Abstract
Mephedrone is a β-ketoamphetamine belonging to the family of synthetic cathinones, an emerging class of designer drugs known for their hallucinogenic and psychostimulant properties as well as for their abuse potential. The aim of this review was to examine the emerging scientific literature on the possible mephedrone-induced neurotoxicity, yet not well defined due to the limited number of experimental studies, mainly carried on animal models. Relevant scientific articles were identified from international literature databases (Medline, Scopus, etc.) using the keywords: “Mephedrone”, “4-MMC,” “neurotoxicity,” “neuropharmacology”, “patents”, “monoamine transporters” and “neurochemical effects”. Of the 498 sources initially found, only 36 papers were suitable for the review. Neurotoxic effect of mephedrone on 5-HT and DA systems remains controversial. Although some studies in animal models reported no damage to DA nerve endings in the striatum and no significant changes in brain monoamine levels, some others suggested a rapid reduction in 5-HT and DA transporter function. Persistent serotonergic deficits were observed after binge like treatment in a warm environment and in both serotonergic and dopaminergic nerve endings at high ambient temperature. Oxidative stress cytotoxicity and an increase in frontal cortex lipid peroxidation were also reported. In vitro cytotoxic properties were also observed, suggesting that mephedrone may act as a reductant agent and can also determine changes in mitochondrial respiration. However, due to the differences in the design of the experiments, including temperature and animal model used, the results are difficult to compare. Further studies on toxicology and pharmacology of mephedrone are therefore necessary to establish an appropriate treatment for substance abuse and eventual consequences for public health.
Keywords: Mephedrone, neurotoxicity, neuropharmacology, monoamine transporters, 5HT, DA
1. Introduction
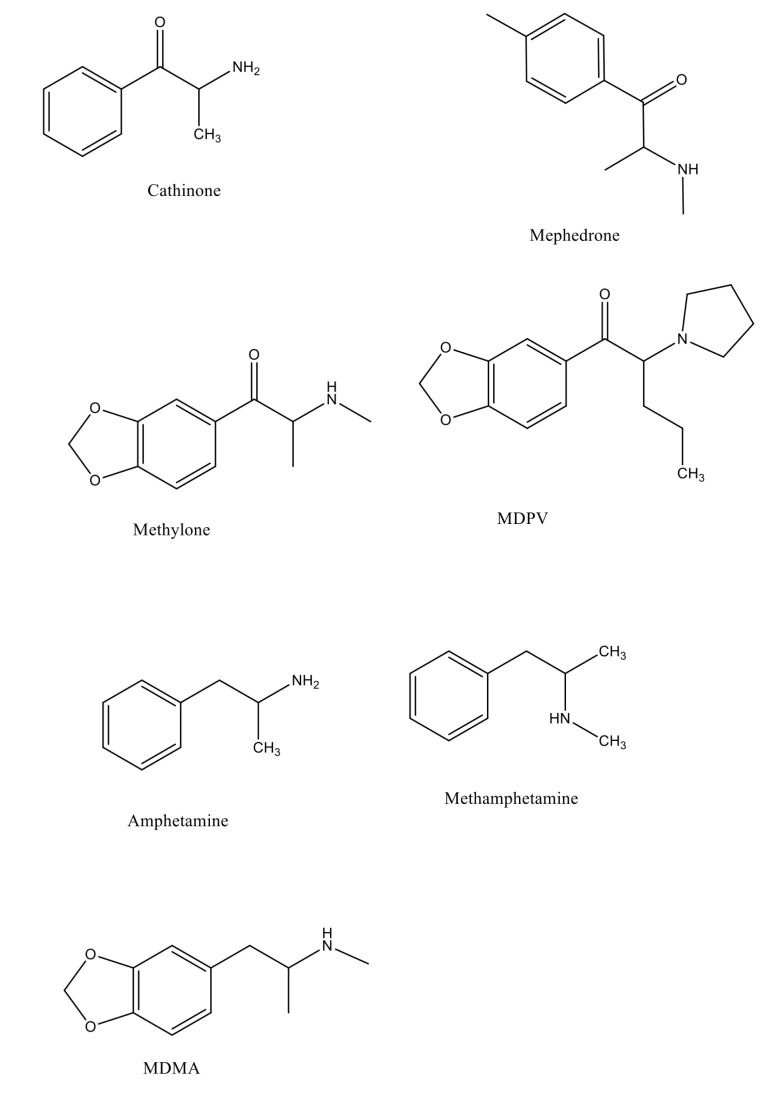
Mephedrone is a β-ketoamphetamine [4-methyl-methcathinone; 4-methylephedrone; 4-MMC; IUPAC: 2-(methylamino) -1-(4-methylphenyl)propan-1-one] belonging to the family of synthetic cathinones [1], an emerging class of designer drugs known for their hallucinogenic and psycho-stimulant properties, some of which they share with 3,4 methylenedioxymethamphetamine (MDMA), methamphetamine, amphetamine and cocaine [2]. Due to those effects, mephedrone is becoming increasingly popular as a recreational drug of abuse, not only among youngsters, but also in other age groups, including but not limited to young and older adults [3-5]. Mephedrone was first synthesized in 1929 as a ring-substituted cathinone (4-methyl aromatic analogue of methcathinone) and its structure, such that of other synthetic cathinones (e.g. methylone or methylenedioxypyrovalerone- MDPV) is somehow related to that of the phenethylamine family [6] (Fig. 1). Mephedrone is available from several selling sources, such as internet suppliers, head shops [7], street drug dealers, as a “legal high” or “bath salt” under different street names including “4-MMC”, “meow meow”, “M-Cat”, “MMCAT”, “bubbles” and “Crab”.
Fig. (1).
Structures of cathinone, mephedrone, methylone, methylenedioxypyrovalerone (MDPV), amphetamine, methamphetamine and methylenedioxymethamphetamine (MDMA).
It can be obtained in fine crystals or as white or yellowish powder and less frequently in tablet or capsule form [8-11]. The remarkable increase of its use in recent years has made it a significant public health threat both in the US and in Europe [9, 12, 13].
Mephedrone and structurally related compounds were categorized as Class B compounds in the UK in April 2010 under the Misuse of the Drugs Act Legislation [14]. Furthermore, in October 2011, the US Drug Enforcement Administration (DEA) issued a temporarily Schedule I for the more frequently encountered substances of the cathinones class, including mephedrone among others, in response to this increasing epidemic of its abuse [15]. In many other European and non-European countries, this drug has also been banned to limit its availability.
Many adverse effects have been reported following the use of “Bath salts” and especially β-ketoamphetamine including sleeplessness, psychosis, anxiety, depression and cardiovascular complications [9, 16, 17]. β-ketoamphetamines are known to block serotonin transporter (SERT) [18-24] and determine the release of monoamines in vivo [25-28] and in vitro [20, 25, 29, 30]. Specifically, vasoconstriction and bruxism observed in acute intoxication with mephedrone [1] and case of a 22-year-old male who displayed diaphoresis, clonus, hypertonia, hyper-reflexia and was tachycardic after mephedrone ingestion supported the idea that substance toxicity may be linked to the serotonin syndrome [31].
Not only severe intoxications have been attributed to mephedrone consumption, but also a number of fatal cases where the drug either alone or in combination to other substances, such as: MDMA, GHB and heroin was identified in biological matrices from deceased [32, 33].
Notwithstanding these evidences on mephedrone hazard, limited and controversial information is currently available on the way mephedrone acts on the central nervous system (CNS) and on its neurotoxicity potential.
