Abstract
Background:
Synthetic cannabinoids (SCs) have become an increasing issue in forensic toxicology. Controlled human studies evaluating pharmacokinetic data of SCs are lacking and only few animal studies have been published. Thus, an interpretation of analytical results found in intoxicated or poisoned individuals is difficult. Therefore, the distribution of two selected SCs, namely 4-ethylnaphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-210) and 2-(4-methoxyphenyl)-1-(1-pentyl-indol-3-yl)methanone (RCS-4) as well as ∆9-tetrahydrocannabinol (THC) as reference were examined in pigs.
Methods:
Pigs (n = 6 per drug) received a single intravenous 200 µg/kg BW dose of JWH-210, RCS-4, or THC. Six hours after administration, the animals were exsanguinated and relevant organs, important body fluids such as bile, and tissues such as muscle and adipose tissue, as well as the bradytrophic specimens dura and vitreous humor were collected. After hydrolysis and solid phase extraction, analysis was performed by LC-MS/MS. To overcome matrix effects of the LC-MS/MS analysis, a standard addition method was applied for quantification.
Results:
The parent compounds could be detected in every analyzed specimen with the exception of THC that was not present in dura and vitreous humor. Moderate concentrations were present in brain, the site of biological effect. Metabolite concentrations were highest in tissues involved in metabolism and/or elimination.
Conclusions:
Besides kidneys and lungs routinely analyzed in postmortem toxicology, brain, adipose, and muscle tissue could serve as alternative sources, particularly if other specimens are not available. Bile fluid is the most appropriate specimen for SCs and THC metabolites detection.
Keywords: Synthetic cannabinoids, tetrahydrocannabinol, pigs, tissue distribution, LC-MS/MS, pharmacokinetics
1. INTRODUCTION
Synthetic cannabinoids (SCs) have become an increasing public health concern since their emergence on the illicit drug market [1]. Unlike their natural pendant ∆9-tetrahydro-cannabinol (THC), which is a partial agonist of cannabinoid (CB) receptors, many SCs are highly potent substances, which frequently act as full agonists on CB1 receptors and can cause unpredictable severe adverse effects even resulting in death [2]. Several fatalities associated with SCs have already been reported [3-9] including e.g. poisoning, psychiatric complications, sudden episodes after ‘partying’, or related liver failure [3-6].
Whereas some derivatives have been controlled, the new generation of SCs was flooding the illicit drug market to circumvent law. However, these structurally slightly modified SCs have even higher potencies and efficiencies [10]. As a consequence, lower doses are needed to obtain the ‘high’ effect and thus unpredictable biological effects and poisonings due to overdosing are more likely. Deaths involving new emerging SCs such as MAB-CHMINACA and 5F-ADB have been reported recently [11-13]. However, the above-mentioned evidences provide only little information on SCs pharmacokinetic (PK) properties such as tissue distribution and persistence, since dose, time of administration, and dosing frequency were unknown. In forensic toxicology, the clarification of PK and pharmacodynamic properties of SCs is important in order to interpret analytical results found e.g. in poisoned individuals, particularly postmortem. As controlled human studies cannot be conducted, animal models have to be established to investigate the SC distribution after controlled administration. Poklis et al. administered 200 mg “Buzz” containing 5.4% (w/w) JWH-018 to six mice. Twenty minutes after exposure JWH-018 was detected in their blood, brain, and liver [14], and 60 min later JWH-018 was found in blood, brain, heart, kidney, liver, lung, and spleen [15]. The same group studied the disposition of JWH-018 and JWH-073 in blood and brain of mice 20 min and 20 h (n = 5 each) after exposure to “Magic Gold” smoke containing 3.6% JWH-018, 5.7% JWH-073, and less than 0.1% JWH-398 (w/w each) [16]. Schaefer et al. investigated the persistence of JWH-210 and JWH-122 in adipose tissue four weeks after gastric intubation of an 20 mg/kg BW dose of these substances to two rats [17]. On the one hand, none of these studies provided comprehensive information on SC whole body distribution including a representative choice of specimens, such as relevant organs, tissues, and important body fluids such as bile. On the other hand, only parent compounds have been studied without considering their metabolites’ distribution. However, in case of a longer survival time, sometimes parent compounds cannot be detected anymore and analyzing and interpreting the distribution pattern of metabolites may become unequivocal to establish the diagnosis.
Small animals such as rodents are less appropriate for extensive sampling due to their small organ size. For example, detailed preparation of the anatomical structures within the brain is difficult, so that possible differences are difficult to determine. Yet, pigs as a large mammalian species allow extensive sampling of different tissues. In addition, pigs are closely related to the human species in terms of e.g. metabolism including cytochrome P450 enzyme pattern [18], anatomical structures as well as physiological properties regarding e.g. cardiovascular, urogenital, and digestive systems [19, 20]. Furthermore, they are omnivores, sensitive to a wide range of drugs and chemicals, and all routes of administration are possible using the pig [19-21]. In addition, they have proven to be suitable for PK and postmortem redistribution studies [22, 23]. Therefore, a PK model has previously been developed for the two selected SCs 4-ethylnaphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-210) and 2-(4-methoxyphenyl)-1-(1-pentyl-indol-3-yl)methanone (RCS-4), and as reference also for THC in pig serum [24]. This model appeared to be an appropriate tool to predict human THC PK leading to the assumption that it is also suitable to predict human SC PK.
Therefore, the aim of the present study was to investigate the distribution of the above-mentioned SCs and THC. In addition, the authors aimed to assess whether there is a difference between adipose and muscle tissue collected from different regions, and if bradytrophic specimens, such as dura and vitreous humor, are viable for SC and THC postmortem analysis.
2. Materials and methods
2.1. Chemicals and Reagents
Glacial acetic acid p.a., acetone Supra Solv, methanol Supra Solv, formic acid EMSURE, di-potassium hydrogen phosphate EMSURE, sodium bicarbonate EMSURE, potassium hydroxide EMSURE, ß-glucuronidase/arylsulfatase (from Helix pomatia), and HPLC grade water were obtained from VWR-International (Darmstadt, Germany), ethanol p.a. and HPLC grade acetonitrile from Sigma-Aldrich (Steinheim, Germany), and Polysorbat 80 from Caesar & Loretz (Hilden, Germany). Methanolic solutions of THC (0.1 mg/mL), THC pharmaceutical grade for drug administration (Dronabinol, DAC 2008, 98.5% purity), JWH-210 (solid), and RCS-4 (solid) were purchased from THC Pharm (Frankfurt/Main, Germany). THC-d3, 11-hydroxy-THC (11-HO-THC), 11-HO-THC-d3, 11-nor-9-carboxy-THC (THC-COOH), and THC-COOH-d3 solution (0.1 mg/mL each) were obtained from LGC/Promochem (Wesel, Germany) and methanolic solutions of JWH-210-d9 (1 mg/mL) and RCS-4-d9 (5 mg/mL), hydroxypentyl-RCS-4 (HO-RCS-4) solution (10 mg/mL in acetonitrile), hydroxypentyl-JWH-210 (HO-JWH-210, solid), JWH-210-pentanoic acid (JWH-210-COOH, solid), and RCS-4-pentanoic acid (RCS-4-COOH, solid) were from Cayman Europe (Tallinn, Estonia). JWH-210 for drug administration was provided by the German Federal Criminal Police Office (Wiesbaden, Germany) and RCS-4 was purchased as ‘research chemical’ from an internet provider. An in-house test of purity using a commercially available reference substance revealed a purity of 96%.
The buffers were prepared as described elsewhere [25]. The phosphate buffer (0.1 M, pH 9) was prepared by dissolving 22.82 g di-potassium hydrogen phosphate in 1 L deionized water. The acetate buffer (pH 4) was prepared with 5.7 mL of glacial acetic acid and 16 mL of aqueous potassium hydroxide (1 M). For the preparation of the sodium bicarbonate solution, 50 g sodium bicarbonate was dissolved in 1 L deionized water.
