral), without performing low-resolution shape analysis. The P(r) plot for RF presents multiple peaks (teal) with Rg of 4.54 ± 0.14 nm and Dmax of 15.4 nm (Fig. 5 E). The low-resolution structural studies performed based on the SAXS data (Fig. 5, C and E) demonstrate that the RF adopts an extended and curved structure in solution (Fig. 5 A). The goodness-of-fit parameter (χ-value) of 1.2 indicates an agreement between the experimentally collected SAXS data and the back-calculated scattering curve derived from individual models. Furthermore, the normalized spatial discrepancy (NSD) of 0.73 ± 0.08 indicates that individual models calculated for RF based on the SAXS data agree well with each other (Table 1).
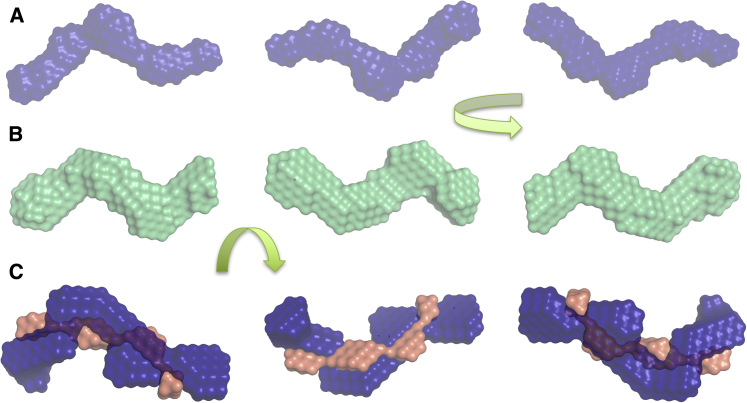
The P(r) function calculated from the SAXS data of the complex differs from that of the RF (Fig. 5 D). The merged SAXS data for the complex were processed using the P(r) analysis to obtain the Rg of 4.85 ± 0.06 nm and Dmax of 15 nm (Fig. 5 E). The increase, albeit relatively small, in the Rg for the complex compared to that of RF (4.85 ± 0.06 nm vs. 4.54 ± 0.14 nm) is indicative of complex formation between HMGA2 and RF. Low-resolution shape reconstruction using the DAMMIN program also suggested an extended and curved structure for the complex (Fig. 6 B) as with the unbound RF. However, as reflected by the shape of the P(r) plot, a distinguishably different pattern of curved domains was observed compared to the low-resolution shape of the RF (Fig. 6 B). The χ-value of 1.0 and the NSD value of 0.83 ± 0.03 indicated that individual models for the complex agree well with the SAXS data and that the models are in good agreement with each other (Table 1).
Figure 6.
Shown here are low-resolution structures of (A) RF (extended shape) and (B) complex determined by DAMMIN. (C) Modeling by MONSA distinguishes HMGA2 (coral) from RF (blue) in the complex, suggesting that HMGA2 interacts with RF in a side-by-side orientation. To see this figure in color, go online.
Having established that HMGA2 and RF form a complex, we wanted to gain further information about the nature of this complex. Theoretical Kratky analysis of the complex based off raw data from the individual components resulted in a distinctly different plot compared to the experimental Kratky analysis (Fig. 5 D, lower panel). This suggests that HMGA2 has adopted a more ordered conformation upon binding to the RF than in the unbound state. Additionally, we employed the program MONSA to deconvolute the location and orientation of HMGA2 and RF in the complex. MONSA is a multiphase bead modeling program that is used to fit multiple curves simultaneously (45). Using the MONSA program, we could observe that HMGA2 wraps around the RF and that HMGA2 has three distinguished AT-hook structures (Fig. 6 C). The χ-value of 1.2, 1.2, and 1 for HMGA2, RF, and complex, respectively, and the NSD value of 0.87 ± 0.06, indicate that the individual models agree well with the SAXS data and the models also agree with each other (Table 1). In summary, the low-resolution structural studies suggest that HMGA2 stretches across the RF to interact in a side-by-side orientation.