Mephedrone shares a number of effects with MDMA and methamphetamine on the CNS, interacting with monoamine plasma membrane transporters for serotonin (5-hydro-xytryptamine, 5-HT) dopamine (DA) and likely norepinephrine (NE) [19, 23, 34-39], blocking their reuptake [19, 34, 35, 37, 39] while stimulating their release [26, 34, 38, 40, 41]. It was originally hypothesized that mephedrone could cause methamphetamine-like neurotoxicity due to the fact that it simultaneously stimulates both DA release and inhibition of its uptake, cause hyperthermia and increase the locomotor activity [42-45]. Moreover, mechanistic and structural similarities with MDMA and methamphetamine led to the hypothesis that mephedrone could also cause toxic effects to DA nerve terminals. However, this hypothesis was ruled out after a number of studies [12, 38, 40, 46-48]. In addition, its effects are evident not only on dopaminergic and -serotoninergic systems, but also on other pathways. As an example, German et al. [41] demonstrated that neuropeptide neurotensin content in the limbic system and basal ganglia was increased after four injections of mephedroneat the dose of 25 mg/kg.
To clarify all the above-reported issues, this review examined the emerging scientific literature on the possible mephedrone-induced neurotoxicity and the neuro-pharmacological profile of this substance yet not well defined due to the limited number of experimental studies mainly carried out in animal models [26, 34, 37, 49, 50].
2. Materials and methods
Relevant scientific articles were searched from Medline, Cochrane Central, Scopus, Web of Science, Science Direct, EMBASE and Google Scholar, up to March 2016 using the following keywords: “Mephedrone”, “4-MMC,” “neurotoxicity,” “neuropharmacology”, “patents”, “monoamine transporters” and “neurochemical effects”. The main keyword “Mephedrone” was individually searched and then again in association to each of the others. The 498 articles initially found were screened to exclude duplicate sources and papers not suitable for the purpose of the review. Only 36 papers (26 experimental studies, 6 review articles, 3 patents and 1 case report) were included in the present review [9, 12, 19, 23, 26, 34, 35, 37-40, 49-73]. All sources have been screened independently by three of the authors, and selected by at least two of them.
3. Results
Selected experimental studies in rodent animal models (mouse and rat) investigating the effects of mephedrone on DA and 5-HT transporters, DA and 5-HT depletion, tyrosine hydroxylase and tryptophan hydroxylase expression, cytotoxic damage and oxidative stress and microglial and astrocytic activation, are summarized in Table 1.
Table 1.
Experimental studies reporting the effects of mephedrone on dopamine, serotonin, monoamine transporters activity, microglia/astrocytic (GFAP) activation, oxidative stress cytotoxicity, etc.
| Ref. | Animal | Experiment Conditions | Route of Administration | Posology | DA | 5-HT | DAT Activity | SERT Activity | NET Activity | TH - TPH | Microglia/ Astrocytic (GFAP) Activation |
Oxidative Stress Ctotoxicity | DA-D2 5-HT Receptors |
Damage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hadlock et al. [34] | Male Rats | warm environment ≥ 27°C | s.c. In vitro experiment. Rats self-administration. |
4 X 10 or 25 mg/kg x injection, every two hours such as to mimic binge-like intake | no effects | reduction assessed 7 days after exposure | Rapid decrease in function (within an hour) in striatum | Rapid decrease in function (within an hour) in hippocampus 7 days after exposure | Persistent serotonergic deficits ↓ SERT activity 5-HT levels ↓ | |||||
| In Vitro | Release in in vitro experiment (striatal suspension) > than MDMA similar to METH |
|||||||||||||
| Baumann et al. [38] | Male Rats | Repeated administrations (22±2 °C) |
s.c. | 3 x 3 or 10 mg/kg 1 every 2 hours | NO long-term change in striatal or cortical amines 2 weeks after intake | |||||||||
| In Vivo microdialysis | i.v. | 0.3 and 1.0 mg/kg | + dose related release (nucleus accumbens) | ++ dose related release (nucleus accumbens) | ||||||||||
| In Vitro | 4-MMC is a non-selective substrate: RELEASE from DAT |
4-MMC is a non-selective substrate: RELEASE from SERT |
4-MMC is a non- selective substrate: RELEASE from NET |
|||||||||||
| Kehr et al. [26] | Rats | s.c. | 1-3 mg/kg-1 | Release in nucleus accumbens (values similar to AMPH but more potent than MDMA ) | Release in nucleus accumbens (values similar to MDMA, more potent than AMPH) | |||||||||
| Angoa-PÉrez et al. [12] | Female Mice | i.p. | 4X 20 or 40 mg/kg every two hours | No permanent decrease after binge like scheme exposure | No permanent activity decrease after binge like scheme exposure | No permanent decrease of TH after binge like scheme exposure | No microglial or astrocytic activation (GFAP) | examination after two or seven days, following administration, showed no neurotoxicity involving DA nerve terminals in the striatum NO permanent reduction in TH, DA, DAT activity | ||||||
| Angoa-PÉrez et al. [56] | Female Mice | i.p. | 4-MMC (10, 20 or 40 mg/kg) 30 minutes before injecting METH 4 x 2.5 or 5.0mg/kg, or AMPH 4x 5.0 mg/kg, or MDMA 4 x 20 mg/kg, every two hours). |
Co-administration enhanced DA levels reduction | Co-administration enhanced DAT protein levels reduction | Co-administration enhanced TH levels reduction | NO protective effects of 4-MMC (as a DAT inhibitor) on METH, MDMA or AMPH co-administration but ENHANCED NEUROTOXICITY on DA nerve terminals |
|||||||
| Angoa-PÉrez et al. [40] | Female Mice | i.p. | 4-MMC (4x 20 mg/kg every 2 hours) alone or 30 minutes before injecting METH 4 x 5.0mg/kg, or MDMA 4 x 20 mg/kg. | No persistent decrease if administered alone. No enhanced decrease in co-administration | No persistent decrease if administered alone. No enhanced decrease in co-administration | No persistent decrease in TPH2 if administered alone. No enhanced decrease in co-administration | NO microglia or astrocytic activation (GFAP) | NO TOXICITY on 5HT nerve endings HIPPOCAMPUS the authors underline that mice are less susceptible to MDMA 5-HT damage than Rats | ||||||
| LÓpez-Arnau et al. [55] | Adolescent Rats | High ambient temperature (26±2 °C) |
s.c. | 3 × 25mg/kg, in a day, with a 2 h interval between doses, for 2 days | 30% density reduction in the frontal cortex | 40% density reduction in frontal cortex and hippocampus, 48% density reduction in striatum | TH and TPH2 expression decrease | NO microgliosis | significant increase in lipid peroxidation in the frontal cortex and increase of glutathione peroxidase levels | down regulation DA D2 receptors in striatum | transporter binding and enzyme markers decrease, oxidative stress: injury at the nerve terminals. Damage in the reference memory. | |||
| Den Hollander et al. [60] 2014 | Male mice | Male mice brain tissue and SH-SY5Y neuroblastoma cells in vitro study |
(2μ-2mM) for 48 hours | Cytotoxic properties in vitro (increase in LDH release due to 4-MMC above 500 μM) |
LDH increase indicating in vitro cytotoxic damage; decrease in mitochondrial respiration; reductant agent (redox electron donor activity) pH increase correlated. | |||||||||
| Den Hollander et al. [48] | Male mice and Rats | temperature, 20-23 °C; |
i.p. | 2x30 mg/kg daily for 4 consecutive days |
no decrease after 15 days | no decrease after 15 days | No changes in neurotransmitters (DA and 5-HT) levels after 15 days from exposure but behavioral effects were observed and working memory was affected | |||||||
| Martinez-Clemente et al. [58] | Mice and in vitro experiment cerebral cortex neurons culture | Schedule 1 temperature (26±2 °C) |
s.c. | 4 x 50 mg/kg 2 h intervals |
loss in DA reuptake sites of 50% |
significant loss in the hippocampus |
no cortical or striatal astrocytic activation hippocampus: apparent increase in GFAP no microglial activation |
in vitro experiment concentration related cytotoxic effect > MDMA |
Persistent (7 days after exposure) neurotoxicity on 5-HT and dopaminergic systems |