2.2. Calibrators for Standard Addition
Standard stock solutions (1 mg/mL) were prepared by dissolving 5 mg of each solid compound in 5 mL of ethanol. Working standard solutions (0.001 mg/mL, 0.01 mg/mL, 0.1 mg/mL) were prepared by diluting stock solution or liquid reference standards with ethanol, respectively. All solutions were stored at -20°C. The concentrations of the calibrators used for standard addition are listed in Table 1.
Table 1.
Calibrator concentrations of the analytes used for standard addition in the different specimens.
| Analyte | Specimen | Calibrator Conc. [ng/g] | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
|
JWH-210 HO-JWH-210 JWH-210-COOH RCS-4 HO-RCS-4 RCS-4-COOH THC HO-THC THC-COOH |
Cerebrum/Cerebellum | 10 | 20 | 30 |
| Liver/Kidney/Heart/ Spleen/Muscle tissues/Dura |
25 | 50 | 75 | |
| Bile | 100 | 200 | 300 | |
| Adipose tissues | 50 | 100 | 150 | |
|
JWH-210 HO-JWH-210 JWH-210-COOH HO-RCS-4 RCS-4-COOH THC HO-THC THC-COOH |
Lung | 25 | 50 | 75 |
| RCS-4 | Lung | 100 | 200 | 300 |
2.3. Animals
As already described in a previous study [25], all experiments were performed in accordance with the German legislation on protection of animals and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Twenty two domestic pigs (Swabian Hall strain; mean body weight 44.7 ± 6.8 kg) were used for the study. The animals had free access to tap water and daily standard chow. They were kept fasting a night before the experiment with free access to water.
2.4. Surgical Procedures
The description of the surgical procedures has already been described elsewhere [25] and is listed in the Electronic Supplementary Material (S1 (121.3KB, pdf) ).
2.5. Study Design
The study included 4 different groups. Pigs in group 1-3 (n = 6 each) received the respective drug in a single intra-venous (i.v.) administration (200 µg/kg BW each) and pigs in group 4 (n = 4) got an i.v. placebo solution (Polysorbat 80 and sodium chloride 0.9%). Analogous to a previous study [25], stock solutions of 100 mg/mL THC (A), 10 mg/mL JWH-210 (B), and 10 mg/mL RCS-4 (C) were first prepared in ethanol for i.v. drug administration to pigs in group 1-3. The appropriate volume of each solution (A: 76.4-104.8 µL, B: 736-1,116 µL, or C: 748-1,216 µL) was used to obtain a 200 µg per kg body weight dose, respectively. The volumes were fortified with about 1 mL Polysorbat 80 for solubilization, filled up with sodium chloride 0.9% to a volume of 10 mL, and administered into the jugular vein. After 360 min, the animals were exsanguinated by opening the vena cava and the following organs, tissues, and body fluids were collected in parts or in toto: brain (cerebrum, cerebellum), heart, lungs (right and left lobe), liver, kidneys (right and left), spleen, muscle tissue (skeletal/psoas), adipose tissue (abdominal, dorsal, perirenal), and bile fluid. In addition, the bradytrophic specimens dura and vitreous humor were collected. All samples were stored at -20°C until analysis.
2.6. Sample Preparation
An amount of 2 g solid tissue (brain, heart, lungs, liver, kidneys, spleen, and muscle tissue) or bile fluid was homogenized (1 amount tissue/bile fluid + 4 amounts water). Subsequently, four 0.5-g portions were prepared with and without addition of different concentrations (Table 1) of JWH-210, HO-JWH-210, JWH-210-COOH, RCS-4, HO-RCS-4, RCS-4-COOH, THC, 11-HO-THC, and THC-COOH to create a standard addition calibration curve. A mixture of 20 µL of an ethanolic stable-isotope-labeled internal standard mixture solution (SIL-IS; 2 ng/20 µL of JWH-210-d9 and RCS-4-d9, 10 ng/20 µL of THC-d3, 11-HO-THC-d3, and THC-COOH-d3) was added together with 500 µL of acetate buffer, and 50 µL ß-glucuronidase/arylsulfatase. After vortexing, the mixture was incubated for 2 h at 60°C for hydrolysis. The specimens were then mixed with 1 mL acetonitrile, vortexed, and centrifuged at 3,500 g for 8 min. An aliquot of 1 mL sodium bicarbonate solution was added to the supernatants and vortexed. A solid phase extraction (SPE) was then carried out as described elsewhere [25]. A detailed description is listed in the Electronic Supplementary Material (S2 (121.3KB, pdf) ).
Adipose tissue specimens were prepared as described elsewhere [17]. An amount of 2 g adipose tissue was homogenized (1 amount tissue + 5 amounts acetonitrile). After centrifugation at 3,500 g for 8 min, 1.5 mL of the supernatants were added to 20 µL SIL-IS and evaporated under nitrogen at 60°C. The dry residues were dissolved in 100 µL of mobile phase A/B (50:50, v/v). Twenty microliters were then injected onto the liquid chromatography-mass spectrometry (LC-MS/MS) system.
Dura specimens were cut into slices, mixed with four amounts acetate buffer and sonicated for 5 min. Subsequently, 50 µL of ß-glucuronidase/arylsulfatase were added and hydrolysis was performed for 2 h at 60°C. Thereafter, the specimens were fortified with 2 mL acetonitrile, vortexed, centrifuged at 3,500 g for 8 min, and the supernatants divided into four 0.5-mL portions. The portions were prepared with and without addition of different concentrations (Table 1) of JWH-210, HO-JWH-210, JWH-210-COOH, RCS-4, HO-RCS-4, RCS-4-COOH, THC, 11-HO-THC, and THC-COOH to create a standard addition calibration curve. A mixture of 20 µL of an SIL-IS was added together with 1 mL of sodium bicarbonate solution and vortexed. SPE was then carried out as described elsewhere [25]. A detailed description is listed in the Electronic Supplementary Material (S2 (121.3KB, pdf) ).
An aliquot of 500 µL vitreous humor was added to a mixture of 20 µL of SIL-IS, 25 µL ethanol, 500 µL acetate buffer, and 50 µL ß-glucuronidase/arylsulfatase. After vortexing, the mixture was incubated for 2 h at 60°C for hydrolysis. Thereafter, the specimens were mixed with 1 mL acetonitrile, vortexed, and centrifuged at 3,500 g for 8 min. The supernatants were fortified with 1 mL sodium bicarbonate solution and vortexed. SPE was then carried out as described elsewhere [25]. A detailed description is given in the Electronic Supplementary Material (S2 (121.3KB, pdf) ).
2.7. LC-MS/MS
LC-MS/MS conditions including instrumentation, chromatographic, and mass spectrometric conditions have already been described elsewhere [25] and are described in the Electronic Supplementary Material (S3 (121.3KB, pdf) ).
2.8. Statistical Tests
A one-way analysis of variance followed by a Bonferroni post-test was performed for the comparison of adipose tissue specimens collected from different regions. An unpaired two-tailed Student’s t-test followed by an f-test was applied to compare cerebrum and cerebellum concentrations, as well as concentrations determined in muscle tissue specimens collected from different regions. Statistics were carried out using GraphPad Prism 5.00 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Standard Addition Approach
To quantify the drugs and their metabolites, a standard addition method was applied. Four portions were prepared of each specimen, one containing no calibrator solution and three containing different concentrations of calibrator solution (Table 1). The slope and intercept ranges that were used to create standard addition calibration equations for the drugs and their metabolites in the specimens viable for quantification are presented in Tables 2-4. Regression coefficients (r2) for JWH-210 and THC as well as their metabolites ranged between 0.96 and 0.99. For RCS-4 and its metabolites r2 laid between 0.95 and 0.99. Because of low sample volume of the vitreous humor specimens, only a qualitative analysis was performed.
Table 2.
