Abstract
Background:
3-Methoxyphencyclidine (3-MeO-PCP) and 3-methoxyrolicyclidine (3-MeO-PCPy) are two new psychoactive substances (NPS). The aims of the present study were the elucidation of their metabolic fate in rat and pooled human liver microsomes (pHLM) the identification of the cytochrome P450 (CYP) isoenzymes involved and the detectability using standard urine screening approaches (SUSA) after intake of common users’ doses using gas chromatography-mass spectrometry (GC-MS) liquid chromatography-multi-stage mass spectrometry (LC-MSn) and liquid chromatography-high-resolution tandem mass spectrometry (LC-HR-MS/MS)
Methods:
For metabolism studies rat urine samples were treated by solid phase extraction or simple precipitation with or without previous enzymatic conjugate cleavage. After analyses via LC-HR-MSn the phase I and II metabolites were identified
Results:
Both drugs showed multiple aliphatic hydroxylations at the cyclohexyl ring and the heterocyclic ring single aromatic hydroxylation carboxylation after ring opening O-demethylation and glucuronidation. The transferability from rat to human was investigated by pHLM incubations where O-demethylation and hydroxylation were observed. The involvement of the individual CYP enzymes in the initial metabolic steps was investigated after single CYP incubations. For 3-MeO-PCP CYP 2B6 was responsible for aliphatic hydroxylations and CYP 2C19 and CYP 2D6 for O-demethylation. For 3-MeO-PCPy aliphatic hydroxylation was again catalyzed by CYP 2B6 and O-demethylation by CYP 2C9 and CYP 2D6
Conclusions:
As only polymorphically expressed enzymes were involved pharmacogenomic variations might occur but clinical data are needed to confirm the relevance. The detectability studies showed that the authors’ SUSAs were suitable for monitoring the intake of both drugs using the identified metabolites
Keywords: New psychoactive substances, 3-MeO-PCP, 3-MeO-PCPy, metabolism, LC-HR-MSn, screening
Introduction
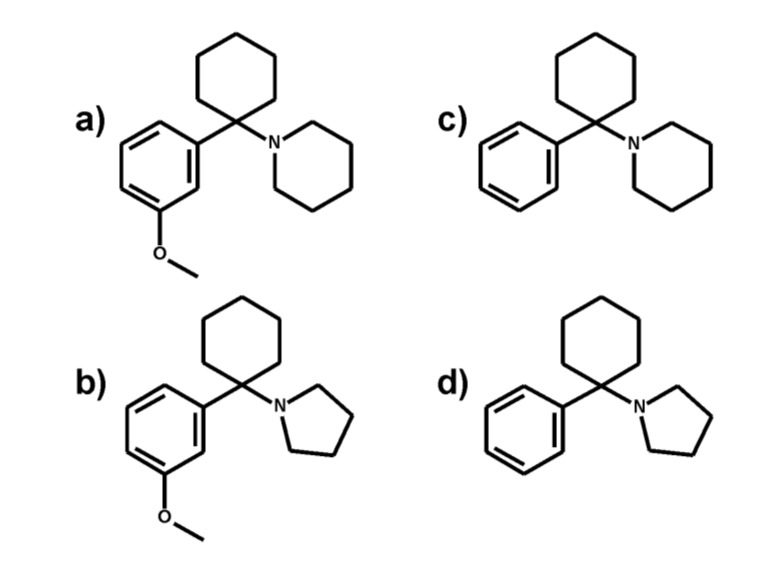
The new psychoactive substances (NPS) 3-methoxyphen-cyclidine (3-MeO-PCP, Fig. 1a) and 3-methoxyrolicyclidine (3-MeO-PCPy, Fig. 1b) are derivatives of phencyclidine (PCP, Fig. 1c), which is known for over 60 years. As reviewed by Morris and Wallach [1], PCP was synthesized first in 1956 and approved in the following years as a non-narcotic anesthetic in veterinary medicine under the trade names Sernyl or Sernylan. The use for human treatment was short-lived and limited due to unfavorable side effects such as agitation, hallucination, and delirium-like conditions. In 1967, phencyclidine appeared as a street drug in the USA under names such as “Peace Pill”, “Angle Dust”, “Blue Dust”, or “Killer Weed”, and in Germany, recreational use appeared in 1977. To overcome national narcotic laws, the structure of phencyclidine was continuously modified and included modifications of the amine moiety, the phenyl, or the piperidine ring [2, 3]. Since the derivative phenylcyclohexylpyrrolidine (also known as rolicyclidine or PCPy, Fig. 1d) has been scheduled in the USA in the 1970s, other modifications were encountered that included alkoxy derivatives [1, 4]. According to reports from the European Monitoring Center for Drugs and Drug Addiction (EMCDDA), 3-MeO-PCP [5] and 4-MeO-PCP have been identified in 2012 and 2011 as NPS, respectively [6, 7]. 3-MeO-PCP and 3-MeO-PCPy are usually snorted, smoked as a PCP-laced marijuana cigarette, ingested as a tablet, or injected intravenously or subcutaneously [1]. Effects such as euphoria and analgesia might share some similarities with PCP [8, 9] although some differences might also exist regarding their psychopharmacological profile [1]. Mechanisms of action include uncompetitive antagonism of the excitatory N-methyl-D-aspartate (NMDA) receptor and reuptake inhibition of noradrenaline, serotonin, and dopamine [10, 11].
Fig. (1).
Structures of 3-MeO-PCP (a), 3-MeO-PCPy (b), PCP (c), and PCPy (d).
However, a current challenge is that such NPS cannot be detected reliably in toxicological urine screening procedures as excretion products are usually unknown. Therefore, the aims of our study were the elucidation of the phase I metabolism of 3-MeO-PCP and 3-MeO-PCPy in rat and human liver microsomes using high resolution LC-MSn (LC-HR-MSn), the identification of the involved CYP isoenzymes, and the investigation of the detectability of common users’ doses in standard urine screening approaches (SUSA) using gas chromatography-mass spectrometry (GC-MS), liquid chromatography-multi-stage mass spectrometry (LC-MSn), and liquid chromatography-high-resolution tandem mass spectrometry (LC-HR-MS/MS).
Experimantal procedures
Chemicals and Reagents
3-MeO-PCP and 3-MeO-PCPy were synthesized and characterized as reported previously [12]. NADP+ and isocitrate dehydrogenase were obtained from Biomol (Hamburg, Germany), Isolute Confirm HCX cartridges (130 mg, 3 mL) from Biotage (Uppsala, Sweden), ammonium sulfate from Fluka (Buches, Switzerland), acetonitrile (LC-MS grade), ammonium formate (analytical grade), dichloromethane, diethyl ether, formic acid (LC-MS grade), isocitrate, magnesium chloride, pyridine, superoxide dismutase, sodium hydroxide, and Tris buffer from Sigma-Aldrich (Taufkirchen, Germany), acetic anhydride, acetic acid, ethyl acetate, glucuronidase/arylsulfatase, hydrochloric acid (37%), isopropanol, methanol (LC-MS grade), aqueous ammonia (32%), and sodium dihydro-gen phosphate and all other chemicals and biochemicals from VWR (Darmstadt, Germany). The baculovirus-infected insect cell microsomes (Supersomes), containing 1 nmol/mL of human cDNA-expressed CYP 1A2, CYP 2A6, CYP 2B6, CYP 2C8, CYP 2C9, CYP 2C19, CYP 2D6, or 2 nmol/mL CYP 2E1, CYP 3A4, CYP 3A5, and pooled human liver microsomes (pHLM, 20 mg microsomal protein/mL, 400 pmol total CYP/mg protein), were obtained from BD Biosciences (Heidelberg, Germany). After delivery, the microsomes were thawed at 37°C, aliquoted, snap-frozen in liquid nitrogen, and stored at -80°C until use.
Urine Samples
Studies were performed using rat urine samples from male Wistar rats (Charles River, Sulzfeld, Germany) for toxicological diagnostic reasons according to the corresponding German law. The compounds were administered once in an aqueous suspension by gastric intubation in a dose of 10 mg/kg body mass (BM) for the identification of the metabolites and once in a 1 mg/kg BM representing common users’ doses, calculated and downscaled using an allometric dose-by-factor approach described by Sharma and McNeill [13].
The rats were housed in metabolism cages for 24 h, having water ad libitum. Urine was collected separately from feces over a 24-h period. Blank urine samples were collected before drug administration to confirm the absence of interfering compounds. The samples were directly analyzed and then stored at -20°C.
Sample Preparation for Identification of Phase I and II Metabolites by LC-HR-MSn
The first of two preparations of high dosed rat urine samples was performed in analogy to a published procedure [14]. A 2-mL aliquot of urine sample was adjusted to pH 5.2 with 1 M acetic acid, a 50-µL aliquot of a mixture (100,000 Fishman units per mL) of glucuronidase (EC No. 3.2.1.31) and arylsulfatase (EC No. 3.1.6.1) from Helix pomatia L was added, and subsequently incubated at 50°C for 2 h. Afterwards, the sample was centrifuged at 5,000 rpm for 5 min, the supernatant loaded onto a cation exchange Confirm HCX solid phase extraction (SPE) cartridge, previously conditioned with 1 mL methanol and 1 mL water. After passing the SPE cartridge, washing steps were performed with 1 mL water, 1 mL 0.01 M hydrochloric acid, 1 mL water, and 1 mL methanol. The retained basic compounds were eluted using 1 mL of a freshly prepared mixture of methanol/32% aqueous ammonia (98:2, v/v). The eluate was subsequently evaporated to dryness under a stream of nitrogen at 70°C and reconstituted with 50 µL of methanol. A 10-µL aliquot was injected for LC-HR-MSn analysis. Conditions were as described below.
The second sample preparation was performed according to Wissenbach et al. [15]. A 100-µL aliquot of the high dosed rat urine sample was mixed with 500 µL of acetonitrile and vortexed for 2 min. Afterwards, the sample was centrifuged for 2 min at 10,000 g, the supernatant evaporated to dryness under a gentle stream of nitrogen at 70°C, and the residue reconstituted with a mixture of mobile phase A and B (1:1, v/v) described below for LC-MSn. A 5-µL aliquot was injected onto the LC-HR-MSn system. Conditions were as described below.
Microsomal Incubations for pHLM and Initial CYP Activity Screening Studies
The incubations were performed according to a published procedure [14] using CYP 1A2, CYP 2A6, CYP 2B6, CYP 2C8, CYP 2C9, CYP 2C19, CYP 2D6, CYP 2E1, CYP 3A4, CYP 3A5, or pHLM for 30 min at 37°C. The final incubation mixture consisted of 90 mM buffer (Tris buffer for CYP 2A6 and CYP 2C9, phosphate buffer for all other incubations), 25 µM drug as substrate, 200 U/mL superoxide dismutase, 75 pmol/mL CYP isoenzyme, and regenerating system consisted of 5 mM magnesium chloride, 5 mM isocitrate, 1.5 mM NADP+, and 0.5 U/mL isocitrate dehydro-genase in a final volume of 50 µL. Reaction was initiated by addition of regenerating system and stopped with 50 µL ice-cold acetonitrile. Afterwards, the sample was centrifuged for 5 min at 10,000 g, the supernatant transferred to a glass vial, and a 10-µL aliquot was injected onto the LC-MSn system. Conditions were as described below.
Identification of Phase I and II Metabolites in Urine by LC-HR-MSn
Phase I and II metabolites in urine were identified by a system consisted of an Orbitrap Velos Pro (Thermo Fischer Scientific, Dreieich, Germany) equipped with a heated electrospray ionization (HESI)-II source coupled to a Dionex Ultimate 3000 LC system. The used conditions and gradient elution were according to a published procedure [14], gradient program was composed according to Wissenbach et al. [15]. A parent mass list was used containing proposed metabolites whereby the list was divided into two separate experiments to reduce the amount of preferred ions for monitoring. The system was running Thermo Scientific Xcalibur 2.2.