Discussion
During the process of DNA replication, various stress inducers can cause the leading strand of a RF to stall the replisome which, unless rescued, can result in their collapse and eventual cell death (57). In ESCs as well as cancer cells, HMGA2 stabilizes RFs (21). This interaction is essential to maintain a high cell proliferation rate for ESCs, but becomes a major concern when it occurs in somatic cells. In addition to stabilizing RFs, the DNA binding capability of HMGA2 has a significant role in other biological processes such as chromatin remodeling, DNA repair pathways, and regulating target genes (1, 2, 58). Although the importance of HMGA proteins in several biological processes is well understood, their solution behavior and interaction with RFs and DNA, in general, is largely unexplored. Previous structural studies using x-ray crystallography and NMR spectroscopy have investigated truncated versions of HMGA1 and their interaction with dsDNA. The results from those studies concluded that the AT-hook domains on HMGA1 are disordered in the apo-state but adapt a defined structure upon binding to the minor groove (59, 60). Furthermore, mouse HMGA2 was also reported to be disordered, similar to HMGA1. However, the mouse HMGA2 forms a dimer in solution (23).
The important role of HMGA2 in stabilizing RFs implies that a knockdown of HMGA2 in cells would have a significant effect on their cell viability. This was evidenced for HEK293T cells used in this study, which is consistent with previous studies on prostate cancer PC3 cells (61), nasopharyngeal cancer cells (62), and breast adenocarcinoma (MDA-MB-468) cells (63) in which a decrease in cell viability upon HMGA2 knockdown was observed. We observed that after 96 h, the effect on cell viability was negligible and cells recovered as they reached confluency. The altered metabolism of cells and growth rates as cells reach confluency affect the results of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (27). However, it is also plausible that over time the cells adapt and activate other cellular pathways to compensate for the lack of HMGA2.
Our biophysical studies revealed that human HMGA2 is monomeric in solution (Table 1), which is contrary to that of a previous study with mouse HMGA2 (23). This can be explained by the presence of a cysteine residue in the murine amino acid sequence that forms a homodimer through a disulphide linkage. As for HMGA2 in humans as well as for many other species, there is no cysteine to form these bridges (64) and therefore the protein exists as a monomer up to concentrations of 10 mg/mL (Fig. 3; Table 1). The absence of this disulphide bridge between AT-hook domains 1 and 2 would allow HMGA2 to have more flexibility in binding to DNA that would otherwise be restricted if in a homodimeric state. It has been previously documented that telomerase activity is higher in mice compared to humans (65) and recently discovered that HMGA2 plays a role in telomere stability (66). As a result, it is possible that for HMGA2 to be successful in stabilizing telomeres in an environment with higher telomerase activity, HMGA2 must be present as a dimer. Alternatively, the disulphide bridge could be required in the binding of mouse HMGA2 to one of its protein binding partners, which is not present in humans. The underlying reason for this difference is unclear, but it does suggest that there is a functional significance that is yet to be revealed.
EMSAs determined that human HMGA2 has a significantly lower affinity to RF (Kd = 740 ± 50 nM) (Fig. 2 C) when compared to previous studies performed using surface plasmon resonance, which determined the affinity of murine HMGA2 to poly(dA)poly(dT) to be between 20 and 30 nM (67). This difference could be attributed to the difference in the HMGA2 source (human in this study versus mouse in the previous study), the differences in the DNA sequence/structure in both studies, and/or buffer composition (e.g., ionic strength) (68, 69). As previously mentioned, mouse HMGA2 is observed as a dimer in solution, which could affect its binding affinity to DNA. Results obtained from Dragan et al. (69) suggested that binding affinities determined from smaller segments of protein and DNA were markedly reduced. The one similarity across all published studies and between mouse and human HMGA2 is that cooperative binding is observed. With a Hill coefficient of 2.0 ± 0.2, our data correlates with previously published data that investigate the cooperative behavior of HMGA2 binding to DNA more thoroughly. Interestingly, it was reported that one AT-hook domain has a high affinity to DNA, bringing the two in contact, which further allows the remaining two AT-hooks to bind (68, 69).