Concentrations of JWH-210 and/or metabolites in solid tissues and body fluids, in which they were quantifiable, including slope range and intercept range; concentrations are the mean of 6 values in ng/g including standard deviation (SD); *indicates less replicates.
| Specimen | Analyte | Mean Conc. |
Slope Range
(a)# |
Intercept Range
(b)# |
|---|---|---|---|---|
| Cerebrum | JWH-210 | 20 ± 6.8 | 0.036-0.058 | 0.55-1.17 |
| Cerebellum | JWH-210 | 32 ± 9.0 | 0.05-0.082 | 1.27-2.27 |
| Lung (right) | JWH-210 | 6.9 ± 2.1 | 0.0563-0.0751 | 0.288-0.66 |
| Lung (left) | JWH-210 | 8.6 ± 4.4 | 0.032-0.0653 | 0.164-0.923 |
| Liver | JWH-210 | 14 ± 6.2 | 0.0264-0.0704 | 0.141-1.28 |
| HO-JWH-210 | 0.67 ± 0.24* | 0.79-0.912 | 0.39-2.98 | |
| JWH-210-COOH | 4.5 ± 2.9 | 0.0347-0.0951 | 0.068-0.415 | |
| Kidney (right) | JWH-210 | 53 ± 45 | 0.0409-0.064 | 0.619-6.06 |
| Kidney (left) | JWH-210 | 43 ± 34 | 0.0312-0.056 | 0.363-3.38 |
| HO-JWH-210 | 1.0 ± 0.2* | 0.327-0.768 | 0.185-1 | |
| Bile | JWH-210 | 26 ± 19 | 0.005-0.0074 | 0.0556-0.448 |
| HO-JWH-210 | 110 ± 123 | 0.0108-1.05 | 0.0398-4.07 | |
| Heart | JWH-210 | 19 ± 6.1 | 0.0489-0.0692 | 0.50-1.64 |
| Spleen | JWH-210 | 11 ± 6.8 | 0.0392-0.0596 | 0.159-0.93 |
| HO-JWH-210 | 1.6 ± 0.49* | 0.2175-0.3275 | 0.2449-0.7294 | |
|
Skeletal Muscle |
JWH-210 | 21 ± 8.0 | 0.0592-0.0736 | 0.495-1.98 |
| HO-JWH-210 | 1.0 ± 0.37* | 0.271-0.333 | 0.181-0.389 | |
| Psoas | JWH-210 | 20 ± 9.5 | 0.0605-0.0729 | 0.792-2.29 |
| HO-JWH-210 | 1.8 ± 0.23* | 0.2545-0.3003 | 0.4656-0.5375 | |
| Dura | JWH-210 | 1.7 ± 0.8 | 0.0473-0.1092 | 0.0296-0.1696 |
#y = a x + b; concentration x can be calculated as minus value.
Table 4.
Concentrations of THC and/or metabolites in solid tissues and body fluids, in which they were quantifiable, including slope range and intercept range; concentrations are the mean of 6 values in ng/g including standard deviation (SD); *indicates less replicates.
| Specimen | Analyte | Mean Conc. |
Slope Range
(a)# |
Intercept Range
(b)# |
|---|---|---|---|---|
| Cerebrum | THC | 12 ± 3.8 | 0.0101-0.0116 | 0.09-0.197 |
| Cerebellum | THC | 24 ± 5.8* | 0.0089-0.0127 | 0.157-0.284 |
| Lung (ri) | THC | 69 ± 37* | 0.0092-0.0434 | 0.169-3.45 |
| Lung (le) | THC | 61 ± 50 | 0.0101-0.0416 | 0.123-3.54 |
| Liver | THC | 18 ± 3.7* | 0.002-0.0118 | 0.0426-0.235 |
| Kidney (ri) | THC | 21 ± 16 | 0.0078-0.133 | 0.122-0.446 |
| THC-COOH | 68 ± 22* | 0.0144-0.018 | 0.691-1.64 | |
| Kidney (le) | THC | 24 ± 11 | 0.0009-0.0133 | 0.0396-0.163 |
| THC-COOH | 57 ± 27* | 0.0029-0.0196 | 0.168-1.46 | |
| Bile | THC | 77 ± 83 | 0.001-0.0016 | 0.0208-0.266 |
| 11-HO-THC | 179 ± 197* | 0.0008-0.0019 | 0.0681-0.416 | |
| THC-COOH | 383 ± 218 | 0.002-0.0029 | 0.329-2.14 | |
| Heart | THC | 17 ± 7.0 | 0.0074-0.105 | 0.1-0.221 |
| Spleen | THC | 7.1 ± 3.8 | 0.0088-0.0131 | 0.012-0.114 |
|
Skeletal Muscle |
THC | 16 ± 0.94 | 0.0096-0.0128 | 0.146-0.229 |
| Psoas | THC | 9.1 ± 3.3 | 0.0104-0.0113 | 0.0446-0.154 |
#y = a x + b; concentration x can be calculated as minus value.
3.2. Distribution Patterns
3.2.1. Parent Compounds
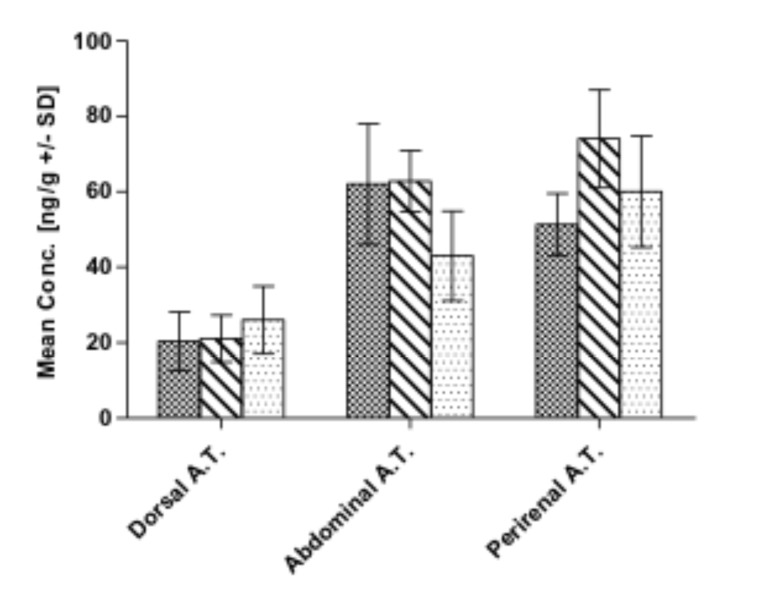
Six hours post-administration, the three parent compounds could be determined in all tissues and body fluids with the exception of THC, which was not detected in dura and vitreous humor specimens. Concerning JWH-210, highest concentrations were found in abdominal and perirenal adipose tissue, followed by the kidneys, cerebellum and bile fluid (Table 2 and Fig. 1). Lowest concentrations were detected in dura, lung, and liver specimens (Table 2). Highest concentrations of RCS-4 were found in the lungs, as well as in abdominal and perirenal adipose tissue, and in bile fluid (Table 3 and Fig. 1). Lowest RCS-4 concentrations were detected in heart, liver, spleen, and dura (Table 3). Highest THC concentrations were found in bile fluid, lungs, perirenal, and abdominal adipose tissue. Lowest THC concentrations were determined in cerebrum, psoas, and spleen (Table 4 and Fig. 1). The analysis of vitreous humor specimens yielded positive results for JWH-210 and RCS-4 (displaying a low abundance of the respective peaks). JWH-210 and THC concentrations in cerebrum were significantly lower than in cerebellum (p<0.05).
Fig. (1).
Mean concentrations including standard deviations (SD) of JWH-210 (squared bars), RCS-4 (striped bars), and THC (dotted bars) in dorsal, abdominal, and perirenal adipose tissue (A.T.) detected 6 h after intravenous administration to pigs.
Table 3.
