Abstract
Introduction:
The aim of the present study was to evaluate whether there are changes in cardiovascular risk factors among noise-exposed workers and to explore the possible mechanisms of a long-term noise exposure leading to cardiovascular disease and the sex differences of cardiovascular risk factors in this population.
Materials and Methods:
Two hundred workers engaged in noise-related work, and a control group of 200 nonnoise-exposed workers hospitalized for occupational health examination were assigned into the study. All workers underwent a medical examination, electrocardiogram recording, blood pressure test, other blood tests, and audiometry. The collected blood was used to detect homocysteine (HCY), renin, angiotensin II, and other markers of cardiovascular risk factors.
Results:
Our study suggests that the type of work with long-term exposure to noise might pose a cardiovascular risk, as evidenced by associated increases in plasma HCY levels, incidence of type 2 diabetes, and incidence of hypertension.
Discussion:
Our research also reveals that among male workers, the levels of triglycerides, uric acid, HCY, renin activity, and the incidence of hypertension are higher than female, while high-density lipoprotein cholesterol is lower than female workers had. Additionally, the study emphasizes again the importance of weight control for reducing cardiovascular risk.
Conclusion:
Our study suggests that noise is a cardiovascular risk factor. Interventions in the work environment could be a preventable and controllable manner for reducing the incidence of cardiovascular disease.
Keywords: Cardiovascular risk factors, homocysteine, hypertension, noise
Introduction
Cardiovascular disease (CVD) remains the major cause of morbidity and death among all racial and ethnic groups.[1] CVDs are a group of disorders of the heart and blood vessels and they include the following diseases: coronary heart disease (CHD), cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, deep vein thrombosis, and pulmonary embolism. In recent years, cardiovascular risk factors continue to expand, in addition to traditional risk factors including age, gender, total cholesterol, high density lipoprotein cholesterol, high blood pressure, blood pressure treatment condition, diabetes, and current smoking status; there are also constantly new cardiovascular risk factors, such as low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), hypersensitive C-reactive protein (hs-CRP), total cholesterol (TC)/high-density lipoprotein cholesterol (HDL-C), body mass index (BMI), HbA1c, family history, social deprivation, and so on.[2,3] Recently, elevated plasma levels of the toxic sulfur-containing amino acid homocysteine (HCY) have received a great deal of interest, primarily due to its prevalence in the general population and strong correlation with the development of a number of cardiovascular related diseases.[4]
Noise is an important occupational hazard in workplace environments in China. The role of noise as an environmental pollutant and its impact on health are being increasingly recognized.[5,6] The association between noise exposure and auditory effects is well documented in the biomedical literature, but the same is not true about extra-auditory effects.[7] Accordingly, the impact of long-term exposure to environmental noise on CVDs is now drawing increasing interests in related areas around the world.[8,9,10]
Evidence of the nonauditory effects of environmental noise exposure on public health is growing. Long-term exposures to noise have been associated with CVDs such as ischemic heart disease and hypertension.[11,12,13] It has been shown that noise causes not only hypertension, myocardial infarction, and stroke but also damage to vasculature.[6,14,15] According to Taylor, Münzel found that in healthy young adults, endothelial function becomes worse after noise exposure, and thus, may provide a link between noise events and the underlying mechanism causing arterial hypertension.[16]
The mechanism for the vascular damage appears to be through non-auditory effects. Noise causes annoyance, anger, or sleep deprivation, and this activates stress hormones.[17,18,19] The result is increased catecholamine and cortisol levels, high blood pressure, and higher heart rate. Together, these factors activate the endothelium so that it produces little nitric oxide and more superoxide, leading to endothelial dysfunction and may, thus, be the one mechanism contributing to the observed association of chronic noise exposure with CVD.[16] Furthermore, acute noise exposure, in both laboratory settings, where traffic noise was simulated and in real-life environments, can cause increases in blood pressure, heart rate, and cardiac output, likely mediated by the release of stress hormones such as catecholamines.[13,20] As shown by field studies, these acute effects occur not only at high sound levels in occupational settings but also at relatively low environmental noise levels when concentration, relaxation, or sleep is disturbed.[21]
Recently, several studies have been conducted on relationships between noise exposure and cardiovascular pathology.[22,23] In clinical practice, it was observed that many young noise workers suffer from high blood pressure or diabetes, which made us think whether such a result has a correlation with long-term adverse noise environmental irritation? We hypothesized that noise exposure would lead to increased levels of cardiovascular risk markers in the blood. We also expect an association of noise with an increased incidence of type 2 diabetes, hypertension, and electrocardiogram (ECG) abnormalities. Therefore, in this study, we focused on a comprehensive analysis of detection index of cardiovascular risk factors in noise-exposed workers; aimed to evaluate whether the category of occupational population is at risk of developing CVDs; and explored the possible mechanisms of long-term noise exposure leading to CVD.
Materials and Methods
All studies were approved by the Institutional Ethics Committee (Approval number: GDHOD MEC2016006), and written informed consent was obtained prior to the examination. All participants agreed to the processing of personal data, and agreed that results from the protocol were treated anonymously and collectively, with methods and purposes in accordance with the scientific principles of the Declaration of Helsinki.