The homogeneity and monomeric state of HMGA2 and RF was confirmed using DLS and SV studies (Fig. 3) where the experimentally calculated Mw of both species agreed with those based on primary sequences. Furthermore, the formation of complex and its purity was evident from the SEC, DLS, and SV experiments (Fig. 3). The Mw of 55.47 for the complex agreed with the sequence determined Mw (53.10) and unequivocally concluded formation of a 1:1 complex. This is contrary to what was observed for HMGA1 binding to dsDNA, where a truncated version of HMGA1 (containing two AT-hook domains) was shown to bind to two dsDNA molecules (59). A decade later, Cui and Leng (70) determined that each AT-hook domain of HMGA2 recognizes a specific type of 15-bp sequence; the first 5 bp are AT-rich, the middle four or five are GC-rich, and the last five or six are AT-rich. Because Huth et al. (59) used an 18-mer DNA segment in their binding studies, it follows that only one of the two AT-hook domains was capable of binding to it, leaving the second one free to bind an additional 18-mer segment of DNA. We designed our RF such that it was large enough to accommodate binding of all three AT-hook domains of HMGA2, and a closer representative to what occurs in vivo during replication. As a result, HMGA2 indeed binds to RF in a 1:1 stoichiometric ratio, suggesting that all three AT-hook domains are involved in stabilizing the RF.
The disordered nature of HMGA2 predicted by bioinformatics analysis of the sequence was confirmed by CD spectropolarimetry, NMR, and SAXS experiments (Fig. 5, A and B). HMGA2 is composed of three AT-hook DNA-binding domains situated in the middle of the protein sequence and attached by linkers (10–20 amino acids in length; Fig. 1 A). These linkers are flexible in nature and do not adopt any distinct structure. Previous NMR studies have also suggested that in the apo state, the AT-hook domains themselves are also unstructured (59, 70). Furthermore, the remaining ∼50% of the protein that could potentially be structured is split up into the N- and C termini with each containing ∼25 amino acids. Structural predictions deem these residues to have a high probability of disorder, which supports the experimental evidence on unbound HMGA2 (Figs. 4 and 5, A, B, and D). It is this disordered and flexible nature of HMGA2 that facilitates its diverse functionality. In addition to the AT-hook domains being used in DNA binding to alter chromatin structure (6) and stabilize stalled and reversed RFs (21), HMGA2 has also been found to have protein binding partners such as telomeric repeat-binding factor 2, which plays a role in telomere dysfunction (66). The composition of the linkers between the AT-hook domains of HMGA2 is critical for its interaction with such protein binding partners. For example, mutations in the AT-hook domains of HMGA2 only reduce its binding, but mutations in the linker regions completely abolish interaction of HMGA2 with telomeric repeat-binding factor 2 (66). This wide range of activities and binding partners requires HMGA2 to be disordered in solution such that it can adapt to the various binding sites present on the same or different molecules (6). Moreover, this enhanced flexibility is inherent in its role in chromatin restructuring and formation of enhanceosomes for regulation of gene expression (19).