Concentrations of RCS-4 and/or metabolites in solid tissues and body fluids, in which they were quantifiable, including slope range and intercept range; concentrations are the mean of 6 values in ng/g including standard deviation (SD); *indicates less replicates.
| Specimen | Analyte | Mean Conc. |
Slope Range
(a)# |
Intercept Range
(b)# |
|---|---|---|---|---|
| Cerebrum | RCS-4 | 14 ± 8.4 | 0.0191-0.0296 | 0.114-0.581 |
| HO-RCS-4 | 1.2 ± 0.86* | 0.0204-0.0545 | 0.01-0.349 | |
| Cerebellum | RCS-4 | 15 ± 8.6 | 0.0166-0.0305 | 0.165-0.607 |
| HO-RCS-4 | 1.2 ± 0.71* | 0.0148-0.0289 | 0.0114-0.0659 | |
| Lung (right) | RCS-4 | 111 ±115* | 0.0026-0.0196 | 0.0971-0.8114 |
| HO-RCS-4 | 13.5 ± 9.2* | 0.0009-0.0184 | 0.0011-0.4253 | |
| Lung (left) | RCS-4 | 107 ± 91 | 0.0023-0.006 | 0.0418-3.54 |
| HO-RCS-4 | 6.8 ± 4.1 | 0.0256-0.0609 | 0.0503-0.4067 | |
| Liver | RCS-4 | 12 ± 7.3 | 0.0135-0.0512 | 0.045-0.83 |
| HO-RCS-4 | 46 ± 45 | 0.0168-0.068 | 0.166-2.22 | |
| RCS-4-COOH | 9 ± 6.5* | 0.002-0.0054 | 0.0117-0.04 | |
| Kidney (right) | RCS-4 | 17 ± 9.0 | 0.0206-0.0304 | 0.07-0.96 |
| HO-RCS-4 | 40 ± 30 | 0.0168-0.0449 | 0.0379-2.37 | |
| RCS-4-COOH | 12 ± 7.4* | 0.0016-0.0021 | 0.0112-0.038 | |
| Kidney (left) | RCS-4 | 16 ± 5.0 | 0.0228-0.0441 | 0.228-0.762 |
| HO-RCS-4 | 25 ± 9.0 | 0.027-0.0456 | 0.162-1.19 | |
| RCS-4-COOH | 12 ± 2.3* | 0.0014-0.0022 | 0.0175-0.0294 | |
| Bile | RCS-4 | 61 ± 41 | 0.0015-0.003 | 0.0418-0.22 |
| HO-RCS-4 | 345 ± 270 | 0.0012-0.036 | 0.0082-9.6 | |
| Heart | RCS-4 | 13 ± 5.0* | 0.032-0.046 | 0.302-0.712 |
| HO-RCS-4 | 1.5 ± 0.07* | 0.029-0.054 | 0.019-0.061 | |
| Spleen | RCS-4 | 8.7 ± 4.8 | 0.0148-0.0227 | 0.058-0.3 |
| HO-RCS-4 | 3.3 ± 1.5* | 0.0265-0.0418 | 0.0444-0.1802 | |
|
Skeletal Muscle |
RCS-4 | 24 ± 8.2 | 0.0223-0.0329 | 0.416-1.05 |
| Psoas | RCS-4 | 14 ± 5.0 | 0.022-0.029 | 0.12-0.51 |
| HO-RCS-4 | 2.0 ± 1.3* | 0.0231-0.0289 | 0.0187-0.0952 | |
| Dura | RCS-4 | 3.3 ± 2.1* | 0.0214-0.0295 | 0.0285-0.1134 |
#y = a x + b; concentration x can be calculated as minus value.
3.2.2. Metabolites
A high interindividual variability in metabolites concentration in different target organs was observed (Tables 2-4). HO-JWH-210 was reliably detected in bile fluid of all pigs at very high concentrations. In liver, kidney, spleen, and muscle tissues only very low concentrations were found (Table 2). It was also present in perirenal adipose tissue of two pigs (4.5 and 9 ng/g) and in abdominal adipose tissue (6 ng/g) of one pig. JWH-210-COOH was detectable only in liver (Table 2) and in two cases in kidney specimens (0.5 and 1 ng/g).
Generally, HO-RCS-4 could be determined in all specimens (Table 3) except for dura, vitreous humor, and adipose tissue, and only in two pigs in skeletal muscle (5.7 and 1.6 ng/g). Highest concentrations were found in bile fluid, liver, and kidneys, and lowest concentrations in the brain (Table 3). RCS-4-COOH was detected in liver and kidney specimens in medium concentrations (Table 3) and in the bile fluid of two pigs (47 and 154 ng/g). HO-THC was only present in bile fluid at very high concentrations and THC-COOH only in bile fluid at very high and in the kidneys at high concentrations (Table 4).
3.3. Comparison of Concentrations in Adipose and Muscle Tissue Specimens Collected from Different Regions
In case of the adipose tissue specimens sampled from different regions, highest RCS-4 and THC concentrations were detected perirenally, followed by abdominal adipose tissue, and lowest in dorsal adipose tissue (Fig. 1). With regard to JWH-210, highest concentrations were detected in abdominal adipose tissue, followed by perirenal and dorsal adipose tissue (Fig. 1). RCS-4 concentrations in dorsal adipose tissue were significantly lower than the respective perirenal and abdominal concentrations (p<0.05). As far as the distribution in muscle tissue is concerned, RCS-4 and THC showed significantly higher concentrations in skeletal than in psoas muscle (p<0.05). The hydroxy metabolites were found in the muscle tissue of only few animals (Tables 2 and 3).
3.4. Disposition in Bradytrophic Specimens
The analysis of the bradythrophic specimens revealed very low JWH-210 and RCS-4 concentrations in dura (Tables 2 and 3) and positive results for JWH-210 and RCS-4 in vitreous humor with low peak abundances. No metabolites could be detected in both specimens.
4. Discussion
4.1. Background and Relevance
JWH-210 and RCS-4 are potent SCs and act with high affinity as agonists on CB1 receptors [26, 27]. Cases of intoxications accompanied with adverse effects such as tachycardia, somnolence, anxiety, nausea, vomiting, seizures, hypokalemia, agitation, and hallucination, resulting in emergency admission - or even in death - after consumption of JWH-210 or RCS-4 have been reported [7, 8, 28]. Possible (neuro- or geno-) toxic effects of the two drugs have also been discussed [29, 30] and their main metabolites have been elucidated [31, 32] as well as their pharmacokinetic properties [24]. However, there is limited information about their distribution in the body. Therefore, we investigated the distribution of the two SCs JWH-210 and RCS-4 and THC as reference in pigs. In addition, it was assessed whether there is a difference between adipose and muscle tissue collected from different regions, and whether bradytrophic specimens such as dura and vitreous humor are viable for SC and THC postmortem analysis.
4.2. Dosage
A 200 µg/kg body weight dose of JWH-210, RCS-4, and THC was administered intravenously to pigs with a mean body weight 44.7 ± 6.8 kg, resulting in a total dose of 9.0 ± 1.5 mg. Regarding THC, this dosage may be compared to a THC quantity that is reported to be consumed by frequent users [22]. Relating to JWH-210 and RCS-4, a common drug users’ dose is in the range of 0.5-8 mg, depending on the route of administration [33]. Thus, a similar dose was chosen in this study to grant the comparability with already published human data and to assure that the animals remained under the influence of measurable substance concentrations [25].
4.3. Standard Addition Approach
In the present study, the standard addition approach was chosen for quantification of the drugs and their metabolites. As various different specimens had to be analyzed, this approach seemed to be the most appropriate tool. Although being more laborious as compared to the conventional method development, standard addition bore the advantage that matrix effects could be overcome. In particular in postmortem toxicology, specimens are altered as compared to antemortem specimens, and frequently even decomposed. In such cases, matrix effects can further be increased.
The concentrations of the calibrators were assessed by conducting a previous screening, whereby the drug concentrations in the different specimens were roughly estimated and the calibrator concentrations subsequently adjusted. The calibration curves were considered to be linear with r2 > 0.95.
4.4. Distribution Patterns and Resulting Recommenda-tions for Routine Analysis
Similar distribution patterns of the parent compounds were observed throughout the tested specimens with the exception of two organs. At first, the lungs showed a discrepancy between very high RCS-4 and THC concentrations and very low JWH-210 concentrations (Tables 2-4). Regarding THC, the storage in and metabolism by the lungs has already been observed [22, 34, 35]. Especially lipophilic and basic substances display a high affinity to lung tissue [36-38]. RCS-4 contains a tertiary amine with weak basic properties, which might be responsible for sequestration or metabolization. The very low JWH-210 concentrations in lung tissue might be explained on the other hand by its very high lipid-soluble nature [39], which may have led to more rapid elimination from lung and distribution to more lipophilic tissues [36].