|||||
| Schedule 2 temperature (26±2 °C) |
s.c. | 4 x 25 mg/kg 2 h intervals |
transient decrease |
no loss | Transient dopaminergic injury | |||||||||
| Schedule 3 temperature (26±2 °C) |
s.c. | 3 x 25 mg/kg 2 h intervals for two days consecutively |
DA transporter loss |
5-HT transporter loss |
TH and TPH expression decrease |
D2 density decrease in striatum 5-HT 2a frontal cortex |
Damage in dopaminergic nerve endings (frontal cortex) and serotonergic (hippocampus) |
|||||||
| Pifl et al. [59] | Human cell lines synaptic vesicles from human striatum | Release from monoamine transporter DAT reverse transport |
Release from monoamine transporter SERT reverse transport |
-DA-DAT uptake inhibition more potent than MDMA -inhibition of vesicular uptake 10 fold less powerful than MDMA | SERT-serotonin uptake inhibition less potent than MDMA | NET-Nor- adrenaline uptake inhibition = to MDMA and release from NET reverse transport |
The less potent(10 folds) effect of 4-MMC on vesicular DA uptake in comparison with MDMA, could suggest an explanation of the lower long term effects observed with 4-MMC compared to MDMA. | |||||||
| Motbey et al. [67] | Male adolescent rats | (21-22±1 °C) | i.v. self-administration |
four doses were tested 0.03-0.1-0.3-1.0 mg/kg | reduction in metabolite 5-HIAA levels in striatum 3 days after treatment | No density changes at the 43th day from the beginning of self administration | No density changes at the 43th day from the beginning of self administration | MMC, but not METH, self-administration decreased striatal 5-hydroxyindolacetic acid (5-HIAA) concentrations in striatum. |
DA = Dopamine 5-HT = Serotonin DAT = Dopamine Transporter SERT = Serotonin Transporter NET = Norepinephrine transporter METH = Methamphetamine 4-MMC = Mephedrone MDMA = 3,4-methylenedioxy-methamphetamine AMPH = Amphetamine LDH = Lactate dehydrogenase TH =Tyrosine Hydroxylase TPH = Tryptophan Hydroxylase 5HIAA = 5-Hydroxyindoleacetic acid GFAP = Glial Fibrillary Acidic Protein s.c. = Subcutaneous injection i.v. = Intravenous i.p. = Intra-peritoneal injection.
3.1. Dopamine and Serotonin Transporters
A number of studies reported that mephedrone acts as an uptake inhibitor of monoamine neurotransmitters in the CNS, suggesting this drug could be a transporter blocker [19, 34, 35, 37, 39, 51]. Hadlock et al. [34] studied the effects of mephedrone in a study on male rats. Rodents were administered 4 x 10 or 25 mg/kg subcutaneously every two hours. The experiment was carried out in a warm ambient temperature at ≥ 27°C. As in case of methamphetamines, methcathinone and MDMA, a rapid decrement (within an hour) in dopamine transporter (DAT) and serotonin transporter (SERT) function was observed in striatum and hippocampus. No damages in the dopaminergic system were noticed while a persistent serotonergic deficit (5-HT depletion and reduction in SERT activity) was observed seven days after exposure. This occurrence was also evidenced with MDMA, but not with methamphetamine. The authors concluded that mephedrone displayed both neurotoxicity and abuse potential, and its toxicity, predominantly exerted on 5-HT terminals, mimicked that of MDMA with which it shares the same subjective sensations on abusers, although mephedrone is more potent on DA release in in vitro experiments. On the contrary, in another study [38] performed at controlled temperature (22±2 °C), no permanent change on striatal or cortical amines was observed, when rats underwent repeated mephedrone subcutaneous (s.c.) injections (3 x 3 or 10 mg/kg every 2 hours). Although data suggest that mephedrone blocks the uptake of [3H] DA, [3H] 5-HT and [3H] NE into rat brains synaptosomes [52], traditional uptake-inhibition assays cannot distinguish between drugs acting as transporter substrates and those operating as blockers, taking into account that both types prevent the accumulation of [3H] neurotransmitters in tissue. Consequently, in vitro release assays have been developed to discriminate between these two types [23, 53, 54]. It has been shown, that mephedrone acts (in vitro) as a non-selective substrate on monoamine transporters, thus stimulating the release of [3H] 5-HT via SERT and release of [3H]1-methyl-4-phenylpyridinium ([3H]MPP+) via DAT and NE transporter (NET) [23, 38]. According to other authors [55], mephedrone, s.c. administered at high ambient temperature (26±2 °C) (3 × 25mg/kg) in a day, with two-hours intervals between doses, for two days) reduces the density of DAT of Table 1: Experimental studies reporting the effects of mephedrone on dopamine, serotonin, monoamine trans-porters activity, microglia/astrocytic (GFAP) activation, oxidative stress cytotoxicity, etc. about 30% in the frontal cortex and of 48 and 40%SERT in the striatum and in the frontal cortex and hippocampus, respectively.
Because of its similarity to methamphetamine and methcathinone, known to determine damages to striatum DA nerve endings, mephedrone harm potential has been investigated in an animal study involving female mice treated with a binge like scheme of the substance [12]. This regimen consisted in four intra-peritoneal (i.p.) injections (20 or 40 mg/kg) every two hours since it was proved that injection of cathinone derivatives and substituted amphetamines determines widespread injury to DA nerve terminals. Although locomotor excitement and hyperthermia were observed, two or seven days after administration, striatum examination showed no neurotoxicity involving DA nerve terminals, as no persistent decrease in DAT activity was observed. This suggested that mephedrone mechanism of action consists in raising DA synaptic amount without causing neurotoxicity. Since mephedrone blocks DAT, Angoa-Pérez et al. [56] suggested that this drug could protect from the toxicity induced by methamphetamines in the same way nomifensine (a DAT inhibitor) does.
For this purpose, the authors treated mice with mephedrone 30 minutes before the administration of neurotoxic doses of methamphetamines, amphetamines or MDMA. Toxicity was observed, ranging from moderate to severe, and essentially increased with the co-administration of mephedrone and each of the other substances. Although mephedrone had previously been described as a substance with poor toxic effects on DA nerve terminals, in this study the drug was characterized by unsafe interaction with methamphetamines, amphetamines and MDMA, commonly co-abused with this synthetic cathinone [9, 57]. Mephedrone did not seem to damage DA nerve terminals although it increased the neurotoxicity of other amphetamine like substances. No increase of typical hyperthermia was observed, therefore the raise of neurotoxic effects should be considered independent from the added raising of body temperature of amphetamines. In the author opinion, its neurotoxicity enhancement could be indicative of an atypical interaction with DAT.
However drug effects on 5-HT nerve endings are controversial and not fully established as some authors report damage and others do not. In this concern, Angoa-Pérez et al. [40] studied the effect of mephedrone alone or in combination with MDMA and methamphetamine on 5-HT nerve terminals of mice hippocampus. They concluded that when mephedrone was administered alone, it caused a non-persistent decrease in 5-HT, SERT, whereas when it was administered together with MDMA or methamphetamine there were no changes on the status of 5-HT nerve endings other than the effects produced by MDMA and methamphetamine alone. It was found that the administration of methamphetamine 30 minutes after that of mephedrone caused a significant decrease in 5-HT metabolite 5-hydroxyindoleacetic acid (5HIAA) concentration when compared to controls and this effect was not observed when mephedrone was administered together with MDMA. However, a limitation of this study could have been that the authors used mice in their experiments and these animals are not so susceptible to the 5-HT damaging of MDMA as rats.