The AUC and DLS data demonstrates the formation of a stable complex between recombinantly produced HMGA2 and RF formed through annealing DNA. An increase in the Rh (4.12–4.65 nm) and s020,w (3.86–4.06 S) of the RF was observed upon binding of RF with HMGA2 (Table 1). Through CD spectropolarimetry, we were able to detect that there are no significant structural changes to the RF upon binding to HMGA2 (Fig. 5 A). Although NMR (59) and x-ray crystallography (60) studies reveal that the minor groove widens and kinks are introduced due to AT-hook binding, such changes would not be visible by CD spectropolarimetry. Additionally, because the CD spectrum of the protein is convoluted with that of the DNA (71), we are unable to directly detect any secondary structural changes of the protein in the complex (Fig. 5 A). However, through NMR studies we could detect the protein in the complex and determine that there were no significant secondary structure changes of HMGA2 upon binding to RF. The resulting HSQC showed a nearly identical chemical shift range to unbound HMGA2 (Fig. 5 B) with decreased peak intensity. The difference in the Kratky analysis of the theoretical and experimental complex suggests that although HMGA2 does not adopt a defined secondary structure upon binding, it becomes more structured. In the theoretical analysis, it presumed that both HMGA2 and RF retained the same structure as in the apo-state, which from the resulting experimental analysis was not true. This can be explained through atomic level data of a truncated protein, which suggests that amino acids in the AT-hook domain adopt a defined structure when bound (59), but the remaining residues of HMGA2 are still largely in a disordered state. Because it is widely known that AT-hooks are involved in binding to DNA (18), it is inherent that W83 is adjacent to an AT-hook. The disappearance of the tryptophan side-chain peak in the complex confirms that AT-hook 2 is involved in RF binding.
SAXS studies provided low-resolution structural information on the complex of HMGA2 and RF (Fig. 6), demonstrating that HMGA2 interacts with the RF along the molecule in a side-by-side orientation. It is known that AT-hooks penetrate deep into the minor groove, with flanking residues forming electrostatic and hydrophobic interactions with the floor of the minor groove (59). Therefore, one could suggest that upon deconvolution, each AT-hook of HMGA2 is represented by a lobe as it interacts with the various AT-rich regions of the RF (Fig. 6 C). With HMGA2 stretched across the RF, it suggests that HMGA2 is not localized at the replicating end of the RF, but rather uses all AT-hook domains to bind to both sides of the replication bubble acting as a clamp until replication can continue. In the process of replication, multiple proteins and protein complexes are assembled onto the DNA surrounding the fork, thus making it difficult for HMGA2 to bind. However, when replication stalls, this is in part due to the lack of necessary machinery bound to the RF, leaving some of the DNA exposed (72). With the disordered nature of HMGA2 and its cooperative binding to RFs, this exposed DNA becomes a prime target for HMGA2 allowing stabilization of the RF until it can resume its replicating process. Although it has been shown that a single AT-hook can bind to DNA (59), our model supports previous mutational data demonstrating that all three AT-hook domains need to be present to be successful in stabilizing the RF (21).
Conclusions
Through our integrated approach to elucidate the interaction between HMGA2 and RF, we have shed light on the mechanism of this biologically relevant process. CD spectropolarimetry, NMR, and SAXS analysis determined that HMGA2 is inherently disordered, which provides it with the flexibility necessary to bind to distorted DNA. Combined DLS and SV data confirm that a single HMGA2 binds to one RF to form a monodisperse complex. Ab initio modeling of SAXS data concludes that all three AT-hook domains of HMGA2 interact with RF in a side-by-side orientation binding on both sides of the point of replication. It is this clamped orientation that indeed allows HMGA2 to stabilize stalled RFs in vivo and provides ESCs and cancer cells the added RF protection that is absent in somatic cells. This added protection aids toward maintaining the cellular viability for increased proliferation.
Author Contributions
N.K. prepared samples for all the experiments. M.M. performed codon optimization for E. coli expression. N.K. performed AUC and DLS data collection. M.M. and T.R.P. analyzed the AUC and DLS data collection. N.K. performed the CD spectropolarimetry experiments and analysis with help from M.M. V.T. and J.D.O. performed NMR data collection and analysis. K.M. ran the SAXS experiments. T.R.P. completed the data analysis. E.P.B. performed the HEK293T cellular work and Western blots with help from S.A.M. N.K., T.R.P., and J.S. wrote the manuscript. J.S. and T.R.P. were both main supervisors of this work.