The second difference could be observed in the kidney. In this organ, high concentrations of JWH-210 were detected as opposed to low concentrations of the other two drugs (Tables 2-4). Previous studies showed that synthetic cannabinoids were mainly excreted into urine in their metabolized form [25, 40]. Due to their high protein binding (unpublished data) and lipophilic nature, they probably underlie tubular reabsorption into the bloodstream and/or persistence in the kidneys. As compared to RCS-4 (LogP = 5.6) and THC (LogP = 6.7), JWH-210 (LogP = 7.5) is more lipophilic [39]. In addition, compared to RCS-4 metabolites, only low concentrations of HO-JWH-210 and JWH-210-COOH could be detected in the analyzed specimens, except for bile fluid (Table 2). This might be explained by the higher JWH-210 lipophilicity resulting in higher persistence in kidneys and higher distribution into adipose tissue. Apart from this, lungs and kidneys are standard specimens and thus, should be analyzed in routine postmortem toxicology.
After 6 h, still moderate concentrations of the parent drugs could be detected in brain, especially in cerebellum (Tables 2-4). The higher concentrations in cerebellum might be explained by the high density of CB1 receptors in this region of the brain [41]. As far as the distribution of the metabolites in brain is concerned, only the hydroxy metabolite of RCS-4 could be determined in the brain of four pigs. At first sight, this is surprising, because RCS-4 has a LogP value of 5.6 and is more hydrophilic than JWH-210 (LogP = 7.5) and HO-RCS-4 is even more hydrophilic (LogP = 4.08, [39]). The presence of HO-RCS-4 in brain is a very important finding, because previous studies showed that monohydroxylated synthetic cannabinoids exhibited high affinities to CB1 receptors and also high intrinsic activity [42, 43]. An additional analysis of brain tissue is therefore highly recommended.
The same applies to bile fluid. Moderate to high concentrations of parent compounds and very high metabolite concentrations (after cleavage of conjugates) were detected in this specimen (Tables 2-4). This suggests an enterohepatic circulation process and a biliary excretion of the three substances. The enterohepatic circulation can lead to a retarded elimination of a parent compound and/or metabolites and thus to a prolonged drug effect. Regarding THC, this phenomenon has already been discussed [44].
The second aim of the study was to assess whether the distribution of the substances and their metabolites varies in adipose and muscle tissue specimens collected from different regions, and if bradytrophic specimens such as dura and vitreous humor are appropriate for SCs and THC postmortem analysis. With respect to the adipose tissue specimens, highest RCS-4 and THC concentrations were determined in perirenal adipose tissue, followed by abdominal and dorsal adipose tissue (Fig. 1). Highest JWH-210 concentrations were found in abdominal adipose tissue, followed by perirenal, and dorsal adipose tissue. Central perirenal and abdominal adipose tissue are supposed to be well supplied by blood vessels as opposed for dorsal adipose tissue. In addition, pig dorsal adipose tissue has a much thicker texture, which probably hampers substances to penetrate. In this context, it also has to be discussed that pig and human adipose tissue composition is different in terms of the triglyceride types. Pig adipose tissue triglycerides are dominated by saturated fatty acids [45]. Therefore, a difference in drug sequestration between the two species is possible. Considering the metabolites, only HO-JWH-210 was present at least in two pigs in perirenal and in one pig in abdominal adipose tissue. As this metabolite is the most lipophilic one compared to the metabolites of RCS-4 and THC [39], it is not surprising that it persisted in adipose tissue. However, the high inter-individual variability suggests that adipose tissue is not viable for SCs’ metabolite detection. On the contrary, it may serve as an alternative matrix for the detection of the parent compounds. This has also been investigated in a previous study [17] and has been suggested by other researchers [3, 5, 46]. In contrast to blood, adipose tissue is available in higher amounts, even if a decedent is already putrefied. In addition, Levisky et al. demonstrated that drugs identified in postmortem adipose tissue prove an antemortem deposition and cannot be attributed to postmortem redistribution or any other change [47]. Furthermore, adipose tissue provides long-term information about a subject’s pharmacological history, whereas a certain date of a particular consumption cannot precisely be determined. The storage in adipose tissue also entails some important issues that have to be considered when interpreting analytical data of forensic toxicological cases. The high affinity to lipophilic tissues can lead to long terminal half-life values [44], due to release of the substance from adipose tissue into the bloodstream [44]. In this context, the usually unknown consumption habits of chronic users have also to be taken into consideration. Especially after chronic consumption longer windows of detection due to accumulation and retarded release from adipose tissue have to be expected. This has particularly been discussed for SCs and THC [44, 48].
As far as the distribution in skeletal and psoas muscle is concerned, moderate concentrations of the parent compounds could be found after 6 h. Thus, this tissue seems to be a helpful tool for the detection of cannabinoids, when standard specimens are not available, i.e. due to decomposition.
Focusing on the bradytrophic specimens, very low concentrations were found in dura and only low abundances of JWH-210 and RCS-4 in vitreous humor. This implicates that these specimens cannot be recommended for the detection of cannabinoids. Vitreous humor has already been identified to be unsuitable for the detection of THC [49].
The tissue concentrations may be compared to corresponding serum concentrations assessed in a previous study, which were found to be 3.1 ± 1.1 ng/mL for JWH-210, 3.8 ± 1.1 ng/mL for RCS-4, and 7.1 ± 4.3 ng/mL for THC six hours after administration [24]. To sum up, the tissue concentrations were considerably higher, except for the bradytrophic dura, reflecting the tissue distribution. Of particular interest is the higher concentration in brain tissue, the site of the main biological effects.
4.5. Comparison with Published Data
4.5.1. SCs
The majority of published human case reports on poisonings with SCs, report only blood concentrations and no attention has been paid to the SCs’ tissue distribution [4, 6, 50]. Just a few case reports provided data on the tissue distribution [11-13]. In one case [3], a man was found dead in his home with three herbal blend sachets. The postmortem interval was estimated to be four days. At autopsy, no evidence was observed neither for violence nor for a disease. The toxicological analysis of heart blood, urine, liver, kidney, brain, and adipose tissue specimens revealed the presence of MAM-2201, which is structurally related to JWH-210. The highest concentration was found in adipose tissue (1,535 ng/g), followed by the liver (18.1 ng/g), the heart blood (12.4 ng/mL), and kidney (11.2 ng/g). Lowest concentrations were detected in brain (4.3 ng/g). A rapid metabolism and high lipophilicity of MAM-2201 was suggested to be the reason for relatively low levels in blood, liver, kidney, and brain as compared to very high adipose tissue levels. However, the specimens have not been analyzed for the metabolites of MAM-2201. Compared to the JWH-210 tissue concentrations determined in the present study, the MAM-2201 concentrations are slightly lower or in the same range except for the adipose tissue levels. Considering that the time interval between consumption and death was shorter than in the pig study, the adipose tissue value is extremely high. This leads to the assumption that the decedent was a frequent user of MAM-2201 and that its concentration was significantly higher due to accumulation.
In order to assess SCs’ tissue distribution patterns, systematic controlled studies are necessary. Poklis et al. analyzed mice blood, brain, and liver [14], as well as blood, brain, heart, kidney, liver, lung, and spleen 20 and 60 min respectively [15] after exposure to 200 mg of the herbal blend “Buzz”, containing 5.4% (w/w) JWH-018. After 60 min, they found highest concentrations in the tissues involved in lung, liver, and kidney [15]. These findings are in good agreement with the findings of the present study (Tables 2-4), although our specimens have been collected after 360 min. Poklis et al. also studied the disposition of JWH-018 and JWH-073 in blood and brain of mice 20 min and 20 h (n = 5 each) after exposure to “Magic Gold” smoke containing 3.6% JWH-018, 5.7% JWH-073, and less than 0.1% JWH-398 (w/w each) [16]. Twenty hours post-exposure they still detected 19 ± 9 ng/g of JWH-018 in the brain specimens, but no of JWH-073.
