Abstract
Background
During inflammation, immune cells produce cytokines, chemokines and prostaglandins. This results in acute or chronic inflammation, which favor the development of degenerative diseases such as diabetes, obesity or cardiovascular diseases. Inflammatory processes are modulated by intrinsic and external factors. External factors are supposed to act via similar modes of action as do endogenous molecules and mediators. Both endogenous ligands and nutrient-derived metabolites might modify the extent and status of the cellular and systemic response during inflammation. Therefore, the biological activity of endogenous mediators was compared with nutrition-derived substances.
Methods
Murine macrophages (RAW264.7 cells), in vitro differentiated human promyeloid THP-1 cells and peripheral blood leukocytes (PBL) were stimulated with LPS in the presence of z-ligustilide (LIG) or the endogenous PPARγ ligand 15deoxyΔ12,14-prostaglandin J2 (15d–PGJ2). Secretion of mediators of inflammation was measured by EIA, the Griess reaction and multiplex ELISA (Luminex®). Gene expression was quantified by real-time PCR. Nuclear translocation of NF-κB was measured by cytometric techniques.
Results
LPS-activated RAW264.7 cells produced nitric oxide (NO), COX2-dependent prostaglandin E2 (PGE2), interleukins and chemokines. LIG concentration-dependently reduced the production of nitric oxide (NO) and PGE2, although it did not match the inhibitory potential of 15d–PGJ2. LIG inhibited the secretion of cytokines (IL-1α, IL-6, TNF-α) and differentiation factors (GM-CSF) in murine macrophages. It blunted the production of CCL2/MCP-1, but did not alter the secretion of CCL5/RANTES. LIG reduced mRNA levels of pro-inflammatory cytokines (e.g. TNF-α, IL-1α, IL-6), chemokines (CCL4/MIP-1β), and pro-inflammatory enzymes (iNOS). Similarly, LIG robustly impaired inflammatory mediators (e.g. CCL2/MCP-1, CCL3-MIP-1α, CCL4/MIP-1β, CXCL10/IP-10, IL-12p70, TNF-α) of LPS-activated human THP-1 cells and PBLs. Unexpectedly, it augmented the production of IL-1β, IL-6 and GM-CSF in PBLs.
Conclusions
LIG diminished the extent of the inflammatory response measured by the production of different mediators or metabolites (NO, PGE2, interleukins, cytokines, chemokines). LIG acted at the transcriptional level and targeted the NF-κB signaling pathway. Since LIG and the anti-inflammatory prostaglandin 15d–PGJ2 share most of the analyzed biological features, we infer that they have similar modes of action. Hence, LIG acts as an anti-inflammatory prostaglandin and modulates cytokine- and chemokine-dependent inflammatory responses.
Background
Inflammatory processes are involved in the etiology of different diseases such as atherosclerosis, diabetes or arthritis. Acute inflammation encompasses distinct phases - initiation, progression and resorption - which are tightly controlled by mediators [1]. Inflammatory stimuli provoke a rapid release of mediators, which trigger the onset of the inflammatory response. Activation and recruitment of cell populations such as macrophages/monocytes or neutrophils are major hallmarks of the systemic response. Eventually, acute inflammation is self-controlled and relies partly on mechanisms similar to those operational during progression [2, 3]. Conversely, in chronic inflammation some of these events are not contained and provoke prolonged production of mediators with concomitant tissue erosion and pain.
At the molecular level, various transcription factors including NF-κB and nuclear receptors are involved in the control of acute and chronic inflammation. Peroxisome proliferator-activated receptors (PPAR) regulate inflammatory responses of macrophages; thus natural PPARγ ligands modulate macrophage activation [4–6]. Endogenous PPARγ ligands are derived from polyunsaturated fatty acids and generated through the action of lipoxygenase and prostaglandin synthase [7, 8]. Oxidized low-density lipoprotein (ox-LDL) and cyclopentenone prostaglandins including 15-deoxyΔ12,14-prostaglandin J2 (15d–PGJ2) were identified as PPARγ ligands with pro-atherogenic and anti-inflammatory properties, respectively [9, 10]. Several studies demonstrated that PPARγ-dependent and PPARγ-independent mechanisms regulate inflammatory processes [11–15].
This study aimed at identifying natural substances that modulate inflammatory processes and therefore might be of use in preventing associated diseases. Secondary plant metabolites such as EGCG [16] and resveratrol [17, 18] were found to bind to PPAR subtypes and thus modulate inflammatory responses. Z-ligustilide (LIG), a phtalide isolated from Ligusticum chuanxiong has anti-diabetic and anti-inflammatory properties [19–22] and was identified as putative novel PPARγ ligand [23]. This prompted us to compare the nutrient-based substance and the endogenously produced anti-inflammatory 15d–PGJ2. The data demonstrate that LIG and 15d–PGJ2 share numerous features in modulating the cellular response to inflammatory stimuli and thus confer LIG the properties of an anti-inflammatory prostaglandin.
Methods
Reagents
LIG was from MicroSource (Gaylordsville, CT), or was prepared by DSM Nutritional Products; it was also isolated from Ligusticum chuanxiong by Kieselgur column fractionation (Fig. 1a). Its purity was >98% (according to the manufacturer’s data sheet). 15-deoxyΔ12,14 prostaglandin J2 (15d–PGJ2) was from Cayman Chemicals (Ann Harbor, MI). Rosiglitazone was purchased from Shanco International, Inc. (Hazlet, NJ). L-NAME (L-NG-Nitroarginine methyl ester) was from Sigma, Saint-Louis, MO. Compounds were dissolved in DMSO and added to the culture medium concomitantly with the stimulus. Final DMSO concentration was 0.5%. Lipopolysaccharide (LPS, E. coli serotype 055:B5) and foetal bovine serum (FBS) were from Sigma. Ficoll-Isopaque was from Nycomed Pharma AS (Oslo, Norway). DMEM, RPMI Medium 1640, PBS, non-essential amino acids (NEAA), β-mercaptoethanol were from Invitrogen (Carlsbad, CA). Human recombinant interferon-γ (IFN-γ) was from Preprotech EC (London, UK). Primers and probes used in RT-PCR were designed with the Primer Express™ program (Applied Biosystems Inc., Foster City, CA;) and synthesized by Sigma.
Fig. 1.