Acknowledgments
N.K. was supported by University of Manitoba Graduate Enhancement of Tri-Council Stipends (GETS) funding. J.D.O. and V.T. were supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant and by the University of Manitoba. E.P.B. and S.A.M. were supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (2015-06142). J.S. was funded by the NSERC Discovery grants (RGPIN 342077-2012 and RGPIN-004954-2017) and the Research Tool and Infrastructure Support (345517 07) from NSERC. T.R.P. was supported by the NSERC Discovery grant (RGPIN-2017-04003).
Editor: Andreas Engel.
Contributor Information
Trushar R. Patel, Email: trushar.patel@uleth.ca.
Jörg Stetefeld, Email: joerg.stetefeld@umanitoba.ca.
References
- 1.Reeves R. High mobility group (HMG) proteins: modulators of chromatin structure and DNA repair in mammalian cells. DNA Repair (Amst.) 2015;36:122–136. doi: 10.1016/j.dnarep.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Reeves R., Adair J.E. Role of high mobility group (HMG) chromatin proteins in DNA repair. DNA Repair (Amst.) 2005;4:926–938. doi: 10.1016/j.dnarep.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Reeves R., Nissen M.S. The AT-DNA-binding domain of mammalian high mobility group I chromosomal proteins. A novel peptide motif for recognizing DNA structure. J. Biol. Chem. 1990;265:8573–8582. [PubMed] [Google Scholar]
- 4.Natarajan S., Hombach-Klonisch S., Klonisch T. HMGA2 inhibits apoptosis through interaction with ATR-CHK1 signaling complex in human cancer cells. Neoplasia. 2013;15:263–280. doi: 10.1593/neo.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copley M.R., Babovic S., Eaves C.J. The lin28B-let-7-HMGA2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nat. Cell Biol. 2013;15:916–925. doi: 10.1038/ncb2783. [DOI] [PubMed] [Google Scholar]
- 6.Pfannkuche K., Summer H., Dröge P. The high mobility group protein HMGA2: a co-regulator of chromatin structure and pluripotency in stem cells? Stem Cell Rev. 2009;5:224–230. doi: 10.1007/s12015-009-9078-9. [DOI] [PubMed] [Google Scholar]
- 7.Ozturk N., Singh I., Barreto G. HMGA proteins as modulators of chromatin structure during transcriptional activation. Front. Cell Dev. Biol. 2014;2:5. doi: 10.3389/fcell.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J., Wei J.-J. HMGA2 and high-grade serous ovarian carcinoma. J. Mol. Med. (Berl.) 2013;91:1155–1165. doi: 10.1007/s00109-013-1055-8. [DOI] [PubMed] [Google Scholar]
- 9.Fedele M., Fidanza V., Fusco A. Haploinsufficiency of the HMGA1 gene causes cardiac hypertrophy and myelo-lymphoproliferative disorders in mice. Cancer Res. 2006;66:2536–2543. doi: 10.1158/0008-5472.CAN-05-1889. [DOI] [PubMed] [Google Scholar]
- 10.Foti D., Chiefari E., Brunetti A. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nat. Med. 2005;11:765–773. doi: 10.1038/nm1254. [DOI] [PubMed] [Google Scholar]
- 11.Papaioannou G., Inloes J.B., Kobayashi T. Let-7 and miR-140 microRNAs coordinately regulate skeletal development. Proc. Natl. Acad. Sci. USA. 2013;110:E3291–E3300. doi: 10.1073/pnas.1302797110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jun K.H., Jung J.H., Chin H.M. HMGA1/HMGA2 protein expression and prognostic implications in gastric cancer. Int. J. Surg. 2015;24(Pt A):39–44. doi: 10.1016/j.ijsu.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y.S., Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pallante P., Sepe R., Fusco A. High mobility group-A proteins as tumor markers. Front. Med. (Lausanne) 2015;2:15. doi: 10.3389/fmed.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloth L., Gottlieb A., Bullerdiek J. HMGA2 expression distinguishes between different types of postpubertal testicular germ cell tumour. J. Pathol. Clin. Res. 2015;1:239–251. doi: 10.1002/cjp2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J., Zhang S., Wang X. Elevated HMGA2 expression is associated with cancer aggressiveness and predicts poor outcome in breast cancer. Cancer Lett. 2016;376:284–292. doi: 10.1016/j.canlet.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- 18.Aravind L., Landsman D. AT-hook motifs identified in a wide variety of DNA-binding proteins. Nucleic Acids Res. 1998;26:4413–4421. doi: 10.1093/nar/26.19.