Abstract
Background:
Akathisia continues to be a significant challenge in current neurological and psychiatric practice. Prompt and accurate detection is often difficult and there is a lack of consensus concerning the neurobiological basis of akathisia. No definitive treatment has been established for akathisia despite numerous preclinical and clinical studies.
Method:
We reviewed antipsychotic-induced akathisia including its clinical presentation, proposed underlying pathophysiology, current and under investigation therapeutic strategies.
Conclusion:
Despite the initial promise that second generation antipsychotics would be devoid of akathisia effects, this has not been confirmed. Currently, there are limited therapeutic options for the clinical practice and the evidence supporting the most widely used treatments (beta blockers, anticholinergic drugs) is still absent or inconsistent.
Keywords: Akathisia, antipsychotics, neuroleptics, movement disorders, agitation, anticholinergics, benzodiazepines, extrapyramidal signs
1. INTRODUCTION
Akathisia is a movement disorder characterized by subjective feelings of internal restlessness or jitteriness with a compelling urge to move leading to the observation of repetitive movements, such as leg crossing, swinging or persistent shifting from one foot to another [1-3]. Although most often considered an adverse effect of certain medications, the first description of akathisia in the medical literature appeared in 1901 when the Czech neuropsychiatrist Ladislav Haskovec described a phenomenon he called “inability to sit”, i.e. a non-drug related akathisia, in two of his patients. The first report of drug-related akathisia did not appear until 1960, when Kruse described three patients who developed “muscular restlessness” while taking phenothiazines [4]. Akathisia was subsequently grouped with other antipsychotic-induced movement disorders, including parkinsonism and dystonia, under the umbrella of extrapyramidal signs (EPS).
Akathisia poses a significant challenge in clinical practice. The clinical presentation of akathisia can be confusing in those patients who often describe vague, non-specific complaints such as nervousness, inner tension, discomfort, restlessness, itching, and/or an inability to relax. As a result, these symptoms are often misdiagnosed as persistent anxiety and/or agitation, and a subsequent dose increase is not only ineffective but often exacerbates antipsychotic- [5, 6] or selective serotonin reuptake inhibitor (SSRIs)-induced akathisia [7]. Failure to correctly identify akathisia can have catastrophic implications, since increasing severity of akathisia has been linked to the emergence and/or worsening of suicidal ideation, aggression, and violence [8]. With these issues in mind, the current manuscript was designed to provide a review of antipsychotic-induced akathisia including its clinical presentation, proposed underlying pathophysiology, and an update of potential treatment strategies
2. CLINICAL CLASSIFICATION OF AKATHISIA
Akathisia is generally classified according to the time frame of symptom development. That is, acute akathisia occurs within a few days to weeks of either initiating or increasing the dose of antipsychotic medication. If akathisia emerges later, it is considered subacute or chronic. Chronic akathisia refers to akathisia that has been present for several months. In contrast, tardive akathisia occurs late (1-3 months) during the course of treatment with antipsychotics and it may even emerge after antipsychotic discontinuation or dosage reduction. Tardive akathisia often is attenuated by increasing the antipsychotic dose but it may also persist for months to years after the antipsychotic is discontinued. However, it is important to note that tardive akathisia, tardive dyskinesia and tardive dystonia are considered distinct entities as illustrated in Table 1 [9, 10].
Table 1.
Differences between tardive syndromes: tardive dyskinesia (TD), tardive akathisia (TA) and tardive dystonia.
| TD | TA | Tardive Dystonia | |
|---|---|---|---|
| Established risk factors | Females Mood disorders Neuroleptics |
Schizophrenia Bipolar disorder Neuroleptics |
Males Bipolar disorder Neuroleptics |
| Nature of movement | Athetoid, choreo-athetoid involuntary movements | Restlessness and jitteriness | Involuntary muscle contractions resulting in repetitive movements and/or abnormal posture |
| Somatic distribution | Lower face and distal extremities | Mainly leg | Eyes, tongue, neck, shoulders and trunk |
Withdrawal akathisia emerges within two weeks of antipsychotic discontinuation or dose reduction and is generally self-limited resolving within 6 weeks. If the akathisia persists for more than 6 weeks, it is no longer considered withdrawal akathisia and instead should be classified as tardive akathisia [11, 12]. The term “pseudoakathisia” has been used for the condition in which there are objective signs of akathisia in the absence of any subjective component such as internal restlessness [13]. Although “pseudoakathisia” remains a controversial entity, Lang (1994) included it in the chronic akathisia classification system [14, 15].
3. EPIDEMIOLOGY
Akathisia is the most common and one of the most distressing of the movement disorders associated with psychotropic medication particularly antipsychotics. In fact, chronic akathisia and “pseudoakathisia” prevalence was estimated at 24% and 18%, respectively [16, 17] in patients with schizophrenia. Akathisia rates were reportedly 39% in clozapine-treated patients, and 45% among patients treated with first generation antipsychotics (FGA) in another report [18]. In a recent study investigating the prevalence of global akathisia in a community-dwelling sample of patients with schizophrenia, akathisia rates ranged from 15 to 35%. Since the study design did not permit changes in antipsychotic medication in the 4 weeks prior to the initial assessment, it is likely that some of these patients had the chronic akathisia subtype. Patients on combination SGAs (second-generation antipsychotic) had a higher risk of akathisia than those prescribed a SGA with a FGA (first generation antipsychotic) (34.2% vs. 14.7%) [19]. Moreover, patients on more than one SGA had a 3-fold increased risk of akathisia compared to SGA monotherapy (34.2% vs. 10.9) [19]. The rate of akathisia reported in this study was consistent with previous estimates in the literature.
Historically, akathisia is most commonly linked to antipsychotic medication [20] in particular FGA agents [21, 22]. For example, results from a recent meta-analysis reported that SGA monotherapy was associated with a significantly lower risk of akathisia as well as extra-pyramidal signs in general in comparison with FGA monotherapy [23, 24]. However, akathisia is not limited to antipsychotic medication. Other psychotropic medications especially SSRI [25], monoamine oxidase inhibitor (MAOI) [26], and tricyclic (TCA) antidepressants [27] have been associated with akathisia. In fact, 10-18% of bipolar I patients taking antidepressants are estimated to develop akathisia [28, 29]. In addition, antibiotics [30], calcium channel blockers [31], and even illicit drug use such as amphetamine, methamphetamine, and cocaine [32] can elicit akathisia.
Akathisia represents not only a treatment challenge in patients suffering from schizophrenia [33] or mood disorders [34], but it may also complicate a wide range of other conditions including parkinsonian syndromes [35] and traumatic brain injury [36]. As reviewed in the next section and despite its impact, the neurobiological basis of akathisia remains considerably controversial.
4. NEUROBIOLOGICAL BASIS OF AKATHISIA
Although the exact pathophysiological mechanisms underlying akathisia have yet to be identified, basal ganglia and/or striatal circuitry dysfunction [37] are most commonly implicated in the development of movement disorders including akathisia. The neurotransmitters most specifically linked to akathisia are gamma-aminobutyric acid (GABA) and serotonin. GABA (mainly via GABAA receptor interactions) exerts an influence on dopamine-dependent signaling, thus, increasing or reducing locomotor activity [38]. With a distinctive distribution pattern, serotonin is also involved in motor regulation through serotonin receptors present in several cortical areas as well as the striatum [39]. More specifically, serotonin regulates dopaminergic motor function in the nigro-striatal system [40].
Classically, an imbalance between dopaminergic and serotonergic/noradrenergic neurotransmitter systems [41, 42] has been most commonly considered as the basis for akathisia, although other theories such as overstimulation of the locus ceruleus leading to a mismatch between the core and the shell of the nucleus accumbens [43] have also been proposed. More recently, a model involving D2/D3 receptor occupancy in the ventral striatum has been linked to the pathogenesis of akathisia [44]. In addition, new patho-physiological mechanisms related to neuroinflammation, damage to the blood brain barrier, and/or impaired neurogenesis have also been implicated in the emergence of akathisia [45].
One of the most intriguing recent theories involves the role of genetics in neuroleptic-induced movement disorders. Preliminary data from both genome wide association studies (GWAS) and candidate gene studies have suggested a genetic susceptibility for development and expression of extrapyramidal syndromes, although these studies require replication and validation in larger samples [46-48].