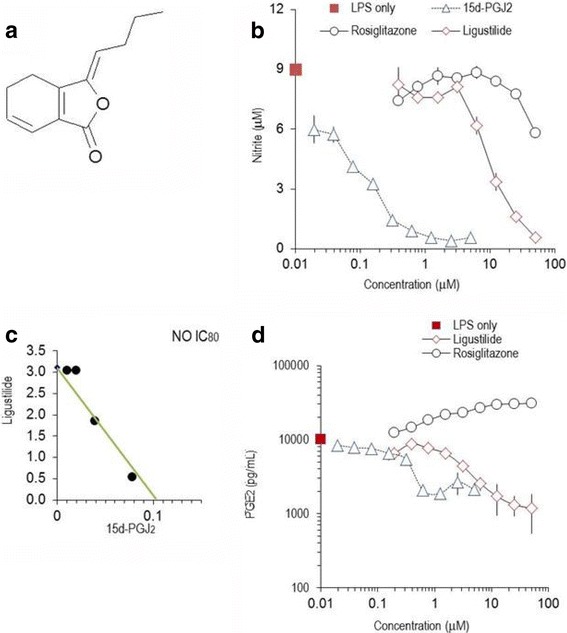
Z-ligustilide and 15d–PGJ2 alter nitric oxide and PGE2 in LPS-activated RAW264.7 cells. a: Molecular structure of z-ligustilide. b: Effect of z-ligustilide, 15d–PGJ2 and rosiglitazone on the production of nitric oxide (NO, measured as nitrite) by LPS-stimulated RAW264.7 cells, which were cultured for 24 h. Mean values (± SEM) of triplicate cultures from three independent experimental series are shown. c: Interactions between z-ligustilide and 15d–PGJ2 displayed in an isobologram (for details see reference [26] and Materials and Methods). A straight line was drawn between the IC80 value for z-ligustilide (in the absence of 15d–PGJ2) and the IC80 value of 15d–PGJ2 (in the absence of z-ligustilide). The values of the substances in combination fall on the straight line and thus reflect additive effects between z-ligustilide and 15d–PGJ2. d: Inhibition of PGE2 production by 15d–PGJ2, z-ligustilide and rosiglitazone in LPS-stimulated RAW264.7 cells which were cultured for 24 h. PGE2 was measured by EIA. Mean values ± SD of triplicates are shown. Similar results were obtained in 3 independent experimental series. Note the logarithmic scale of the axes
Cell culture
RAW264.7 cells were from ATCC (Manassas, VA) and cultured in DMEM supplemented with 10% FBS, 50 units/mL penicillin, 50 μg/mL streptomycin, L-glutamine and non-essential amino acids. Cells were used between passage 10 and 30. For experiments, cells were seeded into 6-well, 12-well or 96-well plates at 2, 1 and 0.05 × 106 cells per well, respectively, and used after 2 days of pre-culture. Cells were starved in complete DMEM medium containing 0.25% FBS 18 h before the treatment. Cells were stimulated with LPS (1 μg/mL) for 4–24 h in phenol-free DMEM containing 0.25% FBS.
THP-1 cells (obtained from ATCC) were cultured in RPMI 1640 medium supplemented with 10% FBS, 50 units/mL penicillin, 50 μg/mL streptomycin, NEAA and 2 × 10−5 M β-mercaptoethanol. Cells were treated with 50 nM phorbol myristate acetate for 3 days. Cells were starved overnight in medium containing 0.25% FBS before being treated. Cells were stimulated with LPS (1 μg/mL) for 2–24 h in phenol-free RPMI containing 0.25% FBS.
Blood was obtained from healthy human volunteers. Peripheral blood leukocytes (PBLs) were also isolated from buffy coats obtained from local blood transfusion centers, using the Dextran sedimentation method to remove erythrocytes. Peripheral blood mononuclear cells were isolated by Ficoll-Isopaque gradient centrifugation and cultured in RPMI 1640 medium, supplemented with 0.25% FBS, NEAA, penicillin / streptomycin (50 U/mL / 50 μg/mL), and 5 × 10−5 M β-mercaptoethanol. Cell viability was determined by the Trypan Blue exclusion test and exceeded 95%. For in vitro cultures, cells were adjusted to 1 × 106 cells/mL. Peripheral blood leukocytes were stimulated with LPS (1 μg/mL) and IFN-γ (20 U/mL) for 2–24 h in phenol-free RPMI containing 0.25% FBS.
Measurements of cell viability
LDH was determined in supernatants from cell cultures immediately after harvesting, using a commercially available cytotoxicity kit (Promega, Madison, WI).
Measurement of production of PGE2 and NO
Concentrations of nitrite, which is generated from cell-released nitric oxide, were determined by the Griess reaction [24]. PGE2 was quantified by EIA [25] according to the manufacturer’s instructions (Cayman Chemicals). All determinations were done in duplicates and at various dilutions of the culture supernatants.
Isobolographic analysis of interaction between substances
The interactions between substances were evaluated using isobologram analysis essentially as described [26]: LIG and 15-PGJ2 were mixed at a fixed ratio, added to RAW264.7 cells at a large concentration range and their effects on LPS-induced NO and PGE2 production determined and expressed as IC50; concomitantly, effect of each individual substance was measured. Alternatively, to cultures containing a given concentration of LIG, 15d–PGJ2 was added at varying concentrations and the IC50 or IC80 was computed for the mixture of substances. The IC80 values were then used to create isobolograms as described [26, 27]. Synergistic effects were also computed with the CalcuSyn Version 2.0 software (Biosoft, Ferguson, MO).
Determination of cytokine and chemokine production
Multiparametric kits were obtained from BIO-RAD Laboratories (Hercules, CA) and used in the LiquiChip Workstation IS200 (Qiagen, Hilden, Germany) according to the manufacturers’ instructions. We used the Bio-Plex Mouse Cytokine 23-Plex Panel and the Bio-Plex Human Group I cytokine Broad Range 27-Plex Panel; the data were acquired with the Luminex IS 2.3 software and evaluated with the LiquiChip Analyser software provided by Qiagen.
Gene expression analysis
The isolation of total RNA, reverse transcription and quantitative real-time PCR have been done as described before [28]. The parameters for quantitative PCR and the calculation of fold changes (i.e. the relative expression of genes) are given in ‚Gene Expression Analysis Using Taqman Assays’ (http://www.thermofisher.com). Expression of 18S RNA was used as internal control (housekeeping gene). Expression values were normalized on the basis of unstimulated cells (where the threshold value is set as 1) and computed as fold changes (based on 2-X, where X returns to threshold value Ct of stimulated cells – threshold value Ct of unstimulated cells). Primer and probe sequences are given in Additional file 1: Table S1.
Measurements of cytoplasmic/nuclear location of NF-κB
Cells were grown in 96-well plates and pre-incubated with various concentrations of LIG for 1 h. Cells were activated with LPS (1 μg/mL) for 20 min. Thereafter, cells were washed, fixed and permeabilized as detailed by Ding et al. [29]. Immunostaining for NF-κBp65 was performed using the Cellomics NF-κB Activation HitKitTM (Thermofisher Scientific Inc., Pittsburgh, PA). Nuclei were counter-stained with Hoechst dye. Immunofluorescence was measured by quantitative cytometric technique, ArrayscanTM, with the Cellomics instrumentation (Cellomics™ Inc.) expressed as Mean_CircRingAvgInten (for detail see: Cellomics HCS application guide). All treatments were done in triplicates.
Statistical analysis
Data were evaluated by statistical tools described previously [28, 30, 31]. A p value <0.05 (calculated by using Student’s t test or one-way ANOVA) was considered to reflect statistically significant differences. Where appropriate, the Tuckey post-hoc test was applied for multiple comparisons. Statistical analysis was conducted with the SPSS software package, version 23.0.0. (SPSS, Munich, Germany).