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattaruzzi G., Altamura S., Manfioletti G. The second AT-hook of the architectural transcription factor HMGA2 is determinant for nuclear localization and function. Nucleic Acids Res. 2007;35:1751–1760. doi: 10.1093/nar/gkl1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summer H., Li O., Dröge P. HMGA2 exhibits dRP/AP site cleavage activity and protects cancer cells from DNA-damage-induced cytotoxicity during chemotherapy. Nucleic Acids Res. 2009;37:4371–4384. doi: 10.1093/nar/gkp375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H., Lim H.H., Dröge P. Chaperoning HMGA2 protein protects stalled replication forks in stem and cancer cells. Cell Reports. 2014;6:684–697. doi: 10.1016/j.celrep.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Branzei D., Foiani M. Maintaining genome stability at the replication fork. Nat. Rev. Mol. Cell Biol. 2010;11:208–219. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- 23.Frost L., Baez M.A., Leng F. The dimerization state of the mammalian high mobility group protein AT-hook 2 (HMGA2) PLoS One. 2015;10:e0130478. doi: 10.1371/journal.pone.0130478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice P., Longden I., Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 25.Booy E.P., Meier M., McKenna S.A. The RNA helicase RHAU (DHX36) unwinds a G4-quadruplex in human telomerase RNA and promotes the formation of the P1 helix template boundary. Nucleic Acids Res. 2012;40:4110–4124. doi: 10.1093/nar/gkr1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Booy E.P., McRae E.K.S., McKenna S.A. RNA helicase associated with AU-rich element (RHAU/DHX36) interacts with the 3′-tail of the long non-coding RNA BC200 (BCYRN1) J. Biol. Chem. 2016;291:5355–5372. doi: 10.1074/jbc.M115.711499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Ryder S.P., Recht M.I., Williamson J.R. Quantitative analysis of protein-RNA interactions by gel mobility shift. Methods Mol. Biol. 2008;488:99–115. doi: 10.1007/978-1-60327-475-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel T.R., Nikodemus D., Stetefeld J. Biophysical analysis of a lethal laminin α-1 mutation reveals altered self-interaction. Matrix Biol. 2016;49:93–105. doi: 10.1016/j.matbio.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Meier M., Patel T.R., McKenna S.A. Binding of G-quadruplexes to the N-terminal recognition domain of the RNA helicase associated with AU-rich element (RHAU) J. Biol. Chem. 2013;288:35014–35027. doi: 10.1074/jbc.M113.512970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stetefeld J., McKenna S.A., Patel T.R. Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys. Rev. 2016;8:409–427. doi: 10.1007/s12551-016-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krahn N., Spearman M., Butler M. Inhibition of glycosylation on a camelid antibody uniquely affects its FcγRI binding activity. Eur. J. Pharm. Sci. 2017;96:428–439. doi: 10.1016/j.ejps.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 33.Dam J., Schuck P. Calculating sedimentation coefficient distributions by direct modeling of sedimentation velocity concentration profiles. Methods Enzymol. 2004;384:185–212. doi: 10.1016/S0076-6879(04)84012-6. [DOI] [PubMed] [Google Scholar]
- 34.Schuck P. Sedimentation analysis of noninteracting and self-associating solutes using numerical solutions to the Lamm equation. Biophys. J. 1998;75:1503–1512. doi: 10.1016/S0006-3495(98)74069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laue T.M., Shah B.D., Pelletier S.L. Computer-aided interpretation of analytical sedimentation data for proteins. In: Harding S.E., Rowe A.J., Horton J.C., editors. Analytical Ultracentrifugation in Biochemistry and Polymer Science. Royal Society of Chemistry; Cambridge, UK: 1992. pp. 90–125. [Google Scholar]
- 36.Patel T.R., Winzor D.J., Scott D.J. Analytical ultracentrifugation: a versatile tool for the characterisation of macromolecular complexes in solution. Methods. 2016;95:55–61. doi: 10.1016/j.ymeth.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Kay L., Keifer P., Saarinen T. Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J. Am. Chem. Soc. 1992;114:10663–10665. [Google Scholar]
- 38.Delaglio F., Grzesiek S., Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 39.Goddard, T. D., and D. G. Kneller. SPARKY 3. https://www.cgl.ucsf.edu/home/sparky/.