To observe the neurotoxic effects of different doses of mephedrone in relation with the exposure time, an animal study involving mice was designed as detailed [58]. Three s.c. drug administration protocols were involved: -1) 50 mg/kg (four doses); -2) 25 mg/kg (four doses); -3) 25 mg/kg, (three doses for two days successively, so as to mimic “week-end intake”). Loss in weight gain, hyperthermia, as well as aggressive behavior, were observed with all three dosages. The first administration program caused seven days lasting neurotoxicity on both serotoninergic and dopaminergic systems. The second administration program resulted in temporary damage on the dopaminergic system, whereas the third one, seven days after exposure, in DAT loss (in striatum and frontal cortex) and SERT (in the hippocampus) loss. After the third administration program, there was a predominant damage in the frontal cortex nerve terminals (dopaminergic) and hippocampus (serotoninergic). It was observed that mephedrone caused a decrease of D2 receptor concentration in the striatum and of 5-HT2 a receptors in the frontal cortex; moreover, there was also a depression-like behavior and this result suggested a greater vulnerability to addictive drugs. All three administration programs caused in some mice the onset of a stereotypy: “repeated self-licking of the ventral base of the neck” followed by aggressive behavior and self-injury.
Pifl et al. [59] carried out experiments to compare the effects of mephedrone and MDMA on the human NET, DAT and SERT monoamine transporters using human cell lines. Both drugs showed similar uptake inhibition potency at the NET, while mephedrone was more potent at the DAT and less potent at SERT compared to MDMA. It was also shown that mephedrone-induced release of [3H]MPP+ from NET, DAT or SERT expressing cells was exclusively caused by carrier-mediated reverse transport through the plasmalemmal transporters. Moreover, mephedrone and MDMA had a different interaction with the human brain vesicular monoamine uptake; with mephedrone displaying a 10 times lower transport inhibitory potency compared to MDMA.
3.2. Dopamine and Serotonin Depletion, Tyrosine Hydroxylase and Tryptophan Hydroxylase Expression
In vitro experiments on striatal suspension of rats, showed that mephedrone [34] was able to determine a DA release, similar to that of methamphetamine, displaying more adverse effects than those of MDMA. Moreover, rodents which self-administered mephedrone, showed a trend towards an increase of the intake, therefore suggesting a certain abuse liability. In vivo microdialysis studies showed that intravenous injection of mephedrone (0.3 or 1.0 mg/kg) increased extracellular levels of 5-HT and DA in rat nucleus accumbens, and the elevation of monoamines was dose related [38]. Some other studies reported a rise of dialysate 5-HT and DA in rat nucleus accumbens after s.c. mephedrone injection (3 mg/kg-1). The rise in 5-HT was similar to that following MDMA intake, but higher than that caused by amphetamine, whereas DA elevation was comparable after mephedrone and amphetamine injection and more marked than after MDMA administration [26, 51]. It is interesting to note that the elevation in extracellular 5-HT was of greater significance in comparison to DA increase after mephedrone or methylone (another abused synthetic cathinone) treatment, suggesting therefore that neuro-chemical effects of the latter substances relate more to those of MDMA and less to those of methamphetamine [26, 38]. However, after repeated s.c. injections of mephedrone or methylone (3 x 3 or 10 mg/kg every 2 hours) Baumann et al. [38] did not observe any long term alteration in striatal or cortical amines. In the study of Lopez-Arnau et al. [55] a decrease in biochemical marker of neuronal integrity tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) (catalysts of DA and 5-HT synthesis respectively) expressions were observed after mephedrone administration along with a down regulation of D2 DA receptors in the striatum suggesting damage of the nerve endings. Conversely, Angoa-Pérez et al. [12] showed no decrease of DA, TH and DAT concentration in murine striatum. In another study, the same authors [40] evaluated tryptophan hydroxylase 2 (TPH2) levels after i.p. administering mephedrone (4x 20 mg/kg every 2 hours) in mice and did not find any decrease in the catalyst expression. In contrast, [58] a decrease of the expression of TPH 2 and TH was demonstrated after administering a “week-end like” regimen of mephedrone at warm temperature to mice (26±2 °C).
3.3. Cytotoxic Damage and Oxidative Stress
Den Hollander et al. [60] assessed cytotoxicity of β-ketoamphetamines in neuroblastoma SH-SY5S cells, evaluating the redox potential and investigating the effect of mephedrone in forming protein adducts. It has been shown that β-ketoamphetamines increase lactate dehydrogenase (LDH) release, which is associated with cytotoxicity in SH-SY5S neuroblastoma cells. It was also proved that these substances are selective and effective reductants of electron acceptors, even at physiological pH. However, at pH from 7.6 to 8.0, the reactivity was six times higher. No formation of protein adducts was observed and the authors suggested that the reactivity was linked to direct electron transfer by β-ketoamphetamines. Both mephedrone and methamphetamine caused a decrease in mitochondrial respiration, but displayed different effects on the electron transport chain. It was concluded that this synthetic cathinone was actually able to determine cytotoxicity in vitro.
Lòpez-Arnau et al. investigated enzymatic and neurological changes occurring in adolescent rats after the administration of mephedrone mimicking human recreational abuse [55]. Changes in memory and spatial learning were also investigated. Glutathione peroxidase values were found to be elevated in the frontal cortex, striatum and hippocampus and a rise in lipid peroxidation was observed in the frontal cortex. These findings suggested an mephedrone-related oxidative stress. Damage of the reference memory was displayed seven days after the end of drug administration, whereas spatial learning was not affected. Other authors [58] performed in vitro experiments on cerebral cortex neuronal cultures, observing a dose-dependent cytotoxic effect of mephedrone on these cells, superior to that of MDMA.
3.4. Microglial and Astrocytic Activation
After the administration of mephedrone mimicking human recreational abuse, Lopéz-Arnau et al. did not observe any microglial activation [55]. These results are in agreement with those of Angoa-Pérez et al. [12], who did not detect microglial activation or a raise in the levels of glial fibrillary acidic protein (GFAP), a measure of astrocytic activation, after a binge like scheme of i.p. injections of the drug. Another study [40], measuring the GFAP levels on mice did not reveal cellular activation in the hippocampus, microglia or astrocytes. Martinez-Clemente et al. [58] showed that mephedrone did not change GFAP values, nor was microglial activation observed. Only in the hippocampus an apparent increase in GFAP was noted.
3.5. Behavioral Effects and Abuse Potential
Conditioned place preference and motor activity tests were carried out on rats to investigate behavioral effects of mephedrone. An increased ambulatory activity after acute drug i.p. injection (3, 5, 10, 30 mg/kg) was observed. The pretreatment with DA D1 receptor antagonist SCH 23390 at the dose of 0.5, 1, 2 mg/kg, ip inhibited mephedrone -nduced ambulation, while pretreatment with D2 DA receptor antagonist sulpiride at the dose of 2 mg/kg, i.p. enhanced the latter activity. The injection of low doses of the synthetic cathinone (0.5 mg/kg, i.p.) for 5 days followed by 10 days of abstinence resulted in a sensitization of ambulatory activity when mephedrone was re-injected at the same concentration. Mephedrone-treated (30 mg/kg, i.p.) rats and mice both displayed a preference shift in the cell penetrating peptide assay. These findings suggest an abuse liability of the drug [50].
Behavioral effects on memory, depression and anxiety were investigated two weeks after the administration of binge-like dose mephedrone or methylone to mice [48]. DA, 5-HT, their metabolites and NE levels were also measured together with SERT and DAT levels. It was found that mephedrone decreased working memory proficiency (T-maze task), but neurotransmitter concentrations were not affected, apart from a 22% reduction in homovanillic acid (HVA, a DA metabolite) concentration in murine striatum. Depression and anxiety-associated behavior did not appear to be affected by mephedrone.