Several preclinical animal models have been used to investigate the underlying pathophysiology of acute akathisia. These models include the rat defecation model that can be used to measure emotional distress [49], and lesions of the ventral tegmental area (VTA) and the medial prefrontal cortex (MPC) that can produce locomotor responses that mimic drug-induced akathisia [50]. Additional pre-clinical animal tests proposed to model akathisia are the SSRI-induced restlessness model [51] and dopamine agonist and antagonist-induced restlessness tests in non-human primates [52, 53]. There is also the hyperkinesia dog model but this is reportedly less optimal for studying akathisia [54]. The primate model of akathisia appears to convey several advantages in that the symptoms elicited (restlessness, compulsive shifting of weight from one foot to the other, rhythmic finger movements, and body rocking) are very similar to those seen in humans and acute neuroleptic challenges can detect subject-to-subject variability in response.
In our opinion, the aforementioned models are limited as they only partially reproduce signs similar to either the emotional (subjective) or the motor (objective) human features of akathisia. In addition, none of the available models has been fully validated to date. Most of the available animal models are limited by other confounding side effects associated with antipsychotic administration such as cataplexy or parkinsonism. There have also been inconsistent results reported. For instance, applicability of the rat defecation model to akathisia has been hampered by the lack of consistent findings across differential doses, habituation schemes and time frame. Even more troubling, pre-treatment with anti-anxiety agents in the rat defection model results in a greatly attenuated response, suggesting that it is much more suited to assess antipsychotic-induced anxiety/dysphoria than akathisia [55].
While lesions in the VTA and MPC regions consistently produce akathisia-like locomotor activity, they also results in other less specific behavioral responses such as reduced exploratory activity, ongoing effects on other behaviors and attention span, loss of fear reactions, and attenuated response to penalties during avoidance conditioning. These additional responses are more consistent with a hypo-arousal state in contrast to the state of heightened emotional distress experienced with akathisia in humans [56].
As previously mentioned, primate models of akathisia appear less problematic, but there are relatively few reports in comparison to non-primate models. Primate models are also not without drawbacks. Acute administration of antipsychotics to monkeys has also been associated with the emergence of dystonic reactions that can also confound the assessment of akathisia.
Conducting akathisia research with human subjects also poses unique challenges and limitations. Exposing normal subjects to antipsychotic medication, especially on a repeated or prolonged basis, is controversial and rife with ethical considerations in that these are not innocuous medications and they are associated with potential morbidity. Similarly, investigating akathisia in acutely ill psychiatric patients is still fraught with many challenges such as the presence of agitation, competency issues, etc.
Despite these many obstacles, there are numerous ongoing and promising clinical trials investigating potential treatments for akathisia as well as involving medications purported to avoid or at least minimize the risk of akathisia (See Supplementary Table regarding trials listed on www.clinicaltrials.gov).
5. CLINICAL EVALUATION AND DIFFERENTIAL DIAGNOSIS
Rating scales have been developed to identify and score the severity of akathisia. Currently, the most commonly used tool for assessment is the Barnes Akathisia-Rating Scale (BARS). The 4-item scale provides a measure of akathisia severity including observation of the subject, the subject’s awareness of the presence of restlessness, any associated emotional distress, and a global clinical assessment [57]. The BARS has demonstrated validity for assessing akathisia [58]. In fact, the BARS was significantly better at identifying antipsychotic-induced akathisia in the lower limbs than conventional actometry, after adjusting for the confounding effects of other associated hyperactivity syndromes (e.g., parkinsonism and tardive dyskinesia) [58]. In contrast, the Extrapyramidal Symptom Rating Scale (ESRS) was developed to evaluate the frequency and severity of akathisia as well as additional movement disorder symptoms including parkinsonism, dyskinesia, and dystonia. The ESRS is comprised of 12 questionnaire items along with a specialized physical exam designed to assess both subjective symptoms and objective findings, especially potential parkinsonian features [59].
Akathisia predominantly affects the lower extremities from the hips to the ankles, and this predilection for the lower extremities can be helpful in distinguishing akathisia from other antipsychotic-induced syndromes. Akathisia is most frequently characterized by constant shifting while standing or continual rocking or feet movements while sitting [60]. Although akathisia is the most common drug-induced movement disorder, it remains woefully under-recognized and even misdiagnosed in clinical settings. Unfortunately there is a substantial burden associated with this lack of accurate identification and prompt intervention. Akathisia can not only result in premature treatment discontinuation, but if it is misinterpreted as worsening of the underlying illness or psychosis, an increase in anti-psychotic dose can worsen the patient’s distress and subsequently contribute to treatment non-compliance with resultant symptom exacerbation, relapse of the primary illness, or even the emergence of aggression and/or suicidal behavior [61].
Hirose classified the primary reasons that contribute to under-reporting and/or non-recognition of akathisia into “clinician” and “patient” -related components. The clinician factors included: emphasis on objective restlessness, failure to routinely assess for akathisia during therapy, failure to implement anti-akathisia treatments in ambiguous cases, and adherence to research diagnostic criteria. The patient factors identified were: mild severity of akathisia, lack of objective restlessness, no or unclear expression of subjective restlessness, presence of restlessness in other body parts rather than the legs, occurrence of other prominent psychiatric symptoms, and absence of other extrapyramidal motor signs [62].
It is important to differentiate akathisia from other conditions, such as anxiety, agitation, or tics as summarized in Table 2, tardive dyskinesia (TD), also from restless leg syndrome (RLS) (Table 3) [63].
Table 2.
Differences among tics, anxiety and agitation.
| Tics | Anxiety | Agitation | |
|---|---|---|---|
| Associations | 25% of children, attention deficit hyperactivity disorder. | Panic attacks, phobias, and obsessive compulsive-disorder. | Mania, schizophrenia, depression, dementia. |
| Nature of movement | Involuntary, sudden, repetitive, non-rhythmic, partially controllable, worse with stress. | Clumsiness, coordination problems | Unintentional, purposeless movements, typically pacing around a room, wringing the hands, uncontrolled tongue movement, pulling off clothing and putting it back on. Can be harmful in extreme cases. |
| Somatic distribution | Motor (any part, mainly eyelids, facial muscles), Vocal. | Lip smacking, picking, along with heart racing, chest discomfort, light headed/dizziness, chest, sweating, blushing, visual disturbance. | Whole body with extreme arousal and tense feeling. |
Table 3.
Differences between akathisia and restless leg syndrome (RLS).
| Akathisia | RLS | |
|---|---|---|
| Prevalence | 15-35% | 3-9% |
| Gender prevalence | Same in both male and females | Tend to occur more in females |
| Motor restlessness | Can be absent | Present |
| Paresthesia | Absent | Present mostly at night and disappears in the morning |
| Time of occurrence | Anytime | Mostly at night |
| Sleep disturbance | Absent | Patient usually cannot sleep |
| Lying down | Somewhat decreases it | Aggravates it |
| Myoclonus | Absent | Present with severe cases |
| Treatment | Anticholinergic and Beta-blockers | Dopamine agonists and opiates |
6. CURRENT TREATMENT STRATEGIES: General Overview
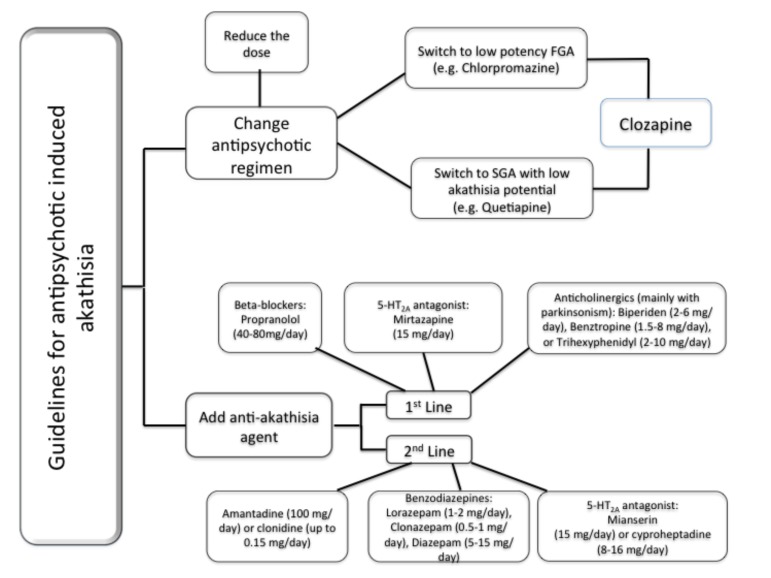
Traditionally, two major treatment strategies for anti-psychotic related akathisia have been proposed: a) change in the antipsychotic medication regimen and/or; b) the addition of an anti-akathisia agent (Fig. 1). The first strategy involves reduction in dose of antipsychotic medication or the potential switch to either a low-potency FGA or to a SGA with low potential to induce akathisia. In cases of intractable or refractory akathisia, clozapine should be considered.