In this study, we evaluated whether chronic exposure to noise can affect cardiovascular risk-associated markers in the blood in 200 exposed workers compared to a group of 200 nonexposed workers, then we further analyzed the discrepancy of cardiovascular risk factors at different genders and the correlation between body weight and cardiovascular risk factors.
Study design and population
The 200 cases of workers engaged in noise-related work and suffering from varying degrees of noise-induced hearing loss constituted the study population. Two hundred cases of non-noise-exposed workers during the same period, hospitalized for occupational health examination, were considered the control group. The proportion of male and female was adjudicated according to real-life situations, and participants with ages ranged between 18 and 60 years were included. The inclusion criteria and exclusion criteria are shown in Figure 1.
Figure 1.
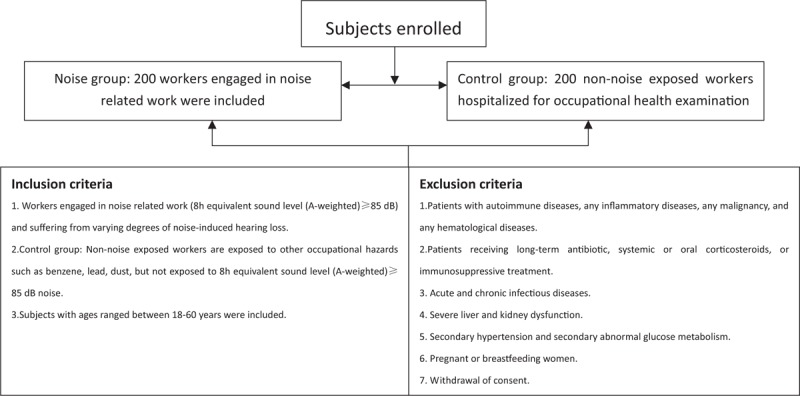
Inclusion and exclusion criteria
Noise exposure in this study is defined as workplace noise intensity exceeding “the occupation exposure limits of workplace harmful factors” according to Occupational Disease Prevention Law of the People’s Republic of China, which corresponds to 8 h equivalent sound level (A-weighted) ≥85 dB. Another 200 cases of non-exposed workers were enrolled who were hospitalized for occupational health examination during the same period of time. These workers are exposed to other occupational hazards such as benzene, lead, dust, but not 8h equivalent sound level (A-weighted) ≥85 dB noise. With regard to benzene, lead, dust, low dosages obtained from the environmental sample, and the use of personal protective equipment greatly reduces the chances of it causing cardiovascular effects. Therefore, the grouping criteria were based solely on the presence or absence of long-term noise exposure.
The present study focused on patients with a clear history of noise exposure, through a comprehensive analysis of cardiovascular risk factors, abnormal ECG, and the incidence of type 2 diabetes and hypertension, to investigate the potential mechanism of long-term environmental noise exposure leading to CVD, then further analyzed the discrepancy of cardiovascular risk factors at different genders and the correlation between body weight and cardiovascular risk factors.
Anthropometric measurements and biochemical analysis
All participants underwent medical examination, starting with body weight evaluation. Blood pressure was then measured in the supine position with a mercury sphygmomanometer.
To avoid situations that could cause artificial variations of blood pressure and other parameters, the workers were asked to fast for 10 h, rest for at least 15 min, and to refrain from smoking for at least 15 min before the medical examination. Up to three measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were taken from participants. When making a diagnosis of hypertension, it is recommended[24] to measure at least 3 consecutive days in a week and one to three measurements in each session in the morning and evening. The flowchart of experimental design of this study is depicted in Figure 2. A blood sample was taken by a trained nurse for subsequent assessment of blood markers, and participants provided information about their health status and drug intake. Fresh blood serum and plasma samples were centrifuged and stored in a cold box at approximately 4°C – until they were frozen at −80°C – after a maximum of 2 h. The collected blood was used to detect blood markers of cardiovascular risk factors including fasting plasma glucose (FPG), blood lipids, HCY, uric acid (UA), and hypertension related indicators: angiotensin II, renin activity, and aldosterone.
Figure 2.
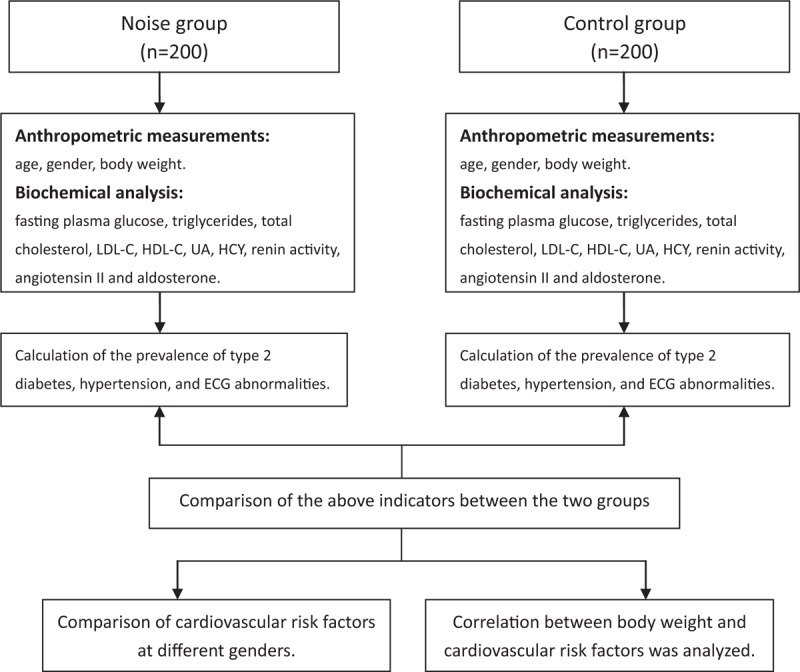
Flowchart of experimental design
All of the above were measured by routine methods, to perform the following examinations: blood glucose [System of International Units (SI) 3.4–6.1 mmol/L], total cholesterol (SI <6.1 mmol/L), triglycerides (SI <1.8 mmol/L), LDL-C (SI <4.11 mmol/L), HDL-C (SI >0.85 mmol/L), UA (SI <450 μmol/L), HCY (SI <15 μmol/L), renin activity (SI 0.15–2.33 ng/mL/h), angiotensin II (SI 32.00–90.00 pg/mL), and aldosterone (SI 40.00–310.00 pg/mL).