The drug seemed to produce long-term effects on biochemical or behavioral pathways in rodents. Marusich et al. [61] examined the acute effects of mephedrone, among other substances, on rotarod (a performance test based on a rotating rod with forced motor activity for evaluation of balance, neurotoxicity and motor coordination), locomotor activity and a functional observational battery. Regarding locomotor activity, mephedrone showed significant effects related to time, dose and interaction with other substances. Significant increases in beam breaks were produced by all drug doses. Dosages of 10, 3 and 30 mg/kg of mephedrone produced an increase in locomotor activity during the first 70 min of the session, 20–40 min post-injection and for the entire session, respectively, when compared to saline. Results of the functional observational battery illustrated that mephedrone significantly increased hyperactivity, stereotyped head weaving and circling and stimulation.
A rat model study was carried out to evaluate the cardiovascular and behavioral effects of mephedrone. It was found that its discriminative stimulus effects were more similar to those of MDMA in comparison with those of methamphetamine and cocaine and not antagonized by haloperidol administration suggesting that the mechanism of action of the drug was not predominantly due to interaction with DA D2 receptors. Nevertheless, its actions on cardio-vascular activity and on acquisition behavior resemble those of methamphetamine [62].
Recreational abusers report that a stimulant drug with short duration of action and rapid onset like cocaine is preferable, since the short duration of action allows successive dosing and therefore more frequent exposures to the desirable effects [63], suggesting that psychoactive drugs sharing the latter characteristics are more prone to be abused. Thus, synthetic cathinones such as mephedrone, MDPV and 4-fluoromethcathinone are likely to have a non-negligible potential of abuse since they reveal a rapid initial increase of locomotor activity, which deteriorates during the session [63, 64]. A limited set of laboratory experiments demonstrated that mephedrone is readily self-administered by male rats [34, 65-67]. The drug showed a relatively high abuse liability if compared to MDMA and methylone, with the latter drugs presenting lower and analogous liability for repetitive use. Ramoz et al. [68] reported that behavioral effects related to psychostimulant drugs such as DA- sensitive stereotyped movements, abstinence-induced withdrawal and environmental place conditioning are all produced by mephedrone. suggesting a strong evidence for an addiction liability [34, 69]. Creehan and collegues [70] were the first to evaluate methylone, MDMA and mephedrone self-administration in female rats. Their findings showed that the intake in mephedrone-trained rodents was significantly higher, compared to that of methylone and MDMA-trained rats. Dose substitution showed that mephedrone-trained rats developed greater intakes of all tested compounds, suggesting higher abuse liability. Moreover, i.p. administration of mephedrone to rats at concentrations of 15 or 30 mg/kg, resulted in an increase in the expression of Fos protein in brain areas which are reward-relevant such as the ventral striatum, ventral tegmental and prefrontal cortex [49]. The fact that mephedrone owns a much faster clearance rate than MDMA, may rise the proclivity for repeated binge use of the drug [26].
Conclusion
Despite the increasing use of mephedrone and the fatalities already attributed to its intoxication [33], little information is still available on the mechanism of action to definitively establish the rate of abuse liability and neurotoxicity.
The literature regarding the neurotoxic effect of mephedrone in 5-HT and DA systems remains controversial. Although some studies on animal models have reported no damage to DA nerve endings in the striatum [12, 58] and no significant changes in brain monoamine levels [48], some others [34] have suggested a rapid reduction in 5-HT and DA transporter function following drug administration. Persistent serotonergic deficits were observed after binge like treatment in a warm environment [34] and in both serotonergic and dopaminergic nerve endings [58], where mice were tested for dose and time dependent neurotoxicity at high ambient temperature. Other authors [55] demonstrated oxidative stress cytotoxicity and an increase in lipid peroxidation in the frontal cortex. In vitro cytotoxic properties were also detected, suggesting that mephedrone may act as a reductant agent and can also determine changes in mitochondrial respiration [60]. Cytotoxic effects were also described by other authors and were found to be dose related [58]. Nevertheless, other studies have failed to show any persistent neurochemical effect of this drug when using long duration dosing protocols [47], while behavioral effects could still be noticed in addition to damages on the working memory. However, due to the differences in the design of the experiments, including temperature and the different species of rodents used, the results are difficult to compare. Moreover, it must be highlighted that damages were more frequently observed in studies carried out at high temperature (≥ 26°C) mimicking the hyperthermia following the exposure to these mephedrone and more in general to amphetamine-like compounds.
Up to now, no clinical trials in humans, nor clinical observations in intoxicated users have been available to clearly describe pharmacology and toxicology of mephedrone in humans. This information is undoubtedly necessary to establish eventual appropriate treatments to limit mephedrone abuse outcomes and eventual consequences the public health.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Coppola M., Mondola R. Synthetic cathinones: chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as bath salts or plant food. Toxicol. Lett. 2012;211(2):144–149. doi: 10.1016/j.toxlet.2012.03.009. [http://dx.doi.org/10.1016/j.toxlet.2012.03.009]. [PMID: 22459606]. [DOI] [PubMed] [Google Scholar]
- 2.McGraw M., McGraw L. Bath salts: not as harmless as they sound. J. Emerg. Nurs. 2012;38(6):582–588. doi: 10.1016/j.jen.2012.07.025. [http://dx.doi.org/ 10.1016/j.jen.2012.07.025]. [PMID: 23040164]. [DOI] [PubMed] [Google Scholar]
- 3.Wood D.M., Davies S., Puchnarewicz M., Button J., Archer R., Ovaska H., Ramsey J., Lee T., Holt D.W., Dargan P.I. Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J. Med. Toxicol. 2010;6(3):327–330. doi: 10.1007/s13181-010-0018-5. [http://dx.doi.org/10.1007/s13181-010-0018-5]. [PMID: 20358417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustavsson D., Escher C. MephedroneInternet drug which seems to have come and stay. Fatal cases in Sweden have drawn attention to previously unknown substance. Lakartidningen. 2009;106(43):2769–2771. [PMID: 19960905]. [PubMed] [Google Scholar]