Fig. (1).
This figure depicts a practical guideline for management of akathisia. The first option is antipsychotic regimen adjustment. When this is not possible and/or not effective, it is a good clinical practice to consider the addition of an anti-akathisia agent. There is no evidence to fully support a specific agent (beta blocker, serotonin-based or anti-cholinergic; commonly used as first line approach) in detriment of others. The selection of an anti-akathisia agent must take into consideration patients’ clinical comorbidities and associated symptoms.
Results from the CATIE (Clinical Antipsychotic Trials of Intervention Effectiveness) trial demonstrated that schizophrenic patients with perphenazine-induced akathisia were still vulnerable to adverse effects such as akathisia when switched to risperidone. Extrapolating from these data, switching from a FGA to a SGA with a relatively elevated risk of akathisia such as risperidone or aripiprazole may yield similar results, but direct controlled data is clearly lacking [64-66].
Propranolol (40–80 mg/day twice daily) and low-dose mirtazapine (15 mg once daily) have the most robust evidence as first-line treatments for akathisia. There are also data suggesting that mianserin (15 mg once daily) and cyproheptadine (8–16 mg/day) may represent alternative strategies. In patient with antipsychotic-induced akathisia with parkinsonism, anticholinergic agents (e.g. biperiden, trihexyphenidyl, benztropine) are reasonable therapeutic options. The anxiolytic and sedative effects of benzodiazepines alone or in combination with propranolol may be beneficial for treating akathisia in selected patients. Though data are very limited, clonidine and amantadine may be considered in patients that have failed to respond to usual widely recommended agents [67].
7. TRADITIONAL TREATMENT STRATEGIES: A Close Look (Table 4)
Table 4.
Levels of evidence for akathisia treatment guidelines.
| Medication Lines | Levels of Evidence* | |
|---|---|---|
| 1 | Beta blockers | B |
| 2 | Anticholinergics | B |
| 3 | Benzodiazepines | C |
| 4 | Serotonin based medications | B |
| 5 | GABA modulators | C |
| 6 | Vitamin B6 | U |
| 7 | NAC | U |
NAC: N-acetylcysteine.
*Evidence level is according to the guidelines of the American Academy of Neurology (AAN).
7.1. Prevention and Psychosocial Intervention
A standardized titration schedule and preferential selection of a SGA over a FGA may be the easiest way to prevent or at least minimize anti-psychotic related akathisia. Once akathisia develops, it usually resolves when the causative agent is either discontinued or dosage is decreased. Again, first step strategies include lowering the dose of antipsychotic drug, switching to lower potency FGA, or switching to an SGA other than risperidone and aripiprazole. Non-medication interventions include psychoeducation about the potential benefits, risks, side effects and possible strategies for treatment-emergent side effects such as akathisia [68, 69].
7.2. Beta-blockers (Propranolol, Metoprolol) and Selective Alpha-blockers (Clonidine)
The therapeutic action of beta-blockers (BB) is linked to their purported action in blocking noradrenergic/serotonergic input into the dopaminergic pathways [70]. BB are generally considered the gold standard of treatment for akathisia [71, 72]. BB can have significant side effects though such as hypotension and sleep disturbances. They are also contra-indicated in patients with comorbid diabetes. There are also data supporting the utility of clonidine, a selective alpha-2 adrenergic agonist, in medication-related akathisia when other strategies fail [68]. Conversely, results from meta-analyses such as the Cochrane Review have reported that the efficacy of BB as well as anticholinergic agents in the treatment of akathisia is poor at best [73, 74].
7.3. Anticholinergics (Biperiden, Trihexyphenidyl, Ben-zotropine)
Anticholinergics are traditionally used to reverse the EPS resulting from acetylcholine/dopamine imbalance at the nigrostriatal level [75]. Nevertheless, they are also associated with potentially significant adverse effects such as cognitive impairment, blurred vision, constipation, and urinary retention. Their limited efficacy also makes them relatively unsuitable for long-term use [67]. Interestingly, anti-cholinergics are still often recommended when patients with parkinsonian symptoms develop akathisia, despite the lack of controlled data supporting this approach [76].
7.4. Benzodiazepines (Lorazepam, Clonazepam, Diazepam)
Benzodiazepines are considered second-line treatment agents for akathisia. Their therapeutic effect has been attributed to a GABA-dependent mechanism. They have proven clinically useful, but do not appear to truly attenuate motor restlessness [77]. Only brief courses of benzodiazepines should be utilized due to their well-documented CNS adverse effects including drowsiness, cognitive impairment, risk of fall and potential for drug abuse or dependence, particularly with long-term use.
7.5. Antihistamines
The antihistamine diphenhydramine crosses the blood brain barrier and can block central muscarinic, serotonergic, and α-adrenergic receptors, in addition to histaminergic H1 receptors. The role of antihistamines in locomotor disorders remains controversial with some trials suggesting reduction in akathisia and other trials reporting that antihistamines worsened akathisia [78-80]. In a randomized controlled trial, the administration of diphenhydramine two minutes after an antipsychotic (Prochlorperazine) infusion resulted in a relative (61%) and absolute (22%) reduction in akathisia rates in comparison to patients treated only with Prochlorperazine followed by placebo [81]. However, antihistamines can also be associated with serious adverse events. Promethazine has an FDA-mandated black box warning regarding its use in young children due to its association with respiratory depression and death in the pediatric age group [82, 83].
Level A:Established as effective, ineffective, or harmful for the given condition in the specified population, it requires at least two consistent Class I studies. Level B: Probably effective, ineffective, or harmful for the given condition in the specified population, it requires at least one Class I study or two consistent Class II studies. Level C: Possibly effective, ineffective, or harmful for the given condition in the specified population, it requires at least one Class II study or two consistent Class III studies. Level U: Data is inadequate or conflicting; given current knowledge, treatment is unproven.
Class I Studies:A randomized, controlled clinical trial of the intervention of interest with masked or objective outcome assessment, in a representative population. Class II studies: A randomized, controlled clinical trial of the intervention of interest with masked, or objective outcome assessment that lacks one criterion a-c in class I, or a prospective matched cohort study with masked or objective outcome assessment in a representative population. Class III studies: All other controlled trials (including well-defined natural history controls or patients serving as their own controls) in a representative population, where outcome is independently assessed, or independently derived by objective outcome measurements. Class IV studies: Studies not meeting Class I, II or III criteria, including consensus, or expert opinion.
It is worth mentioning that despite the absence of robust scientific evidence supporting the efficacy of BB and anticholinergic agents, they remain the most prescribed agents in clinical practice for the treatment of medication-related akathisia.
8. NEW TREATMENT STRATEGIES
8.1. Serotonin Based Strategies
Both mianserin and cyproheptadine have demonstrated significant efficacy and adequate tolerability in small randomized, placebo-controlled trials conducted in patients with FGA-induced akathisia [60]. The therapeutic effects have been linked to antagonist activities at the 5-HT2a/c receptor. This activity is thought to counteract antipsychotic-induced dopamine D2 receptor blockade with subsequent enhancement in dopamine neurotransmission. Since mianserin in contrast to cyproheptadine lacks anticholinergic effects it may be better tolerated.