Results
Z-ligustilide inhibits the production of nitric oxide and PGE2 in murine RAW264.7 cells
Macrophages respond to inflammatory stimuli by the exuberant secretion of cytokines, chemokines and other inflammatory mediators or enzymes. LPS-stimulated RAW264.7 cells produced significant quantities of nitric oxide (NO) and PGE2 within 24 h of culture [24, 25], whereas unstimulated cells produced >10-fold less mediators. LIG reduced NO production (Fig. 1b) with IC50 of 12.8 ± 1.4 μM (Table 1). L-nitroso-arginine-methyl ester (L-NAME) or resveratrol revealed to be less potent inhibitors with IC50 of 150 ± 12 μM and 28.5 ± 1.7 μM, respectively. 15d–PGJ2 abrogated NO production at >2 μM (IC50 2.2 ± 0.6 μM). Rosiglitazone only marginally impaired NO production at concentrations >25 μM (Fig. 1b).
Table 1.
IC50 values (in μM) for substances tested in LPS-activated RAW264.7 cells
| PGE2 | Nitric Oxide | |||
|---|---|---|---|---|
| Mean ± S.E.M. | N | Mean ± S.E.M. | N | |
| Z-ligustilide | 9.3 ± 1.6 | 33 | 12.8 ± 1.4 | 58 |
| 15d–PGJ2 | 2.2 ± 0.5 | 16 | 2.3 ± 0.6 | 25 |
| Rosiglitazone | >50 | 2 | 26.9 ± 1.9 | 16 |
| L-NAME | >500 | 2 | 150 ± 12 | 2 |
Cells (in triplicates) were incubated with graded amounts of substances, stimulated with 1 μg/mL LPS and cultured for 24 h. PGE2 and NO were measured by EIA and the Griess reaction, respectively, and the IC50 values were calculated for each experimental series. N: number of independent experimental series. L-NAME: L-NG-Nitroarginine methyl ester
In order to test the hypothesis that substances might interact, we stimulated macrophages in the presence of combination of substances and visualized the effects on NO production by isobolographic analysis [26, 27]. LIG combined with 15d–PGJ2 led to inhibition curves, which reflected additive interaction as displayed in the isobologram (Fig. 1c). The points were on a straight line and thus indicated additive effects between the substances. Rosiglitazone did not reverse the inhibitory effect of LIG or 15d–PGJ2 nor was the effect of the combined compounds cumulative (not shown). Next, we explored the effect of LIG and 15d–PGJ2 on PGE2 production by LPS-simulated RAW264.7 cells. LIG (at >20 μM) abrogated PGE2 production (Fig. 1d), but it was a less potent inhibitor than 15d–PGJ2 (IC50 of 9.3 ± 1.6 μM and IC50 2.2 ± 0.5 μM, respectively). When the two substances were combined and the inhibitory effect on PGE2 production analyzed, isobologram analysis revealed additive effects similar to those observed on NO production (data not shown). It should be noted that rosiglitazone concentration-dependently increased COX2-dependent PGE2 production (Fig. 1d). LIG and 15d–PGJ2 had only insignificant effects in unstimulated cells, which produced <10% of mediators secreted after LPS activation.
We further inspected whether the treatment of cells with compounds affected cell viability. LIG did not exert significant cytotoxic effects within a concentration range of <100 μM (Additional file 1: Figure S1) and was comparable to other micronutrients such as resveratrol or the catechin EGCG, which did not impair cell viability. Similarly, rosiglitazone or 15d–PGJ2 had no cytotoxic effects at concentrations <50 μM and <2.5 μM, respectively.
Z-ligustilide modulates the inflammatory response in human THP-1 cells and peripheral blood leukocytes
We hypothesized that LIG modulated the production of inflammatory mediators in a similar way in different species. Therefore, THP-1 cells, a human monocytic leukemia cell line, were induced to differentiate in vitro, stimulated with LPS [32] and the effect of substances on eicosanoid synthesis was determined. LIG and 15d–PGJ2 blunted PGE2 production, whereas rosiglitazone augmented COX-2 dependent PGE2 levels in THP-1 cells (Fig. 2a). Likewise, human peripheral blood leukocytes (PBLs, consisting of mononuclear and polymorphonuclear cells) were stimulated with LPS/IFN-γ in the presence of various concentrations of LIG and the PGE2 secretion was determined. LIG dose-dependently decreased the inflammatory response as measured by the PGE2 production (Fig. 2b); 15d–PGJ2 had stronger inhibitory effects than LIG (not shown). Effects of LIG and 15d–PGJ2 on unstimulated cells were not significant. Collectively, murine macrophages and human cell lines or primary cells (PBLs) displayed a similar responsiveness to LIG.
Fig. 2.
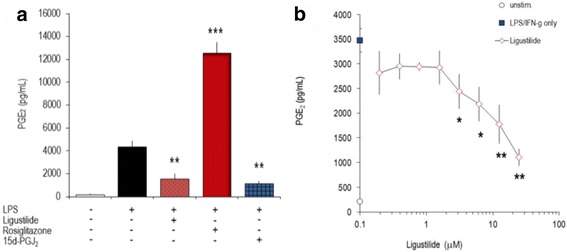
Z-ligustilide modulates PGE2 production in human THP-1 cells and PBLs. a: Effect of z-ligustilide, 15d–PGJ2 and rosiglitazone on THP-1 cells: Cells were differentiated in vitro with phorbol myristate acetate (20 nm/L) for 3 days, stimulated with LPS (1 μg/mL) in the absence or presence of z-ligustilide (25 μM), rosiglitazone (25 μM), or 15d–PGJ2 (2.5 μM). Secreted PGE2 was determined after 24 h by EIA. b: Freshly isolated peripheral blood leukocytes were stimulated with LPS/IFN-γ in the presence of graded amounts of z- ligustilide. Secreted PGE2 was determined after 24 h by EIA
Z-ligustilide alters the production of cytokines, chemokines and differentiation factors secreted by murine macrophages and human peripheral blood leukocytes
Next, we investigated the effect of LIG and 15d–PGJ2 on inflammatory proteins produced by murine macrophages. The secretion of 9 (of 14 measured) cytokines and chemokines was increased 4-fold to ~4000-fold in LPS-activated RAW264.7 cells. Among the analyzed differentiation factors and chemokines, LIG concentration-dependently impaired the secretion of GM-CSF, CCL2/MCP-1 and CCL4/MIP-1β; CCL5/RANTES was only affected by high phtalide concentrations (25 μM) (Table 2 and Fig. 3). It exerted similar inhibitory effects on IL-1α, IL-6 and TNF-α, whereas IFN-γ and IL-12p70 were virtually unaltered. 15d–PGJ2 shared with LIG all features of the inhibition pattern, except for GM-CSF, CCL4/MIP-1α, CCL5/RANTES and TNF-α (Table 2 and Fig. 3). It should be noted, however, that the two substances differed in their biological potency: on a stoichiometric basis, 15d–PGJ2 was ~ 10-fold more efficient than LIG.
Table 2.
