Abstract
Modified measles virus (MV) has effective oncolytic activity preclinically and is currently being investigated in clinical trials for various types of cancer. We investigated the use of cystine knot proteins (CKPs) to direct MV activity. CKPs are short polypeptides that bind their targets with high affinity. We used a CKP that binds αvβ3, αvβ5, and α5β1 integrins with single-digit nanomolar affinity to retarget MV to the integrins (MV-CKPint). MV-CKPint infected, replicated in, and killed human glioblastoma, medulloblastoma, diffuse intrinsic pontine glioma (DIPG), and melanoma cancer cells in vitro, all of which express the target integrins. MV-CKPint activity was competitively blocked by echistatin, an integrin binding peptide. When the CKP was cleaved from the viral H protein at an included protease site, virus activity was abrogated. When delivered intravenously (i.v.), the retargeted virus reached a subcutaneous glioblastoma tumor bed and produced cytopathic effects similar to that shown by intratumoral injection of the virus. Because these target integrins are overexpressed by tumor vascular endothelium, MV-CKPint may allow for effective therapy with i.v. injection. These results indicate for the first time that CKPs can be used to retarget MV for a receptor of choice. In addition, MV-CKPint provides proof of principle for the use of a CKP of interest to retarget any enveloped virus for both oncolytic and gene therapy purposes.
Keywords: measles virus, cystine knot protein, knottin, integrin, oncolytic virus
Introduction
Standard therapy for malignant brain tumors has failed to achieve meaningful prolongation of survival. For example, combined radiation therapy and chemotherapy with temozolomide, the current standard of care for glioblastoma multiforme (GBM), has been shown to prolong overall survival only 2.5 months over irradiation alone, and most patients still progress to death caused by the tumor.1 While the success of treating the most common pediatric malignant brain tumor, medulloblastoma, is better, with 5-year survival rates of 70% or more, most children are left with significant cognitive and endocrine deficits after therapy that result in poor quality of life.2, 3 Clearly, new strategies that have a greater impact on survival and that have fewer long-term side effects are needed for the treatment of malignant brain tumors in adults and children.
The use of replication-competent oncolytic viruses (OVs) as anticancer agents has promise as a tumor-specific therapy. In our laboratory, we have been investigating a vaccine strain of measles virus (MV) as a potential therapy for pediatric brain tumors. We have demonstrated efficacy in the treatment of intraparenchymal and cerebrospinal fluid (CSF)-disseminated murine xenograft models of medulloblastoma and in the treatment of intraparenchymal atypical teratoid/rhabdoid tumor.4, 5, 6 Similarly, MV has proven efficacious in the treatment of a murine xenograft model of intracerebral GBM.7 Animal safety studies have demonstrated no evidence of toxicity from injection of MV into the brain or CSF, leading to open phase 1 trials for the treatment of recurrent intracerebral GBM and recurrent intraparenchymal and/or CSF-disseminated medulloblastoma and atypical teratoid/rhabdoid tumor (AT/RT).8, 9
One of the advantages of MV is the ease with which the virus can be retargeted to cell surface antigens of choice. The production of such viruses has proven laborious, as the receptor-binding motif used is often a single-chain antibody. An alternative receptor-binding motif may be cystine knot proteins (CKPs). These small peptides of 20–50 amino acids containing two or three disulfide bonds bind to a specific target with single-digit nanomolar to picomolar binding coefficients.10 In this paper, we manufacture a retargeted MV that uses a CKP that binds αvβ3, αvβ5, and α5β1 integrins with single-digit nanomolar affinity to retarget MV to the integrins (MV-CKPint). The CKP used expresses the integrin-binding motif arginine-glycine-aspartic acid (RGD) in the solvent-exposed loop of the peptide.11 This CKP was specifically selected for restricted binding to the tumor-specific integrins αvβ3, αvβ5, and α5β1, and binds to these integrins with single-digit nanomolar affinity. We demonstrate that MV can be retargeted to these integrins by the CKP and that MV-CKPint binds to, replicates in, and kills tumor cells in vitro. Proteolytic removal of CKP abrogates infection and cell killing, indicating that specific binding of the CKP motif mediates cellular entry of the virus. In addition, we show that this retargeted virus infects tumor cells in a murine xenograft model when delivered by intravascular injection. These results suggest the possible use of any CKP of interest to retarget MV and, indeed, suggest that CKPs can be used to retarget any enveloped virus.