Exposed workers included in the study were compared with control participants who had not been exposed, by age, gender, body weight, FPG, triglycerides, total cholesterol, LDL-C, HDL-C, UA, renin activity, angiotensin II and aldosterone.
Hypertension diagnostic criteria: according to the “Chinese Hypertension Prevention Guide 2010”.[24] Diagnostic criteria set at SBP ≥140 mmHg (1 mmHg = 0.133 kPa), and (or) DBP ≥90 mmHg. The diagnosis of diabetes was according to American Diabetes Association. Diagnostic criteria: FPG ≥126 mg/dL (7.0 mmol/L), or 2-h plasma glucose ≥200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test (OGTT), or HbA1c (Hemoglobin A1c), A1C ≥6.5% (48 mmol/mol), or in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥200 mg/dL (11.1 mmol/L).[25]
All workers underwent ECG recording and electrocardiographic abnormalities were classified as follows: left axis deviation for fascicular disorder, disorders of ventricular repolarization, grade I atrioventricular conduction disturbances, right and left intraventricular conduction disturbances, atrial and ventricular arrhythmias (sporadic and multiple), serious disturbances of rhythm (bigeminal rhythm due to ventricular extrasystoles), left or right ventricular effort, II and III level atrioventricular block, coronary insufficiency, and previous myocardial necrosis.[26]
The discrepancy of cardiovascular risk factors at different genders and the correlation between body weight and cardiovascular risk factors were also evaluated.
Statistical analysis
Data were expressed as the mean ± standard deviation. Data analysis was performed using the Statistical Package for the Social Sciences software (SPSS Inc., Illinois, USA). The difference between mean values was evaluated using Student t-test for unpaired data, for hypothesis testing on the mean of the population. Statistical methods also included descriptive analysis and correlation analysis to explore the role of related influencing factors. Differences were considered significant when P was <0.05.
Results
Influence of noise on homocysteine levels and cardiovascular disease
To investigate health effects of exposure to noise, we took measurements of cardiovascular risk factors on workers with a clear history of noise exposure. In the group exposed to noise, elevated blood HCY, increased incidence of type 2 diabetes, and hypertension were observed [Figure 3], while the ECG abnormality was no significant difference compared with the control group [Figure 3]. HCY reference in our labs was <15.00 μmol/L. It was significantly increased in noise exposure group (15.12 ± 7.77 μmol/L) compared with controls (13.00 ± 2.43 μmol/L). In addition, noise exposure time was significantly associated with renin activity, while controlled for age, gender and other variables. Pearson correlation coefficient was = 0.201, data not shown. Influence of noise on cardiovascular risk factors and CVD are shown in Table 1 and Figure 3.
Figure 3.
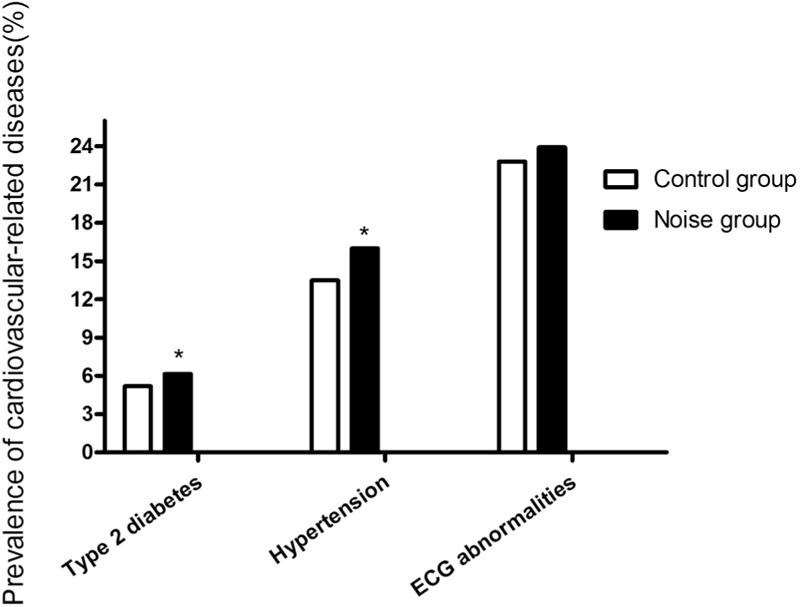
The prevalence of type 2 diabetes, hypertension, and ECG abnormalities. *P < 0.05 versus the control group.