- 40.Deo S., Patel T.R., McKenna S.A. Activation of 2′ 5′-oligoadenylate synthetase by stem loops at the 5′-end of the West Nile Virus genome. PLoS One. 2014;9:e92545. doi: 10.1371/journal.pone.0092545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dzananovic E., Patel T.R., McKenna S.A. Recognition of viral RNA stem-loops by the tandem double-stranded RNA binding domains of PKR. RNA. 2013;19:333–344. doi: 10.1261/rna.035931.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konarev P.V., Volkov V.V., Svergun D.I. PRIMUS—a Windows-PC based system for small-angle scattering data analysis. J. Appl. Crystallogr. 2003;36:1277–1282. [Google Scholar]
- 43.Svergun D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 1992;25:495–503. [Google Scholar]
- 44.Franke D., Svergun D.I. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J. Appl. Crystallogr. 2009;42:342–346. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svergun D.I. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 1999;76:2879–2886. doi: 10.1016/S0006-3495(99)77443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volkov V.V., Svergun D.I. Small-angle scattering techniques. J. Appl. Crystallogr. 2003;36:860–864. [Google Scholar]
- 47.Ortega A., Amorós D., García de la Torre J. Prediction of hydrodynamic and other solution properties of rigid proteins from atomic- and residue-level models. Biophys. J. 2011;101:892–898. doi: 10.1016/j.bpj.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voss N.R., Gerstein M. Calculation of standard atomic volumes for RNA and comparison with proteins: RNA is packed more tightly. J. Mol. Biol. 2005;346:477–492. doi: 10.1016/j.jmb.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 49.Ishida T., Kinoshita K. PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res. 2007;35:W460–W464. doi: 10.1093/nar/gkm363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchan D.W., Minneci F., Jones D.T. Scalable web services for the PSIPRED protein analysis workbench. Nucleic Acids Res. 2013;41:W349–W357. doi: 10.1093/nar/gkt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones D.T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- 52.Yang Z.R., Thomson R., Esnouf R.M. RONN: the bio-basis function neural network technique applied to the detection of natively disordered regions in proteins. Bioinformatics. 2005;21:3369–3376. doi: 10.1093/bioinformatics/bti534. [DOI] [PubMed] [Google Scholar]
- 53.Kelly S.M., Jess T.J., Price N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Rambo R.P., Tainer J.A. Characterizing flexible and intrinsically unstructured biological macromolecules by SAS using the Porod-Debye law. Biopolymers. 2011;95:559–571. doi: 10.1002/bip.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kypr J., Kejnovská I., Vorlícková M. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res. 2009;37:1713–1725. doi: 10.1093/nar/gkp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amato N.J., Mwai C.N., Bryant-Friedrich A.C. Thermodynamic and structural analysis of DNA damage architectures related to replication. J. Nucleic Acids. 2013;2013:867957. doi: 10.1155/2013/867957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cortez D. Preventing replication fork collapse to maintain genome integrity. DNA Repair (Amst.) 2015;32:149–157. doi: 10.1016/j.dnarep.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li O., Li J., Dröge P. DNA architectural factor and proto-oncogene HMGA2 regulates key developmental genes in pluripotent human embryonic stem cells. FEBS Lett. 2007;581:3533–3537. doi: 10.1016/j.febslet.2007.06.072. [DOI] [PubMed] [Google Scholar]