Results
The Integrin-Binding CKP Motif Is Expressed at the C Terminus of the H Protein
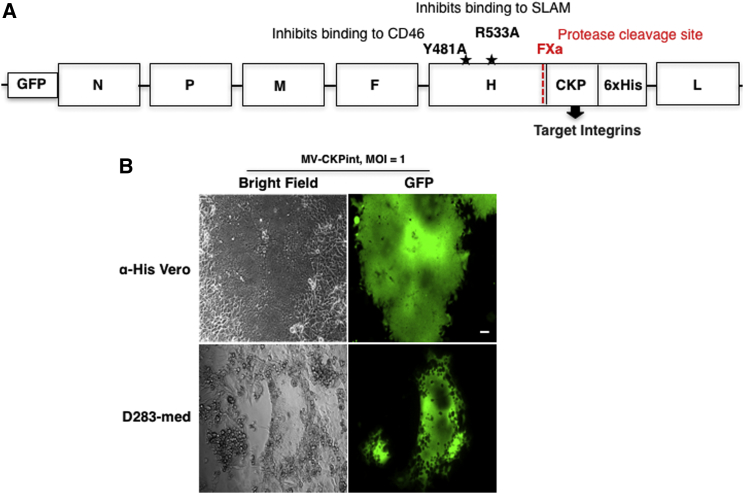
MV normally binds to its receptors via the H protein. The H protein encoded by the viral genome used to manufacture MV-CKPint has two mutations (Y481A and R533A) that eliminate binding to CD46 and SLAM, the normal MV receptors. The integrin-binding CKP was expressed at the carboxyl end of the H protein, resulting in its exposure on the surface of the virus (Figure 1A). The functionality of the MV-CKPint was verified by infecting anti-His (αHis)-Vero cells. These modified cells express membrane-bound single-chain antibody for hexahistidine and hence facilitate viral entry through binding of the 6xHis tag at the C terminus of the MV-CKPint virus (Figure 1B). We also tested this virus on the D283-medulloblastoma cell line (Figure 1B). The MV-CKPint virus successfully infected αHis-Vero and D283-med cells, and large syncytia fluorescing with the GFP were formed in both cell lines. In D283-med cells, GFP florescence is clearly visible in the perinuclear space, which is typical for MV. This finding indicates that adding the integrin-binding CKP to the H protein is functional and allows for infection, and replication of, the virus.
Figure 1.
Structure and Function of MV-CKPint
(A) Genomic organization of the modified MV-CKPint virus. The integrin-binding cystine knot protein (CKP) motif is cloned at the C terminus of the double mutant H protein that cannot bind to its normal receptors, CD46 and SLAM (mutations are shown with a star symbol). A 6xHis tag expressed at the terminal carboxyl end of the recombinant H protein allows virus preparation using α-His Vero cells. (B) The infection ability of the recombinant virus was tested on α-His Vero cells and human medulloblastoma (D283-med) cells. When infected at an MOI of 1, both cell lines show the formation of green florescent multinucleated syncytia. Scale bar, 50 μm.