Table 1.
Influence of noise on cardiovascular risk factors
| Variable | Noise group (n = 200) | Control group (n = 200) | P-value |
|---|---|---|---|
| Gender [n (%)] | 0.476 | ||
| Female | 51 (25.5%) | 42 (21%) | |
| Male | 149 (74.5%) | 158 (79%) | |
| Age [years (min–max)] | 39.69 ± 7.49 (22–56) | 39.74 ± 5.33 (20–62) | 0.173 |
| Body weight (kg) | 65.71 ± 10.93 | 64.15 ± 6.22 | 0.437 |
| FPG (mmol/L) | 4.68 ± 1.14 | 4.67 ± 0.44 | 0.633 |
| Triglycerides (mmol/L) | 1.56 ± 1.07 | 1.74 ± 2.01 | 0.821 |
| Total cholesterol (mmol/L) | 4.80 ± 0.89 | 5.11 ± 1.29 | 0.674 |
| LDL-C (mmol/L) | 2.96 ± 0.73 | 3.11 ± 0.75 | 0.218 |
| HDL-C (mmol/L) | 1.13 ± 0.28 | 1.21 ± 0.28 | 0.180 |
| UA (μmol/L) | 328.07 ± 97.46 | 345.66 ± 81.93 | 0.718 |
| Renin activity (PRA) (ng/mL/h) | 1.55 ± 1.47 | 1.71 ± 0.10 | 0.880 |
| Angiotensin II (pg/mL) | 60.75 ± 18.23 | 66.69 ± 3.83 | 0.647 |
| ALD (pg/mL) | 155.67 ± 136.90 | 278.88 ± 215.45 | 0.211 |
| HCY (μmol/L) | 15.12 ± 7.77* | 13.00 ± 2.43 | 0.012 |
FPG = fasting plasma glucose, HCY = homocysteine, LDL-C = low-density lipoprotein cholesterol, HDL-C = high-density lipoprotein cholesterol, UA = uric acid, ALD = aldosterone. Data are presented as the mean ± SD and number (%). *P < 0.05 versus the control group.
Analysis of gender-related differences
In this research, we were interested in the discrepancy of cardiovascular risk factors at different genders. To explore the relationship between gender and cardiovascular risk factors in these workers, participants were divided into male and female groups using independent samples t-test. Results obtained between gender and cardiovascular risk factors showed: the levels of triglycerides, UA, HCY, renin activity and the incidence of hypertension in male workers is higher than female, while high-density lipoprotein cholesterol is lower than female. The fasting blood glucose, total cholesterol, low-density lipoprotein cholesterol, angiotensin II, aldosterone and the incidence of type 2 diabetes, abnormal ECG showed no significant differences between the sexes (P > 0.05). Data are shown in Table 2.
Table 2.
Differences in cardiovascular risk factors among different genders
| Variable | Male (n = 307) | Female (n = 93) | P-value |
|---|---|---|---|
| Age [years (min–max)] | 40.76 ± 8.97 (21–60) | 40.44 ± 9.20 (20–62) | 0.820 |
| Body weight (kg) | 67.35 ± 9.81** | 54.81 ± 8.89 | <0.001 |
| Systolic blood pressure (mmHg) | 125.07 ± 13.25** | 116.53 ± 16.59 | 0.001 |
| Diastolic blood pressure (mmHg) | 77.17 ± 11.35* | 72.78 ± 10.48 | 0.032 |
| FPG (mmol/L) | 4.73 ± 0.95 | 4.85 ± 1.09 | 0.440 |
| Triglycerides (mmol/L) | 1.62 ± 1.22** | 1.02 ± 0.41 | <0.001 |
| Total cholesterol (mmol/L) | 4.85 ± 0.97 | 4.58 ± 0.91 | 0.085 |
| LDL-C (mmol/L) | 2.96 ± 0.76 | 2.83 ± 0.75 | 0.254 |
| HDL-C (mmol/L) | 1.15 ± 0.28** | 1.29 ± 0.31 | 0.002 |
| UA (μmol/L) | 338.32 ± 85.83** | 232.62 ± 62.97 | <0.001 |
| HCY (μmol/L) | 15.47 ± 9.77* | 11.35 ± 3.62 | 0.035 |
| Renin activity (PRA) (ng/mL/h) | 1.65 ± 1.55** | 1.08 ± 0.60 | 0.004 |
| Angiotensin II (pg/mL) | 61.94 ± 17.63 | 56.09 ± 19.83 | 0.155 |
| Aldosterone (ALD) (pg/mL) | 164.19 ± 143.90 | 118.51 ± 85.51 | 0.144 |
| Type 2 diabetes [n (%)] | 17 (5.53%) | 5 (5.37%) | 0.304 |
| Hypertension [n (%)] | 47 (15.3%)* | 10 (10.75%) | 0.012 |
| ECG abnormalities [n (%)] | 72 (23.45%) | 19 (20.43%) | 0.097 |
Data are presented as the mean ± SD and number (%). *P < 0.05, **P < 0.01 versus female group.