Inflammatory proteins secreted by RAW264.7 cells
| Protein | Secretion by unstimulated cells [pg/mL] |
LPS-induced protein secretion
(ratio LPS-stim./unstim.) |
% inhibition by LIG (25 μM) |
% inhibition by
15-dPGJ 2 (1.25 μM) |
|---|---|---|---|---|
| CCL2/MCP-1 | 4005 | 65 | 86 ± 5 ***) | 58 ± 39 **) |
| CCL4/MIP-1β | 39,250 | 7.0 | 40 ± 13 *) | -9 ± 22 |
| CCL5/RANTES | 268 | 219 | 61 ± 4 **) | 2 ± 27 |
| IFN-γ | 22 | 4.2 | 32 ± 3 **) | 24 ± 4 *) |
| GM-CSF | 134 | 16 | 94 ± 5 **) | 91 ± 2 ***) |
| IL-1α | 9 | 233 | 95 ± 4 ***) | 50 ± 1 **) |
| IL-6 | 28 | 1442 | 90 ± 0 ***) | 76 ± 15 *) |
| IL-12p70 | 84 | 4.6 | 38 ± 2 **) | 27 ± 7 *) |
| TNF-α | 40 | 3820 | 68 ± 5 **) | 67 ± 2 **) |
RAW264.7 cells were stimulated with LPS and cultured for 24 h in the presence of substances. Secreted proteins were measured by multiparametric analysis (Luminex technology). The LPS-induced increase of secreted proteins is given as ratio of values obtained in stimulated versus unstimulated cells. * p < 0.05; ** p < 0.01; *** p < 0.005 (‘LPS + substance’ treated versus ‘LPS-only’ treated cells)
Fig. 3.
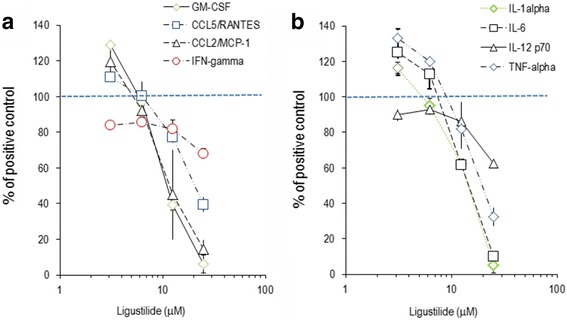
Production of cytokines and chemokines by RAW264.7 cells. RAW264.7 cells were stimulated for 24 h with LPS in the presence of indicated amounts of LIG. Secreted chemokines, cytokines and differentiation factors were measured by multiparametric analysis (i.e. GM-CSF, CCL5/RANTES,CCL2/MCP-1, IFN-gamma (in a); IL-1alpha, IL-6, IL-12p70, TNF-alpha (in b)). Data are expressed in % of the values obtained for LPS-stimulated cells and are the means (± CV) of triplicates from three independent experimental series. The straight dotted line indicates the level of mediators produced by ‘LPS alone’-treated cells. All values <80% of positive control were significantly lower (p < 0.01 [versus 100% positive control])
We extended this analysis to human PBLs, where the activation with LPS/IFN-γ led to >4-fold increased secretion of inflammatory proteins, which included chemokines (CCL3/MIP-3α, CXCL8/IL-8, CXCL10/IP-10) and cytokines (TNF-α, IL-1β, IL-6, IL-12p70). CCL5/RANTES and CCL2/MCP-1 were constituently expressed at high levels presumably by polymorphonuclear cells (PMNL), contained in PBLs. The secretion of GM-CSF, CCL2/MCP-1 and TNF-α was drastically decreased by raising concentrations of LIG (Fig. 4 and Table 3). Conversely, IFN-γ, CXCL/10IP-10, IL-1β, IL-6 and CCL5/RANTES were only marginally influenced. This further revealed that the phtalide specifically affected the secretion of distinct cytokines and chemokines. LIG and 15d–PGJ2 differed in two important aspects in their anti-inflammatory effects on PBLs: CCL2/MCP-1 and TNF-α production were not impaired by the cyclopentenone prostaglandin, whereas LIG strongly altered them (Fig. 4). It should be noted that GM-CSF and IL-6 secretion was markedly enhanced when LPS/INF-γ activated PBLs were treated with LIG or 15d–PGJ2. This was substantially more potent than LIG in its biological effects.
Fig. 4.
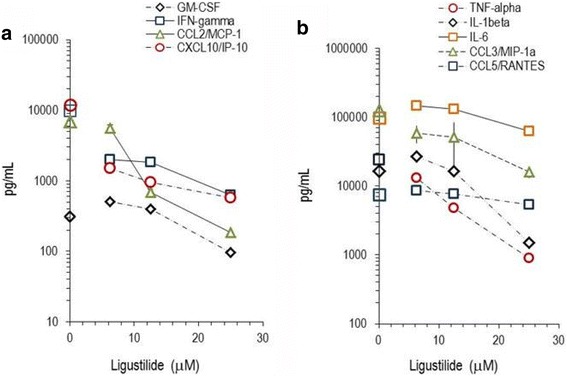
Production of cytokines and chemokines by PBLs. Freshly isolated peripheral blood leukocytes were stimulated for 24 h with LPS/INF-γ in the presence of indicated amounts of LIG. Secreted chemokines, cytokines and differentiation factors (i.e. GM-CSF, IFN-gamma, CCL2/MCP-1, CXCL10/IP-105/RANTES (in a); TNF-alpha, IL-1beta, IL-6, CCL3/MIP1alpha, CCL5/RANTES (in b)) were measured by multiparametric analysis and are indicated in pg/mL. ‘LPS/INF-γ alone’ values for the molecules are indicated on the y-axis. Data are means ± SD of triplicate cultures of PBLs
Table 3.