- 5.Regan L., Mitchelson M., Macdonald C. Mephedrone toxicity in a Scottish emergency department. Emerg. Med. J. 2011;28(12):1055–1058. doi: 10.1136/emj.2010.103093. [http://dx.doi.org/10.1136/emj.2010.103093]. [PMID: 21183522]. [DOI] [PubMed] [Google Scholar]
- 6.Gerace E., Petrarulo M., Bison F., Salomone A., Vincenti M. Toxicological findings in a fatal multidrug intoxication involving mephedrone. Forensic Sci. Int. 2014;243:68–73. doi: 10.1016/j.forsciint.2014.04.038. [http://dx.doi. org/10.1016/j.forsciint.2014.04.038]. [PMID: 24846124]. [DOI] [PubMed] [Google Scholar]
- 7.Loeffler G., Hurst D., Penn A., Yung K. Spice, bath salts, and the U.S. military: the emergence of synthetic cannabinoid receptor agonists and cathinones in the U.S. Armed Forces. Mil. Med. 2012;177(9):1041–1048. doi: 10.7205/milmed-d-12-00180. [http://dx.doi.org/10.7205/MILMED-D-12-00180]. [PMID: 23025133]. [DOI] [PubMed] [Google Scholar]
- 8.Dickson A.J., Vorce S.P., Levine B., Past M.R. Multiple-drug toxicity caused by the coadministration of 4-methylmethcathinone (mephedrone) and heroin. J. Anal. Toxicol. 2010;34(3):162–168. doi: 10.1093/jat/34.3.162. [http://dx.doi.org/10.1093/jat/34.3.162]. [PMID: 20406541]. [DOI] [PubMed] [Google Scholar]
- 9.Schifano F., Albanese A., Fergus S., Stair J.L., Deluca P., Corazza O., Davey Z., Corkery J., Siemann H., Scherbaum N., Farre M., Torrens M., Demetrovics Z., Ghodse A.H. Mephedrone (4-methylmethcathinone; meow meow): chemical, pharmacological and clinical issues. Psychopharmacology (Berl.) 2011;214(3):593–602. doi: 10.1007/s00213-010-2070-x. [http://dx.doi.org/10.1007/s00213-010-2070-x]. [PMID: 21072502]. [DOI] [PubMed] [Google Scholar]
- 10.Archer R.P. Fluoromethcathinone, a new substance of abuse. Forensic Sci. Int. 2009;185(1-3):10–20. doi: 10.1016/j.forsciint.2008.11.013. [http://dx.doi.org/10. 1016/j.forsciint.2008.11.013]. [PMID: 19195800]. [DOI] [PubMed] [Google Scholar]
- 11.Busardò F.P., Kyriakou C., Tittarelli R., Mannocchi G., Pantano F., Santurro A., Zaami S., Baglìo G. Assessment of the stability of mephedrone in ante-mortem and post-mortem blood specimens. Forensic Sci. Int. 2015;256:28–37. doi: 10.1016/j.forsciint.2015.07.021. [http://dx.doi.org/10.1016/ j.forsciint.2015.07.021]. [PMID: 26295910]. [DOI] [PubMed] [Google Scholar]
- 12.Angoa-Pérez M., Kane M.J., Francescutti D.M., Sykes K.E., Shah M.M., Mohammed A.M., Thomas D.M., Kuhn D.M. Mephedrone, an abused psychoactive component of bath salts and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J. Neurochem. 2012;120(6):1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [PMID: 22191803]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vardakou I., Pistos C., Spiliopoulou Ch. Drugs for youth via Internet and the example of mephedrone. Toxicol. Lett. 2011;201(3):191–195. doi: 10.1016/j.toxlet.2010.12.014. [http://dx.doi.org/10.1016/j.toxlet.2010.12.014]. [PMID: 21187132]. [DOI] [PubMed] [Google Scholar]
- 14.Winstock A., Mitcheson L., Marsden J. Mephedrone: still available and twice the price. Lancet. 2010;376(9752):1537. doi: 10.1016/S0140-6736(10)62021-1. [http:// dx.doi.org/10.1016/S0140-6736(10)62021-1]. [PMID: 21056754]. [DOI] [PubMed] [Google Scholar]
- 15.Fass J.A., Fass A.D., Garcia A.S. Synthetic cathinones (bath salts): legal status and patterns of abuse. Ann. Pharmacother. 2012;46(3):436–441. doi: 10.1345/aph.1Q628. [http://dx.doi.org/10.1345/aph.1Q628]. [PMID: 22388331]. [DOI] [PubMed] [Google Scholar]
- 16.Wood D.M., Dargan P.I. Mephedrone (4-methylmethcathinone): what is new in our understanding of its use and toxicity. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39(2):227–233. doi: 10.1016/j.pnpbp.2012.04.020. [http://dx.doi.org/10.1016/j.pnpbp.2012.04.020]. [PMID: 22564711]. [DOI] [PubMed] [Google Scholar]
- 17.Prosser J.M., Nelson L.S. The toxicology of bath salts: a review of synthetic cathinones. J. Med. Toxicol. 2012;8(1):33–42. doi: 10.1007/s13181-011-0193-z. [http://dx.doi.org/10.1007/s13181-011-0193-z]. [PMID: 22108839]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozzi N.V., Foley K.F. Methcathinone is a substrate for the serotonin uptake transporter. Pharmacol. Toxicol. 2003;93(5):219–225. doi: 10.1046/j.1600-0773.2003.pto930504.x. [http://dx.doi.org/10.1046/j.1600-0773.2003.pto930504.x]. [PMID: 14629733]. [DOI] [PubMed] [Google Scholar]
- 19.Cozzi N.V., Sievert M.K., Shulgin A.T., Jacob P., III, Ruoho A.E. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur. J. Pharmacol. 1999;381(1):63–69. doi: 10.1016/s0014-2999(99)00538-5. [http://dx.doi.org/10.1016/S0014-2999(99)00538-5]. [PMID: 10528135]. [DOI] [PubMed] [Google Scholar]
- 20.Rothman R.B., Vu N., Partilla J.S., Roth B.L., Hufeisen S.J., Compton-Toth B.A., Birkes J., Young R., Glennon R.A. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J. Pharmacol. Exp. Ther. 2003;307(1):138–145. doi: 10.1124/jpet.103.053975. [http://dx.doi.org/10.1124/jpet.103.053975]. [PMID: 12954796]. [DOI] [PubMed] [Google Scholar]
- 21.Metzger R.R., Hanson G.R., Gibb J.W., Fleckenstein A.E. 34-Methylenedioxymethamphetamine-induced acute changes in dopamine transporter function. Eur. J. Pharmacol. 1998;349(2-3):205–210. doi: 10.1016/s0014-2999(98)00196-4. [http://dx.doi.org/10.1016/S0014-2999(98)00196-4]. [PMID: 9671099]. [DOI] [PubMed] [Google Scholar]
- 22.Fleckenstein A.E., Gibb J.W., Hanson G.R. Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur. J. Pharmacol. 2000;406(1):1–13. doi: 10.1016/s0014-2999(00)00639-7. [http://dx.doi.org/10.1016/S0014-2999(00)00639-7]. [PMID: 11011026]. [DOI] [PubMed] [Google Scholar]
- 23.Nagai F., Nonaka R., Satoh H.K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol. 2007;559(2-3):132–137. doi: 10.1016/j.ejphar.2006.11.075. [http://dx.doi.org/ 10.1016/j.ejphar.2006.11.075]. [PMID: 17223101]. [DOI] [PubMed] [Google Scholar]
- 24.Meltzer P.C., Butler D., Deschamps J.R., Madras B.K. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J. Med. Chem. 2006;49(4):1420–1432. doi: 10.1021/jm050797a. [http://dx.doi.org/10.1021/ jm050797a]. [PMID: 16480278]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gygi M.P., Fleckenstein A.E., Gibb J.W., Hanson G.R. Role of endogenous dopamine in the neurochemical deficits induced by methcathinone. J. Pharmacol. Exp. Ther. 1997;283(3):1350–1355. [PMID: 9400010]. [PubMed] [Google Scholar]
- 26.Kehr J., Ichinose F., Yoshitake S., Goiny M., Sievertsson T., Nyberg F., Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011;164(8):1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [http://dx.doi.org/10.1111/j.1476-5381. 2011.01499.x]. [PMID: 21615721]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pehek E.A., Schechter M.D., Yamamoto B.K. Effects of cathinone and amphetamine on the neurochemistry of dopamine in vivo. Neuropharmacology. 1990;29(12):1171–1176. doi: 10.1016/0028-3908(90)90041-o. [http://dx. doi.org/10.1016/0028-3908(90)90041-O]. [PMID: 2293059]. [DOI] [PubMed] [Google Scholar]