Analysis of the Metabolites in Microsomal Incubations by LC-MSn
LC-MSn analysis of the metabolites in microsomal incubations was performed according to a published procedure [14]. The system consisted of a LXQ linear ion trap MS equipped with an HESI-II source coupled to an Accela LC system consisting of a degasser, a quaternary pump, and an autosampler (all from ThermoFisher Scientific, Dreieich, Germany). The gradient program was composed according to Wissenbach et al. [15] and the same as described for LC-HR-MSn. The system was running Thermo Scientific Xcalibur 2.0.7.
GC-MS SUSA
According to a published procedure [16], a 5-mL aliquot of the low dosed rat urine sample was divided into two aliquots, the first was submitted to acid hydrolysis for 15 min and basified to pH 9, followed by combining with the second and subsequent extraction with a dichloromethane-isopropanol-ethyl acetate mixture (1:1:3, v/v/v). After evaporation, the residue was acetylated with an acetic anhydride-pyridine mixture (3:2, v/v) under microwave irradiation, again evaporated and reconstituted in 100 µL of methanol. A 1-µL aliquot was injected onto the GC-MS system, consisted of a Hewlett-Packard (HP; Agilent, Waldbronn, Germany) 5890 Series II gas chromatograph combined with an Agilent 5972 MSD mass spectrometer running HP MS ChemStation (DOS series) B.02.05. GC and MS conditions were according to a published procedure [16].
The full scan data files were evaluated by use of the automated mass spectral deconvolution and identification system (AMDIS, http://chemdata.nist.gov/mass-spc/amdis/) in simple mode. The target library was a modified version of the Maurer/Pfleger/Weber MPW_2016 library [17]. The deconvolution settings were according to Meyer et al. [18].
LC-MSN SUSA
In accordance to Wissenbach et al. [19], a 100-µL aliquot of the low dosed urine sample was worked up as described for the identification of phase I and II metabolites in urine. A 10-µL aliquot was injected onto the LC-MSn system. Conditions were as described for analysis of the metabolites in microsomal incubations.
For data acquisition, ThermoFisher ToxID 2.1.1 for automatic target screening in the MS2 screening mode was used. The settings were according to Wissenbach et al. [19]. ToxID was run automatically after file acquisition by using an Xcalibur processing method starting the software tool. The target library was a modified version of the Maurer/Wissenbach/Weber MWW_2014 library [20].
LC-HR-MS/MS SUSA
In accordance to Helfer et al. [21], a 100-µL aliquot of the low dosed rat urine sample was mixed with 500 µL of acetonitrile and vortexed for 2 min. After centrifugation for 2 min at 10,000 g, the supernatant was evaporated to dryness under a gentle stream of nitrogen at 70°C and the residue reconstituted with a mixture of mobile phase A and B (1:1, v/v) described below. A 10-µL aliquot was injected onto the LC-HR-MS/MS system, consisted of a Q-Exactive system equipped with an HESI-II source (ThermoFisher Scientific, Dreieich, Germany) coupled to an Accela LC system, consisting of a degasser, a quaternary pump, and an HTC PAL auto-sampler (CTC Analytics AG, Zwingen, Switzerland). Gradient elution and MS conditions were according to a published procedure [21]. The system was running Thermo Scientific Xcalibur 3.0.63. For data acquisition, ThermoFisher TraceFinder Clinical Research 3.2 software was used as described by Helfer et al. [21].
Determination of the Detection Limits of the Parent Drugs in Urine for SUSA
For assessing the general performance of the SUSA, the determination of the limits of detection (LOD) for the parent drugs were determined [22]. For this purpose, 3-MeO-PCP and 3-MeO-PCPy were spiked in rat urine in increasing concentrations, respectively, and analyzed via GC-MS, LC-MSn, and LC-HR-MS/MS SUSA. The concentration level with a signal-to-noise of three was defined as LOD.
Results and discussion
Identification of Phase I and II Metabolites by LC-HR-MSn
With the SPE-based sample preparation, only basic compounds were retained leading to cleaner extracts. For the non-basic compounds and the phase II metabolites, the second sample preparation was performed after simple precipitation.
The proposed structural formulas were deduced by comparing the spectra of the metabolites with those of the parent compounds. Precursor masses (PM) are from MS1 spectra, fragment ions from MS2 spectra. All masses are given with the calculated exact masses. The phase I metabolites found for 3-MeO-PCP are shown in Table 1, those for 3-MeO-PCPy in Table 2. The phase II metabolites found for 3-MeO-PCP are shown in Table 3, those for 3-MeO-PCPy in Table 4. Overall, for 3-MeO-PCP, 30 phase I and seven phase II metabolites, and for 3-MeO-PCPy, 26 phase I and eight phase II metabolites were detected.
Table 1.
3-MeO-PCP and its phase I metabolites detected in rat urine by LC-HR-MSn with protonated precursor mass (PM), characteristic fragment ions (FI), calculated exact masses, proposed elemental composition, mass error, relative intensity, and retention times (RT).
| No. | Metabolite |
Measured Accurate
Mass (m/z) |
Calculated Exact
Mass (m/z) |
Error (ppm) | Elemental Composition | Relative Intensity (%) | RT (min) |
|---|---|---|---|---|---|---|---|
| 1 | 3-MeO-PCP | 10.6 | |||||
| 274.2166 | 274.2171 | -1.82 | C18H28NO | 100 | |||
| 86.0963 | 86.0970 | -8.13 | C5H12N | 100 | |||
| 189.1277 | 189.1279 | -1.06 | C13H17O | 40 | |||
| 2 | 3-MeO-PCP-M (O-demethyl-) | 7.3 | |||||
| 260.2006 | 260.2014 | -3.07 | C17H26NO | 100 | |||
| 86.0964 | 86.0970 | -6.97 | C5H12N | 100 | |||
| 175.1122 | 175.1123 | -0.57 | C12H15O | 26 | |||
| 3 | 3-MeO-PCP-M (O-demethyl-piperidine-HO-) | 6.1 | |||||
| 276.1955 | 276.1964 | -3.26 | C17H26NO2 | 100 | |||
| 84.0808 | 84.0813 | -5.95 | C5H10N | 8 | |||
| 102.0914 | 102.0919 | -4.90 | C5H12NO | 100 | |||
| 175.1122 | 175.1123 | -0.57 | C12H15O | 30 | |||
| 4 | 3-MeO-PCP-M (O-demethyl-cyclohexyl-HO-) | 5.1 | |||||
| 276.1959 | 276.1964 | -1.81 | C17H26NO2 | 100 | |||
| 86.0964 | 86.0970 | -6.97 | C5H12N | 100 | |||
| 173.0965 | 173.0966 | -0.58 | C12H13O | 30 | |||
| 191.1071 | 191.1072 | -0.52 | C12H15O2 | 6 | |||
| 5 | 3-MeO-PCP-M (O-demethyl-cyclohexyl-HO-dehydro-oxo-piperidine-) | 5.7 | |||||
| 288.1598 | 288.1600 | -0.69 | C17H22NO3 | 100 | |||
| 80.0497 | 80.0500 | -3.75 | C5H6N | 5 | |||
| 98.0601 | 98.0606 | -5.10 | C5H8NO | 100 | |||
| 173.0965 | 173.0966 | -0.58 | C12H13O | 6 | |||
| 191.1073 | 191.1072 | 0.52 | C12H15O2 | 1 | |||
| 6 | 3-MeO-PCP-M (piperidine-HO-) | 8.9 | |||||
| 290.2115 | 290.2120 | -1.72 | C18H28NO2 | 28 | |||
| 84.0808 | 84.0813 | -5.95 | C5H10N | 1 | |||
| 102.0914 | 102.0919 | -4.90 | C5H12NO | 18 | |||
| 189.1278 | 189.1279 | -0.53 | C13H17O | 8 | |||
| 7 | 3-MeO-PCP-M (cyclohexyl-HO-) | 7.6 | |||||
| 290.2177 | 290.2170 | 2.41 | C18H28NO2 | 60 | |||
| 86.0964 | 86.0970 | -6.97 | C5H12N | 100 | |||
| 187.1122 | 187.1123 | -0.53 | C13H15O | 52 | |||
| 205.1227 | 205.1229 | -0.98 | C13H17O2 | 14 | |||
| 8 | 3-MeO-PCP-M (O-demethyl-piperidine-di-HO-) isomer 1 | 7.3 | |||||
| 292.1906 | 292.1913 | -2.40 | C17H26NO3 | 30 | |||
| 101.0598 | 101.0603 | -4.95 | C5H9O2 | 18 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 100 | |||
| 175.1121 | 175.1123 | -1.14 | C12H15O | 20 | |||
| 9 | 3-MeO-PCP-M (O-demethyl-piperidine-di-HO-) isomer 2 | 8.1 | |||||