Inflammatory proteins secreted by human PBLs
| Protein | Unstimulated cells 24 h) [pg/mL] |
Protein secretion
(ratio LPS-stim./ unstim.) |
% inhibition by Lig (6.25 μM) | % inhibition by 15d–PGJ 2 (1.25 μM) |
|---|---|---|---|---|
| CCL2/MCP-1 | 8540 | 0.8 | 87 ± 3 **) | - 37 ± 34 |
| CCL3/MIP-1α | 6055 | 20.6 | 61 ± 4 *) | 67 ± 36 **) |
| CCL4/MIP-1β | 35,000 | 7.0 | 68 ± 7 **) | 51 ± 14 *) |
| CCL5/RANTES | 9225 | 0.8 | < 2 | <2 |
| CXCL8/IL-8 | 20,700 | 21.1 | <2 | <2 |
| CXCL10/IP-10 | 529 | 22.6 | 89 ± 3 **) | 86 ± 12 **) |
| IFN-γ | 22 | 10.4 | 84 ± 1 **) | 85 ± 9 **) |
| TNF-α | 982 | 24.8 | 46 ± 4 *) | −16 ± 18 |
| IL-1β | 1055 | 15.5 | −57 ± 5 *) | 38 ± 0 *) |
| IL-6 | 11,400 | 8.6 | −51 ± 3 **) | −135 ± 32 **) |
| IL-12p70 | 12 | 21.1 | 89 ± 2 **) | 85 ± 12 **) |
Peripheral blood leukocytes were isolated and cultured for 24 h at the indicated treatments. The proteins secreted into the culture supernatants were measured by multiparametric analysis. * p < 0.05; ** p < 0.01; *** p < 0.005 (‘LPS + substance’ treated versus ‘LPS-only’ treated cells)
Using quantitative real-time PCR technology, we have evaluated the influence of LIG on inflammatory genes. Since macrophages drastically up-regulated inflammatory gene mRNA expression within 1–6 h following LPS-stimulation, we have chosen to analyze the influence of compounds in cells after 4 h of culture. There were notable differences in the basal expression levels in unstimulated macrophages with weakly (e.g. COX-2, iNOS, prostaglandin E synthase [PGES], IL-6, IL-1β, PPARγ1), moderately (e.g. COX-1, prostaglandin EP-2 receptor [EP-2], hematopoietic-type prostaglandin D synthase [PGDS], IL-1α) and abundantly expressed genes (e.g. TNF-α, fibronectin receptor-α [FNR-α], PPARβ, CCL4/MIP-1β) (data not shown). LPS induced a substantial increase of mRNA of interleukins and cytokines (e.g. IL-6, IL-1α and TNF-α) but also of iNOS and COX-2, whereas other genes were down-regulated (e.g. PGDS, EP-2, PPARγ1) (Fig. 5, Additional file 1: Figure S2). Unlike LIG, 15d–PGJ2 modulated PPARγ expression and shifted it towards pre-homeostatic/inflammatory levels. LIG reduced expression levels of iNOS, IL-1α, IL-1β, TNF-α and CCL4/MIP-1β by up to 90% in the concentration range of 6.25–50 μM. Interestingly, LPS-inducible COX-2 gene expression did not significantly change in the presence of LIG.
Fig. 5.

Effect of z-ligustilide on inflammatory genes expression of murine macrophages. Unstimulated or LPS-stimulated RAW264.7 cells were cultured for 4 h in the presence of graded amounts of LIG; mRNA levels were determined by RT-PCR. mRNA levels are indicated as fold change (y axis), which was calculated as indicated in Materials and Methods. Bars represent mean values of fold change +/− errors (see [28]) of triplicates (versus unstimulated cells). * p < 0.05; ** p < 0.01 (versus ‘LPS alone’ stimulated cells). a: COX-2; b: TNF-α; c: iNOS; d: IL-6; e: IL-1β; f: IL-1α
Since the expression of inflammatory genes in macrophages is in part PPARγ dependent [4, 5, 15], we compared the effects of PPARγ ligands like rosiglitazone or 15d–PGJ2 with LIG (Fig. 6). LIG (at 25 μM) significantly down-regulated 3 of 10 genes, while it up-regulated PPARβ and the receptor of PGE2 and EP-2 [33]. 15d–PGJ2 modulated inflammatory gene expression in a similar pattern, as did LIG, yet its effects were substantially stronger. Remarkably, it shifted expression of PPARγ1 and PGDS to base-line homeostasis (Additional file 1: Figure S2). Rosiglitazone shared some features with LIG (and thus also with 15d–PGJ2), but unlike LIG, it increased the expression of IL-1α, IL-6 and COX-2. Taken together, in murine macrophages the biological effects of LIG matched those of 15d–PGJ2 but differed from rosiglitazone.
Fig. 6.
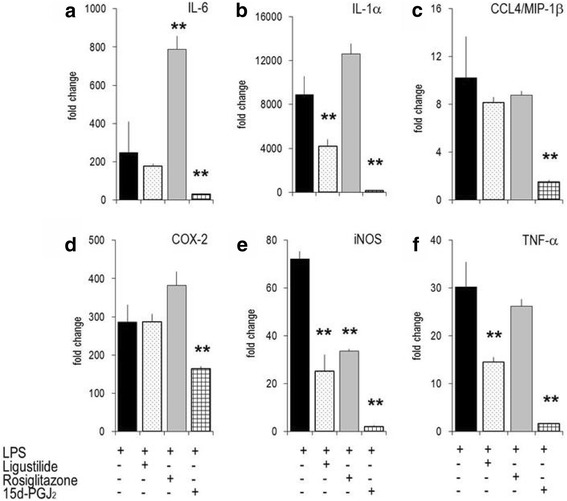
Modulation of gene expression by z-ligustilide, rosiglitazone and 15d–PGJ2 in RAW 264.7 cells. Unstimulated or LPS-stimulated RAW264.7 cells were cultured for 4 h in the presence of graded amounts of LIG (25 μM), rosiglitazone (25 μM) or 15d–PGJ2 (2.5 μM). mRNA levels were determined by RT-PCR. Bars represent mean values of fold change +/− errors of triplicates (versus unstimulated cells; set at 1). ** p-value <0.01 (versus ‘LPS-alone’ stimulated cells). a: IL-6; b: IL-1α; c: CCL4/MIP-1β; d: COX-2; e: iNOS; f: TNF-α
Z-ligustilide modulates expression of inflammatory genes in human THP-1 cells
Similar to murine macrophages, THP-1 cells responded to LPS stimulation by extensively up-regulated interleukins (IL-1α, IL-6), chemokines (IL-8, CXCL2/MIP-2, CCL20/MIP3α) or cytokines (e.g. TNF-α) (Fig. 7). All three compounds tested altered the gene expression pattern in a similar way, and their effects could be ranked as for RAW264.7 cells (i.e. 15d–PGJ2 > LIG > rosiglitazone). Other genes involved in inflammatory pathways including PPARα, 5-LOX and MMP − 9 were refractory to LIG, 15d–PGJ2 and rosiglitazone (data not shown).
Fig. 7.

Effect of z-ligustilide on the expression of genes involved in the inflammatory response of THP-1 cells. Quantitative RT-PCR was made with RNA obtained from THP-1 cells that were stimulated with LPS for 4 h without or with LIG (25 μM), rosiglitazone (25 μM), 15d–PGJ2 (2.5 μM). a: COX-2; b: IL-1α; c: IL-6; d: TNF-α; e: CCL20/MIP-3α; f: MIP-2; 7 g: CXCL8/IL-8; h: MMP-9. Bars represent mean values of fold change +/− errors of triplicates (versus unstimulated cells). * p < 0.05; ** p < 0.01 (versus ‘LPS-only’ stimulated cells)
Z-ligustilide inhibits nuclear translocation of NF-κBp65
In order to further detect early effects of LIG on cell activation, the nuclear translocation of NF-κB during LPS-activation of RAW264.7 cells was measured by quantitative cytometric techniques. Cells responded to stimulation within 20 min by a substantial accumulation of NF-κBp65 in the nucleus; this was reflected by the shift in the ratio of nuclear/cytoplasmic fluorescence as shown in Fig. 8. In cells, which were pre-treated with LIG, the translocation of NF-κBp65 into the nucleus was significantly reduced in a concentration-dependent way.
Fig. 8.