- 28.Banjaw M.Y., Schmidt W.J. Catha edulis extract and its active principle cathinone induce ipsilateral rotation in unilaterally lesioned rats. Behav. Pharmacol. 2006;17(7):615–620. doi: 10.1097/01.fbp.0000236273.10418.2b. [http://dx. doi.org/10.1097/01.fbp.0000236273.10418.2b]. [PMID: 17021394]. [DOI] [PubMed] [Google Scholar]
- 29.Kalix P. Effect of the alkaloid (-)-cathinone on the release of radioactivity from rat striatal tissue prelabelled with 3H-serotonin. Neuropsychobiology. 1984;12(2-3):127–129. doi: 10.1159/000118124. [http://dx.doi.org/ 10.1159/000118124]. [PMID: 6527753]. [DOI] [PubMed] [Google Scholar]
- 30.Kalix P., Glennon R.A. Further evidence for an amphetamine-like mechanism of action of the alkaloid cathinone. Biochem. Pharmacol. 1986;35(18):3015–3019. doi: 10.1016/0006-2952(86)90380-1. [http://dx.doi.org/10.1016/0006-2952 (86)90380-1]. [PMID: 3753515]. [DOI] [PubMed] [Google Scholar]
- 31.Garrett G., Sweeney M. The serotonin syndrome as a result of mephedrone toxicity. BMJ Case Rep. 2010:bcr0420102925. doi: 10.1136/bcr.04.2010.2925. [http://dx.doi.org/10.1136/bcr.04.2010.2925]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busardò F.P., Zaami S., Baglio G., Indorato F., Montana A., Giarratana N., kyriakou C., Marinelli E., Romano G. Assessment of the stability of exogenous gamma hydroxybutyric acid (GHB) in stored blood and urine specimens. Eur. Rev. Med. Pharmacol. Sci. 2015;19(21):4187–4194. [PubMed] [Google Scholar]
- 33.Busardò F.P., Kyriakou C., Napoletano S., Marinelli E., Zaami S. Mephedrone related fatalities: a review. Eur. Rev. Med. Pharmacol. Sci. 2015;19(19):3777–3790. [PMID: 26502870]. [PubMed] [Google Scholar]
- 34.Hadlock G.C., Webb K.M., McFadden L.M., Chu P.W., Ellis J.D., Allen S.C., Andrenyak D.M., Vieira-Brock P.L., German C.L., Conrad K.M., Hoonakker A.J., Gibb J.W., Wilkins D.G., Hanson G.R., Fleckenstein A.E. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 2011;339(2):530–536. doi: 10.1124/jpet.111.184119. [http://dx.doi.org/10.1124/jpet.111.184119]. [PMID: 21810934]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmler L.D., Buser T.A., Donzelli M., Schramm Y., Dieu L.H., Huwyler J., Chaboz S., Hoener M.C., Liechti M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [http://dx.doi.org/10. 1111/j.1476-5381.2012.02145.x]. [PMID: 22897747]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sogawa C., Sogawa N., Ohyama K., Kikura-Hanajiri R., Goda Y., Sora I., Kitayama S. Methylone and monoamine transporters: correlation with toxicity. Curr. Neuropharmacol. 2011;9(1):58–62. doi: 10.2174/157015911795017425. [http://dx.doi.org/10.2174/157015911795017425]. [PMID: 21886563]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.López-Arnau R., Martínez-Clemente J., Pubill D., Escubedo E., Camarasa J. Comparative neuropharmacology of three psycho-stimulant cathinone derivatives: butylone, mephedrone and methylone. Br. J. Pharmacol. 2012;167(2):407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [http://dx.doi.org/10. 1111/j.1476-5381.2012.01998.x]. [PMID: 22509960]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumann M.H., Ayestas M.A., Jr, Partilla J.S., Sink J.R., Shulgin A.T., Daley P.F., Brandt S.D., Rothman R.B., Ruoho A.E., Cozzi N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192–1203. doi: 10.1038/npp.2011.304. [http:// dx.doi.org/10.1038/npp.2011.304]. [PMID: 22169943]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martínez-Clemente J., Escubedo E., Pubill D., Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur. Neuropsychopharmacol. 2012;22(3):231–236. doi: 10.1016/j.euroneuro.2011.07.009. [http:// dx.doi.org/10.1016/j.euroneuro.2011.07.009]. [PMID: 21824752]. [DOI] [PubMed] [Google Scholar]
- 40.Angoa-Pérez M., Kane M.J., Herrera-Mundo N., Francescutti D.M., Kuhn D.M. Effects of combined treatment with mephedrone and methamphetamine or 3,4-methylenedioxymethamphetamine on serotonin nerve endings of the hippocampus. Life Sci. 2014;97(1):31–36. doi: 10.1016/j.lfs.2013.07.015. [http://dx.doi.org/10.1016/j.lfs.2013.07.015]. [PMID: 23892197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.German C.L., Hoonakker A.H., Fleckenstein A.E., Hanson G.R. Mephedrone alters basal ganglia and limbic neurotensin systems. J. Neurochem. 2014;130(3):402–407. doi: 10.1111/jnc.12727. [http://dx.doi.org/10.1111/ jnc.12727]. [PMID: 24678634]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto B.K., Bankson M.G. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit. Rev. Neurobiol. 2005;17(2):87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [http://dx.doi.org/10.1615/CritRevNeurobiol. v17.i2.30]. [PMID: 16808729]. [DOI] [PubMed] [Google Scholar]
- 43.Cadet J.L., Krasnova I.N., Jayanthi S., Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox. Res. 2007;11(3-4):183–202. doi: 10.1007/BF03033567. [http://dx.doi.org/10. 1007/BF03033567]. [PMID: 17449459]. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn D.M., Francescutti-Verbeem D.M., Thomas D.M. Dopamine disposition in the presynaptic process regulates the severity of methamphetamine-induced neurotoxicity. Ann. N. Y. Acad. Sci. 2008;1139:118–126. doi: 10.1196/annals.1432.026. [http://dx.doi.org/10.1196/annals.1432.026]. [PMID: 18991856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleckenstein A.E., Volz T.J., Riddle E.L., Gibb J.W., Hanson G.R. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [http://dx. doi.org/10.1146/annurev.pharmtox.47.120505.105140]. [PMID: 17209801]. [DOI] [PubMed] [Google Scholar]
- 46.Shortall S.E., Macerola A.E., Swaby R.T., Jayson R., Korsah C., Pillidge K.E., Wigmore P.M., Ebling F.J., Richard Green A., Fone K.C., King M.V. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur. Neuropsychopharmacol. 2013;23(9):1085–1095. doi: 10.1016/j.euroneuro.2012.09.005. [http://dx.doi.org/10.1016/j.euroneuro. 2012.09.005]. [PMID: 23051939]. [DOI] [PubMed] [Google Scholar]
- 47.Motbey C.P., Karanges E., Li K.M., Wilkinson S., Winstock A.R., Ramsay J., Hicks C., Kendig M.D., Wyatt N., Callaghan P.D., McGregor I.S. Mephedrone in adolescent rats: residual memory impairment and acute but not lasting 5-HT depletion. PLoS One. 2012;7(9):e45473. doi: 10.1371/journal.pone.0045473. [http://dx.doi.org/10.1371/journal. pone.0045473]. [PMID: 23029034]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.den Hollander B., Rozov S., Linden A.M., Uusi-Oukari M., Ojanperä I., Korpi E.R. Long-term cognitive and neurochemical effects of bath salt designer drugs methylone and mephedrone. Pharmacol. Biochem. Behav. 2013;103(3):501–509. doi: 10.1016/j.pbb.2012.10.006. [http://dx. doi.org/10.1016/j.pbb.2012.10.006]. [PMID: 23099177]. [DOI] [PubMed] [Google Scholar]
- 49.Motbey C.P., Hunt G.E., Bowen M.T., Artiss S., McGregor I.S. Mephedrone (4-methylmethcathinone, meow): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict. Biol. 2012;17(2):409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [http://dx.doi.org/10.1111/j.1369-1600. 2011.00384.x]. [PMID: 21995495]. [DOI] [PubMed] [Google Scholar]