In a double blind, controlled comparison with propranolol, mirtazapine (15 mg/day) was reported to be as effective as propranolol in controlling akathisia over a 7 day period with better tolerability. Trazodone at a dose of 100 mg/day has also been reported to have potent anti-akathisia effects at least in one report [84]. These data, if replicated, suggest that agents with 5-HT2a antagonism properties may represent a promising anti-akathisia strategy [68, 85, 86]. One study reported that Zolmitriptan, a selective agonist on 5-HT1d receptor currently used for migraine treatment, had a similar efficacy of propranolol for akathisia [87]. While these results are encouraging, the potential of side effects such as sedation and orthostatic hypotension may end up hampering the widespread clinical use of serotonin-based strategies for akathisia.
8.2. Dopamine Agonist Strategies
Amantadine enhances dopamine release and acts as a dopamine reuptake inhibitor through its antagonism of the glutamate receptor. Since it has been associated with the emergence of psychosis, its utility in akathisia is very limited. In fact amantadine generally relegated to a last resort in the treatment of akathisia [88]. Similarly, ropinirole, a potent D2, D3, and D4 dopamine receptor agonist, also is generally avoided in patients with psychosis although it was found to be effective in a case of aripiprazole-induced tardive akathisia [89].
8.3. GABA Receptors Directed Therapy
Agents that increase GABA levels have demonstrated efficacy for movement disorders [90]. Pregabalin and gabapentin, for instance, were shown to effectively treat patients with antipsychotic-induced akathisia and also helped in reducing tardive akathisia [91, 92]. Pregabalin, an analogue of GABA, does not bind directly to GABAA, GABAB, or benzodiazepine receptors but does bind to the α2δ subunit, whereas gabapentin binds to α2δ-1subunit modifier. This specific binding activity results in augmented GABA neurotransmission by both increasing the density of GABA transporter proteins and also enhancing the rate of functional GABA transport [93, 94]. The FDA has already approved gabapentin for the indication of restless leg syndrome (RLS), so it is not surprising that it may be efficacious for akathisia as well since it enhances the inhibitory effects of GABA throughout the CNS [95-97].
8.4. Vitamin B6
Vitamin B6 serves as a cofactor in the metabolic reactions for synthesis of several neurotransmitter including dopamine, serotonin, and GABA. In two studies of patients with acute antipsychotic-induced akathisia, high doses of vitamin B6 daily for 5 days resulted in significant improvement of akathisia [98, 99]. The exact mechanism of action underlying the observed anti-akathisia effects reported remains unknown at this time.
8.5. N-acetylcysteine (NAC)
NAC was shown to have a moderate positive effect on akathisia in a randomized control trial after 24 weeks of treatment. If replicated these findings suggest that NAC may be effective as a neuroprotective strategy for akathisia and other extrapyramidal syndromes [100].
CONCLUSION AND FUTURE PERSPECTIVES
Akathisia remains a significant clinical problem in patients treated with psychotropic agents, particularly antipsychotic regimens. Despite the initial promise that SGAs would be devoid of akathisia effects, this has not been demonstrated in controlled trials. Prompt and accurate detection of akathisia continues to be a problem in most clinical settings with frequent dire consequences. Even when akathisia is correctly diagnosed, there are limited therapeutic options and the evidence supporting the most widely used treatments is still absent or inconsistent. While more recent treatment strategies have involved additional mechanisms of therapeutic action such as serotonin-based, GABAergic, and/or dopamine agonist strategies, more comprehensive work is clearly needed for this critical problem.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflicts of interest associated with this work. Authors did not receive any sort of financial support for completion of this work.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Web site along with the published article.
REFERENCES
- 1.Brüne M. Ladislav Haskovec and 100 Years of Akathisia. Am. J. Psychiatry. 2002;159(5):727. [http://dx.doi.org/10.1176/appi. ajp.159.5.727]. [Google Scholar]
- 2.Mohr P, Volavka J. Ladislav Haskovec and akathisia: 100th anniversary. Br. J. Psychiatry. 2002;181(6) doi: 10.1192/bjp.181.6.537-a. [DOI] [PubMed] [Google Scholar]
- 3.Rummel-Kluge C., Komossa K., Schwarz S., Hunger H., Schmid F., Kissling W., Davis J.M., Leucht S. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr. Bull. 2012;38(1):167–177. doi: 10.1093/schbul/sbq042. [http://dx.doi.org/10.1093/ schbul/sbq042]. [PMID: 20513652]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruse W. Treatment of drug-induced extrapyramidal symptoms. (A comparative study of three antiparkinson agents). Dis. Nerv. Syst. 1960;21:79–81. [PMID: 14412284]. [PubMed] [Google Scholar]
- 5.Peitl M.V., Prološčić J., Blažević-Zelić S., Skarpa-Usmiani I., Peitl V. Symptoms of agitated depression and/or akathisia. Psychiatr. Danub. 2011;23(1):108–110. [PMID: 21448111]. [PubMed] [Google Scholar]
- 6.Young S.L., Taylor M., Lawrie S.M. First do no harm. A systematic review of the prevalence and management of antipsychotic adverse effects. J. Psychopharmacol. (Oxford) 2015;29(4):353–362. doi: 10.1177/0269881114562090. [http://dx.doi.org/10.1177/0269881114562090]. [PMID: 25516373]. [DOI] [PubMed] [Google Scholar]
- 7.Lane R.M. SSRI-induced extrapyramidal side-effects and akathisia: implications for treatment. J. Psychopharmacol. (Oxford) 1998;12(2):192–214. doi: 10.1177/026988119801200212. [http://dx.doi.org/10.1177/026988119801200212]. [PMID: 9694033]. [DOI] [PubMed] [Google Scholar]
- 8.Lipinski J.F., Jr, Mallya G., Zimmerman P., Pope H.G. Jr Fluoxetine-induced akathisia: clinical and theoretical implications. J. Clin. Psychiatry. 1989;50(9):339–342. [PMID: 2549018]. [PubMed] [Google Scholar]
- 9.Leong G.B., Silva J.A. Neuroleptic-induced akathisia and violence: a review. J. Forensic Sci. 2003;48(1):187–189. [http://dx.doi. org/10.1520/JFS2002173]. [PMID: 12570226]. [PubMed] [Google Scholar]
- 10.Bratti I.M., Kane J.M., Marder S.R. Chronic restlessness with antipsychotics. Am. J. Psychiatry. 2007;164(11):1648–1654. doi: 10.1176/appi.ajp.2007.07071150. [http:// dx.doi.org/10.1176/appi.ajp.2007.07071150]. [PMID: 17974927]. [DOI] [PubMed] [Google Scholar]
- 11.Bhidayasiri R., Boonyawairoj S. Spectrum of tardive syndromes: clinical recognition and management. Postgrad. Med. J. 2011;87(1024):132–141. doi: 10.1136/pgmj.2010.103234. [http://dx.doi.org/10.1136/pgmj.2010.103234]. [PMID: 21131613]. [DOI] [PubMed] [Google Scholar]