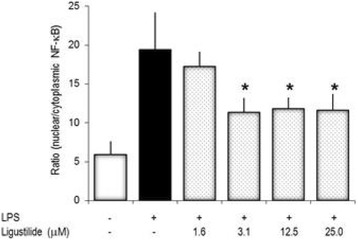
Early events in cell activation are impaired by z-ligustilide. RAW264.7 cells were pre-treated with LIG, activated with 1 μg/mL LPS for 20 min and the cytoplasmic-nuclear translocation of NF-κBp65 determined by quantitative cytometric technique. Y-axis: Ratio of nuclear versus cytoplasmic immunofluorescence. * p < 0.05 (relative to LPS-treated cells)
Discussion
The coordinated responses to inflammatory stimuli include synthesis and secretion of mediators during the progression of inflammation and its resolution by orchestrated production of endogenous molecules that terminate inflammation. Among these, cyclopentenone prostaglandins, lipoxins, NF-κB and mediators of apoptosis have recently attracted considerable interest (reviewed in [2]. Any deviation in these fine-tuned interactions can lead to an excessive and uncontrolled response that might result in chronic inflammatory processes. Given the fact that inflammatory responses are vital for the host’s appropriate defense against pathogens and external insults, the major challenge relies in the containment of inflammation i.e. its appropriate timing and extent of resolution. In this study, we have shown that LIG behaves to a large extent like anti-inflammatory prostaglandins and thus might contribute to the adequate resolution of inflammation as well as to the attenuation of chronic inflammatory processes (Additional file 1: Table S2).
Our experimental approach was instigated by the identification of LIG, a natural substance with anti-diabetic properties [23]. We hypothesized that LIG and PPARγ agonists like rosiglitazone interfere with inflammatory responses in a similar manner and compared their impact on hallmarks of inflammation including the gene expression and production of nitric oxide, PGE2, chemokines, interleukins and cytokines. Whereas rosiglitazone moderately impaired NO production and even enhanced PGE2 secretion, LIG potently diminished the production of both metabolites and performed like 15d–PGJ2. Previously, 15d–PGJ2 had been identified as a PPARγ ligand that induced adipogenesis [34, 35]. More recent data showed that PPARγ ligands regulated inflammatory responses by interfering with the expression of iNOS and cytokines including IL-1, TNF-α or gelatinase B (MMP-9) in macrophages, which express the respective PPAR isoform [4, 5]. Yet, cyclopentenone prostaglandins influenced the inflammatory responses via different signaling pathways in macrophages from PPARγ−/− mice [36, 37]. Conceivably, insufficient expression of PPAR isoforms in the studied cellular systems might account for the unresponsiveness to PPARγ ligands. Yet, PPARβ and PPARγ mRNA were readily detected in RAW264.7 cells, THP-1 cells and peripheral blood leukocytes (not shown). Collectively, the data support the notion that there is an association of LIG activity and PPARγ expression in macrophages; yet, this interaction is not causally related to inflammatory pathways [10, 38].
Experimental data provide a mechanistic explanation for PPARγ-independent suppression of NF-κB by 15d–PGJ2 [15, 39]. LIG and 15d–PGJ2 possess α, β or α, β, γ unsaturated carbonyl structures that react with nucleophiles including free sulfhydryl groups of glutathione or cysteine. This prevents the binding of p65 homodimers of the NF-κB complexes [15, 40] and subsequent gene activation. In the NF-κB signaling cascade, IκB-kinase also contains cysteine residues that are prone to covalent modification. Indeed, cyclopentenone prostaglandins specifically inhibit IκB degradation [15]. Importantly, since LIG and 15d–PGJ2 both have unsaturated carbonyl structures, they might share similar modes of action, as has been described for other natural substances [40, 41]. By structural analogy to 15d–PGJ2, there is evidence for a structure-activity relationship between LIG and the effect on NF-κB. LIG and 15d–PGJ2 might differ in their biological half-life and the propensity to bind to reactive groups, which would explain their different IC50 values. The observation that LIG reduced nuclear translocation of NF-κBp65 in LPS-stimulated cells provides additional evidence that it modulated cellular activities along the NF-κB signaling pathway. It should be noted that sensu stricto the immunofluoerescence data only show that LIG impaired the cytoplasmic-nuclear translocation of NF-κB.
The production of most of the cytokines and chemokines was blunted by LIG in macrophages, monocytic leukemia cells (THP-1) and PBLs. Notable exceptions were IL-1β and IL-6, which are assigned pro-inflammatory properties. LIG increased the expression of these ILs in the blood compartment, but not in macrophages or monocytic cells. We have made similar observations with other nutrients like ω-3 PUFAs, resveratrol and tomato aqueous extracts [28, 30, 31]. This emphasizes the dichotomic effects of nutrients in distinct cellular compartments. Conceivably, an enhanced expression of IL-6 and IL-1β ameliorate the adaptive immune response and might act on the differentiation of the M1 and M2 macrophage subtypes.
The biological consequences of this mode of action are far-reaching and give a plausible explanation for the observed pleiotropic activity pattern of LIG. The genes that were down-regulated by 15d–PGJ2 and LIG (i.e. IL-1α, IL-1β, IL-6, IL-8, TNF-α, iNOS, CCL4/MIP-1β) possess NF-κB regulatory sequences/binding sites or are susceptible to the NF-κB signalling pathway (see also [42, 43]. Transcriptional activation in response to inflammatory stimuli can further be mediated by combined action with other factors [44, 45]. Given the importance of NF-κB in inflammatory diseases (reviewed in e.g. [46], even minor shifts in the amount, cellular localization or association of NF-κB elements drastically influence the outcome of the response. Admittedly, NF-κB ablation is not a panacea for inflammation, since it can result in severe apoptotic tissue damage [47]. A notable exception is the COX-2 expression in RAW264.7 cells, which remains unaffected by LIG (Fig. 5), while PGE2 production was inhibited (Fig. 1b). Concomitantly, PGES expression decreased in LIG treated cells, whereas mRNA levels of COX-2, EP-2 and PGDS were maintained. Consequently, LIG appears not to affect the part of the eicosanoid synthesis pathway that is required for the resorption of inflammation. Preserved COX-2 expression, mediated by LIG, ought to be beneficial during the resorption of inflammation when COX-2 is required for the concurrent PGDS-dependent synthesis of cyclopentenone prostaglandin PGJ2 [48].
We have demonstrated in this study that LIG, and to a larger extent 15d–PGJ2, markedly diminished the secretion of chemokines such as CXCL8/IL-8 and macrophage inflammatory proteins, CCL4/MIP-3α. Quantitative RT-PCR analysis revealed that the compounds attenuated the expression of these and other chemokines (CXCL1/MIP-1, CXCL2/MIP-2) by activated cells of the macrophage lineage. The CXC chemokines (e.g. murine CXCL2/MIP-2, human CXCL8/IL-8) play a role in recruitment of neutrophils, while the CC chemokine CCL4/MIP-3α recruits macrophages and lymphocytes. Chemokines and cytokines are mutually induced by LPS in a specific temporal pattern with pro-inflammatory cytokines usually preceding chemokine expression. This cross-talk is controlled by NF-κB and relies on NF-κB consensus sequences in the promoter region of chemokine and cytokine genes [49–52]. Conceivably, LIG and related compounds have an indirect effect on chemokine expression e.g. through inhibition of TNF-α expression, which in turn affects chemokine activation. As a consequence of the versatile in vitro effects of LIG described in this study, we anticipate that LIG has in vivo activities in acute inflammation models. Indeed, LIG proved to attenuate inflammation in the carrageenan-induced paw edema model (D. Raederstorff, unpublished results). At this time, comparable studies done in human have not been published. Therefore, the effects on acute and chronic inflammation in humans needs to be established in appropriate nutritional intervention trials.