- 50.Lisek R., Xu W., Yuvasheva E., Chiu Y.T., Reitz A.B., Liu-Chen L.Y., Rawls S.M. Mephedrone (bath salt) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126(1-2):257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [http://dx.doi.org/ 10.1016/j.drugalcdep.2012.04.021]. [PMID: 22652295]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumann M.H., Partilla J.S., Lehner K.R. Psychoactive bath salts: not so soothing. Eur. J. Pharmacol. 2013;698(1-3):1–5. doi: 10.1016/j.ejphar.2012.11.020. [http://dx.doi.org/10.1016/j.ejphar.2012.11.020]. [PMID: 23178799]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baumann M.H., Partilla J.S., Lehner K.R., Thorndike E.B., Hoffman A.F., Holy M., Rothman R.B., Goldberg S.R., Lupica C.R., Sitte H.H., Brandt S.D., Tella S.R., Cozzi N.V., Schindler C.W. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive bath salts products. Neuropsychopharmacology. 2013;38(4):552–562. doi: 10.1038/npp.2012.204. [http://dx.doi.org/10.1038/npp.2012.204]. [PMID: 23072836]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothman R.B., Baumann M.H., Dersch C.M., Romero D.V., Rice K.C., Carroll F.I., Partilla J.S. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39(1):32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [http://dx.doi.org/10.1002/1098-2396(20010101)39:1<32: AID-SYN5>3.0.CO;2-3]. [PMID: 11071707]. [DOI] [PubMed] [Google Scholar]
- 54.Rothman R.B., Baumann M.H. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479(1-3):23–40. doi: 10.1016/j.ejphar.2003.08.054. [http://dx.doi.org/10.1016/j.ejphar.2003.08.054]. [PMID: 14612135]. [DOI] [PubMed] [Google Scholar]
- 55.López-Arnau R., Martínez-Clemente J., Rodrigo T., Pubill D., Camarasa J., Escubedo E. Neuronal changes and oxidative stress in adolescent rats after repeated exposure to mephedrone. Toxicol. Appl. Pharmacol. 2015;286(1):27–35. doi: 10.1016/j.taap.2015.03.015. [http://dx.doi.org/10.1016/ j.taap.2015.03.015]. [PMID: 25817894]. [DOI] [PubMed] [Google Scholar]
- 56.Angoa-Pérez M., Kane M.J., Briggs D.I., Francescutti D.M., Sykes C.E., Shah M.M., Thomas D.M., Kuhn D.M. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J. Neurochem. 2013;125(1):102–110. doi: 10.1111/jnc.12114. [http://dx.doi. org/10.1111/jnc.12114]. [PMID: 23205838]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feyissa A.M., Kelly J.P. A review of the neuropharmacological properties of khat. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(5):1147–1166. doi: 10.1016/j.pnpbp.2007.12.033. [http://dx.doi.org/10.1016/j.pnpbp.2007. 12.033]. [PMID: 18561890]. [DOI] [PubMed] [Google Scholar]
- 58.Martínez-Clemente J., López-Arnau R., Abad S., Pubill D., Escubedo E., Camarasa J. Dose and time-dependent selective neurotoxicity induced by mephedrone in mice. PLoS One. 2014;9(6):e99002. doi: 10.1371/journal.pone.0099002. [http://dx.doi.org/10.1371/journal.pone.0099002]. [PMID: 24892744]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pifl C., Reither H., Hornykiewicz O. The profile of mephedrone on human monoamine transporters differs from 3,4-methylenedioxy-methamphetamine primarily by lower potency at the vesicular monoamine transporter. Eur. J. Pharmacol. 2015;755:119–126. doi: 10.1016/j.ejphar.2015.03.004. [http://dx.doi.org/10.1016/j.ejphar.2015.03.004]. [PMID: 25771452]. [DOI] [PubMed] [Google Scholar]
- 60.den Hollander B., Sundström M., Pelander A., Ojanperä I., Mervaala E., Korpi E.R., Kankuri E. Keto amphetamine toxicity-focus on the redox reactivity of the cathinone designer drug mephedrone. Toxicol. Sci. 2014;141(1):120–131. doi: 10.1093/toxsci/kfu108. [http://dx.doi. org/10.1093/toxsci/kfu108]. [PMID: 24913801]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marusich J.A., Grant K.R., Blough B.E., Wiley J.L. Effects of synthetic cathinones contained in bath salts on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33(5):1305–1313. doi: 10.1016/j.neuro.2012.08.003. [http://dx.doi.org/10.1016/j.neuro.2012.08.003]. [PMID: 22922498]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varner K.J., Daigle K., Weed P.F., Lewis P.B., Mahne S.E., Sankaranarayanan A., Winsauer P.J. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology (Berl.) 2013;225(3):675–685. doi: 10.1007/s00213-012-2855-1. [http://dx.doi.org/10.1007/s00213-012-2855-1]. [PMID: 22972412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischman M.W. Relationship between self-reported drug effects and their reinforcing effects: studies with stimulant drugs. NIDA Res. Monogr. 1989;92:211–230. [PMID: 2512494]. [PubMed] [Google Scholar]
- 64.Calabrese E.J. Addiction and dose response: the psychomotor stimulant theory of addiction reveals that hormetic dose responses are dominant. Crit. Rev. Toxicol. 2008;38(7):599–617. doi: 10.1080/10408440802026315. [http://dx. doi.org/10.1080/10408440802026315]. [PMID: 18709568]. [DOI] [PubMed] [Google Scholar]
- 65.Watterson L.R., Hood L., Sewalia K., Tomek S.E., Yahn S., Johnson C.T. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts”. J. Addict. Res. Ther. 2012;(Suppl 9):Pii–002. doi: 10.4172/2155-6105.S9-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aarde S.M., Angrish D., Barlow D.J., Wright M.J., Jr, Vandewater S.A., Creehan K.M., Houseknecht K.L., Dickerson T.J., Taffe M.A. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in sprague-dawley and wistar rats. Addict. Biol. 2013;18(5):786–799. doi: 10.1111/adb.12038. [http://dx.doi.org/10.1111/adb. 12038]. [PMID: 23363010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Motbey C.P., Clemens K.J., Apetz N., Winstock A.R., Ramsey J., Li K.M., Wyatt N., Callaghan P.D., Bowen M.T., Cornish J.L., McGregor I.S. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine. J. Psychopharmacol. (Oxford) 2013;27(9):823–836. doi: 10.1177/0269881113490325. [http://dx.doi.org/10.1177/ 0269881113490325]. [PMID: 23739178]. [DOI] [PubMed] [Google Scholar]
- 68.Tallarida R.J., Raffa R.B., Rawls S.M. Mephedrone (bath salt) pharmacology: insights from invertebrates. Neuroscience. 2012;208:79–84. doi: 10.1016/j.neuroscience.2012.01.019. [http://dx.doi.org/10.1016/j.neuroscience.2012.01.019]. [PMID: 22300981]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blum K., Foster O.M., Wang K.K., Febo M., Borsten J., Giordano J., Hauser M., Gold M.S. Hypothesizing that designer drugs containing cathinones (bath salts) have profound neuro-inflammatory effects and dangerous neurotoxic response following human consumption. Med. Hypotheses. 2013;81(3):450–455. doi: 10.1016/j.mehy.2013.06.007. [http://dx.doi.org/10.1016/j.mehy.2013.06.007]. [PMID: 23845561]. [DOI] [PubMed] [Google Scholar]
- 70.Creehan K.M., Vandewater S.A., Taffe M.A. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015;92:90–97. doi: 10.1016/j.neuropharm.2015.01.003. [http://dx.doi.org/ 10.1016/j.neuropharm.2015.01.003]. [PMID: 25600245]. [DOI] [PMC free article] [PubMed] [Google Scholar]