Correlation between body weight and cardiovascular risk factors
In addition, we tested whether body mass can influence some biomarkers of cardiovascular risk. Correlation analysis of body weight and cardiovascular risk factors suggested that in this study population, body weight was significantly associated with the concentration of triglycerides, high density lipoprotein cholesterol, UA, blood pressure, and the incidence of hypertension (P < 0.01); but there was no significant correlation between total cholesterol, low density lipoprotein cholesterol and body weight (P > 0.05). Data are shown in Table 3.
Table 3.
Correlation between body weight and cardiovascular risk related factors
| Triglycerides (mmol/L) | Total cholesterol (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) | |
|---|---|---|---|---|
| Pearson correlation coefficients | 0.482 | 0.156 | 0.044 | −0.452 |
| P-value | <0.001 | 0.064 | 0.603 | <0.001 |
| UA (umol/L) | Systolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | Hypertension (0 = N, 1 = Y) | |
| Pearson correlation coefficients | 0.505 | 0.387 | 0.343 | 0.252 |
| P-value | <0.001 | <0.001 | <0.001 | 0.003 |
Discussion
The main results that our study has produced are the following: in workers of long-term exposure to noise, the blood indexes related to cardiovascular risk factors were higher than in the control group, in particular, the elevated level of blood HCY, increased incidence of type 2 diabetes and hypertension, while the ECG abnormality was found similar as compared to the control group. Our research found that noise exposure period was significantly associated with renin activity. The triglycerides, UA, HCY, renin activity, and the incidence of hypertension in male workers are higher than female, while high-density lipoprotein cholesterol is lower than female. There was a significant association between body weight with triglycerides, high-density lipoprotein cholesterol, UA, and hypertension in the present study population.
Elevated plasma HCY concentration is considered a risk factor for CVD and may also be associated with hypertension.[27] A number of epidemiologic studies[28,29,30] show that moderately elevated plasma HCY levels are highly prevalent in the general population and are associated with an increased risk for fatal and nonfatal CVD, independent of the classic cardiovascular risk factors.[30] Over the past 40 years, there have been more than 100 clinical studies that have unequivocally established the relationship between HCY and atherosclerotic vascular diseases.[31,32] Although links have been established between hyperhomocysteinemia and elevated risk for CVD, plasma HCY is affected by health-related behaviors, including diet, smoking, and sedentary lifestyle.[33,34] Genetic factors also contribute to plasma HCY.[27] Our results suggest that in long-term exposure to noise workers, the blood HCY was elevated. This may indicate the existence of a relevance of noise, elevated blood HCY, and CVD or hypertension.
Although the mechanism of the action by which chronic exposure to noise affects the cardiovascular system has not been well studied and documented in literature, it can be assumed that noise causes a chronic sympathetic activation. This event causes a high initial catecholamine release, followed by catecholamine depletion and/or reduced catecholamine response, also due to β-receptor desensitization, with a reduced tissue density which is reversible for short time.[35,36] From the analysis of our results, noise exposure time was significantly associated with renin activity but was controlled for age, gender, and other variables. The Pearson correlation coefficient was <0.5, which means that the variable were not closely related, but it still represents the potential evidence that chronic noise exposure may give rise to physiological effects in terms of raised blood pressure.
Body weight is closely related to several known cardiovascular risk factors, but it may also have an independent effect on the risk of CHD.[37,38,39] BMI is the most commonly used indicator of obesity in population studies. It seems that increased central or visceral fat, independent of relative body weight, is associated with a variety of metabolic disorders and increased cardiovascular mortality. Furthermore, weight is usually positively related to increased morbidity and mortality, whereas height is often associated with good health.[37] Therefore, among obese participants, BMI can reflect the negative effects of both fatness and shortness. But the risks of fatness and shortness are most likely mediated via different mechanisms. Some studies suggested that serum cholesterol and both diastolic and systolic BPs had a positive association with BMI.[37] In the current study, we also tested whether body mass may influence some cardiovascular risk factors in the blood. Correlation analysis of body weight and cardiovascular risk factors suggested that body weight, not the BMI, was significantly associated with triglycerides, high density lipoprotein cholesterol, UA, SBP, DBP, and hypertension incidence, but there was no significant correlation between total cholesterol, low density lipoprotein cholesterol and body weight. These results are, in part, consistent with previous studies[38,39] involving BMI and cardiovascular risk factors, but also indicate a potential difference in body weight and BMI for the evaluation of cardiovascular risk research.