| 292.1911 | 292.1913 | -0.68 | C17H26NO3 | 100 | |||
| 101.0598 | 101.0603 | -4.95 | C5H9O2 | 38 | |||
| 118.0865 | 118.0868 | -2.54 | C5H12NO2 | 100 | |||
| 175.1121 | 175.1123 | -1.14 | C12H15O | 28 | |||
| 10 | 3-MeO-PCP-M (O-demethyl-piperidine-di-HO-) isomer 3 | 9.2 | |||||
| 292.1912 | 292.1913 | -0.34 | C17H26NO3 | 26 | |||
| 101.0600 | 101.0603 | -2.97 | C5H9O2 | 1 | |||
| 118.0865 | 118.0868 | -2.54 | C5H12NO2 | 1 | |||
| 175.1119 | 175.1123 | -2.28 | C12H15O | 1 | |||
| 11 | 3-MeO-PCP-M (O-demethyl-cyclohexyl-HO-piperidine-HO-) isomer 1 | 2.4 | |||||
| 292.1909 | 292.1913 | -1.37 | C17H26NO3 | 100 | |||
| 84.0807 | 84.0813 | -7.14 | C5H10N | 1 | |||
| 102.0914 | 102.0919 | -4.90 | C5H12NO | 100 | |||
| 173.0965 | 173.0966 | -0.58 | C12H13O | 14 | |||
| 191.1071 | 191.1072 | -0.52 | C12H15O2 | 2 | |||
| 12 | 3-MeO-PCP-M (O-demethyl-cyclohexyl-HO-piperidine-HO-) isomer 2 | 4.1 | |||||
| 292.1909 | 292.1913 | -1.37 | C17H26NO3 | 78 | |||
| 84.0808 | 84.0813 | -5.95 | C5H10N | 1 | |||
| 102.0913 | 102.0919 | -5.88 | C5H12NO | 100 | |||
| 173.0963 | 173.0966 | -1.73 | C12H13O | 20 | |||
| 191.1070 | 191.1072 | -1.05 | C12H15O2 | 3 | |||
| 13 | 3-MeO-PCP-M (piperidine-di-HO-) | 10.2 | |||||
| 306.2064 | 306.2069 | -1.63 | C18H28NO3 | 50 | |||
| 101.0597 | 101.0603 | -5.94 | C5H9O2 | 16 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 100 | |||
| 189.1276 | 189.1279 | -1.59 | C13H17O | 56 | |||
| 14 | 3-MeO-PCP-M (cyclohexyl-HO-piperidine-HO-) isomer 1 | 4.2 | |||||
| 306.2065 | 306.2069 | -1.31 | C18H28NO3 | 100 | |||
| 84.0806 | 84.0813 | -8.33 | C5H10N | 1 | |||
| 102.0913 | 102.0919 | -5.88 | C5H12NO | 100 | |||
| 187.1120 | 187.1123 | -1.60 | C13H15O | 24 | |||
| 205.1226 | 205.1229 | -1.46 | C13H17O2 | 8 | |||
| 15 | 3-MeO-PCP-M (cyclohexyl-HO-piperidine-HO-) isomer 2 | 5.8 | |||||
| 306.2065 | 306.2069 | -1.31 | C18H28NO3 | 38 | |||
| 102.0914 | 102.0919 | -4.90 | C5H12NO | 100 | |||
| 187.1122 | 187.1123 | -0.53 | C13H15O | 30 | |||
| 205.1228 | 205.1229 | -0.49 | C13H17O2 | 10 | |||
| 16 | 3-MeO-PCP-M (O-demethyl-carboxy-) methyl artifact | 8.7 | |||||
| 306.2062 | 306.2069 | -2.29 | C18H28NO3 | 100 | |||
| 115.0756 | 115.0759 | -2.61 | C6H11O2 | 30 | |||
| 132.1022 | 132.1025 | -2.27 | C6H14NO2 | 100 | |||
| 175.1122 | 175.1123 | -0.57 | C12H15O | 18 | |||
| 17 | 3-MeO-PCP-M (O-demethyl-cyclohexyl-HO-piperidine-di-HO-) isomer 1 | 4.9 | |||||
| 308.1856 | 308.1862 | -1.95 | C17H26NO4 | 100 | |||
| 101.0598 | 101.0603 | -4.95 | C5H9O2 | 1 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 100 | |||
| 173.0965 | 173.0966 | -0.58 | C12H13O | 14 | |||
| 191.1070 | 191.1072 | -1.05 | C12H15O2 | 2 | |||
| 18 | 3-MeO-PCP-M (O-demethyl-cyclohexyl-HO-piperidine-di-HO-) isomer 2 | 5.1 | |||||
| 308.1858 | 308.1862 | -1.30 | C17H26NO4 | 20 | |||
| 101.0598 | 101.0603 | -4.95 | C5H9O2 | 1 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 4 | |||
| 191.1068 | 191.1072 | -2.09 | C12H15O2 | 1 | |||
| 19 | 3-MeO-PCP-M (O-demethyl-cyclohexyl-HO-piperidine-di-HO-) isomer 3 | 5.5 | |||||
| 308.1857 | 308.1862 | -1.62 | C17H26NO4 | 78 | |||
| 101.0597 | 101.0603 | -5.94 | C5H9O2 | 1 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 100 | |||
| 173.0964 | 173.0966 | -1.16 | C12H13O | 20 | |||
| 191.1070 | 191.1072 | -1.05 | C12H15O2 | 3 | |||
| 20 | 3-MeO-PCP-M (carboxy-) methyl artifact | 11.7 | |||||
| 320.2216 | 320.2226 | -3.12 | C19H39NO3 | 100 | |||
| 115.0756 | 115.0759 | -2.61 | C6H11O2 | 30 | |||
| 132.1022 | 132.1025 | -2.27 | C6H14NO2 | 100 | |||
| 189.1278 | 189.1279 | -0.53 | C13H17O | 40 | |||
| 21 | 3-MeO-PCP-M (O-demethyl-carboxy-cyclohexyl-HO-) methyl artifact isomer 1 | 5.2 | |||||
| 322.2016 | 322.2018 | -0.62 | C18H28NO4 | 100 | |||
| 115.0755 | 115.0759 | -3.48 | C6H11O2 | 28 | |||
| 132.1021 | 132.1025 | -3.03 | C6H14NO2 | 100 | |||
| 173.0963 | 173.0966 | -1.73 | C12H13O | 6 | |||
| 191.1069 | 191.1072 | -1.57 | C12H15O2 | 1 | |||
| 22 | 3-MeO-PCP-M (O-demethyl-carboxy-cyclohexyl-HO-) methyl artifact isomer 2 | 5.8 | |||||
| 322.2012 | 322.2018 | -1.86 | C18H28NO4 | 100 | |||
| 115.0755 | 115.0759 | -3.48 | C6H11O2 | 26 | |||
| 132.1021 | 132.1025 | -3.03 | C6H14NO2 | 100 | |||
| 173.0964 | 173.0966 | -1.16 | C12H13O | 14 | |||
| 191.1070 | 191.1072 | -1.05 | C12H15O2 | 2 | |||
| 23 | 3-MeO-PCP-M (O-demethyl-carboxy-cyclohexyl-HO-) methyl artifact isomer 3 | 6.2 | |||||
| 322.2011 | 322.2018 | -2.17 | C18H28NO4 | 58 | |||
| 115.0755 | 115.0759 | -3.48 | C6H11O2 | 30 | |||
| 132.1022 | 132.1025 | -2.27 | C6H14NO2 | 100 | |||
| 173.0965 | 173.0966 | -0.58 | C12H13O | 20 | |||
| 191.1071 | 191.1072 | -0.52 | C12H15O2 | 2 | |||
| 24 | 3-MeO-PCP-M (cyclohexyl-HO-piperidine-di-HO-) | 3.8 | |||||
| 322.2016 | 322.2018 | -0.62 | C18H28NO4 | 100 | |||
| 100.0758 | 100.0762 | -4.00 | C5H10NO | 1 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 100 | |||
| 187.1120 | 187.1123 | -1.60 | C13H15O | 24 | |||
| 205.1226 | 205.1229 | -1.46 | C13H17O2 | 8 | |||
| 25 | 3-MeO-PCP-M (carboxy-cyclohexyl-HO-) methyl artifact isomer 1 | 7.2 | |||||
| 336.2175 | 336.2175 | 0.00 | C19H30NO4 | 36 | |||
| 115.0756 | 115.0759 | -2.61 | C6H11O2 | 34 | |||
| 132.1023 | 132.1025 | -1.51 | C6H14NO2 | 100 | |||
| 187.1124 | 187.1123 | 0.53 | C13H15O | 20 | |||
| 205.1229 | 205.1229 | 0.00 | C13H17O2 | 4 | |||
| 26 | 3-MeO-PCP-M (carboxy-cyclohexyl-HO-) methyl artifact isomer 2 | 8.0 | |||||
| 336.2173 | 336.2175 | -0.59 | C19H30NO4 | 52 | |||
| 115.0755 | 115.0759 | -3.48 | C6H11O2 | 30 | |||
| 132.1021 | 132.1025 | -3.03 | C6H14NO2 | 100 | |||
| 187.1121 | 187.1123 | -1.07 | C13H17O | 24 | |||
| 205.1226 | 205.1229 | -1.46 | C13H17O2 | 8 | |||
| 27 | 3-MeO-PCP-M (carboxy-cyclohexyl-HO-) methyl artifact isomer 3 | 8.5 | |||||
| 336.2172 | 336.2175 | -0.89 | C19H30NO4 | 80 | |||
| 115.0756 | 115.0759 | -2.61 | C6H11O2 | 10 | |||
| 132.1022 | 132.1025 | -2.27 | C6H14NO2 | 34 | |||
| 187.1122 | 187.1123 | -0.53 | C13H15O | 20 | |||
| 205.1228 | 205.1229 | -0.49 | C13H17O2 | 4 | |||
| No. | Metabolite |
Measured Accurate Mass (m/z) |
Calculated Exact Mass (m/z) |
Error (ppm) | Elemental Composition | Relative Intensity (%) | RT (min) |
| 28 | 3-MeO-PCP-M (carboxy-cyclohexyl-HO-) methyl artifact isomer 4 | 8.9 | |||||
| 336.2172 | 336.2175 | -0.89 | C19H30NO4 | 24 | |||
| 115.0755 | 115.0759 | -3.48 | C6H11O2 | 32 | |||
| 132.1022 | 132.1025 | -2.27 | C6H14NO2 | 100 | |||
| 187.1122 | 187.1123 | -0.53 | C13H15O | 18 | |||
| 205.1227 | 205.1229 | -0.98 | C13H17O2 | 2 | |||
| 29 | 3-MeO-PCP-M (carboxy-cyclohexyl-HO-) methyl artifact isomer 5 | 9.1 | |||||
| 336.2171 | 336.2175 | -1.19 | C19H30NO4 | 45 | |||
| 115.0755 | 115.0759 | -3.48 | C6H11O2 | 20 | |||
| 132.1021 | 132.1025 | -3.03 | C6H14NO2 | 60 | |||
| 187.1122 | 187.1123 | -0.53 | C13H15O | 8 | |||
| 205.1226 | 205.1229 | -1.46 | C13H17O2 | 100 | |||
| 30 | 3-MeO-PCP-M (carboxy-alkyl-HO-) | 10.5 | |||||
| 336.2172 | 336.2175 | -0.89 | C19H30NO4 | 50 | |||
| 99.0439 | 99.0446 | -7.07 | C5H7O2 | 1 | |||
| 131.0704 | 131.0708 | -3.05 | C6H11O3 | 6 | |||
| 148.0970 | 148.0974 | -2.70 | C6H14NO3 | 100 | |||
| 189.1277 | 189.1279 | -1.06 | C13H17O | 28 | |||
Table 2.
3-MeO-PCPy and its phase I metabolites detected in rat urine by LC-HR-MSn with protonated precursor mass (PM), characteristic fragment ions (FI), calculated exact masses, proposed elemental composition, mass error, relative intensity, and retention times (RT).