MV-CKPint Infects and Produces Cytopathic Effect in Cells of Different Tumor Types
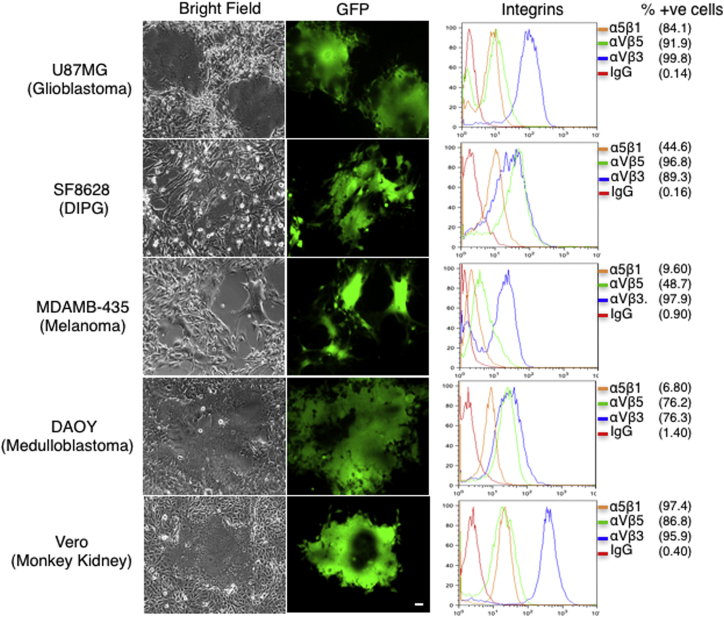
To investigate CKP-mediated cellular infection, we analyzed tumor cell lines of different origin, glioblastoma, diffuse intrinsic pontine glioma (DIPG), medulloblastoma, and melanoma for expression of target integrins. Flow cytometry confirmed that all tested cell lines expressed αvβ3, αvβ5, and α5β1 integrins on the membrane (Figure 2, right panel). The percentages of cells positive for each integrin dimers are shown in parentheses. The expression of the GFP protein in the MV-CKPint virus was used as a marker of infection. After infection with the virus, cell lines of all tested tumor types showed multiple green florescent syncytia (Figure 2, left panel). Syncytia formation became visible as early as 48 hr post-infection. The formation of syncytia indicates that MV-CKPint infects and grows in cells expressing target integrins.
Figure 2.
MV-CKPint Virus Infects Cells of Different Tumor Types
Human glioblastoma, DIPG, melanoma and medulloblastoma cells, and Vero cells (monkey kidney) were infected with the modified virus at an MOI of 1 for 3 hr at 37°C. The infection of cells and formation of syncytia were monitored by GFP florescence. Scale bar, 50 μm. The expression of target integrins of CKP on live cells was determined by flow cytometry after incubation with monoclonal antibody for each integrin dimer. The percentages of cells expressing each dimer are shown in parentheses (% +ve cells). The IgG isotype control antibody (IgG) was used as a reference for staining.
MV-CKPint Replicates in and Kills Cells of Different Tumor Types
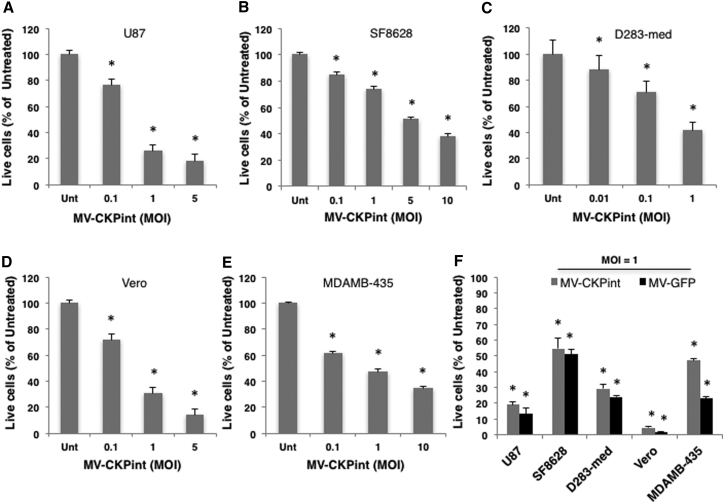
Human glioblastoma, DIPG, medulloblastoma and melanoma cells, and Vero cells were infected with increasing MOIs of MV-CKPint virus. The virus killed all these cell types in a dose-dependent manner (Figures 3A–3E). When infected at an MOI of 1, 75% glioblastoma cells and 60% medulloblastoma cells died after 96 hr, whereas 53% of melanoma cells were killed by the virus in that period. Because this virus targets three integrin dimers, a precise correlation between a particular integrin expression and cell killing by MV-CKPint could not be established. In a separate set of experiments, the oncolytic efficacy of the retargeted MV-CKPint virus was compared side by side with the parental Edmonston strain of the virus (MV-GFP). When infected at an MOI of 1, the killing of all these cell types by both viruses was comparable and statistically significant compared to the untreated control at 96 hr post-infection (Figure 3F).
Figure 3.
