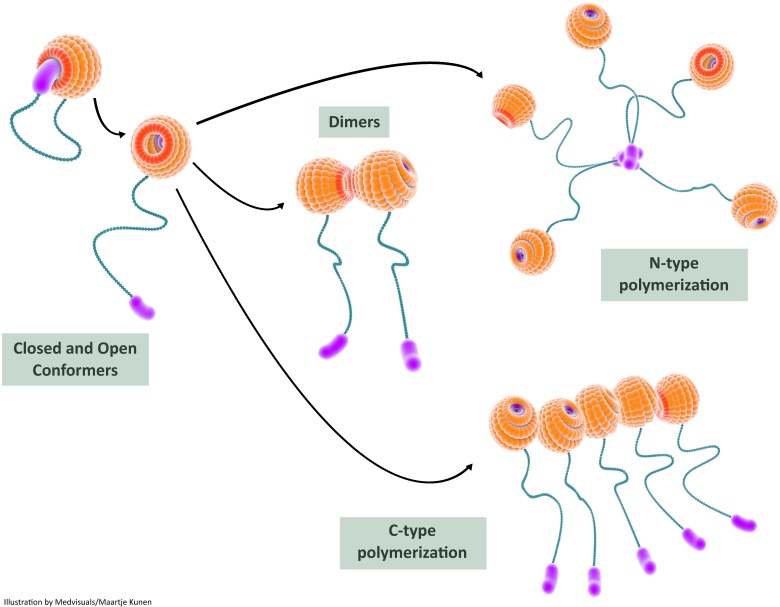
FIGURE 5.
In this illustration, we depict the role of self-interactions in galectin-3 bioactivation. Intramolecular interactions between the carbohydrate recognition domain (CRD) and the N-terminal render the galectin-3 molecule relatively inert in the closed conformer state; the galectin-3 molecule can still bind S-face ligands such as lactose in this state. Release of the N-terminal from the F-face results in the open conformer which is biologically more active. The open conformer can bind to various ligands (both S-face ligands and F-face ligands) and can also undergo dimerization or oligomerization. Two types of intermolecular interactions, N-terminal interactions and CRD-CRD interactions, are usually observed during multimerization and this results in increased biological activity of galectin-3. CRD: carbohydrate recognition domain