| No. | Metabolite |
Measured Accurate
Mass (m/z) |
Calculated Exact
Mass (m/z) |
Error (ppm) | Elemental Composition | Relative Intensity (%) | RT (min) |
|---|---|---|---|---|---|---|---|
| 31 | 3-MeO-PCPy | 10.0 | |||||
| 260.2010 | 260.2014 | -1.54 | C17H26NO | 42 | |||
| 189.1276 | 189.1279 | -1.59 | C13H17O | 100 | |||
| 32 | 3-MeO-PCPy-M (O-demethyl-) | 6.6 | |||||
| 246.1850 | 246.1858 | -3.25 | C16H24NO | 100 | |||
| 72.0807 | 72.0813 | -8.32 | C4H10N | 68 | |||
| 175.1121 | 175.1123 | -1.14 | C12H15O | 100 | |||
| 33 | 3-MeO-PCPy-M (O-demethyl-pyrrolidine-HO-) | 6.1 | |||||
| 262.1802 | 262.1807 | -1.91 | C16H24NO2 | 100 | |||
| 70.0651 | 70.0657 | -8.56 | C4H8N | 1 | |||
| 88.0756 | 88.0762 | -6.81 | C4H10NO | 100 | |||
| 175.1120 | 175.1123 | -1.71 | C12H15O | 24 | |||
| 34 | 3-MeO-PCPy-M (O-demethyl-cyclohexyl-HO-) isomer 1 | 4.4 | |||||
| 262.1801 | 262.1807 | -2.29 | C16H24NO2 | 70 | |||
| 173.0963 | 173.0966 | -1.73 | C12H13O | 100 | |||
| 191.1069 | 191.1072 | -1.57 | C12H15O2 | 18 | |||
| 35 | 3-MeO-PCPy-M (O-demethyl-cyclohexyl-HO-) isomer 2 | 4.6 | |||||
| 262.1801 | 262.1807 | -2.29 | C16H24NO2 | 100 | |||
| 173.0963 | 173.0966 | -1.73 | C12H13O | 100 | |||
| 191.1062 | 191.1072 | -5.23 | C12H15O2 | 14 | |||
| 36 | 3-MeO-PCPy-M (O-demethyl-cyclohexyl-HO-) isomer 3 | 5.6 | |||||
| 262.1806 | 262.1807 | -0.38 | C16H24NO2 | 64 | |||
| 173.0966 | 173.0966 | 0.00 | C12H13O | 4 | |||
| 191.1070 | 191.1072 | -1.05 | C12H15O2 | 20 | |||
| 37 | 3-MeO-PCPy-M (pyrrolidine-HO-) | 8.8 | |||||
| 276.1958 | 276.1964 | -2.17 | C17H26NO2 | 60 | |||
| 70.0659 | 70.0657 | 2.85 | C4H8N | 2 | |||
| 88.0756 | 88.0762 | -6.81 | C4H10NO | 100 | |||
| 189.1277 | 189.1279 | -1.06 | C13H17O | 70 | |||
| 38 | 3-MeO-PCPy-M (O-demethyl-pyrrolidine-di-HO-) | 6.5 | |||||
| 278.1754 | 278.1756 | -0.72 | C16H24NO3 | 84 | |||
| 87.0440 | 87.0446 | -6.89 | C4H7O2 | 14 | |||
| 104.0706 | 104.0712 | -5.77 | C4H10NO2 | 100 | |||
| 175.1120 | 175.1123 | -1.71 | C12H15O | 50 | |||
| 39 | 3-MeO-PCPy-M (pyrrolidine-di-HO-) | 9.5 | |||||
| 292.1905 | 292.1913 | -2.74 | C17H26NO3 | 18 | |||
| 87.0440 | 87.0446 | -6.89 | C4H7O2 | 10 | |||
| 104.0707 | 104.0712 | -4.80 | C4H10NO2 | 94 | |||
| 189.1277 | 189.1279 | -1.06 | C13H17O | 100 | |||
| 40 | 3-MeO-PCPy-M (O-demethyl-carboxy-) methyl artifact | 8.0 | |||||
| 292.1907 | 292.1913 | -2.05 | C17H26NO3 | 100 | |||
| 101.0598 | 101.0603 | -4.95 | C5H9O2 | 38 | |||
| 118.0865 | 118,0868 | -2.54 | C5H12NO2 | 100 | |||
| 175.1122 | 175.1123 | -0.57 | C12H15O | 30 | |||
| 41 | 3-MeO-PCPy-M (O-demethyl-cyclohexyl-HO-pyrrolidine-di-HO-) isomer 1 | 3.0 | |||||
| 294.1710 | 294.1705 | 1.70 | C16H24NO4 | 22 | |||
| 87.0440 | 87.0446 | -6.89 | C4H7O2 | 10 | |||
| 104.0706 | 104.0712 | -5.77 | C4H10NO2 | 100 | |||
| 173.0963 | 173.0966 | -1.73 | C12H13O | 20 | |||
| 191.1071 | 191.1072 | -0.52 | C12H15O2 | 4 | |||
| 42 | 3-MeO-PCPy-M (O-demethyl-cyclohexyl-HO-pyrrolidine-di-HO-) isomer 2 | 4.0 | |||||
| 294.1703 | 294.1705 | -0.68 | C16H24NO4 | 94 | |||
| 87.0440 | 87.0446 | -6.89 | C4H7O2 | 10 | |||
| 104.0706 | 104.0712 | -5.77 | C4H10NO2 | 100 | |||
| 173.0964 | 173.0966 | -1.16 | C12H13O | 44 | |||
| 191.1069 | 191.1072 | -1.57 | C12H15O2 | 12 | |||
| 43 | 3-MeO-PCPy-M (O-demethyl-cyclohexyl-HO-pyrrolidine-di-HO-) isomer 3 | 4.4 | |||||
| 294.1701 | 294.1705 | -1.36 | C16H24NO4 | 92 | |||
| 87.0441 | 87.0446 | -5.74 | C4H7O2 | 12 | |||
| 104.0707 | 104.0712 | -4.80 | C4H10NO2 | 100 | |||
| 173.0965 | 173.0966 | -0.58 | C12H13O | 30 | |||
| 191.1072 | 191.1072 | 0.00 | C12H15O2 | 6 | |||
| 44 | 3-MeO-PCPy-M (carboxy-) | 11.0 | |||||
| 306.2058 | 306.2069 | -3.59 | C18H28NO3 | 100 | |||
| 101.0597 | 101.0603 | -5.94 | C5H9O2 | 40 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 100 | |||
| 189.1277 | 189.1279 | -1.06 | C13H17O | 70 | |||
| 45 | 3-MeO-PCPy-M (cyclohexyl-HO-pyrrolidine-di-HO-) isomer 1 | 4.7 | |||||
| 308.1856 | 308.1862 | -1,95 | C17H26NO4 | 20 | |||
| 86.0440 | 87.0446 | -6,89 | C4H7O2 | 6 | |||
| 45 | 3-MeO-PCPy-M (cyclohexyl-HO-pyrrolidine-di-HO-) isomer 1 | 4.7 | |||||
| 104.0707 | 104.0712 | -4,80 | C4H10NO2 | 100 | |||
| 187.1121 | 187.1123 | -1,07 | C13H15O | 42 | |||
| 205.1227 | 205.1229 | -0,98 | C13H17O2 | 14 | |||
| 46 | 3-MeO-PCPy-M (cyclohexyl-HO-pyrrolidine-di-HO-) isomer 2 | 6.3 | |||||
| 308.1858 | 308.1862 | -1.30 | C17H26NO4 | 82 | |||
| 87.0441 | 87.0446 | -5.74 | C4H7O2 | 2 | |||
| 104.0707 | 104.0712 | -4.80 | C4H10NO2 | 44 | |||
| 187.1121 | 187.1123 | -1.07 | C13H15O | 20 | |||
| 205.1227 | 205.1229 | -0.98 | C13H17O2 | 6 | |||
| 47 | 3-MeO-PCPy-M (O-demethyl-carboxy-cyclohexyl-HO-) methyl artifact isomer 1 | 5.0 | |||||
| 308.1855 | 308.1862 | -2.27 | C17H26NO4 | 100 | |||
| 101.0598 | 101.0603 | -4.95 | C5H9O2 | 30 | |||
| 118.0865 | 118.0868 | -2.54 | C5H12NO2 | 100 | |||
| 173.0966 | 173.0966 | 0.00 | C12H13O | 20 | |||
| 191.1072 | 191.1072 | 0.00 | C12H15O2 | 4 | |||
| 48 | 3-MeO-PCPy-M (O-demethyl-carboxy-cyclohexyl-HO-) methyl artifact isomer 2 | 5.4 | |||||
| 308.1852 | 308.1862 | -3.24 | C17H26NO4 | 100 | |||
| 101.0597 | 101.0603 | -5.94 | C5H9O2 | 40 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 100 | |||
| 173.0964 | 173.0966 | -1.16 | C12H13O | 18 | |||
| 191.1070 | 191.1072 | -1.05 | C12H15O2 | 2 | |||
| 49 | 3-MeO-PCPy-M (O-demethyl-carboxy-cyclohexyl-HO-) methyl artifact isomer 3 | 6.3 | |||||
| 308.1857 | 308.1862 | -1.62 | C17H26NO4 | 88 | |||
| 87.0440 | 87.0446 | -6.89 | C4H7O2 | 1 | |||
| 101.0598 | 101.0603 | -4.95 | C5H9O2 | 38 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 100 | |||
| 173.0963 | 173.0966 | -1.73 | C12H13O | 6 | |||
| 191.1070 | 191.1072 | -1.05 | C12H15O2 | 16 | |||
| 50 | 3-MeO-PCPy-M (O-demethyl-carboxy-cyclohexyl-HO-) methyl artifact isomer 4 | 10.2 | |||||
| 308.1859 | 308.1862 | -0.97 | C17H26NO4 | 34 | |||
| 101.0599 | 101.0603 | -3.96 | C5H9O2 | 8 | |||
| 118.0864 | 118.0868 | -3.39 | C5H12NO2 | 24 | |||
| 173.0965 | 173.0966 | -0.58 | C12H13O | 4 | |||
| 51 | 3-MeO-PCPy-M (carboxy-alkyl-HO-) methyl artifact | 9.7 | |||||
| 322.2015 | 322.2018 | -0.93 | C18H28NO4 | 80 | |||
| 102.0553 | 102.0555 | -1.96 | C4H8NO2 | 1 | |||
| 117.0548 | 117.0551 | -2.56 | C5H9O3 | 26 | |||
| 134.0814 | 134.0817 | -2.24 | C5H12NO3 | 100 | |||
| 189.1278 | 189.1279 | -0.53 | C13H17O | 30 | |||
| 52 | 3-MeO-PCPy-M (carboxy-cyclohexyl-HO-) methyl artifact isomer 1 | 6.1 | |||||
| 322.2012 | 322.2018 | -1.86 | C18H28NO4 | 34 | |||
| 101.0597 | 101.0603 | -5.94 | C5H9O2 | 30 | |||
| 118.0863 | 118.0868 | -4.23 | C5H12NO2 | 100 | |||
| 187.1119 | 187.1123 | -2.14 | C13H15O | 20 | |||
| 205.1225 | 205.1229 | -1.95 | C13H17O2 | 6 | |||
| 53 | 3-MeO-PCPy-M carboxy-cyclohexyl-HO-) methyl artifact isomer 2 | 7.1 | |||||
| 322.2014 | 322.2018 | -1.24 | C17H28NO4 | 32 | |||
| 101.0597 | 101.0603 | -5.94 | C5H9O2 | 30 | |||
| 118.0863 | 118.0868 | -4.23 | C5H12NO2 | 100 | |||
| 187.1119 | 187.1123 | -2.14 | C13H15O | 48 | |||
| 205.1225 | 205.1229 | -1.95 | C13H17O2 | 12 | |||
| 54 | 3-MeO-PCPy-M (carboxy-cyclohexyl-HO-) methyl artifact isomer 3 | 7.8 | |||||
| 322.2016 | 322.2018 | -0.62 | C18H28NO4 | 100 | |||
| 101.0598 | 101.0603 | -4.95 | C5H9O2 | 32 | |||
| 118.0865 | 118.0868 | -2.54 | C5H12NO2 | 100 | |||
| 187.1122 | 187.1123 | -0.53 | C13H15O | 30 | |||
| 205.1228 | 205.1229 | -0.49 | C13H17O2 | 6 | |||
| 55 | 3-MeO-PCPy-M (carboxy-cyclohexyl-HO-) methyl artifact isomer 4 | 8.3 | |||||
| 322.2016 | 322.2018 | -0.62 | C18H28NO4 | 100 | |||
| 101.0598 | 101.0603 | -4.95 | C5H9O2 | 10 | |||
| 118.0865 | 118.0868 | -2.54 | C5H12NO2 | 30 | |||
| 187.1120 | 187.1123 | -1.60 | C13H15O | 8 | |||
| 205.1227 | 205.1229 | -0.98 | C13H17O2 | 100 | |||
| 56 | 3-MeO-PCPy-M (carboxy-cyclohexyl-di-HO-) methyl artifact | 5.4 | |||||
| 338.1958 | 338.1967 | -2.66 | C18H28NO5 | 4 | |||
| 101.0597 | 101.0603 | -5.94 | C5H9O2 | 24 | |||
| 118.0863 | 118.0868 | -4.23 | C5H12NO2 | 100 | |||
| 203.1069 | 203.1072 | -1.48 | C13H15O2 | 40 | |||
| 221.1174 | 221.1178 | -1.81 | C13H17O3 | 70 | |||
Table 3.