In adults, age-related changes in structural and functional arterial properties have been well described and are jointly known as vascular aging.[40] These changes include arterial stiffening, luminal dilatation, and wall thickening and are known to have a heterogeneous distribution between central and peripheral arteries.[41,42,43] In contrast, the existence of gender-related differences in arterial properties and cardiovascular risk is a well-known and well-described fact for adults.[44,45,46,47] In this context, we tried to identify the presence of gender-related differences in cardiovascular risk factors. We found that the triglycerides, UA, HCY, renin activity, and the incidence of hypertension in male workers are higher than what female participants had, while high-density lipoprotein cholesterol is lower than female. No gender differences existed in noise exposure intensity and age, where the expected physiological differences determined that males were significantly heavier than females. Therefore, these findings revealed that there are gender differences in blood indicators of cardiovascular risk factors. However, the normal reference values which we normally use does not reflect gender differences, thus may lead to some deficiencies in our clinical evaluation process of patients, at least in a small portion of Chinese population. Moreover, these results may indicate the need for setting different reference values of cardiovascular risk factors for different gender, and future studies of large randomized controlled trials should be designed.In addition to lifestyle changes, there are uncontrollable factors, namely age (aging), gender, race, family history, etc, for the incidence of CVDs. Evidence from epidemiologic studies[48,49,50,51,52,53] demonstrates that environmental noise is associated with an increased incidence of arterial hypertension, myocardial infarction, and stroke. Hearing impairments due to noise are a direct consequence of the effects of sound energy on the inner ear.[54] It is well known that noise may seriously damage hearing. However, its role in arterial hypertension and CVDs has only been noticed recently. The majority of authors found that noise causes an increase in heart rate, blood pressure, and ECG abnormalities.[22,26,55,56] Münzel[19] wanted to discover whether night time aircraft noise impaired vascular function. He found that in healthy young adults, endothelial function becomes worse after noise exposure. Additionally, Dr. Münzel hopes that the European Society of Cardiology takes into account these new findings concerning noise and CVD to help establish noise as a significant cardiovascular risk factor.[16] Our study suggests that noise is a cardiovascular risk factor. It can be assumed that changes, both hearing and cardiovascular, are related to levels of noise and exposure time. Interventions in the work environment could be a preventable and controllable manner for reducing the incidence of CVD. Using appropriate ways to monitor and treat cardiovascular risk factors in noise workers and giving them primary prevention recommendations might have important meaning for the prevention and treatment of noise (environmental factors)-associated CVD.
Limitations of the study
There are some limitations to the study, including the fact that it did not include the sedentary office workers or the unemployed, and, therefore, it may not be representative of the general population. Second, the sample size of our study was relatively small, and although several findings were statistically significant, some real differences could have been missed. Third, there are differences between the work of the control group and the selected workers investigated, and possible differences in socioeconomic status. Finally, in view of applicability, for workers underwent medical examination, BMI, rather than only body weight, should be used as an index of health status. However, this precisely revealed a potential difference between body weight and BMI for the evaluation of cardiovascular risk research, which we always wanted to know.
Conclusions
This project, by studying the relationship between long-term environmental noise exposure and cardiovascular risk factors, aimed to clarify the influence of environmental factors on CVD and revealed the potential pathogenic mechanism of adverse environmental noise. In conclusion, our findings suggest that exposure to noise at the workplace might pose a cardiovascular health risk, as evidenced by associated increases in HCY, and the incidence of type 2 diabetes and hypertension. It is, therefore, important to make efforts to reduce these exposures. For this reason, it is necessary to educate workers with proper information and training on more effective prevention techniques that aim to reduce levels of noise in the working environment. On the one hand, this study may also serve to increase workers’ awareness about the occupational effects on health. On the other hand, our research also reveals the presence of gender-related differences in cardiovascular risk factors and emphasizes the importance of weight control for reducing cardiovascular risk. However, we must admit that the results found are in line with the literature but are not novel.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular diseases: A historical perspective. Lancet. 2014;383:999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kavousi M, Leening MJ, Nanchen D, Greenland P, Graham IM, Steyerberg EW, et al. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA. 2014;311:1416–23. doi: 10.1001/jama.2014.2632. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Colantonio LD, Cushman M, Goff DC, Jr, Howard G, Howard VJ, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–15. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal. 2011;15:1927–43. doi: 10.1089/ars.2010.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M, et al. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin Res Cardiol. 2015;104:23–30. doi: 10.1007/s00392-014-0751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Münzel T, Gori T, Babisch W, Basner M. Cardiovascular effects of environmental noise exposure. Eur Heart J. 2014;35:829–36. doi: 10.1093/eurheartj/ehu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Souza TC, Périssé AR, Moura M. Noise exposure and hypertension: Investigation of a silent relationship. BMC Public Health. 2015;15:328. doi: 10.1186/s12889-015-1671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halonen JI, Hansell AL, Gulliver J, Morley D, Blangiardo M, Fecht D, et al. Road traffic noise is associated with increased cardiovascular morbidity and mortality and all-cause mortality in London. Eur Heart J. 2015;36:2653–61. doi: 10.1093/eurheartj/ehv216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stansfeld S, Clark C. Health effects of noise exposure in children. Curr Environ Health Rep. 2015;2:171–8. doi: 10.1007/s40572-015-0044-1. [DOI] [PubMed] [Google Scholar]
- 10.Swinburn TK, Hammer MS, Neitzel RL. Valuing quiet: An economic assessment of U.S. Environmental Noise as a cardiovascular health hazard. Am J Prev Med. 2015;49:345–53. doi: 10.1016/j.amepre.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 12.Babisch W, Wolf K, Petz M, Heinrich J, Cyrys J, Peters A. Associations between traffic noise, particulate air pollution, hypertension, and isolated systolic hypertension in adults: The KORA study. Environ Health Perspect. 2014;122:492–8. doi: 10.1289/ehp.1306981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babisch W. Cardiovascular effects of noise. Noise Health. 2011;13:201–4. doi: 10.4103/1463-1741.80148. [DOI] [PubMed] [Google Scholar]
- 14.Floud S, Blangiardo M, Clark C, de Hoogh K, Babisch W, Houthuijs D, et al. Exposure to aircraft and road traffic noise and associations with heart disease and stroke in six European countries: A cross-sectional study. Environ Health. 2013;12:89. doi: 10.1186/1476-069X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sørensen M, Hvidberg M, Andersen ZJ, Nordsborg RB, Lillelund KG, Jakobsen J, et al. Road traffic noise and stroke: A prospective cohort study. Eur Heart J. 2011;32:737–44. doi: 10.1093/eurheartj/ehq466. [DOI] [PubMed] [Google Scholar]
- 16.Taylor J. Noise: A new cardiovascular risk factor. Eur Heart J. 2014;35:821–2. doi: 10.1093/eurheartj/ehu089. [DOI] [PubMed] [Google Scholar]
- 17.Miedema HM, Oudshoorn CG. Annoyance from transportation noise: Relationships with exposure metrics DNL and DENL and their confidence intervals. Environ Health Perspect. 2001;109:409–16. doi: 10.1289/ehp.01109409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muzet A. Environmental noise, sleep and health. Sleep Med Rev. 2007;11:135–42. doi: 10.1016/j.smrv.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt FP, Basner M, Kröger G, Weck S, Schnorbus B, Muttray A, et al. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J. 2013;34:3508–14a. doi: 10.1093/eurheartj/eht269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babisch W. Stress hormones in the research on cardiovascular effects of noise. Noise Health. 2003;5:1–11. [PubMed] [Google Scholar]
- 21.Basner M, Samel A, Isermann U. Aircraft noise effects on sleep: Application of the results of a large polysomnographic field study. J Acoust Soc Am. 2006;119:2772–84. doi: 10.1121/1.2184247. [DOI] [PubMed] [Google Scholar]
- 22.Aydin Y, Kaltenbach M. Noise perception, heart rate and blood pressure in relation to aircraft noise in the vicinity of the Frankfurt airport. Clin Res Cardiol. 2007;96:347–58. doi: 10.1007/s00392-007-0507-y. [DOI] [PubMed] [Google Scholar]
- 23.Raggam RB, Cik M, Höldrich RR, Fallast K, Gallasch E, Fend M, et al. Personal noise ranking of road traffic: Subjective estimation versus physiological parameters under laboratory conditions. Int J Hyg Environ Health. 2007;210:97–105. doi: 10.1016/j.ijheh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Liu LS. Guidelines for prevention and treatment of hypertension in China 2010. Chin J Hypertens. 2011;19:701–43. [Google Scholar]
- 25.American Diabetes Association. Standards of medical care in diabetes – 2016 abridged for primary care providers. Clin Diabetes. 2016;34:3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assunta C, Ilaria S, Simone de S, Gianfranco T, Teodorico C, Carmina S, et al. Noise and cardiovascular effects in workers of the sanitary fixtures industry. Int J Hyg Environ Health. 2015;218:163–8. doi: 10.1016/j.ijheh.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Dinavahi R, Falkner B. Relationship of homocysteine with cardiovascular disease and blood pressure. J Clin Hypertens (Greenwich) 2004;6:494–8. doi: 10.1111/j.1524-6175.2004.03643.x. Quiz 499-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 29.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part II: Variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855–64. doi: 10.1161/hc4701.099488. [DOI] [PubMed] [Google Scholar]
- 30.Eikelboom JW, Lonn E, Genest J, Jr, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: A critical review of the epidemiologic evidence. Ann Intern Med. 1999;131:363–75. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- 31.Williams KT, Schalinske KL. Homocysteine metabolism and its relation to health and disease. Biofactors. 2010;36:19–24. doi: 10.1002/biof.71. [DOI] [PubMed] [Google Scholar]
- 32.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 33.Ho CH, Kuo BI, Kong CW, Chau WK, Hsu HC, Gau JP, et al. Influence of methylene tetrahydrofolate reductase (MTHFR) C677T polymorphism, B vitamins and other factors on plasma homocysteine and risk of thromboembolic disease in Chinese. J Chin Med Assoc. 2005;68:560–5. doi: 10.1016/S1726-4901(09)70094-2. [DOI] [PubMed] [Google Scholar]