Conclusions
LIG is a potent anti-inflammatory natural substance that modulates the inflammatory response at various levels. Both by its structure and activity profile it is closely related to the cyclopentenone prostaglandins 15d–PGJ2. This confers LIG the profile of an anti-inflammatory prostaglandin that regulates the resorption of acute inflammation and blunts chronic inflammation.
Acknowledgments
We thank Nicole Seifert for performing the quantitative cytometric analysis for NF-κB.
Funding
Not applicable.
Availability of data and materials
These can be requested from the corresponding author.
Abbreviations
- CCL
Ligand of the C-C family of chemokines
- CXCL
Ligand of the C-X-C family of chemokines
- IC50
50% (half-maximal) inhibitory concentration
- IC80
80% maximal inhibitor concentration
- IL
Interleukin
- INF
Interferon
- iNOS
inducible nitric oxide synthase
- LIG
Ligustilde
- LPS
Lipopolysaccharide
- NF-κB
Nuclear factor-kappaB
- PGE2
Prostaglandin E2
- PGJ2
Prostaglandin J2
- PPAR
Peroxisome proliferator-activator receptor
- TNF-α
Tumor necrosis factor-α
Additional file
Viability of RAW 264.7 cells after incubation with graded amounts of ligustilide, EGCG, resveratrol, 15d–PGJ2 and rosiglitazone for 24 h. Figure S2. Regulation of inflammatory gene expression in RAW264.7 cells by ligustilide (25 μM) rosiglitazone (25 μM) and 15d–PGJ2 (2.5 μM). Table S1. Synopsis of effects on protein secretion by (murine) macrophages or peripheral blood leukocytes (human). Table S1. Sequences of primers and probes used in quantitative real-time PCR. Table S2. Comparison of effects of PGJ2 and ligustilide on cytokines and chemokine expression. (DOCX 465 kb)
Authors’ contributions
Conceived and planned the experiments: JS, PW and IB. Performed the experiment: LG, NR and JS. Analysed and compiled the data: LG and JS. Identified and provided the natural substances: WS. Reviewed and evaluated all data and wrote the manuscript: JS. All authors approved the final manuscript. This is part of LG’s master work in Pharmacy.
Ethics approval and consent to participate
Not applicable.
Consent of publication
Not applicable.
Competing interest
The authors declare that they have no conflict of interest. They were employees of DSM Nutritional Products, which supported the study in the frame of a corporate program of screening for natural anti-inflammatory ingredients.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12986-018-0239-1) contains supplementary material, which is available to authorized users.
Contributor Information
Joseph Schwager, Phone: +41-79-488-09-05, Email: jpschwager@gmail.com.
Lidia Gagno, Email: lidia.gagno@hotmail.com.
Nathalie Richard, Email: nathalie.richard@dsm.com.
Werner Simon, Email: simondowe@bluewin.ch.
Igor Bendik, Email: igor.bendik@dsm.com.
References
- 1.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 2.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nature Rev Drug Discovery. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 3.Willoughby DA, Moore AR, Colville-Nash PR, Gilroy D. Resolution of inflammation. Int J Immunopharmacol. 2000;22:1131–1135. doi: 10.1016/S0192-0561(00)00064-3. [DOI] [PubMed] [Google Scholar]
- 4.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 5.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 6.Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev. 2001;21:185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 7.Colville-Nash PR, Qureshi SS, Willis D, Willoughby DA. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. J Immunol. 1998;161:978–984. [PubMed] [Google Scholar]
- 8.Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 9.Fajas L, Debril MB, Auwerx J. PPARgamma: An essential role in metabolic control. Nutr Metab Cardiovasc Dis. 2001;11:64–69. [PubMed] [Google Scholar]
- 10.Inoue M, Itoh H, Tanaka T, Chun TH, Doi K, Fukunaga Y, Sawada N, Yamshita J, Masatsugu K, Saito T, et al. Oxidized LDL regulates vascular endothelial growth factor expression in human macrophages and endothelial cells through activation of peroxisome proliferator-activated receptor-gamma. Arteriosclerosis thrombosis Vasc Biol. 2001;21:560–566. doi: 10.1161/01.ATV.21.4.560. [DOI] [PubMed] [Google Scholar]
- 11.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 12.Hinz B, Brune K, Pahl A. 15-deoxy-delta(12,14)-prostaglandin J2 inhibits the expression of proinflammatory genes in human blood monocytes via a ppar-gamma-independent mechanism. Biochem Biophys Res Comm. 2003;302:415–420. doi: 10.1016/S0006-291X(03)00195-5. [DOI] [PubMed] [Google Scholar]
- 13.Hortelano S, Castrillo A, Alvarez AM, Bosca L. Contribution of cyclopentenone prostaglandins to the resolution of inflammation through the potentiation of apoptosis in activated macrophages. J Immunol. 2000;165:6525–6531. doi: 10.4049/jimmunol.165.11.6525. [DOI] [PubMed] [Google Scholar]
- 14.Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARamma. J Biol Chem. 2000;275:28028–28032. doi: 10.1074/jbc.M001387200. [DOI] [PubMed] [Google Scholar]
- 15.Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappab signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ, Jia Y. The effect of bioactive compounds in tea on lipid metabolism and obesity through regulation of peroxisome proliferator-activated receptors. Current Opinion in Lipidol. 2015;26:3–9. doi: 10.1097/MOL.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 17.Ge H, Zhang JF, Guo BS, He Q, Wang BY, He B, Wang CQ. Resveratrol inhibits macrophage expression of emmprin by activating ppargamma. Vasc Pharmacol. 2007;46:114–121. doi: 10.1016/j.vph.2006.08.412. [DOI] [PubMed] [Google Scholar]
- 18.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 19.YW S, Chiou WF, Chao SH, Lee MH, Chen CC, Tsai YC. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol. 2011;11:1166–1172. doi: 10.1016/j.intimp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Chung JW, Choi RJ, Seo EK, Nam JW, Dong MS, Shin EM, Guo LY, Kim YS. Anti-inflammator effects of (Z)-ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. Arch Pharmaceutical Res. 2012;35:723–732. doi: 10.1007/s12272-012-0417-z. [DOI] [PubMed] [Google Scholar]
- 21.Zhao LX, Jiang BC, XB W, Cao DL, Gao YJ. Ligustilide attenuates inflammatory pain via inhibition of NFκB-mediated chemokines production in spinal astrocytes. Eur J. Neuroscience. 2014;39:1391–1402. doi: 10.1111/ejn.12502. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Ning ZQ, Shan S, Zhang K, Deng T, XP L, Cheng YY. Phthalide lactones from ligusticum chuanxiong inhibit lipopolysaccharide-induced TNF-alpha production and TNF-alpha-mediated NF-kappab activation. Planta Med. 2005;71:808–813. doi: 10.1055/s-2005-871231. [DOI] [PubMed] [Google Scholar]
- 23.D’Orazio D,; De Saizieu A, Raederstorff D, Schueler G, Teixeira S, Wang Y, Weber P, Wolfram S. Use of phthalide derivative for the preparation of a pharmaceutical or dietary composition for preventing or treating diabetes mellitus. International Publication Number WO-2004/100945 A1. Publication date 25.Nov. 2004.