3-MeO-PCP and its phase II metabolites detected in rat urine by LC-HR-MSn with protonated precursor mass (PM), characteristic fragment ions (FI), calculated exact masses, proposed elemental composition, mass error, relative intensity, and retention times (RT).
| No. | Metabolite |
Measured Accurate
Mass (m/z) |
Calculated Exact
Mass (m/z) |
Error (ppm) | Elemental Composition | Relative Intensity (%) | RT (min) |
|---|---|---|---|---|---|---|---|
| 57 | 3-MeO-PCP-M (O-demethyl-) glucuronide | 5.3 | |||||
| 436.2320 | 436.2335 | -3.44 | C23H34NO7 | 100 | |||
| 175.1117 | 175.1123 | -3.43 | C12H15O | 100 | |||
| 351.1436 | 351.1444 | -2.28 | C18H23O7 | 3 | |||
| 58 | 3-MeO-PCP-M (O-demethyl-aryl-HO-) glucuronide | 4.6 | |||||
| 452.2272 | 452.2284 | -2.65 | C23H34NO8 | 100 | |||
| 175.1117 | 175.1123 | -3.43 | C12H15O | 100 | |||
| 191.1066 | 191.1072 | -3.14 | C12H15O2 | 30 | |||
| 367.1386 | 367.1393 | -1.91 | C18H23O8 | 60 | |||
| 59 | 3-MeO-PCP-M (O-demethyl-piperidine-HO-) glucuronide isomer 1 | 5.7 | |||||
| 452.2272 | 452.2284 | -2.65 | C23H24NO8 | 100 | |||
| 175.1119 | 175.1123 | -2.28 | C12H15O | 3 | |||
| 278.1235 | 278.1240 | -1.80 | C11H20NO7 | 100 | |||
| 60 | 3-MeO-PCP-M (O-demethyl-piperidine-HO-) glucuronide isomer 2 | 5.9 | |||||
| 452.2275 | 452.2284 | -1.99 | C23H34NO8 | 100 | |||
| 175.1118 | 175.1123 | -2.86 | C12H15O | 3 | |||
| 278.1234 | 278.1240 | -2.16 | C11H20NO7 | 100 | |||
| 61 | 3-MeO-PCP-M (piperidine-HO-) glucuronide | 8.1 | |||||
| 466.2432 | 466.2441 | -1.93 | C24H36NO8 | 100 | |||
| 189.1275 | 189.1279 | -2.11 | C13H17O | 6 | |||
| 278.1235 | 278.1240 | -1.80 | C11H20NO7 | 100 | |||
| 62 | 3-MeO-PCP-M (O-demethyl-piperidine-di-HO-) glucuronide | 5.5 | |||||
| 468.2226 | 468.2234 | -1.71 | C23H34NO9 | 10 | |||
| 175.1117 | 175.1123 | -3.43 | C12H15O | 2 | |||
| 351.1440 | 351.1444 | -1.14 | C18H23O7 | 100 | |||
| 63 | 3-MeO-PCP-M (O-demethyl-cyclohexyl-HO-piperidine-HO-) glucuronide | 4.3 | |||||
| 468.2263 | 468.2234 | 6.19 | C23H34NO9 | 14 | |||
| 191.1067 | 191.1072 | -2.62 | C12H15O2 | 90 | |||
| 367.1389 | 367.1393 | -1.09 | C18H23O8 | 100 | |||
of m/z 123.0440 (C7H7O2) in LC-HR-MS/MS. Furthermore, the other three glucuronides (nos. 68-70) showed keto groups at the pyrrolidine ring. The fact, that the corresponding phase I metabolites could not be detected after conjugate cleavage with subsequent SPE, non-basic structures could be proposed, which led to the suggestion of lactam ring formation following hydroxylation at the α-position and further oxidation.
Table 4.
3-MeO-PCPy and its phase II metabolites detected in rat urine by LC-HR-MSn with protonated precursor mass (PM), characteristic fragment ions (FI), calculated exact masses, proposed elemental composition, mass error, relative intensity, and retention times (RT).
| No. | Metabolite |
Measured Accurate
Mass (m/z) |
Calculated Exact
Mass (m/z) |
Error (ppm) | Elemental Composition | Relative Intensity (%) | RT (min) |
|---|---|---|---|---|---|---|---|
| 64 | 3-MeO-PCPy-M (O-demethyl-) glucuronide | 5.1 | |||||
| 422.2169 | 422.2179 | -2.37 | C22H32NO7 | 100 | |||
| 175.1118 | 175.1123 | -2.86 | C12H15O | 100 | |||
| 351.1411 | 351.1444 | -9.40 | C18H23O7 | 1 | |||
| 65 | 3-MeO-PCPy-M (O-demethyl-pyrrolidine-HO-) glucuronide | 5.8 | |||||
| 438.2121 | 438.2128 | -1.60 | C22H32NO8 | 60 | |||
| 175.1118 | 175.1123 | -2.86 | C12H15O | 1 | |||
| 264.1078 | 264.1083 | -1.89 | C10H18NO7 | 100 | |||
| 66 | 3-MeO-PCPy-M (O-demethyl-aryl-HO-) glucuronide | 4.5 | |||||
| 438.2119 | 438.2128 | -2.05 | C22H32NO8 | 100 | |||
| 191.1067 | 191.1072 | -2.62 | C12H15O2 | 50 | |||
| 367.1388 | 367.1393 | -1.36 | C18H23O8 | 100 | |||
| 67 | 3-MeO-PCPy-M (O-demethyl-aryl-HO-pyrrolidine-2-oxo-) glucuronide | 10.1 | |||||
| 452.1915 | 452.1920 | -1.11 | C22H30NO9 | 30 | |||
| 191.1067 | 191.1072 | -2.62 | C12H15O2 | 100 | |||
| 367.1387 | 367.1393 | -1.63 | C18H23O8 | 72 | |||
| 68 | 3-MeO-PCPy-M (cyclohexyl-HO-pyrrolidine-2-oxo-) glucuronide | 10.8 | |||||
| 466.2072 | 466.2077 | -1.07 | C23H32NO9 | 50 | |||
| 205.1222 | 205.1229 | -3.41 | C13H17O2 | 100 | |||
| 381.1543 | 381.1549 | -1.57 | C19H25O8 | 12 | |||
| 69 | 3-MeO-PCPy-M (cyclohexyl-di-HO-pyrrolidine-2-oxo-) glucuronide isomer 1 | 4.6 | |||||
| 482.2020 | 482.2026 | -1.24 | C23H32NO10 | 10 | |||
| 203.1068 | 203.1072 | -1.97 | C13H15O2 | 40 | |||
| 221.1173 | 221.1178 | -2.26 | C13H17O3 | 100 | |||
| 397.1492 | 397.1499 | -1.76 | C19H25O9 | 8 | |||
| 70 | 3-MeO-PCPy-M (cyclohexyl-di-HO-pyrrolidine-2-oxo-) glucuronide isomer 2 | 5.2 | |||||
| 482.2020 | 482.2026 | -1.24 | C23H32NO10 | 14 | |||
| 203.1065 | 203.1072 | -3.45 | C13H15O2 | 44 | |||
| 221.1170 | 221.1178 | -3.62 | C13H17O3 | 100 | |||
| 397.1482 | 397.1499 | -4.28 | C19H25O9 | 4 | |||
| 71 | 3-MeO-PCPy-M (cyclohexyl-HO-pyrrolidine-di-HO-) glucuronide | 8.0 | |||||
| 484.2178 | 484.2183 | -1.03 | C23H34NO10 | 60 | |||
| 205.1224 | 205.1229 | -2.44 | C13H17O2 | 100 | |||
| 381.1545 | 381.1549 | -1.05 | C19H25O8 | 20 | |||
Proposed Fragmentation Patterns for Identification of the Phase I and II Metabolites by LC-HR-MSn
3-MeO-PCP
In general, the spectrum of the parent compound (no. 1 in Table 1) revealed a characteristic fragmentation pattern, whereby a benzylic cleavage led to a 3-methoxy-phenyl-cyclohexyl fragment represented by the fragment ion of m/z 189.1279 and a piperidine fragment represented by the fragment ion of m/z 86.0967. In cases where the 3-methoxy-phenylcyclohexyl fragment was unchanged, fragment ions could be observed at m/z 189.1279 (nos. 6, 13, 20, and 30), if monohydroxylated at m/z 205.1229 (nos. 7, 14, 15, and 24-29), if O-demethylated at m/z 175.1123 (nos. 2, 3, 8-10, and 16), and if hydroxylated and O-demethylated at m/z 191.1072 (nos. 4, 5, 11, 12, 17-19, and 21-23). In cases where the piperidine fragment was unchanged, fragment ions could be observed at m/z 86.0970 (nos. 2, 4, and 7), if monohydro-xylated at m/z 102.0919 (nos. 3, 6, 11, 12, 14, and 15), and if dihydroxylated at m/z 118.0868 (nos. 8-10, 13, 17-19, and 24). Aliphatic hydroxylations were proposed by the elimination of water resulting in unsaturated fragments at m/z 187.1123 (205.1229 – 18.0100 u; nos. 7, 14, 15, and 24-29), 173.0966 (191.1072 – 18.0100 u; nos. 4, 5, 11, 12, 17, 19, and 21-23), and 84.0813 (102.0919 – 18.0100 u; nos. 3, 6, 11, 12, and 14).
A metabolite with PM of m/z 260.2014 revealed a spectrum of an O-demethyl metabolite (no. 2) with a fragment ion of m/z 175.1123. Two hydroxy metabolites were revealed by PM of m/z 290.2120. One isomer revealed a piperidine-hydroxy metabolite (no. 6) and one a cyclohexyl-hydroxy metabolite (no. 7). Four dihydroxy metabolites were revealed by PM of m/z 306.2069, one piperidine-dihydroxy (no. 13) and two isomeric cyclohexyl-hydroxy piperidine-hydroxy metabolites (nos. 14 and 15). One trihydroxy metabolite was revealed by PM of m/z 322.2018 representing a cyclohexyl-hydroxy piperidine-dihydroxy metabolite (no. 24). In addition, a hydroxylation at the α-position to the amine at the piperidine ring would led to a rather instable hemiaminal species followed by ring opening and oxidation to an aliphatic carboxy metabolite. This mechanism had already been described for PCP [23] and for diverse PCP derivatives by Sauer et al. [24-29]. The observed corresponding metabolites were a carboxy metabolite (no. 20) with PM of m/z 320.1862, five isomeric methyl artifacts of carboxy cyclohexyl-hydroxy metabolites (nos. 25-29) with PM of m/z 336.2175, and one carboxy alkyl-hydroxy metabolite (no. 30) with PM of m/z 336.2175. For metabolite no. 7, only one isomer was detected most probably due to the lower formation rate in contrast to the metabolites nos. 25-29. Moreover, in combination with O-demethylations ring opened methyl artifacts of carboxy metabolites could be observed with PM of m/z 306.2069 (no. 16) and after additional cyclohexyl-hydroxylation (methyl artifact) with PM of m/z 322.2018 (nos. 21-23). The existence of some carboxy metabolites was proposed after finding artificially formed methyl ester structures. One explanation for these formations could be a methylation of the carboxy group and nucleophilic attack during the work-up procedure, where the samples were evaporated in methanol at 70°C. Further confirmation was obtained when methanol was replaced with ethanol, leading to ethyl ester structures giving spectra with methylene shifts of the corresponding fragment ions.
Combinations of O-demethylation and hydroxylation could also be proposed. Two O-demethyl hydroxy isomers occurred with PM of m/z 276.1964, in particular one piperidine- (no. 3) and one cyclohexyl-hydroxy metabolite (no. 4), and five O-demethyl dihydroxy metabolites with PM of m/z 292.1913. Three isomers were observed with both hydroxy groups at the piperidine ring (nos. 8-10) and two isomers with one hydroxy group at the piperidine and the cyclohexyl ring, respectively (nos. 11 and 12). Spectra with PM of m/z 308.1862 were revealed after threefold hydroxylation, represented by three O-demethyl cyclohexyl-hydroxy piperidine-dihydroxy metabolites (nos. 17-19). One product of the metabolites nos. 8-10 after oxidation and elimination of water could be observed with PM of m/z 288.1600 (no. 5).
Concerning phase II metabolism, seven glucuronides were found (nos. 57-63 in Table 3). These conjugates were identified by the PM shift of +176.0322 u. The fragment ions were identical to those of the underlying phase I metabolites. One of these glucuronides was postulated as O-demethyl aryl-hydroxy glucuronide (no. 58). The corresponding phase I metabolite could not be detected, either due to low concentrations or insufficient conjugate cleavage during work-up procedure for this particular metabolite. The position in the aromatic ring system could be confirmed with performed LC-HR-MS/MS analysis, revealing a spectrum with an additional fragment ion of m/z 123.0440 (C7H7O2), representing a dihydroxy tropylium ion.