4.5.2. THC
Although THC is considered to be a drug with minor toxicity, some fatalities involved THC at least as a contributing factor in causing death [51, 52]. Investigating the distribution of cannabinoids in five postmortem cases, Gronewold et al. [51] detected a peak concentration of THC (40 ng/g) in muscle tissue of one decedent. However, the THC muscle tissue concentrations determined in the other cases were in the same range than those found in our pig study. In good correlation to the findings of the present study, THC metabolites could hardly be detected in muscle tissue as well as in brain, but were highest in bile fluid. Gronewold et al. suggested muscle tissue to be an alternative source for cannabinoid detection. A storage of THC in muscle tissue and a following accumulation after multiple administrations has already been discussed [53]. THC muscle concentrations detected in the present study are moderate and lower than in the corresponding adipose tissue specimens. This was also true for the SCs. With regard to the fact that muscle tissue is higher perfused than adipose tissue, the highly lipophilic cannabinoids might preferably incorporate in adipose tissue. In a fatal fall from the height [52], toxicological analysis was performed to clarify whether the decedent had been under the influence. THC concentrations were low in liver, moderate in kidneys, spleen, muscle, and brain, and highest in lung and adipose tissue. Even if dosage, time, and consumption habits were unknown in these case reports, it is astonishing that these findings were in overall agreement with the THC results of the present study.
Brunet et al. [22] performed a systematic pig study applying a similar protocol than in the present study. After 6 h, they detected lower THC concentrations in the analyzed specimens compared to our study, except for bile fluid. This might be owed to their sampling protocol. They administered 200 µg/kg THC i.v. to eight pigs. Two animals were sacrificed 30 min after injection and the concentrations detected in these specimens were set as initial levels (100%). Two further pigs were sacrificed after 2, 4, 6, and 24 h, respectively. The concentrations determined were given as percentage of THC remaining as compared to the initial levels. However, it has to be taken into consideration that the values represented the mean of only two analyses, respectively. In addition, the determination of the THC concentration at the different time-points was related to the initial levels found after 30 min, though, these levels derived from two different animals. Thus, it is not surprising that the results of the two studies differ.
4.6. Summary
Taken the findings of our study together, besides lungs and kidneys routinely analyzed in postmortem toxicology, brain, adipose and muscle tissue could serve as alternative matrices for the detection of SCs. Bile fluid was the most appropriate specimen for detection of SCs and THC metabolites. In addition, kidney was suitable for THC-COOH, HO-RCS-4, and RCS-4-COOH detection. With respect to the high potency of SCs, blood concentrations are expected to be very low. Especially with long postmortem intervals, the detection in blood might be difficult e.g. due to possible drug degradation. In such cases, the analysis of the recommended specimens would be advisable.
CONCLUSION
Distribution patterns of the two SCs JWH-210 and RCS-4 as well as THC after i.v. administration were investigated in domestic pigs. In terms of the parent compounds, the distribution patterns were similar with two exceptions. RCS-4 and THC persisted in lung tissue, while JWH-210 was stored in the kidneys. Retention of the three drugs in brain and adipose tissue could be observed, especially in abdominal and perirenal ones. Besides routinely analyzed specimens, brain and adipose tissue, as well as muscle tissue could serve as alternative sources, particularly if other specimens are not available. In addition, bile fluid is highly recommended as additional matrix, because it was the most appropriate specimen for SC and THC metabolite detection. Bradytrophic specimens such as dura and vitreous humor are neither suitable for the detection of parent compounds nor for metabolite detection.
ACKNOWLEDGEMENTS
The authors thank Markus R. Meyer, Benjamin Peters, Dietmar Bregel, and the staff of the Institute for Clinical & Experimental Surgery in Homburg for their support and help during the study.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
CONFLICT OF INTEREST
There are no financial or other relations that could lead to a conflict of interest.
REFERENCES
- 1.Castaneto M.S., Gorelick D.A., Desrosiers N.A., Hartman R.L., Pirard S., Huestis M.A. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005. [http://dx.doi.org/10.1016/j.drugalcdep. 2014.08.005]. [PMID: 25220897]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auwärter V., Kneisel S., Hutter M., Thierauf A. Synthetische Cannabinoide. Rechtsmedizin. 2012;22:259–271. [http://dx.doi. org/10.1007/s00194-012-0810-4]. [Google Scholar]
- 3.Saito T., Namera A., Miura N., Ohta S., Miyazaki S., Osawa M., Inokuchi S. A fatal case of MAM-2201 poisoning. Forensic Toxicol. 2013;31:333–337. [http://dx.doi.org/10.1007/s11419-013-0190-9]. [Google Scholar]
- 4.Patton A.L., Chimalakonda K.C., Moran C.L., McCain K.R., Radominska-Pandya A., James L.P., Kokes C., Moran J.H. K2 toxicity: fatal case of psychiatric complications following AM2201 exposure. J. Forensic Sci. 2013;58(6):1676–1680. doi: 10.1111/1556-4029.12216. [http://dx.doi. org/10.1111/1556-4029.12216]. [PMID: 23822805]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa K., Wurita A., Minakata K., Gonmori K., Nozawa H., Yamagishi I., Watanabe K., Suzuki O. Postmortem distribution of AB-CHMINACA, 5-fluoro-AMB, and diphenidine in body fluids and solid tissues in a fatal poisoning case: usefulness of adipose tissue for detection of the drugs in unchanged forms. Forensic Toxicol. 2015;33:45–53. [http://dx.doi.org/10.1007/s11419-014-0245-6]. [Google Scholar]
- 6.Behonick G., Shanks K.G., Firchau D.J., Mathur G., Lynch C.F., Nashelsky M., Jaskierny D.J., Meroueh C. Four postmortem case reports with quantitative detection of the synthetic cannabinoid, 5F-PB-22. J. Anal. Toxicol. 2014;38(8):559–562. doi: 10.1093/jat/bku048. [http://dx.doi.org/10.1093/jat/bku048]. [PMID: 24876364]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronstrand R., Roman M., Andersson M., Eklund A. Toxicological findings of synthetic cannabinoids in recreational users. J. Anal. Toxicol. 2013;37(8):534–541. doi: 10.1093/jat/bkt068. [http://dx.doi.org/ 10.1093/jat/bkt068]. [PMID: 23970540]. [DOI] [PubMed] [Google Scholar]
- 8.Labay L.M., Caruso J.L., Gilson T.P., Phipps R.J., Knight L.D., Lemos N.P., McIntyre I.M., Stoppacher R., Tormos L.M., Wiens A.L., Williams E., Logan B.K. Synthetic cannabinoid drug use as a cause or contributory cause of death. Forensic Sci. Int. 2016;260:31–39. doi: 10.1016/j.forsciint.2015.12.046. [http://dx.doi.org/10.1016/j.forsciint.2015.12. 046]. [PMID: 26795398]. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer N., Peters B., Bregel D., Kneisel S., Auwärter V., Schmidt P., Ewald A. A fatal case involving several synthetic cannabinoids. Toxichem Krimtech. 2013;80:248–251. [Google Scholar]