- 59.Huth J.R., Bewley C.A., Clore G.M. The solution structure of an HMG-I(Y)-DNA complex defines a new architectural minor groove binding motif. Nat. Struct. Biol. 1997;4:657–665. doi: 10.1038/nsb0897-657. [DOI] [PubMed] [Google Scholar]
- 60.Fonfría-Subirós E., Acosta-Reyes F., Campos J.L. Crystal structure of a complex of DNA with one AT-hook of HMGA1. PLoS One. 2012;7:e37120. doi: 10.1371/journal.pone.0037120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cai J., Shen G., Meng Q. Downregulation of HMGA2 inhibits cellular proliferation and invasion, improves cellular apoptosis in prostate cancer. Tumour Biol. 2016;37:699–707. doi: 10.1007/s13277-015-3853-9. [DOI] [PubMed] [Google Scholar]
- 62.Xia Y.Y., Yin L., He X. Downregulating HMGA2 attenuates epithelial-mesenchymal transition-induced invasion and migration in nasopharyngeal cancer cells. Biochem. Biophys. Res. Commun. 2015;463:357–363. doi: 10.1016/j.bbrc.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 63.Mansoori B., Mohammadi A., Baradaran B. Silencing of high mobility group isoform I-C (HMGI-C) enhances paclitaxel chemosensitivity in breast adenocarcinoma cells (MDA-MB-468) Adv. Pharm. Bull. 2016;6:171–177. doi: 10.15171/apb.2016.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altschul S.F., Madden T.L., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horikawa I., Chiang Y.J., Barrett J.C. Differential cis-regulation of human versus mouse TERT gene expression in vivo: identification of a human-specific repressive element. Proc. Natl. Acad. Sci. USA. 2005;102:18437–18442. doi: 10.1073/pnas.0508964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Natarajan S., Begum F., Klonisch T. High mobility group A2 protects cancer cells against telomere dysfunction. Oncotarget. 2016;7:12761–12782. doi: 10.18632/oncotarget.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miao Y., Cui T., Wilson W.D. Inhibition of high-mobility-group A2 protein binding to DNA by netropsin: a biosensor-surface plasmon resonance assay. Anal. Biochem. 2008;374:7–15. doi: 10.1016/j.ab.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui T., Wei S., Leng F. Energetics of binding the mammalian high mobility group protein HMGA2 to poly(dA-dT)2 and poly(dA)-poly(dT) J. Mol. Biol. 2005;352:629–645. doi: 10.1016/j.jmb.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 69.Dragan A.I., Liggins J.R., Privalov P.L. The energetics of specific binding of AT-hooks from HMGA1 to target DNA. J. Mol. Biol. 2003;327:393–411. doi: 10.1016/s0022-2836(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 70.Cui T., Leng F. Specific recognition of AT-rich DNA sequences by the mammalian high mobility group protein AT-hook 2: a SELEX study. Biochemistry. 2007;46:13059–13066. doi: 10.1021/bi701269s. [DOI] [PubMed] [Google Scholar]
- 71.Kelly S.M., Price N.C. The use of circular dichroism in the investigation of protein structure and function. Curr. Protein Pept. Sci. 2000;1:349–384. doi: 10.2174/1389203003381315. [DOI] [PubMed] [Google Scholar]
- 72.Sabatinos S.A. Replication fork stalling and the fork protection complex. Nature Education. 2010;3:40. [Google Scholar]