3-MeO-PCPy
The parent compound spectrum (no. 31 in Table 2) showed a characteristic fragmentation pattern analogous to 3-MeO-PCP. The 3-methoxy-phenyl-cyclohexyl fragment was represented by the fragment ion of m/z 189.1279, but the corresponding pyrrolidine fragment at m/z 72.0813 was not detected. In analogy to 3-MeO-PCP, an unchanged 3-methoxy-phenyl-cyclohexyl fragment was represented at m/z 189.1279 (nos. 37, 39, 44, and 51), after monohydroxylation at m/z 205.1229 (nos. 45, 46, and 52-55), after O-demethylation at m/z 175.1123 (nos. 32, 33, 38, and 40), and after hydro-xylation and O-demethylation at m/z 191.1072 (nos. 34-36, 41-43, and 47-49). Although the pyrrolidine fragment was not detectable in the spectrum of the unchanged molecule, the corresponding fragments were observed in the spectra of the metabolites. When the pyrrolidine fragment was unchanged, fragment ions could be observed at m/z 72.0813 (no. 32), if monohydroxylated at m/z 88.0762 (nos. 33 and 37), and if dihydroxylated at m/z 104.0712 (nos. 38, 39, 41-43, 45, and 46). Again, an aliphatic hydroxylation was proposed if water elimination was observed. These fragments were represented at m/z 187.1123 (205.1229 – 18.0100 u; nos. 45, 46, and 52-55), 173.0966 (191.1072 – 18.0100 u; nos. 34-36, 41-43, and 47-50), and 70.0657 (88.0762 – 18.0100 u; nos. 33 and 37).
A metabolite with PM of m/z 246.1858 revealed an O-demethyl metabolite (no. 32). One pyrrolidine-hydroxy metabolite was revealed by PM of m/z 276.1958 (no. 37), one pyrrolidine-dihydroxy metabolite by PM of m/z 292.1913 (no. 39), and two trihydroxy metabolite by PM of m/z 308.1862, both representing cyclohexyl-hydroxy pyrrolidine-dihydroxy isomers (nos. 45 and 46). In contrast to 3-MeO-PCP, monohydroxylation at the cyclohexyl ring could not be detected being an intermediate to further steps. In accordance to 3-MeO-PCP metabolism, aliphatic carboxy metabolites could be observed as carboxy metabolite (no. 44) with PM of m/z 306.2069, as four isomeric methyl artifacts of carboxy cyclohexyl-hydroxy metabolites (nos. 52-55) with PM of m/z 322.2018, as one methyl artifact of a carboxy cyclohexyl-dihydroxy metabolite (no. 56) with PM of m/z 338.1967, and as one carboxy alkyl-hydroxy metabolite (no. 51) with PM of m/z 322.2018. O-Demethylation and hydroxylation reactions could be observed for four O-demethyl hydroxy isomers with PM of m/z 262.1807, whereby one isomer is postulated as the O-demethyl pyrrolidine-hydroxy metabolite (no. 33) and three isomers as O-demethyl cyclohexyl-hydroxy metabolites (nos. 34-36). Moreover, after dihydroxylation, one O-demethyl pyrrolidine-dihydroxy metabolite with PM of m/z 278.1756 (no. 38) and after trihydroxylation three O-demethyl cyclohexyl-hydroxy pyrrolidine-dihydroxy meta-bolites with PM of m/z 294.1705 (nos. 41-43) could be observed. Furthermore, one methyl artifact of an O-demethylated carboxy metabolite could be observed with PM of m/z 292.1949 (no. 40) and four isomers after additional cyclohexyl-hydroxylation with PM of m/z 308.1862 (nos. 47-50). In analogy to 3-MeO-PCP metabolism studies, the methyl artifacts, postulated as methyl ester structures, were confirmed as described above.
Concerning phase II metabolism, eight glucuronides were found, whereby corresponding phase I metabolites of five of them could not be detected (nos. 66-70 in Table 4). The reason for the lack of corresponding phase I metabolites for the two aryl hydroxylated metabolites (nos. 66 and 67) were the same already described above. Again, the position of the hydroxy groups were confirmed by additional fragment ions
Proposed Metabolic Pathways
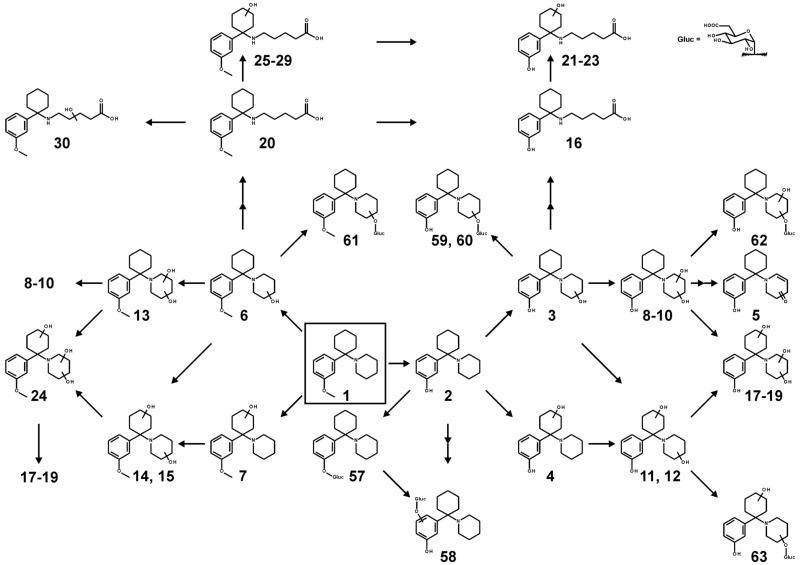
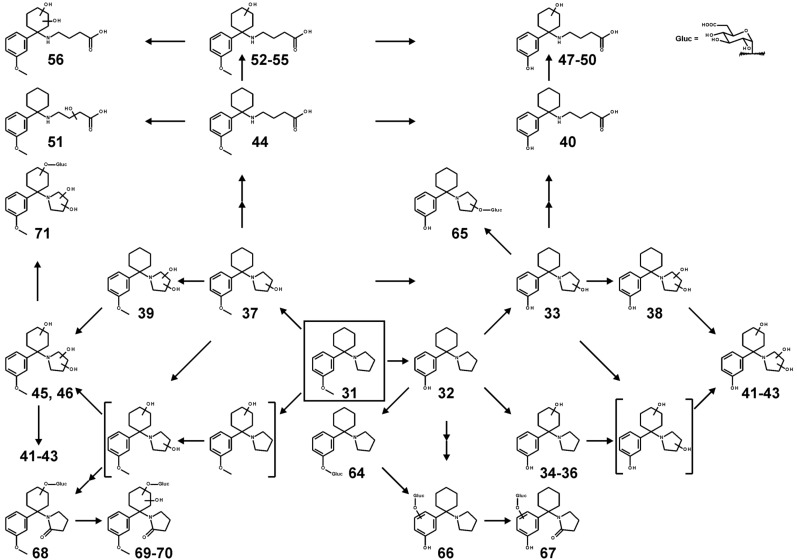
The metabolic pathways, which could be proposed according to the identified metabolites, are given in Fig. (2) for 3-MeO-PCP and in Fig. (3) for 3-MeO-PCPy, respectively. Numbers correspond to those given in Tables 1-4.
Fig. (2).
Metabolic pathways of 3-MeO-PCP. Undefined positions of hydroxylation are indicated by unspecific bonds. Parent compound is marked by a box. Two arrows indicate a pathway that contains multiple metabolism steps.
Fig. (3).
Metabolic pathways of 3-MeO-PCPy. Undefined positions of hydroxylation are indicated by unspecific bonds. Parent compound is marked by a box. Two arrows indicate a pathway that contains multiple metabolism steps. Structures in brackets are postulated intermediate metabolites.
3-MeO-PCP
Hydroxylation steps could be detected at the cyclohexyl ring (no. 7 in Fig. 2), at the piperidine ring (no. 6), or at both rings forming two isomeric metabolites (nos. 14 and 15). The metabolite with monohydroxylated piperidine ring (no. 6) as well as both isomers of cyclohexyl-hydroxy piperidine-hydroxy (nos. 14 and 15) could further get hydroxylated at the piperidine ring to a piperidine-dihydroxy metabolite (no. 13) or to isomers of cyclohexyl-hydroxy piperidine-dihydroxy metabolite, whereby only one isomer was detected (no. 24). If hydroxylation of metabolite no. 6 took place at α-position to the amine, the resulting hemiaminal could perform a ring opening, and the formed aldehyde could be further oxidized to the corresponding carboxylic acid (no. 20). Further hydroxylation of this metabolite at the alkyl side chain (no. 30) or at the cyclohexyl ring to five isomeric structures (nos. 25-29) could be observed. O-Demethylation (no. 2) could be detected as another pathway, followed by hydroxylation at the cyclohexyl ring (no. 4), at the piperidine ring (no. 3), or at both rings, again into two isomeric metabolites (nos. 11 and 12). In analogy to the pathway without O-demethylation, the metabolite with the monohydroxylated piperidine ring (no. 3) as well as both cyclohexyl-hydroxy piperidine-hydroxy isomers (nos. 11 and 12) could further get hydroxylated at the piperidine ring to three isomeric piperidine-dihydroxy metabolites (nos. 8-10) or to three isomeric cyclohexyl-hydroxy piperidine-dihydroxy metabolites (nos. 17-19). Meta-bolite no. 5 could be explained as a product of one of the metabolites nos. 17-19 after oxidation of one hydroxy group and elimination of water. If hydroxylation of metabolite no. 3 led to a hemiaminal, one ring opened carboxy metabolite (no. 16) and after further hydroxylation at the cyclohexyl ring three isomers (nos. 21-23) could be detected.
Glucuronidation could be observed for metabolites nos. 6 (to 61), 2 (to 57), 3 (to 59 or 60), 11 or 12 (to 63), and 8-10 (to 62). For the glucuronide no. 58, the corresponding precursor should be an aryl-hydroxy of metabolite no. 2, which could not be detected possibly for reasons already described above.
3-MeO-PCPy
The metabolic pathways were similar to those of 3-MeO-PCP. Hydroxylation could be detected at the pyrrolidine ring after monohydroxylation (no. 37 in Fig. (3)) and dihydro-xylation (no. 39). At the cyclohexyl ring, monohydroxylation was not observed but two isomers in combination with a dihydroxy pyrrolidine ring (nos. 45 and 46). Again, after ring opening, one carboxylic acid metabolite (no. 44), one after further hydroxylation at the alkyl side chain (no. 51), four cyclohexyl-monohydroxy isomers (nos. 52-55), and one cyclohexyl-dihydroxy isomer (no. 56) were detected. O-Demethylation (no. 32) followed by hydroxylation at the piperidine ring led to one monohydroxy (no. 33) and one dihydroxy isomer (no. 38). At the cyclohexyl ring, three monohydroxy (nos. 34-36), but no dihydroxy isomers were detected. Combinations only occurred with dihydroxy pyrrolidine ring, again resulting in three isomeric structures (nos. 41-43) fitting with the three precursor isomers nos. 34-36. O-Demethylation in combination with ring opening led to metabolite no. 40 and to the three isomers nos. 47-50 after further cyclohexyl-monohydroxylation.
Glucuronidation could be observed for metabolites nos. 45 or 46 (to 71), 32 (to 64), and 33 (to 65). The precursor of no. 68 was a pyrrolidine-monohydroxy metabolite in α-position (no. 37) followed by oxidation to the lactams, those of nos. 69 and 70 after further cyclohexyl-hydroxylation. Metabolite no. 66 resulted by aryl-hydroxylation of the O-demethyl metabolite no. 32 followed by glucuronidation. Metabolite no. 67 resulted from metabolite no. 66 after additional lactam formation.
Microsomal Incubations and Initial CYP Activity Screening
Incubations with pHLM were carried out for comparison of the formed rat phase I metabolites with those of humans. For 3-MeO-PCP, the metabolites nos. 2, 3, 4, 7, and 13 from Table 1, for 3-MeO-PCPy, the metabolites nos. 32 and 39 from Table 2 were detected after pHLM incubation. Essentially, O-demethylation and hydroxylation at the pyrrolidine and/or the cyclohexyl ring were observed for both species. However, ring opening steps could not be detected in pHLM, what could either be explained by low formation rates or by interindividual isomeric variability that could lead to the absence of hydroxylation products in the α-position in humans, and thus, absence of detectable carboxy metabolites.