- 10.Banister S.D., Moir M., Stuart J., Kevin R.C., Wood K.E., Longworth M., Wilkinson S.M., Beinat C., Buchanan A.S., Glass M., Connor M., McGregor I.S., Kassiou M. Pharmacology of indole and indazole synthetic cannabinoid designer drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem. Neurosci. 2015;6(9):1546–1559. doi: 10.1021/acschemneuro.5b00112. [http://dx.doi.org/10. 1021/acschemneuro.5b00112]. [PMID: 26134475]. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa K., Wurita A., Minakata K., Gonmori K., Nozawa H., Yamagishi I., Watanabe K., Suzuki O. Postmortem distribution of MAB-CHMINACA in body fluids and solid tissues of a human cadaver. Forensic Toxicol. 2015;33(2):380–387. doi: 10.1007/s11419-015-0272-y. [http://dx.doi. org/10.1007/s11419-015-0272-y]. [PMID: 26257834]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasegawa K., Wurita A., Minakata K., Gonmori K., Yamagishi I., Nozawa H., Watanabe K., Suzuki O. Identification and quantitation of 5-fluoro-ADB, one of the most dangerous synthetic cannabinoids, in the stomach contents and solid tissues of a human cadaver and in some herbal products. Forensic Toxicol. 2015;33:112–121. doi: 10.1007/s11419-015-0272-y. [http://dx.doi.org/10.1007/s11419-014-0259-0]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trecki J., Gerona R.R., Schwartz M.D. Synthetic cannabinoid-related illnesses and deaths. N. Engl. J. Med. 2015;373(2):103–107. doi: 10.1056/NEJMp1505328. [http://dx.doi.org/10.1056/NEJMp1505328]. [PMID: 26154784]. [DOI] [PubMed] [Google Scholar]
- 14.Poklis J.L., Amira D., Wise L.E., Wiebelhaus J.M., Haggerty B.J., Lichtman A.H., Poklis A. Determination of naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in mouse blood and tissue after inhalation exposure to buzz smoke by HPLC/MS/MS. Biomed. Chromatogr. 2012;26(11):1393–1398. doi: 10.1002/bmc.2710. [http://dx.doi.org/ 10.1002/bmc.2710]. [PMID: 22407432]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiebelhaus J.M., Poklis J.L., Poklis A., Vann R.E., Lichtman A.H., Wise L.E. Inhalation exposure to smoke from synthetic marijuana produces potent cannabimimetic effects in mice. Drug Alcohol Depend. 2012;126(3):316–323. doi: 10.1016/j.drugalcdep.2012.05.034. [http://dx.doi.org/10. 1016/j.drugalcdep.2012.05.034]. [PMID: 22776442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poklis J.L., Amira D., Wise L.E., Wiebelhaus J.M., Haggerty B.J., Poklis A. Detection and disposition of JWH-018 and JWH-073 in mice after exposure to Magic Gold smoke. Forensic Sci. Int. 2012;220(1-3):91–96. doi: 10.1016/j.forsciint.2012.02.003. [http://dx.doi.org/10.1016/j.forsciint. 2012.02.003]. [PMID: 22405481]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer N., Peters B., Bregel D., Maurer H.H., Schmidt P.H., Ewald A.H. Can JWH-210 and JWH-122 be detected in adipose tissue four weeks after single oral drug administration to rats? Biomed. Chromatogr. 2014;28(8):1043–1047. doi: 10.1002/bmc.3137. [http://dx.doi.org/ 10.1002/bmc.3137]. [PMID: 24474420]. [DOI] [PubMed] [Google Scholar]
- 18.Anzenbacher P., Soucek P., Anzenbacherová E., Gut I., Hrubý K., Svoboda Z., Kvĕtina J. Presence and activity of cytochrome P450 isoforms in minipig liver microsomes. Comparison with human liver samples. Drug Metab. Dispos. 1998;26(1):56–59. [PMID: 9443853]. [PubMed] [Google Scholar]
- 19.Swindle M.M., Makin A., Herron A.J., Clubb F.J., Jr, Frazier K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012;49(2):344–356. doi: 10.1177/0300985811402846. [http://dx.doi.org/ 10.1177/0300985811402846]. [PMID: 21441112]. [DOI] [PubMed] [Google Scholar]
- 20.Bode G., Clausing P., Gervais F., Loegsted J., Luft J., Nogues V., Sims J. The utility of the minipig as an animal model in regulatory toxicology. J. Pharmacol. Toxicol. Methods. 2010;62(3):196–220. doi: 10.1016/j.vascn.2010.05.009. [http://dx.doi.org/10.1016/j.vascn.2010.05.009]. [PMID: 20685310]. [DOI] [PubMed] [Google Scholar]
- 21.Svendsen O. The minipig in toxicology. Exp. Toxicol. Pathol. 2006;57(5-6):335–339. doi: 10.1016/j.etp.2006.03.003. [http://dx.doi.org/10.1016/j.etp.2006.03. 003]. [PMID: 16725317]. [DOI] [PubMed] [Google Scholar]
- 22.Brunet B., Doucet C., Venisse N., Hauet T., Hébrard W., Papet Y., Mauco G., Mura P. Validation of large white pig as an animal model for the study of cannabinoids metabolism: application to the study of THC distribution in tissues. Forensic Sci. Int. 2006;161(2-3):169–174. doi: 10.1016/j.forsciint.2006.04.018. [http://dx.doi.org/10.1016/j.forsciint.2006.04.018]. [PMID: 16859848]. [DOI] [PubMed] [Google Scholar]
- 23.Brunet B., Hauet T., Hébrard W., Papet Y., Mauco G., Mura P. Postmortem redistribution of THC in the pig. Int. J. Legal Med. 2010;124(6):543–549. doi: 10.1007/s00414-009-0403-2. [http://dx.doi.org/10.1007/s00414-009-0403-2]. [PMID: 20052591]. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer N., Wojtyniak J.G., Kettner M., Schlote J., Laschke M.W., Ewald A.H., Lehr T., Menger M.D., Maurer H.H., Schmidt P.H. Pharmacokinetics of (synthetic) cannabinoids in pigs and their relevance for clinical and forensic toxicology. Toxicol. Lett. 2016;253:7–16. doi: 10.1016/j.toxlet.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer N., Kettner M., Laschke M.W., Schlote J., Peters B., Bregel D., Menger M.D., Maurer H.H., Ewald A.H., Schmidt P.H. Simultaneous LC-MS/MS determination of JWH-210, RCS-4, ∆(9)-tetrahydrocannabinol, and their main metabolites in pig and human serum, whole blood, and urine for comparing phar-macokinetic data. Anal. Bioanal. Chem. 2015;407(13):3775–3786. doi: 10.1007/s00216-015-8605-6. [http://dx.doi.org/10.1007/s00216-015-8605-6]. [PMID: 25772567]. [DOI] [PubMed] [Google Scholar]
- 26.Huffman J.W., Zengin G., Wu M-J., Lu J., Hynd G., Bushell K., Thompson A.L., Bushell S., Tartal C., Hurst D.P., Reggio P.H., Selley D.E., Cassidy M.P., Wiley J.L., Martin B.R. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg. Med. Chem. 2005;13(1):89–112. doi: 10.1016/j.bmc.2004.09.050. [http://dx.doi.org/10.1016/j.bmc.2004.09.050]. [PMID: 15582455]. [DOI] [PubMed] [Google Scholar]
- 27.Banister S.D., Stuart J., Conroy T., Longworth M., Manohar M., Beinat C., Wilkinson S.M., Kevin R.C., Hibbs D.E., Glass M., Connor M., McGregor I.S., Kassiou M. Structure–activity relationships of synthetic cannabinoid designer drug RCS-4 and its regioisomers and C4 homologues. Forensic Toxicol. 2015;33:355–366. [http://dx.doi.org/10.1007/s11419-015-0282-9]. [Google Scholar]
- 28.Hermanns-Clausen M., Kithinji J., Spehl M., Angerer V., Franz F., Eyer F., Auwärter V. Adverse effects after the use of JWH-210 – a case series from the EU Spice II plus project. Drug Test. Anal. 2016 doi: 10.1002/dta.1936. [DOI] [PubMed] [Google Scholar]
- 29.Cha H.J., Seong Y-H., Song M-J., Jeong H-S., Shin J., Yun J., Han K., Kim Y-H., Kang H., Kim H.S. Neurotoxicity of Synthetic Cannabinoids JWH-081 and JWH-210. Biomol. Ther. (Seoul) 2015;23(6):597–603. doi: 10.4062/biomolther.2015.057. [http://dx.doi.org/10.4062/biomolther. 2015.057]. [PMID: 26535086]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferk F., Gminski R., Al-Serori H., Mišík M., Nersesyan A., Koller V.J., Angerer V., Auwärter V., Tang T., Arif A.T., Knasmüller S. Genotoxic properties of XLR-11, a widely consumed synthetic cannabinoid, and of the benzoyl indole RCS-4. Arch. Toxicol. 2016;90(12):3111–3123. doi: 10.1007/s00204-016-1664-4. [http://dx.doi.org/10. 1007/s00204-016-1664-4]. [PMID: 26856714]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutter M., Broecker S., Kneisel S., Auwärter V. Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in herbal mixtures using LC-MS/MS techniques. J. Mass Spectrom. 2012;47(1):54–65. doi: 10.1002/jms.2026. [http://dx.doi.org/10.1002/jms.2026]. [PMID: 22282090]. [DOI] [PubMed] [Google Scholar]
- 32.Gandhi A.S., Zhu M., Pang S., Wohlfarth A., Scheidweiler K.B., Huestis M.A. Metabolite profiling of RCS-4, a novel synthetic cannabinoid designer drug, using human hepatocyte metabolism and TOF-MS. Bioanalysis. 2014;6(11):1471–1485. doi: 10.4155/bio.14.13. [http://dx.doi.org/10.4155/bio.14.13]. [PMID: 25046048]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. www.eve-rave.ch/Forum/viewtopic.php?t=28044.