- 12.Lee M.J., Lin P.Y., Chang Y.Y., Chong M.Y., Lee Y. Antipsychotics-induced tardive syndrome: a retrospective epidemiological study. Clin. Neuropharmacol. 2014;37(4):111–115. doi: 10.1097/WNF.0000000000000040. [http://dx.doi.org/10.1097/WNF.0000000000000040]. [PMID: 24992086]. [DOI] [PubMed] [Google Scholar]
- 13.Munetz M.R., Cornes C.L. Akathisia, pseudoakathisia and tardive dyskinesia: clinical examples. Compr. Psychiatry. 1982;23(4):345–352. doi: 10.1016/0010-440x(82)90084-0. [http://dx.doi.org/10.1016/0010-440X(82)90084-0]. [PMID: 6126315]. [DOI] [PubMed] [Google Scholar]
- 14.Lang A.E. Withdrawal akathisia: case reports and a proposed classification of chronic akathisia. Mov. Disord. 1994;9(2):188–192. doi: 10.1002/mds.870090211. [http://dx.doi.org/10.1002/mds.870090211]. [PMID: 8196681]. [DOI] [PubMed] [Google Scholar]
- 15.Stubbs J.H., Halstead S.M. Pseudoakathisia: a review and two case reports. Compr. Psychiatry. 2000;41(1):70–72. doi: 10.1016/s0010-440x(00)90134-2. [http://dx.doi. org/10.1016/S0010-440X(00)90134-2]. [PMID: 10646622]. [DOI] [PubMed] [Google Scholar]
- 16.Halstead S.M., Barnes T.R., Speller J.C. Akathisia: prevalence and associated dysphoria in an in-patient population with chronic schizophrenia. Br. J. Psychiatry. 1994;164(2):177–183. doi: 10.1192/bjp.164.2.177. [http://dx. doi.org/10.1192/bjp.164.2.177]. [PMID: 7909711]. [DOI] [PubMed] [Google Scholar]
- 17.Janno S., Holi M., Tuisku K., Wahlbeck K. Prevalence of neuroleptic-induced movement disorders in chronic schizophrenia inpatients. Am. J. Psychiatry. 2004;161(1):160–163. doi: 10.1176/appi.ajp.161.1.160. [http:// dx.doi.org/10.1176/appi.ajp.161.1.160]. [PMID: 14702266]. [DOI] [PubMed] [Google Scholar]
- 18.Sahoo S., Ameen S. Acute nocturnal akathisia induced by clozapine. J. Clin. Psychopharmacol. 2007;27(2):205. doi: 10.1097/01.jcp.0000248618.49789.55. [http://dx. doi.org/10.1097/01.jcp.0000248618.49789.55]. [PMID: 17414247]. [DOI] [PubMed] [Google Scholar]
- 19.Berna F., Misdrahi D., Boyer L., Aouizerate B., Brunel L., Capdevielle D., Chereau I., Danion J.M., Dorey J.M., Dubertret C., Dubreucq J., Faget C., Gabayet F., Lancon C., Mallet J., Rey R., Passerieux C., Schandrin A., Schurhoff F., Tronche A.M., Urbach M., Vidailhet P., Llorca P.M., Fond G. Akathisia: prevalence and risk factors in a community-dwelling sample of patients with schizophrenia. Results from the FACE-SZ dataset. Schizophr. Res. 2015;169(1-3):255–261. doi: 10.1016/j.schres.2015.10.040. [http://dx.doi.org/10. 1016/j.schres.2015.10.040]. [PMID: 26589388]. [DOI] [PubMed] [Google Scholar]
- 20.Saddichha S., Kumar R., Babu G.N., Chandra P. Aripiprazole associated with acute dystonia, akathisia, and parkinsonism in a single patient. J. Clin. Pharmacol. 2012;52(9):1448–1449. doi: 10.1177/0091270011414573. [http:// dx.doi.org/10.1177/0091270011414573]. [PMID: 21903892]. [DOI] [PubMed] [Google Scholar]
- 21.Spielmans G.I., Berman M.I., Linardatos E., Rosenlicht N.Z., Perry A., Tsai A.C. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. doi: 10.1371/journal.pmed.1001403. [http://dx.doi.org/10.1371/journal.pmed.1001403]. [PMID: 23554581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh G.H., Yu J-C., Choi K-S., Joo E-J., Jeong S-H. Simultaneous comparison of efficacy and tolerability of second-generation antipsychotics in schizophrenia: mixed-treatment comparison analysis based on head-to-head trial data. Psychiatry Investig. 2015;12(1):46–54. doi: 10.4306/pi.2015.12.1.46. [http://dx.doi.org/10.4306/pi.2015. 12.1.46]. [PMID: 25670945]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J.P., Gallego J.A., Robinson D.G., Malhotra A.K., Kane J.M., Correll C.U. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2013;16(6):1205–1218. doi: 10.1017/S1461145712001277. [http://dx.doi.org/ 10.1017/S1461145712001277]. [PMID: 23199972]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddad P.M., Das A., Keyhani S., Chaudhry I.B. Antipsychotic drugs and extrapyramidal side effects in first episode psychosis: a systematic review of head-head comparisons. J. Psychopharmacol. (Oxford) 2012;26(5) Suppl.:15–26. doi: 10.1177/0269881111424929. [http://dx.doi.org/10.1177/0269881111424929]. [PMID: 22057019]. [DOI] [PubMed] [Google Scholar]
- 25.Hawthorne J.M., Caley C.F. Extrapyramidal reactions associated with serotonergic antidepressants. Ann. Pharmacother. 2015;49(10):1136–1152. doi: 10.1177/1060028015594812. [http://dx.doi.org/10.1177/1060028015594812]. [PMID: 26185277]. [DOI] [PubMed] [Google Scholar]
- 26.Anderson H.D., Pace W.D., Libby A.M., West D.R. Valuck, RJ Rates of 5 common antidepressant side effects among new adult and adolescent cases of depression: a retrospective US claims study. Clin. Ther. 2012;34(1):113–123. doi: 10.1016/j.clinthera.2011.11.024. [http://dx.doi.org/10.1016/ j.clinthera.2011.11.024]. [DOI] [PubMed] [Google Scholar]
- 27.Madhusoodanan S., Alexeenko L., Sanders R., Brenner R. Extrapyramidal symptoms associated with antidepressantsa review of the literature and an analysis of spontaneous reports. Ann. Clin. Psychiatry. 2010;22(3):148–156. [PMID: 20680187]. [PubMed] [Google Scholar]
- 28.Dag E., Gokce B., Buturak S.V., Tiryaki D., Erdemoglu A.K. Pregabalin-induced akathisia. Ann. Pharmacother. 2013;47(4):592–593. doi: 10.1345/aph.1R699. [http://dx.doi.org/10.1345/aph.1R699]. [PMID: 23535811]. [DOI] [PubMed] [Google Scholar]
- 29.Pondé M.P., Freire A.C. Increased anxiety, akathisia, and suicidal thoughts in patients with mood disorder on aripiprazole and lamotrigine. Case Report. Psychiatry, 2015;2015 doi: 10.1155/2015/419746. [http://dx.doi.org/10.1155/2015/419746] [PMID: 26509095] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riesselman A., El-Mallakh R.S. Akathisia with azithromycin. Ann. Pharmacother. 2015;49(5):609. doi: 10.1177/1060028015570728. [http://dx.doi.org/10.1177/ 1060028015570728]. [PMID: 25870444]. [DOI] [PubMed] [Google Scholar]
- 31.Dressler D. Tardive dystonic syndrome induced by the calcium-channel blocker amlodipine. J. Neural Transm. (Vienna) 2014;121(4):367–369. doi: 10.1007/s00702-013-1108-8. [http://dx.doi.org/10.1007/s00702-013-1108-8]. [PMID: 24169926]. [DOI] [PubMed] [Google Scholar]
- 32.Asser A., Taba P. Psychostimulants and movement disorders. Front. Neurol. 2015;6:75. doi: 10.3389/fneur.2015.00075. [http://dx.doi.org/10.3389/fneur.2015. 00075]. [PMID: 25941511]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammad S.S., Wallace G., Ramanathan S., Brilot F., Dale R.C. Antipsychotic-induced akathisia and neuroleptic malignant syndrome in anti-NMDAR encephalitis. Ann. Clin. Psychiatry. 2014;26(4):297–298. [PMID: 25166486]. [PubMed] [Google Scholar]