To test the involvement of single CYP enzymes in the initial metabolic steps, the proposed metabolites were detected after incubations with the ten most important human hepatic CYP enzymes. The involvement of the corresponding CYPs is shown in Table 5 for 3-MeO-PCP and for 3-MeO-PCPy. The relative involvement of individual CYPs was defined in relation to the highest peak abundances during precursor ion monitoring of the formed metabolites. CYPs forming the relative highest peak abundances are given with “++” and all others with “+”.
Table 5.
General involvement of human CYP isoenzymes in initial metabolic steps of 3-MeO-PCP and 3-MeO-PCPy.
|
CYP
1A2 |
CYP
2A6 |
CYP
2B6 |
CYP
2C8 |
CYP
2C9 |
CYP
2C19 |
CYP
2D6 |
CYP
2E1 |
CYP
3A4 |
CYP
3A5 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 3-MeO-PCP | ||||||||||
| O-Demethylation | + | ++ | + | |||||||
| Piperidine-hydroxylation | ++ | + | ||||||||
| Cyclohexyl-hydroxylation | ++ | |||||||||
| 3-MeO-PCPy | ||||||||||
| O-Demethylation | + | + | ++ | |||||||
| Pyrrolidine-hydroxylation | ++ |
For 3-MeO-PCP, CYP 2B6 was involved in the formation of hydroxylation at the cyclohexyl ring as well as at the piperidine ring. O-Demethylation was performed by CYP 2C19, 2B6, and CYP 2D6. Regarding 3-MeO-PCPy, hydro-xylation at the pyrrolidine ring was again catalyzed by CYP 2B6. However, cyclohexyl-hydroxylation was not detected. Concerning O-demethylation, CYP 2C9, 2B6, and CYP 2D6 were involved. As most involved enzymes were polymor-phically expressed, pharmacogenomic variations might occur, but clinical data are needed for further studies.
Toxicological Detection by GC-MS, LC-MSn, and LC-HR-MS/MS
To test for toxicological detectability after common users’ doses of approximately 10 mg (https://www.erowid.org, http://bluelight.org), rat urines were screened after administration of compound doses scaled by the dose-by-factor approach of Sharma and McNeill [13] via GC-MS, LC-MSn, and LC-HR-MS/MS SUSA. The detected targets for both compounds are given in Tables 6-8.
Table 6.
Proposed targets for GC-MS SUSA monitoring 3-MeO-PCP or 3-MeO-PCPy, with molecular masses, most abundant fragment ions, their relative intensities, and retention indices (RI) according to Kovats [16]. The numbers correspond to those in Figs. (2 and 3).
| No. | Target for SUSA |
Molecular
Mass (u) |
GC-MS Fragment Ions (m/z),
and Relative Intensity (%) |
RI |
|---|---|---|---|---|
| 2 | 3-MeO-PCP | 273 | 273 (40), 230 (100), 161 (52), 121 (41) | 2120 |
| 2 | 3-MeO-PCP-M (O-demethyl-) AC | 301 | 84 (18), 166 (21), 244 (16), 258 (100), 301 (28) | 2210 |
| 3 | 3-MeO-PCP-M (O-demethyl-piperidine-HO-) 2 AC | 359 | 164 (16), 258 (21), 300 (100), 316 (12), 359 (15) | 2510 |
| 32 | 3-MeO-PCPy-M (O-demethyl-) AC | 287 | 70 (38), 107 (60), 152 (38), 244 (100), 287 (22) | 2160 |
Table 8.
Proposed targets for LC-HR-MS/MS SUSA monitoring 3-MeO-PCP or 3-MeO-PCPy, with protonated precursor ions, characteristic MS2 fragment ions, and retention times (RT). The numbers correspond to those in Figs. (2 and 3).
| No. | Target for SUSA |
Precursor
Ions (m/z) |
MS2 Fragment Ions (m/z)
and Relative Intensity (%) |
RT
(min) |
|---|---|---|---|---|
| 2 | 3-MeO-PCP-M (O-demethyl-) | 260.2014 | 81.0701 (15), 86.0966 (100), 107.0490 (73), 175.1111 (37), 260.2001 (4) |
4.4 |
| 3 | 3-MeO-PCP-M (O-demethyl-piperidine-HO-) | 276.1964 | 81.0701 (20), 84.0810 (18), 102.0913 (99), 107.0490 (100), 175.1111 (54) |
4.0 |
| 8 | 3-MeO-PCP-M (O-demethyl-piperidine-di-HO-) | 292.1913 | 79.0545 (16), 84.0810 (18), 102.0914 (100), 107.0491 (41), 173.0954 (58) |
3.1 |
| 13 | 3-MeO-PCP-M (piperidine-di-HO-) | 306.2069 | 81.0701 (23), 101.0597 (14), 118.0860 (25), 121.0645 (100), 189.1266 (48) |
5.1 |
| 24 | 3-MeO-PCP-M (cyclohexyl-HO-piperidine-di-HO-) | 322.2018 | 79.0545 (41.88), 101.0597 (34), 118.0861 (66), 121.0645 (68), 187.1111 (100) |
4.3 |
| 57 | 3-MeO-PCP-M (O-demethyl-) glucuronide | 436.2335 | 81.0702 (10), 86.0966 (100), 107.0491 (23), 141.0176 (9), 175.1112 (22) |
4.0 |
| 58 | 3-MeO-PCP-M (O-demethyl-aryl-HO-) glucuronide | 452.2284 | 81.0701 (8), 86.0966 (55), 123.0438 (100), 191.1059 (76), 367.1369 (3) |
3.8 |
| 59 | 3-MeO-PCP-M (O-demethyl-piperidine-HO-) glucuronide | 452.2284 | 84.0810 (56), 102.0914 (52), 107.0491 (90), 175.1111 (37), 278.1225 (100) |
4.0 |
| 61 | 3-MeO-PCP-M (piperidine-HO-) glucuronide | 466.2441 | 84.0810 (45), 102.0914 (38), 121.0646 (100), 189.1268 (41), 278.1225 (79) |
4.7 |
| 32 | 3-MeO-PCPy-M (O-demethyl-) | 246.1858 | 72.0812 (100), 81.0702 (16), 107.0491 (86), 175.1112 (45), 246.1844 (4) |
4.3 |
| 38 | 3-MeO-PCPy-M (O-demethyl-pyrrolidine-di-HO-) | 278.1756 | 70.0656 (14), 79.0546 (15), 88.0759 (100), 107.0491 (38), 173.0956 (64) |
3.1 |
| 39 | 3-MeO-PCPy-M (pyrrolidine-di-HO-) | 292.1913 | 81.0702 (24), 87.0443 (9), 104.0706 (18), 121.0646 (100), 189.1268 (46) |
5.0 |
| 45 | 3-MeO-PCPy-M (cyclohexyl-HO-pyrrolidine-di-HO-) | 308.1862 | 79.0545 (42), 87.0443 (29), 104.0706 (57), 121.0646 (66), 187.1111 (100) |
4.1 |
| 64 | 3-MeO-PCPy-M (O-demethyl-) glucuronide | 422.2179 | 72.0812 (100), 81.0701 (11), 107.0491 (27), 175.1112 (26), 422.2160 (13) |
3.7 |
| 66 | 3-MeO-PCPy-M (O-demethyl-aryl-HO-) glucuronide | 438.2128 | 72.0812 (43), 81.0701 (8), 123.0438 (100), 191.1059 (78), 367.1375 (3) |
3.5 |
| 67 | 3-MeO-PCPy-M (O-demethyl-aryl-HO-pyrrolidine-2-oxo-) glucuronide | 452.1920 | 81.0702 (8), 86.0603 (22), 123.0439 (100), 149,0592 (9), 191.1060 (78) |
6.2 |
For 3-MeO-PCP, detection was possible via the parent compound (no. 1) and metabolites nos. 2 and 3 by GC-MS, via metabolites nos. 8, 13, 57, 58, and 61 by LC-MSn, and nos. 2, 3, 8, 13, 24, 57, 58, 59, and 61 by LC-HR-MS/MS. Administration of 3-MeO-PCPy could be monitored via the metabolites no. 32 by GC-MS, nos. 32, 38, 39, 64, and 67 by LC-MSn, and nos. 32, 38, 39, 45, 64, 66, and 67 by LC-HR-MS/MS. The reason why not all types of metabolites were found for both drugs could be explained by different formation rates, influence of ion suppression, chromato-graphic and/or ionization properties. The risk of overlooking a drug consumption caused by ion suppression could be minimized by screening for several targets e.g. metabolites.
For general performance of the SUSAs, increasing concentrations of the parent drugs were analyzed although they were not the main targets chosen for urine analysis. The LODs were determined at a signal-to-noise ratio of 3. In GC-MS, LC-MSn, and LC-HR-MS/MS, the LODs were 5, 10, and 0.1 ng/mL for 3-MeO-PCP and 10, 10, and 0.1 ng/mL for 3-MeO-PCPy, respectively.
Conclusion
The PCP analogues 3-MeO-PCP and 3-MeO-PCPy were extensively metabolized in rats via aliphatic and aromatic hydroxylation, carboxylation after ring opening, O-demethylation, and glucuronidation. The initial steps could be confirmed by detection of the corresponding metabolites in pHLM incubations. The CYP enzymes involved in the metabolism of both compounds were CYP 2B6 and CYP 2D6. In addition, CYP 2C19 was involved in 3-MeO-PCP O-demethylation and piperidine-hydroxylation whereas CYP 2C9 in 3-MeO-PCPy O-demethylation. As only polymor-phically expressed enzymes were involved, pharmaco-genomic variations might occur, but clinical data are needed to confirm the relevance. Detectability studies showed that all tested SUSAs were able to monitor consumptions of both drugs considering that metabolites were the main targets.
Table 7.
Proposed targets for LC-MSn SUSA monitoring 3-MeO-PCP or 3-MeO-PCPy, with protonated precursor ions, characteristic MS2 and MS3 fragment ions, and retention times (RT). The numbers correspond to those in Figs. (2 and 3).
| No. | Target for SUSA |
Precursor
Ions (m/z) |
MS2 Fragment Ions (m/z), and Relative Intensity (%) | MS3 Fragment Ions (m/z), and Relative Intensity (%) | RT (min) |
|---|---|---|---|---|---|
| 8 | 3-MeO-PCP-M (O-demethyl-piperidine-di-HO-) | 292 | 101 (20), 118, (100), 175 (25) | 175: 81 (10), 107 (100) | 8.6 |
| 13 | 3-MeO-PCP-M (piperidine-di-HO-) | 306 | 101 (20), 118 (100), 189 (56) | 189: 81 (20), 121 (100) | 11.6 |
| 57 | 3-MeO-PCP-M (O-demethyl-) glucuronide | 436 | 175 (100), 315 (70), 391 (10) | 175: 81 (20), 107 (100) | 5.5 |
| 58 | 3-MeO-PCP-M (O-demethyl-aryl-HO-) glucuronide | 452 | 175 (20), 191 (90), 367 (100) | 191: 81 (6), 123 (100) | 4.6 |
| 61 | 3-MeO-PCP-M (piperidine-HO-) glucuronide | 466 | 189 (6), 278 (100) | 278: 84 (53), 102 (90), 242 (100) | 9.0 |
| 32 | 3-MeO-PCPy-M (O-demethyl-) | 246 | 72 (100), 175 (60) | 175: 81 (15), 107 (100) | 8.2 |
| 38 | 3-MeO-PCPy-M (O-demethyl-pyrrolidine-di-HO-) | 278 | 87 (14), 104 (100), 175 (50), 232 (30) |
175: 81 (20), 107 (100) | 7.3 |
| 39 | 3-MeO-PCPy-M (pyrrolidine-di-HO-) | 292 | 87 (10), 104 (94), 189 (100) | 189: 81 (20), 121 (100) | 10.9 |
| 64 | 3-MeO-PCPy-M (O-demethyl-) glucuronide | 422 | 175 (100), 315 (1) | 175: 81 (30), 107 (100) | 4.9 |
| 67 | 3-MeO-PCPy-M (O-demethyl-aryl-HO-pyrrolidine-2-oxo-) glucuronide |
452 | 191 (70), 367 (100) | 191: 81 (6), 123 (100) | 10.6 |
ACKNOWLEDGEMENTS
The authors like to thank Markus R. Meyer, Andreas G. Helfer, Lilian H. J. Richter, Lea Wagmann, Carsten Schröder, Gabriele Ulrich, and Armin A. Weber for their support.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Morris H., Wallach J. From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs. Drug Test. Anal. 2014;6(7-8):614–632. doi: 10.1002/dta.1620. [http://dx.doi.org/10.1002/dta.1620]. [PMID: 24678061]. [DOI] [PubMed] [Google Scholar]
- 2.Lodge B.A., Duhaime R., Zamecnik J., MacMurray P., Brousseau R. New street analogs of phencyclidine. Forensic Sci. Int. 1992;55(1):13–26. [http://dx.doi.org/10.1016/0379-0738(92) 90090-J]. [Google Scholar]
- 3.Soine W.H., Balster R.L., Berglund K.E., Martin C.D., Agee D.T. Identification of a new phencyclidine analog, 1-(1-phenylcyclohexyl)-4-methylpiperidine, as a drug of abuse. J. Anal. Toxicol. 1982;6(1):41–43. doi: 10.1093/jat/6.1.41. [http://dx.doi.org/10.1093/jat/6.1.41]. [PMID: 7078106]. [DOI] [PubMed] [Google Scholar]
- 4.Roesner P., Junge T., Fritschi G., Klein B., Thielert K., Kozlowski M. Neue synthetische Drogen: Piperazin-, Procyclidin- und alpha-Aminopropiophenonderivate. Toxichem. Krimtech. 1999;66(2):81–90. [Google Scholar]
- 5.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2012 Annual report on the state of the drugs problem in Europe. http://www.emcdda.europa.eu/attachements.cfm/att_ 190854_EN_TDAC12001ENC_ pdf . 2012.