- 24.Chan MM, Fong D, Ho CT, Huang HI. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem Pharmacol. 1997;54:1281–1286. doi: 10.1016/S0006-2952(97)00504-2. [DOI] [PubMed] [Google Scholar]
- 25.D'Acquisto F, Iuvone T, Rombola L, Sautebin L, Di Rosa M, Carnuccio R. Involvement of NF-kappab in the regulation of cyclooxygenase-2 protein expression in LPS-stimulated J774 macrophages. FEBS Lett. 1997;418:175–178. doi: 10.1016/S0014-5793(97)01377-X. [DOI] [PubMed] [Google Scholar]
- 26.Bednarski JJ, Lyssiotis CA, Roush R, Boitano AE, Glick GD, Opipari AW., Jr A novel benzodiazepine increases the sensitivity of B cells to receptor stimulation with synergistic effects on calcium signaling and apoptosis. J Biol Chem. 2004;279:29615–29621. doi: 10.1074/jbc.M403507200. [DOI] [PubMed] [Google Scholar]
- 27.Bitler CM, Viale TM, Damaj B, Crea R. Hydrolyzed olive vegetation water in mice has anti-inflammatory activity. J Nutr. 2005;135:1475–1479. doi: 10.1093/jn/135.6.1475. [DOI] [PubMed] [Google Scholar]
- 28.Richard N, Porath D, Radspieler A, Schwager J. Effects of resveratrol, piceatannol, tri-acetoxystilbene, and genistein on the inflammatory response of human peripheral blood leukocytes. Mol Nutr & Food Res. 2005;49:431–442. doi: 10.1002/mnfr.200400099. [DOI] [PubMed] [Google Scholar]
- 29.Ding GJ, Fischer PA, Boltz RC, Schmidt JA, Colaianne JJ, Gough A, Rubin RA, Miller DK. Characterization and quantitation of NF-kappab nuclear translocation induced by interleukin-1 and tumor necrosis factor-alpha. Development and use of a high capacity fluorescence cytometric system. J Biol Chem. 1998;273:28897–28905. doi: 10.1074/jbc.273.44.28897. [DOI] [PubMed] [Google Scholar]
- 30.Schwager J, Richard N, Mussler B, Raederstorff D. Tomato aqueous extract modulates the inflammatory profile of immune cells and endothelial cells. Molecules. 2016;21(2):168. doi: 10.3390/molecules21020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwager J, Richard N, Riegger C, Salem N Jr. Omega-3 PUFAs and resveratrol differently modulate acute and chronic inflammatory processes. Biomed Res Int. 2015;535189 10.1155/2015/535189. [DOI] [PMC free article] [PubMed]
- 32.Perez-Perez GI, Shepherd VL, Morrow JD, Blaser MJ. Activation of human THP-1 cells and rat bone marrow-derived macrophages by helicobacter pylori lipopolysaccharide. Inf & Immunity. 1995;63:1183–1187. doi: 10.1128/iai.63.4.1183-1187.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arakawa T, Laneuville O, Miller CA, Lakkides KM, Wingerd BA, DeWitt DL, Smith WL. Prostanoid receptors of murine NIH 3T3 and RAW 264.7 cells. Structure and expression of the murine prostaglandin EP4 receptor gene. J Biol Chem. 1996;271:29569–29575. doi: 10.1074/jbc.271.47.29569. [DOI] [PubMed] [Google Scholar]
- 34.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy-delta 12, 14-prostaglandinJ2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 35.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JMA. Prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 36.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of Ikappab kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 37.Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Nat Acad Sci (USA) 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naitoh T, Kitahara M, Tsuruzoe N. The effect of activation of peroxisome proliferator-activated receptor gamma (PPARgamma) on human monocyte function: PPARgamma ligands do not inhibit tumor necrosis factor-alpha release in human monocytic cell line THP-1. Cell Biol Toxicol. 2000;16:131–135. doi: 10.1023/A:1007694110835. [DOI] [PubMed] [Google Scholar]
- 39.Cernuda-Morollon E, Pineda-Molina E, Canada FJ, Perez-Sala D. 15-deoxy-delta 12,14-prostaglandin J2 inhibition of NF-kappab-DNA binding through covalent modification of the p50 subunit. J Biol Chem. 2001;276:35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Pineres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, Merfort I. Cysteine 38 in p65/nf-kappab plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- 41.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa b is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 42.Harant H, de Martin R, Andrew PJ, Foglar E, Dittrich C, Lindley IJ. Synergistic activation of interleukin-8 gene transcription by all-trans-retinoic acid and tumor necrosis factor-alpha involves the transcription factor NF-kappab. J Biol Chem. 1996;271:26954–26961. doi: 10.1074/jbc.271.43.26954. [DOI] [PubMed] [Google Scholar]
- 43.Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon & Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- 44.Lorsbach RB, Murphy WJ, Lowenstein CJ, Snyder SH, Russell SW. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- 45.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa b/rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 46.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]b activity. Ann Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 47.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappab in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 48.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 49.Chandrasekar B, Melby PC, Sarau HM, Raveendran M, Perla RP, Marelli-Berg FM, Dulin NO, Singh IS. Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-kappa b pathway. J Biol Chem. 2003;278:4675–4686. doi: 10.1074/jbc.M207006200. [DOI] [PubMed] [Google Scholar]
- 50.Sobota RM, Mueller PJ, Heinrich PC, Schaper F. Prostaglandin E1 inhibits IL-6-induced MCP-1 expression by interfering specifically in IL-6-dependent ERK1/2, but not STAT3, activation Biochem J. 2008;412;65–72. [DOI] [PubMed]
- 51.Cooper JA., Jr, Parks JM, Carcelen R, Kahlon SS, Sheffield M, Culbreth, R. Attenuation of interleukin-8 production by inhibiting nuclear factor-kappab translocation using decoy oligonucleotides. Biochem Pharmacol 2000;59:605–613. [DOI] [PubMed]
- 52.Sugita S, Kohno T, Yamamoto K, Imaizumi Y, Nakajima H, Ishimaru T, Matsuyama T. Induction of macrophage-inflammatory protein-3alpha gene expression by TNF-dependent NF-kappab activation. J Immunol. 2002;168:5621–5628. doi: 10.4049/jimmunol.168.11.5621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These can be requested from the corresponding author.