- 34.Kemp D.E. Managing the side effects associated with commonly used treatments for bipolar depression. J. Affect. Disord. 2014;169(Suppl. 1):S34–S44. doi: 10.1016/S0165-0327(14)70007-2. [http://dx.doi.org/10.1016/S0165-0327 (14)70007-2]. [PMID: 25533913]. [DOI] [PubMed] [Google Scholar]
- 35.Marras C., Chaudhuri K.R. Nonmotor features of Parkinsons disease subtypes. Mov. Disord. 2016;31(8):1095–1102. doi: 10.1002/mds.26510. [http://dx.doi.org/10.1002/mds.26510]. [PMID: 26861861]. [DOI] [PubMed] [Google Scholar]
- 36.Nordström P., Michaëlsson K., Gustafson Y., Nordström A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann. Neurol. 2014;75(3):374–381. doi: 10.1002/ana.24101. [http://dx.doi. org/10.1002/ana.24101]. [PMID: 24812697]. [DOI] [PubMed] [Google Scholar]
- 37.Shuen J.A., Chen M., Gloss B., Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J. Neurosci. 2008;28(11):2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [http://dx.doi.org/ 10.1523/JNEUROSCI.5492-07.2008]. [PMID: 18337395]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bateup H.S., Santini E., Shen W., Birnbaum S., Valjent E., Surmeier D.J., Fisone G., Nestler E.J., Greengard P. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc. Natl. Acad. Sci. USA. 2010;107(33):14845–14850. doi: 10.1073/pnas.1009874107. [http://dx.doi.org/10.1073/pnas.1009874107]. [PMID: 20682746]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shukla R., Watakabe A., Yamamori T. mRNA expression profile of serotonin receptor subtypes and distribution of serotonergic terminations in marmoset brain. Front. Neural Circuits. 2014;8:52. doi: 10.3389/fncir.2014.00052. [http://dx.doi.org/10.3389/fncir.2014.00052]. [PMID: 24904298]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Matteo V., Cacchio M., Di Giulio C., Esposito E. Role of serotonin(2C) receptors in the control of brain dopaminergic function. Pharmacol. Biochem. Behav. 2002;71(4):727–734. doi: 10.1016/s0091-3057(01)00705-5. [http://dx.doi.org/10.1016/S0091-3057(01)00705-5]. [PMID: 11888564]. [DOI] [PubMed] [Google Scholar]
- 41.Adler L.A., Angrist B., Reiter S., Rotrosen J. Neuroleptic-induced akathisia: a review. Psychopharmacology (Berl.) 1989;97(1):1–11. doi: 10.1007/BF00443404. [PubMed: 2565586]. [http://dx.doi.org/10.1007/ BF00443404]. [PMID: 2565586]. [DOI] [PubMed] [Google Scholar]
- 42.Poyurovsky M., Weizman A. Serotonin-based pharmacotherapy for acute neuroleptic-induced akathisia: a new approach to an old problem. Br. J. Psychiatry. 2001;179:4–8. doi: 10.1192/bjp.179.1.4. [http://dx.doi.org/10. 1192/bjp.179.1.4]. [PMID: 11435260]. [DOI] [PubMed] [Google Scholar]
- 43.Loonen A.J., Stahl S.M. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7–10. [http://dx.doi.org/10. 1017/S1092852912000107]. [PubMed] [Google Scholar]
- 44.Kim J.H., Son Y.D., Kim H.K., Lee S.Y., Cho S.E., Kim Y.B., Cho Z.H. Antipsychotic-associated mental side effects and their relationship to dopamine D2 receptor occupancy in striatal subdivisions: a high-resolution PET study with [11C]raclopride. J. Clin. Psychopharmacol. 2011;31(4):507–511. doi: 10.1097/JCP.0b013e318222353a. [http://dx.doi.org/ 10.1097/JCP.0b013e318222353a]. [PMID: 21694619]. [DOI] [PubMed] [Google Scholar]
- 45.Goncalves J., Baptista S., Silva A.P. Psychostimulants and brain dysfunction: a review of the relevant neurotoxic effects. Neuropharmacology. 2014;87:135–149. doi: 10.1016/j.neuropharm.2014.01.006. [http://dx.doi.org/10.1016/ j.neuropharm.2014.01.006]. [DOI] [PubMed] [Google Scholar]
- 46.Åberg K., Adkins D.E., Bukszár J., Webb B.T., Caroff S.N., Miller D.D., Sebat J., Stroup S., Fanous A.H., Vladimirov V.I., McClay J.L., Lieberman J.A., Sullivan P.F., van den Oord E.J. Genomewide association study of movement-related adverse antipsychotic effects. Biol. Psychiatry. 2010;67(3):279–282. doi: 10.1016/j.biopsych.2009.08.036. [http:// dx.doi.org/10.1016/j.biopsych.2009.08.036]. [PMID: 19875103]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowley J.J., Kim Y., Szatkiewicz J.P., Pratt A.L., Quackenbush C.R., Adkins D.E., van den Oord E., Bogue M.A., Yang H., Wang W., Threadgill D.W., de Villena F.P., McLeod H.L., Sullivan P.F. Genome-wide association mapping of loci for antipsychotic-induced extrapyramidal symptoms in mice. Mamm. Genome. 2012;23(5-6):322–335. doi: 10.1007/s00335-011-9385-8. [http://dx.doi.org/10.1007/ s00335-011-9385-8]. [PMID: 22207321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakker P.R., Bakker E., Amin N., van Duijn C.M., van Os J., van Harten P.N. Candidate gene-based association study of antipsychotic-induced movement disorders in long-stay psychiatric patients: a prospective study. PLoS One. 2012;7(5):e36561. doi: 10.1371/journal.pone.0036561. [http:// dx.doi.org/10.1371/journal.pone.0036561]. [PMID: 22615781]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broadhurst P.L. Determinants of emotionality in the rat. I. Situational factors. Br. J. Psychol. 1957;48(1):1–12. doi: 10.1111/j.2044-8295.1957.tb00594.x. [http://dx.doi. org/10.1111/j.2044-8295.1957.tb00594.x]. [PMID: 13413179]. [DOI] [PubMed] [Google Scholar]
- 50.Le Moal M., Cardo B., Stinus L. Influence of ventral mesencephalic lesions on various spontaneous and conditional behaviours in the rat. Physiol. Behav. 1969;4:567–574. [http://dx. doi.org/10.1016/0031-9384(69)90156-5]. [Google Scholar]
- 51.Teicher M.H., Klein D.A., Andersen S.L., Wallace P. Development of an animal model of fluoxetine akathisia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1995;19(8):1305–1319. doi: 10.1016/0278-5846(95)00268-5. [http://dx.doi.org/10.1016/0278-5846(95)00268-5]. [PMID: 8868211]. [DOI] [PubMed] [Google Scholar]
- 52.Casey D.E. Extrapyramidal syndromes in nonhuman primates: typical and atypical neuroleptics. Psychopharmacol. Bull. 1991;27(1):47–50. [PMID: 1677773]. [PubMed] [Google Scholar]
- 53.Lublin H., Gerlach J., Mørkeberg F. Long-term treatment with low doses of the D1 antagonist NNC 756 and the D2 antagonist raclopride in monkeys previously exposed to dopamine antagonists. Psychopharmacology (Berl.) 1994;114(3):495–504. doi: 10.1007/BF02249341. [http://dx. doi.org/10.1007/BF02249341]. [PMID: 7855208]. [DOI] [PubMed] [Google Scholar]
- 54.Bruhwyler J., Chleide E., Houbeau G., Waegeneer N., Mercier M. Differentiation of haloperidol and clozapine using a complex operant schedule in the dog. Pharmacol. Biochem. Behav. 1993;44(1):181–189. doi: 10.1016/0091-3057(93)90297-7. [http://dx.doi.org/10.1016/0091-3057(93)90297-7]. [PMID: 8430121]. [DOI] [PubMed] [Google Scholar]
- 55.Sachdev P.S., Brüne M. Animal models of acute drug-induced akathisia - a review. Neurosci. Biobehav. Rev. 2000;24(3):269–277. doi: 10.1016/s0149-7634(99)00069-x. [http://dx.doi.org/10.1016/S0149-7634(99)00069-X]. [PMID: 10781691]. [DOI] [PubMed] [Google Scholar]