- 34.Waśkiewicz A, Piotrowski W, Broda G, Sobczyk-Kopcioł A, Płoski R. Impact of MTHFR C677T gene polymorphism and vitamins intake on homocysteine concentration in the Polish adult population. Kardiol Pol. 2011;69:1259–64. [PubMed] [Google Scholar]
- 35.LaRovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 36.Chester MR, Barnett DB. Cardiac chamber-specific beta-adrenoceptor regulation and sympathetic innervation. Lancet. 1995;345:553–5. doi: 10.1016/s0140-6736(95)90467-0. [DOI] [PubMed] [Google Scholar]
- 37.Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality. 15-Year follow-up of middle-aged men and women in eastern Finland. Circulation. 1996;93:1372–9. doi: 10.1161/01.cir.93.7.1372. [DOI] [PubMed] [Google Scholar]
- 38.Murphy MM, Barraj LM, Bi X, Stettler N. Body weight status and cardiovascular risk factors in adults by frequency of candy consumption. Nutr J. 2013;12:53. doi: 10.1186/1475-2891-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan S, Copeland KC, Bright BC, Gardner AW, Blackett PR, Fields DA. Impact of type 1 diabetes and body weight status on cardiovascular risk factors in adolescent children. J Clin Hypertens (Greenwich) 2011;13:351–6. doi: 10.1111/j.1751-7176.2010.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichols WW, O’Rourke MF, Vlachlopoulos C. McDonald’s Blood Flow in Arteries. London, UK: Hodder Arnold; 2011. [Google Scholar]
- 41.Nilsson PM, Boutouyrie P, Cunha P, Kotsis V, Narkiewicz K, Parati G, et al. Early vascular ageing in translation: From laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens. 2013;31:1517–26. doi: 10.1097/HJH.0b013e328361e4bd. [DOI] [PubMed] [Google Scholar]
- 42.van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HA, van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: A population study. Hypertension. 2000;35:637–42. doi: 10.1161/01.hyp.35.2.637. [DOI] [PubMed] [Google Scholar]
- 43.Curcio S, García-Espinosa V, Arana M, Farro I, Chiesa P, Giachetto G, et al. Growing-related changes in arterial properties of healthy children, adolescents, and young adults nonexposed to cardiovascular risk factors: Analysis of gender-related differences. Int J Hypertens. 2016;2016:4982676. doi: 10.1155/2016/4982676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vermeersch SJ, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, VanBortel LM, et al. Age and gender related patterns in carotid-femoral PWV and carotid and femoral stiffness in a large healthy, middle-aged population. J Hypertens. 2008;26:1411–9. doi: 10.1097/HJH.0b013e3282ffac00. [DOI] [PubMed] [Google Scholar]
- 45.Cunha PG, Cotter J, Oliveira P, Vila I, Boutouyrie P, Laurent S, et al. Pulse wave velocity distribution in a cohort study: From arterial stiffness to early vascular aging. J Hypertens. 2015;33:1438–45. doi: 10.1097/HJH.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 46.Yao F, Liu Y, Liu D, Wu S, Lin H, Fan R, et al. Sex differences between vascular endothelial function and carotid intima-media thickness by Framingham Risk Score. J Ultrasound Med. 2014;33:281–6. doi: 10.7863/ultra.33.2.281. [DOI] [PubMed] [Google Scholar]
- 47.Juonala M, Kähönen M, Laitinen T, Hutri-Kähönen N, Jokinen E, Taittonen L, et al. Effect of age and sex on carotid intima-media thickness, elasticity and brachial endothelial function in healthy adults: The cardiovascular risk in Young Finns Study. Eur Heart J. 2008;29:1198–206. doi: 10.1093/eurheartj/ehm556. [DOI] [PubMed] [Google Scholar]
- 48.Beelen R, Hoek G, Houthuijs D, van den Brandt PA, Goldbohm RA, Fischer P, et al. The joint association of air pollution and noise from road traffic with cardiovascular mortality in a cohort study. Occup Environ Med. 2009;66:243–50. doi: 10.1136/oem.2008.042358. [DOI] [PubMed] [Google Scholar]
- 49.Davies HW, Teschke K, Kennedy SM, Hodgson MR, Hertzman C, Demers PA. Occupational exposure to noise and mortality from acute myocardial infarction. Epidemiology. 2005;16:25–32. doi: 10.1097/01.ede.0000147121.13399.bf. [DOI] [PubMed] [Google Scholar]
- 50.Fuks K, Moebus S, Hertel S, Viehmann A, Nonnemacher M, Dragano N, et al. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environ Health Perspect. 2011;119:1706–11. doi: 10.1289/ehp.1103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huss A, Spoerri A, Egger M, Röösli M Swiss National Cohort Study Group. Aircraft noise, air pollution, and mortality from myocardial infarction. Epidemiology. 2010;21:829–36. doi: 10.1097/EDE.0b013e3181f4e634. [DOI] [PubMed] [Google Scholar]
- 52.Dzhambov AM, Dimitrova DD. Heart disease attributed to occupational noise, vibration and other co-exposure: Self-reported population-based survey among Bulgarian workers. Med Pr. 2016;67:435–45. doi: 10.13075/mp.5893.00437. [DOI] [PubMed] [Google Scholar]
- 53.Tomei G, Fioravanti M, Cerratti D, Sancini A, Tomao E, Rosati MV, et al. Occupational exposure to noise and the cardiovascular system: A meta-analysis. Sci Total Environ. 2010;408:681–9. doi: 10.1016/j.scitotenv.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 54.Stansfeld SA, Matheson MP. Noise pollution: Non-auditory effects on health. Br Med Bull. 2003;68:243–57. doi: 10.1093/bmb/ldg033. [DOI] [PubMed] [Google Scholar]
- 55.Nawaz SK, Hasnain S. Noise induced hypertension and prehypertension in Pakistan. Bosn J Basic Med Sci. 2010;10:239–44. doi: 10.17305/bjbms.2010.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomei F, Rosati MV, Baccolo TP, Morelli A, Anzelmo V, Ciarrocca M, et al. Occupational exposure to urban pollutants and plasma growth hormone (GH) J Environ Sci Health A Tox Hazard Subst Environ Eng. 2003;38:1017–24. doi: 10.1081/ese-120019860. [DOI] [PubMed] [Google Scholar]