- 34.Nahas G.G. Toxicology and pharmacology of cannabis sativa with special reference to delta-9-THC. Bull. Narc. 1972;24:11–27. [Google Scholar]
- 35.Law F.C. Metabolism and disposition of alpha1-tetrahydro-cannabinol by the isolated perfused rabbit lung. Drug Metab. Dispos. 1978;6(2):154–163. [PMID: 26531]. [PubMed] [Google Scholar]
- 36.Bend J.R., Serabjit-Singh C.J., Philpot R.M. The pulmonary uptake, accumulation, and metabolism of xenobiotics. Annu. Rev. Pharmacol. Toxicol. 1985;25:97–125. doi: 10.1146/annurev.pa.25.040185.000525. [http://dx.doi.org/10.1146/ annurev.pa.25.040185.000525]. [PMID: 3890717]. [DOI] [PubMed] [Google Scholar]
- 37.Bakhle Y.S. Pharmacokinetic and metabolic properties of lung. Br. J. Anaesth. 1990;65(1):79–93. doi: 10.1093/bja/65.1.79. [http://dx.doi.org/10.1093/bja/65.1. 79]. [PMID: 2200487]. [DOI] [PubMed] [Google Scholar]
- 38.Ryan U.S., Grantham C.J. Metabolism of endogenous and xenobiotic substances by pulmonary vascular endothelial cells. Pharmacol. Ther. 1989;42(2):235–250. doi: 10.1016/0163-7258(89)90037-5. [http://dx.doi.org/10. 1016/0163-7258(89)90037-5]. [PMID: 2664822]. [DOI] [PubMed] [Google Scholar]
- 39. http://www.molinspiration.com.
- 40.Wohlfarth A., Scheidweiler K.B., Castaneto M., Gandhi A.S., Desrosiers N.A., Klette K.L., Martin T.M., Huestis M.A. Urinary prevalence, metabolite detection rates, temporal patterns and evaluation of suitable LC-MS/MS targets to document synthetic cannabinoid intake in US military urine specimens. Clin. Chem. Lab. Med. 2015;53(3):423–434. doi: 10.1515/cclm-2014-0612. [http://dx.doi.org/10.1515/ cclm-2014-0612]. [PMID: 25263309]. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K.A., Linden D.J. Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J. Neurophysiol. 2000;83(3):1167–1180. doi: 10.1152/jn.2000.83.3.1167. [PMID: 10712447]. [DOI] [PubMed] [Google Scholar]
- 42.Chimalakonda K.C., Seely K.A., Bratton S.M., Brents L.K., Moran C.L., Endres G.W., James L.P., Hollenberg P.F., Prather P.L., Radominska-Pandya A., Moran J.H. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab. Dispos. 2012;40(11):2174–2184. doi: 10.1124/dmd.112.047530. [http://dx. doi.org/10.1124/dmd.112.047530]. [PMID: 22904561]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brents L.K., Reichard E.E., Zimmerman S.M., Moran J.H., Fantegrossi W.E., Prather P.L., Phase I. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6(7):e21917. doi: 10.1371/journal.pone.0021917. [http://dx.doi.org/10.1371/journal. pone.0021917]. [PMID: 21755008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huestis M.A. Cannabis (Marihuana) - effects on human behavior and performance. Forensic Sci. Rev. 2002;14:15–60. [PMID: 26256486]. [PubMed] [Google Scholar]
- 45.Christie W.W., Moore J.H. A comparison of the structures of triglycerides from various pig tissues. BBA - Lipid Lipid Met, 1970;210:46–56. doi: 10.1016/0005-2760(70)90060-3. [DOI] [PubMed] [Google Scholar]
- 46.Johansson E., Norén K., Sjövall J., Halldin M.M. Determination of delta 1-tetrahydrocannabinol in human fat biopsies from marihuana users by gas chromatography-mass spectrometry. Biomed. Chromatogr. 1989;3(1):35–38. doi: 10.1002/bmc.1130030109. [http://dx.doi.org/ 10.1002/bmc.1130030109]. [PMID: 2539872]. [DOI] [PubMed] [Google Scholar]
- 47.Levisky J.A., Bowerman D.L., Jenkins W.W., Johnson D.G., Karch S.B. Drugs in postmortem adipose tissues: evidence of antemortem deposition. Forensic Sci. Int. 2001;121(3):157–160. doi: 10.1016/s0379-0738(01)00397-8. [http:// dx.doi.org/10.1016/S0379-0738(01)00397-8]. [PMID: 11566418]. [DOI] [PubMed] [Google Scholar]
- 48.Kneisel S., Teske J., Auwärter V. Analysis of synthetic cannabinoids in abstinence control: long drug detection windows in serum and implications for practitioners. Drug Test. Anal. 2014;6(1-2):135–136. doi: 10.1002/dta.1445. [http://dx.doi.org/10.1002/dta.1445]. [PMID: 23315922]. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins A.J., Oblock J. Phencyclidine and cannabinoids in vitreous humor. Leg. Med. (Tokyo) 2008;10(4):201–203. doi: 10.1016/j.legalmed.2008.01.002. [http:// dx.doi.org/10.1016/j.legalmed.2008.01.002]. [PMID: 18294895]. [DOI] [PubMed] [Google Scholar]
- 50.Shanks K.G., Dahn T., Terrell A.R. Detection of JWH-018 and JWH-073 by UPLC-MS-MS in postmortem whole blood casework. J. Anal. Toxicol. 2012;36(3):145–152. doi: 10.1093/jat/bks013. [http://dx.doi.org/10. 1093/jat/bks013]. [PMID: 22417829]. [DOI] [PubMed] [Google Scholar]
- 51.Gronewold A., Skopp G. A preliminary investigation on the distribution of cannabinoids in man. Forensic Sci. Int. 2011;210(1-3):e7–e11. doi: 10.1016/j.forsciint.2011.04.010. [http://dx.doi.org/10.1016/j.forsciint.2011.04.010]. [PMID: 21570784]. [DOI] [PubMed] [Google Scholar]
- 52.Kudo K., Nagata T., Kimura K., Imamura T., Jitsufuchi N. Sensitive determination of delta 9-tetrahydrocannabinol in human tissues by GC-MS. J. Anal. Toxicol. 1995;19(2):87–90. doi: 10.1093/jat/19.2.87. [http:// dx.doi.org/10.1093/jat/19.2.87]. [PMID: 7769793]. [DOI] [PubMed] [Google Scholar]
- 53.Leighty E.G. Metabolism and distribution of cannabinoids in rats after different methods of administration. Biochem. Pharmacol. 1973;22(13):1613–1621. doi: 10.1016/0006-2952(73)90028-2. [http://dx.doi.org/10.1016/0006-2952(73) 90028-2]. [PMID: 4729809]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.