- 6.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) EMCDDA–Europol 2011 Annual Report on the implementation of Council Decision 2005/387/JHA. , http://www. emcdda.europa.eu/attachements.cfm/att_155113_EN_EMCDDAEuropol% 20Annual%20Report%202011_2012_final.pdf . 2012.
- 7. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) New drugs in Europe, 2012 - EMCDDA–Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA.http://www.emcdda.europa.eu/attachements.cfm/att_212366_EN_E MCDDA-Europol%202012%20Annual%20Report_final.pdf . 2012.
- 8.Bäckberg M., Beck O., Helander A. Phencyclidine analog use in Swedenintoxication cases involving 3-MeO-PCP and 4-MeO-PCP from the STRIDA project. Clin. Toxicol. (Phila.) 2015;53(9):856–864. doi: 10.3109/15563650.2015.1079325. [http://dx.doi.org/10.3109/15563650.2015.1079325]. [PMID: 26295489]. [DOI] [PubMed] [Google Scholar]
- 9.Pradhan S.N. Phencyclidine (PCP): some human studies. Neurosci. Biobehav. Rev. 1984;8(4):493–501. doi: 10.1016/0149-7634(84)90006-x. [http://dx.doi.org/10.1016/ 0149-7634(84)90006-X]. [PMID: 6514253]. [DOI] [PubMed] [Google Scholar]
- 10.Bey T., Patel A. Phencyclidine intoxication and adverse effects: a clinical and pharmacological review of an illicit drug. Cal. J. Emerg. Med. 2007;8(1):9–14. [PMID: 20440387]. [PMC free article] [PubMed] [Google Scholar]
- 11.Roth B.L., Gibbons S., Arunotayanun W., Huang X.P., Setola V., Treble R., Iversen L. The ketamine analogue methoxetamine and 3- and 4-methoxy analogues of phencyclidine are high affinity and selective ligands for the glutamate NMDA receptor. PLoS One. 2013;8(3):e59334. doi: 10.1371/journal.pone.0059334. [http://dx.doi.org/10.1371/journal.pone.0059334]. [PMID: 23527166]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallach J., De Paoli G., Adejare A., Brandt S.D. Preparation and analytical characterization of 1-(1-phenylcyclohexyl)piperidine (PCP) and 1-(1-phenylcyclohexyl)pyrrolidine (PCPy) analogues. Drug Test. Anal. 2014;6(7-8):633–650. doi: 10.1002/dta.1468. [http://dx.doi.org/10.1002/ dta.1468]. [PMID: 23554350]. [DOI] [PubMed] [Google Scholar]
- 13.Sharma V., McNeill J.H. To scale or not to scale: the principles of dose extrapolation. Br. J. Pharmacol. 2009;157(6):907–921. doi: 10.1111/j.1476-5381.2009.00267.x. [http:// dx.doi.org/10.1111/j.1476-5381.2009.00267.x]. [PMID: 19508398]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welter J., Meyer M.R., Wolf E.U., Weinmann W., Kavanagh P., Maurer H.H. 2-methiopropamine, a thiophene analogue of methamphetamine: studies on its metabolism and detectability in the rat and human using GC-MS and LC-(HR)-MS techniques. Anal. Bioanal. Chem. 2013;405(10):3125–3135. doi: 10.1007/s00216-013-6741-4. [http://dx.doi. org/10.1007/s00216-013-6741-4]. [PMID: 23361230]. [DOI] [PubMed] [Google Scholar]
- 15.Wissenbach D.K., Meyer M.R., Remane D., Philipp A.A., Weber A.A., Maurer H.H. Drugs of abuse screening in urine as part of a metabolite-based LC-MSn screening concept. Anal. Bioanal. Chem. 2011;400(10):3481–3489. doi: 10.1007/s00216-011-5032-1. [http://dx.doi.org/10.1007/s00216-011-5032-1]. [PMID: 21533799]. [DOI] [PubMed] [Google Scholar]
- 16.Maurer H.H., Pfleger K., Weber A.A. Mass Spectral and GC Data of Drugs, Poisons, Pesticides, Pollutants and their Metabolites. 4th ed. Weinheim, Germany: Wiley-VCH; 2011. [Google Scholar]
- 17.Maurer H.H., Pfleger K., Weber A.A. Mass Spectral Library of Drugs, Poisons, Pesticides, Pollutants and their Metabolites. 5th Rev. ed. Weinheim: Wiley-VCH; 2016. [Google Scholar]
- 18.Meyer M.R., Peters F.T., Maurer H.H. Automated mass spectral deconvolution and identification system for GC-MS screening for drugs, poisons, and metabolites in urine. Clin. Chem. 2010;56(4):575–584. doi: 10.1373/clinchem.2009.135517. [http://dx.doi.org/10.1373/clinchem.2009.135517]. [PMID: 20185625]. [DOI] [PubMed] [Google Scholar]
- 19.Wissenbach D.K., Meyer M.R., Remane D., Weber A.A., Maurer H.H. Development of the first metabolite-based LC-MS(n) urine drug screening procedure-exemplified for antidepressants. Anal. Bioanal. Chem. 2011;400(1):79–88. doi: 10.1007/s00216-010-4398-9. [http://dx.doi.org/10. 1007/s00216-010-4398-9]. [PMID: 21079926]. [DOI] [PubMed] [Google Scholar]
- 20.Maurer H.H., Wissenbach D.K., Weber A.A. Maurer/ Wissenbach/Weber MWW LC-MSn Library of Drugs, Poisons, and their Metabolites. Weinheim, Germany: Wiley-VCH; 2014. [Google Scholar]
- 21.Helfer A.G., Michely J.A., Weber A.A., Meyer M.R., Maurer H.H. Orbitrap technology for comprehensive metabolite-based liquid chromatographic-high resolution-tandem mass spectrometric urine drug screening - exemplified for cardiovascular drugs. Anal. Chim. Acta. 2015;891:221–233. doi: 10.1016/j.aca.2015.08.018. [http://dx.doi.org/10.1016/j.aca. 2015.08.018]. [PMID: 26388381]. [DOI] [PubMed] [Google Scholar]
- 22.Michely J.A., Helfer A.G., Brandt S.D., Meyer M.R., Maurer H.H. Metabolism of the new psychoactive substances N,N-diallyltryptamine (DALT) and 5-methoxy-DALT and their detectability in urine by GC-MS, LC-MSn, and LC-HR-MS-MS. Anal. Bioanal. Chem. 2015;407(25):7831–7842. doi: 10.1007/s00216-015-8955-0. [http://dx.doi. org/10.1007/s00216-015-8955-0]. [PMID: 26297461]. [DOI] [PubMed] [Google Scholar]
- 23.Holsztynska E.J., Domino E.F. Biotransformation of phencyclidine. Drug Metab. Rev. 1985-1986;16(3):285–320. doi: 10.3109/03602538508991437. [http://dx.doi.org/ 10.3109/03602538508991437]. [PMID: 3914938]. [DOI] [PubMed] [Google Scholar]
- 24.Sauer C., Peters F.T., Staack R.F., Fritschi G., Maurer H.H. New designer drug (1-(1-phenylcyclohexyl)-3-ethoxypropylamine (PCEPA): Studies on its metabolism and toxicological detection in rat urine using gas chromatography/mass spectrometry. J. Mass Spectrom. 2006;41(8):1014–1029. doi: 10.1002/jms.1058. [http://dx.doi.org/10.1002/jms. 1058]. [PMID: 16817170]. [DOI] [PubMed] [Google Scholar]
- 25.Sauer C., Peters F.T., Schwaninger A.E., Meyer M.R., Maurer H.H. Identification of cytochrome P450 enzymes involved in the metabolism of the designer drugs N-(1-phenylcyclohexyl)-3-ethoxypropanamine and N-(1-phenylcyclohexyl)-3-methoxypro-panamine. Chem. Res. Toxicol. 2008;21(10):1949–1955. doi: 10.1021/tx8001302. [http:// dx.doi.org/10.1021/tx8001302]. [PMID: 18778087]. [DOI] [PubMed] [Google Scholar]
- 26.Sauer C., Peters F.T., Staack R.F., Fritschi G., Maurer H.H. Metabolism and toxicological detection of a new designer drug, N-(1-phenylcyclohexyl)propanamine, in rat urine using gas chromato-graphy-mass spectrometry. J. Chromatogr. A. 2008;1186(1-2):380–390. doi: 10.1016/j.chroma.2007.11.002. [http://dx.doi.org/10.1016/j.chroma.2007.11.002]. [PMID: 18035363]. [DOI] [PubMed] [Google Scholar]
- 27.Sauer C., Peters F.T., Staack R.F., Fritschi G., Maurer H.H. Metabolism and toxicological detection of the designer drug N-(1-phenylcyclohexyl)-3-methoxypropanamine (PCMPA) in rat urine using gas chromatography-mass spectrometry. Forensic Sci. Int. 2008;181(1-3):47–51. doi: 10.1016/j.forsciint.2008.09.001. [http://dx.doi.org/10.1016/j.forsciint.2008. 09.001]. [PMID: 18922655]. [DOI] [PubMed] [Google Scholar]
- 28.Sauer C., Peters F.T., Staack R.F., Fritschi G., Maurer H.H. New designer drugs N-(1-phenylcyclohexyl)-2-ethoxyethanamine (PCEEA) and N-(1-phenylcyclohexyl)-2-methoxyethanamine (PCMEA): Studies on their metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques. J. Mass Spectrom. 2008;43(3):305–316. doi: 10.1002/jms.1312. [http://dx.doi.org/10. 1002/jms.1312]. [PMID: 17968862]. [DOI] [PubMed] [Google Scholar]
- 29.Sauer C., Peters F.T., Schwaninger A.E., Meyer M.R., Maurer H.H. Investigations on the cytochrome P450 (CYP) isoenzymes involved in the metabolism of the designer drugs N-(1-phenyl cyclohexyl)-2-ethoxyethanamine and N-(1-phenylcyclohexyl)-2-methoxyethanamine. Biochem. Pharmacol. 2009;77(3):444–450. doi: 10.1016/j.bcp.2008.10.024. [http://dx.doi.org/10.1016/j.bcp.2008.10.024]. [PMID: 19022226]. [DOI] [PubMed] [Google Scholar]