- 56.LeMoal M., Stinus L., Galey D. Radiofrequency lesion of the ventral mesencephalic tegmentum: neurological and behavioral considerations. Exp. Neurol. 1976;50(3):521–535. doi: 10.1016/0014-4886(76)90024-8. [http://dx. doi.org/10.1016/0014-4886(76)90024-8]. [PMID: 943305]. [DOI] [PubMed] [Google Scholar]
- 57.Barnes T.R. A rating scale for drug-induced akathisia. Br. J. Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [http://dx.doi.org/10.1192/bjp.154. 5.672]. [PMID: 2574607]. [DOI] [PubMed] [Google Scholar]
- 58.Janno S., Holi M.M., Tuisku K., Wahlbeck K. Actometry and Barnes Akathisia Rating Scale in neuroleptic-induced akathisia. Eur. Neuropsychopharmacol. 2005;15(1):39–41. doi: 10.1016/j.euroneuro.2004.05.003. [http://dx.doi. org/10.1016/j.euroneuro.2004.05.003]. [PMID: 15572272]. [DOI] [PubMed] [Google Scholar]
- 59.Hirschfeld R.M., Keck P.E., Jr, Kramer M., Karcher K., Canuso C., Eerdekens M., Grossman F. Rapid antimanic effect of risperidone monotherapy: a 3-week multicenter, double-blind, placebo-controlled trial. Am. J. Psychiatry. 2004;161(6):1057–1065. doi: 10.1176/appi.ajp.161.6.1057. [http://dx.doi.org/10.1176/appi.ajp.161.6.1057]. [PMID: 15169694]. [DOI] [PubMed] [Google Scholar]
- 60.Forcen F.E. Akathisia: Is restlessness a primary condition or an adverse drug effect? Curr. Psychiatr. 2015;14(1):14–18. [Google Scholar]
- 61.Kane J.M., Barnes T.R., Correll C.U., Sachs G., Buckley P., Eudicone J., McQuade R., Tran Q.V., Pikalov A., III, Assunção-Talbott S. Evaluation of akathisia in patients with schizophrenia, schizoaffective disorder, or bipolar I disorder: a post hoc analysis of pooled data from short- and long-term aripiprazole trials. J. Psychopharmacol. (Oxford) 2010;24(7):1019–1029. doi: 10.1177/0269881109348157. [http://dx. doi.org/10.1177/0269881109348157]. [PMID: 20008446]. [DOI] [PubMed] [Google Scholar]
- 62.Hirose S. The causes of underdiagnosing akathisia. Schizophr. Bull. 2003;29(3):547–558. doi: 10.1093/oxfordjournals.schbul.a007027. [http://dx.doi.org/10.1093/oxfordjournals. schbul.a007027]. [PMID: 14609248]. [DOI] [PubMed] [Google Scholar]
- 63.Lohr J.B., Eidt C.A., Abdulrazzaq A.A., Soliman M.A. The clinical challenges of akathisia. CNS Spectr. 2015;20(Suppl. 1):1–14. doi: 10.1017/S1092852915000838. [http://dx.doi.org/10.1017/S1092852915000838]. [PMID: 26683525]. [DOI] [PubMed] [Google Scholar]
- 64.Lieberman J.A., Stroup T.S., McEvoy J.P., Swartz M.S., Rosenheck R.A., Perkins D.O., Keefe R.S., Davis S.M., Davis C.E., Lebowitz B.D., Severe J., Hsiao J.K. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [http://dx.doi.org/10.1056/ NEJMoa051688]. [PMID: 16172203]. [DOI] [PubMed] [Google Scholar]
- 65.Kasper S., Winkler D. Addressing the limitations of the CATIE study. World J. Biol. Psychiatry. 2006;7(2):126–127. doi: 10.1080/15622970600685424. [http://dx. doi.org/10.1080/15622970600685424]. [PMID: 16684687]. [DOI] [PubMed] [Google Scholar]
- 66.Stip E., Anselmo K. Effectiveness of antipsychotics: is the CATIE trial a tsunami? Can. Fam. Phys. 2007;53(1):97–98. [PMID: 17872617]. [PMC free article] [PubMed] [Google Scholar]
- 67.Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br. J. Psychiatry. 2010;196(2):89–91. doi: 10.1192/bjp.bp.109.070540. [http://dx.doi.org/10.1192/ bjp.bp.109.070540]. [PMID: 20118449]. [DOI] [PubMed] [Google Scholar]
- 68.Poyurovsky M., Pashinian A., Weizman R., Fuchs C., Weizman A. Low-dose mirtazapine: a new option in the treatment of antipsychotic-induced akathisia. A randomized, double-blind, placebo- and propranolol-controlled trial. Biol. Psychiatry. 2006;59(11):1071–1077. doi: 10.1016/j.biopsych.2005.12.007. [http://dx.doi.org/10.1016/j.biopsych.2005.12. 007]. [PMID: 16497273]. [DOI] [PubMed] [Google Scholar]
- 69.Kane J.M., Fleischhacker W.W., Hansen L., Perlis R., Pikalov A., III, Assunção-Talbott S. Akathisia: an updated review focusing on second-generation antipsychotics. J. Clin. Psychiatry. 2009;70(5):627–643. doi: 10.4088/JCP.08r04210. [http://dx.doi.org/10.4088/JCP.08r04210]. [PMID: 19389331]. [DOI] [PubMed] [Google Scholar]
- 70.Stryjer R., Strous R.D., Bar F., Poyurovsky M., Weizman A., Kotler M. Treatment of neuroleptic-induced akathisia with the 5-HT2A antagonist trazodone. Clin. Neuropharmacol. 2003;26(3):137–141. doi: 10.1097/00002826-200305000-00006. [http://dx.doi.org/10.1097/00002826-200305000-00006]. [PMID: 12782915]. [DOI] [PubMed] [Google Scholar]
- 71.Lima A.R., Weiser K.V., Bacaltchuk J., Barnes T.R. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst. Rev. 2004;(1):CD003727. doi: 10.1002/14651858.CD003727.pub2. [PMID: 14974032]. [DOI] [PubMed] [Google Scholar]
- 72.Iqbal N., Lambert T., Masand P. Akathisia: problem of history or concern of today. CNS Spectr. 2007;12(9) Suppl. 14:1–13. doi: 10.1017/s1092852900026201. [http:// dx.doi.org/10.1017/S1092852900026201]. [PMID: 17805218]. [DOI] [PubMed] [Google Scholar]
- 73.Lima A.R., Bacalcthuk J., Barnes T.R., Soares-Weiser K. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst. Rev. 2004;(4):CD001946. doi: 10.1002/14651858.CD001946.pub2. [PMID: 15495022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rathbone J., Soares-Weiser K. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst. Rev. 2006;(4):CD003727. doi: 10.1002/14651858.CD003727.pub3. [PMID: 17054182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baskak B., Atbasoglu E.C., Ozguven H.D., Saka M.C., Gogus A.K. The effectiveness of intramuscular biperiden in acute akathisia: a double-blind, randomized, placebo-controlled study. J. Clin. Psychopharmacol. 2007;27(3):289–294. doi: 10.1097/jcp.0b013e3180582439. [http://dx.doi.org/ 10.1097/jcp.0b013e3180582439]. [PMID: 17502777]. [DOI] [PubMed] [Google Scholar]
- 76.Barnes T.R., McPhillips M.A. Critical analysis and comparison of the side-effect and safety profiles of the new antipsychotics. Br. J. Psychiatry Suppl. 1999;174(38):34–43. [PMID: 10884898]. [PubMed] [Google Scholar]
- 77.Lima A.R., Soares-Weiser K., Bacaltchuk J., Barnes T.R. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst. Rev. 2002;CD001950(1):CD001950. doi: 10.1002/14651858.CD001950. [http://dx. doi.org/10.1002/ 14651858.CD001950]. [PMID: 11869614]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lapa G.B., Mathews T.A., Harp J., Budygin E.A., Jones S.R. Diphenylpyraline, a histamine H1 receptor antagonist, has psychostimulant properties. Eur. J. Pharmacol. 2005;506(3):237–240. doi: 10.1016/j.ejphar.2004.11.017. [http://dx.doi.org/10.1016/j.ejphar.2004.11.017]. [PMID: 15627433]. [DOI] [PubMed] [Google Scholar]
- 79.Tanda G., Kopajtic T.A., Katz J.L. Cocaine-like neurochemical effects of antihistaminic medications. J. Neurochem. 2008;106(1):147–157. doi: 10.1111/j.1471-4159.2008.05361.x. [http://dx.doi.org/10.1111/j.1471-4159.2008.05361.x]. [PMID: 18363822]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oleson E.B., Ferris M.J., España R.A., Harp J., Jones S.R. Effects of the histamine H1 receptor antagonist and benztropine analog diphenylpyraline on dopamine uptake, locomotion and reward. Eur. J. Pharmacol. 2012;683(1-3):161–165. doi: 10.1016/j.ejphar.2012.03.003. [http://dx.doi. org/10.1016/j.ejphar.2012.03.003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vinson D.R., Drotts D.L. Diphenhydramine for the prevention of akathisia induced by prochlorperazine: a randomized, controlled trial. Ann. Emerg. Med. 2001;37(2):125–131. doi: 10.1067/mem.2001.113032. [http://dx.doi.org/ 10.1067/mem.2001.113032]. [PMID: 11174228]. [DOI] [PubMed] [Google Scholar]
- 82.Simons F.E. Advances in H1-antihistamines. N. Engl. J. Med. 2004;351(21):2203–2217. doi: 10.1056/NEJMra033121. [http://dx.doi.org/10.1056/NEJMra033121]. [PMID: 15548781]. [DOI] [PubMed] [Google Scholar]
- 83.Simons F.E., Akdis C.A. Histamine and antihistamines. Middleton’s allergy principles and practice. St Louis, MO: Mosby, Inc; 2008. pp. 1517–1547. [an affiliate of Elsevier Science] [Google Scholar]
- 84.Stryjer R., Rosenzcwaig S., Bar F., Ulman A.M., Weizman A., Spivak B. Trazodone for the treatment of neuroleptic-induced acute akathisia: a placebo-controlled, double-blind, crossover study. Clin. Neuropharmacol. 2010;33(5):219–222. doi: 10.1097/WNF.0b013e3181ee7f63. [http://dx.doi.org/10. 1097/WNF.0b013e3181ee7f63]. [DOI] [PubMed] [Google Scholar]
- 85.Poyurovsky M., Fuchs C., Weizman A. Low-dose mianserin in treatment of acute neuroleptic-induced akathisia. J. Clin. Psychopharmacol. 1998;18(3):253–254. doi: 10.1097/00004714-199806000-00013. [http://dx.doi.org/10.1097/ 00004714-199806000-00013]. [PMID: 9617987]. [DOI] [PubMed] [Google Scholar]
- 86.Kumar R., Sachdev P.S. Akathisia and second-generation antipsychotic drugs. Curr. Opin. Psychiatry. 2009;22(3):293–299. doi: 10.1097/yco.0b013e32832a16da. [http://dx.doi.org/10.1097/YCO.0b013e32832a16da]. [PMID: 19378382]. [DOI] [PubMed] [Google Scholar]
- 87.Avital A., Gross-Isseroff R., Stryjer R., Hermesh H., Weizman A., Shiloh R. Zolmitriptan compared to propranolol in the treatment of acute neuroleptic-induced akathisia: a comparative double-blind study. Eur. Neuropsychopharmacol. 2009;19(7):476–482. doi: 10.1016/j.euroneuro.2009.02.006. [http://dx.doi.org/10.1016/j.euroneuro.2009.02.006]. [PMID: 19342206]. [DOI] [PubMed] [Google Scholar]
- 88.Blaisdell G.D. Akathisia: a comprehensive review and treatment summary. Pharmacopsychiatry. 1994;27(4):139–146. doi: 10.1055/s-2007-1014294. [http://dx. doi.org/10.1055/s-2007-1014294]. [PMID: 7972345]. [DOI] [PubMed] [Google Scholar]
- 89.Hettema J.M., Ross D.E. A case of aripiprazole-related tardive akathisia and its treatment with ropinirole. J. Clin. Psychiatry. 2007;68(11):1814–1815. doi: 10.4088/jcp.v68n1124e. [http://dx.doi.org/10.4088/JCP.v68n1124e]. [PMID: 18052582]. [DOI] [PubMed] [Google Scholar]
- 90.Garcia-Borreguero D., Larrosa O., Williams A.M., Albares J., Pascual M., Palacios J.C., Fernandez C. Treatment of restless legs syndrome with pregabalin: a double-blind, placebo-controlled study. Neurology. 2010;74(23):1897–1904. doi: 10.1212/WNL.0b013e3181e1ce73. [http://dx.doi.org/10. 1212/WNL.0b013e3181e1ce73]. [PMID: 20427750]. [DOI] [PubMed] [Google Scholar]
- 91.Pfeffer G., Chouinard G., Margolese H.C. Gabapentin in the treatment of antipsychotic-induced akathisia in schizophrenia. Int. Clin. Psychopharmacol. 2005;20(3):179–181. doi: 10.1097/00004850-200505000-00011. [http://dx.doi.org/ 10.1097/00004850-200505000-00011]. [PMID: 15812271]. [DOI] [PubMed] [Google Scholar]
- 92.De Berardis D., Serroni N., Moschetta F.S., Martinotti G., Di Giannantonio M. Reversal of aripiprazole-induced tardive akathisia by addition of pregabalin. J. Neuropsychiatry Clin. Neurosci. 2013;25(2):E9–E10. doi: 10.1176/appi.neuropsych.12030069. [http://dx.doi.org/10.1176/appi. neuropsych.12030069]. [PMID: 23686043]. [DOI] [PubMed] [Google Scholar]
- 93.Dolphin A.C. Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond. Nat. Rev. Neurosci. 2012;13(8):542–555. doi: 10.1038/nrn3311. [http://dx.doi.org/10.1038/nrn3311. PMID 22805911]. [DOI] [PubMed] [Google Scholar]
- 94.Kukkar A., Bali A., Singh N., Jaggi A.S. Implications and mechanism of action of gabapentin in neuropathic pain. Arch. Pharm. Res. 2013;36(3):237–251. doi: 10.1007/s12272-013-0057-y. [http://dx.doi.org/10.1007/ s12272-013-0057-y]. [PMID: 23435945]. [DOI] [PubMed] [Google Scholar]
- 95.Hendrich J., Van Minh A.T., Heblich F., Nieto-Rostro M., Watschinger K., Striessnig J., Wratten J., Davies A., Dolphin A.C. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc. Natl. Acad. Sci. USA. 2008;105(9):3628–3633. doi: 10.1073/pnas.0708930105. [http://dx.doi.org/10.1073/pnas.0708930105]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eroglu C., Allen N.J., Susman M.W., O’Rourke N.A., Park C.Y., Ozkan E., Chakraborty C., Mulinyawe S.B., Annis D.S., Huberman A.D., Green E.M., Lawler J., Dolmetsch R., Garcia K.C., Smith S.J., Luo Z.D., Rosenthal A., Mosher D.F., Barres B.A. Gabapentin pharmacotherapy for antipsychotic-induced akathisia: single-patient experiment and case report. Ther. Adv. Psychopharmacol. 2014;4(2):100–102. doi: 10.1177/2045125314525433. [http://dx.doi.org/10.1177/ 2045125314525433]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sullivan M.A., Wilbur R. Gabapentin pharmacotherapy for antipsychotic-induced akathisia: single-patient experiment and case report. Ther. Adv. Psychopharmacol. 2014;4(2):100–102. doi: 10.1177/2045125314525433. [http://dx.doi.org/10.1177/2045125314525433]. [PMID: 24688760]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lerner V., Bergman J., Statsenko N., Miodownik C. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry. 2004;65(11):1550–1554. doi: 10.4088/jcp.v65n1118. [http://dx.doi.org/10.4088/JCP.v65n1118]. [PMID: 15554771]. [DOI] [PubMed] [Google Scholar]
- 99.Miodownik C., Lerner V., Statsenko N., Dwolatzky T., Nemets B., Berzak E., Bergman J. Vitamin B6 versus mianserin and placebo in acute neuroleptic-induced akathisia: a randomized, double-blind, controlled study. Clin. Neuropharmacol. 2006;29(2):68–72. doi: 10.1097/00002826-200603000-00002. [http://dx.doi.org/10.1097/00002826-200603000-00002]. [PMID: 16614537]. [DOI] [PubMed] [Google Scholar]
- 100.Berk M., Copolov D., Dean O., Lu K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Judd F., Katz F., Katz P., Ording-Jespersen S., Little J., Conus P., Cuenod M., Do K.Q., Bush A.I. N-acetyl cysteine as a glutathione precursor for schizophreniaa double-blind, randomized, placebo-controlled trial. Biol. Psychiatry. 2008;64(5):361–368. doi: 10.1016/j.biopsych.2008.03.004. [http://dx.doi.org/10.1016/j. biopsych.2008.03.004]. [PMID: 18436195]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publishers Web site along with the published article.