MV-CKPint Infects and Kills Tumor Cells in a Dose-Dependent Manner
(A–E) Human glioblastoma (A), DIPG (B), medulloblastoma (C) and melanoma (E), and Vero (D) cell lines (shown above the graph) were infected with the retargeted virus at increasing MOIs, and the number of live cells was counted 96 hr post-infection using the trypan blue exclusion method. (F) A comparison of killing of cell lines with the retargeted virus (MV-CKPint) and the parental Edmonston strain of virus (MV-GFP) at an MOI of 1 for 3 hr at 37°C. The number of live cells was counted after 96 hr using the trypan blue exclusion method. The graphs represent data from at least three independent experiments, and the error bars show SEM. *p < 0.05.
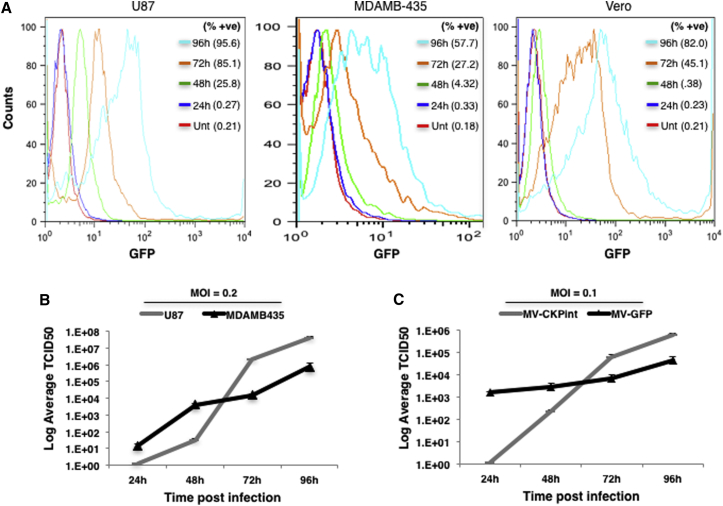
To determine the replication competency of MV-CKPint, we infected human glioblastoma, melanoma, and Vero cells with 0.2 MOI of the virus. Because MV-CKPint expresses GFP as a marker, the replication of the virus in live infected cells was measured in a time course by flow cytometry. The intensity of GFP florescence in all these cell lines increased over time, and the percentages of cells positive for GFP expression are shown in parentheses for each time point (Figure 4A). The GFP fluorescence in untreated control cells was used as a baseline. As a second approach, the titer of cell-associated virus was quantified daily after infection of U87 and MDAMB-435 cells with 0.2 MOI of the virus. As shown in Figure 4B, the titer of infectious virus particles in these cells increased gradually over the period of 4 days post-infection, demonstrating replication of the virus in these cell types. The replication efficiency of the retargeted MV-CKPint virus was compared with that of the parental Edmonston virus strain (MV-GFP). Human U87 glioblastoma cells were infected in parallel with both viruses at an MOI of 0.1, and the titer of cell-associated virus was quantified daily on Vero cells for 4 days post-infection. After 24 hr of infection, the titer of MV-GFP was higher with formation of many florescent syncytia, whereas no syncytia were observed and the titer of infectious particles was significantly lower for MV-CKPint virus (Figure 4C). Nevertheless, the titer of the infectious MV-CKPint virus particles increased over time and rose slightly higher than that of MV-GFP at 72 and 96 hr time points. This suggests that the initial replication and intracellular assembly of the retargeted MV-CKPint virus in U87 glioblastoma cells is slower than the parental MV, but the virus efficiently replicates and the titer of infectious virus increases over time. These results show that the MV-CKPint virus efficiently replicates in and kills tumor cells in vitro.
Figure 4.
MV-CKPint Replicates in Tumor Cells
Human glioblastoma (U87), melanoma (MDAMB-435), and Vero cells were infected with the recombinant virus at an MOI of 0.2 for 3 hr. After removing the unbound virus, cells were incubated to grow for 96 hr post-infection. (A) The intensity of GFP florescence in live cells that represents the cellular viral load was determined by flow cytometry. The percentages of cells positive for GFP fluorescence are shown in parentheses (% +ve). (B) Cells were infected with MV-CKPint virus at an MOI of 0.2 for 3 hr at 37°C. The cell-associated virus was harvested at intervals of 24 hr, cleared from cellular components, and the titer of infectious virus particles was determined by TCID50 quantitation assay. (C) A comparison of replication efficiency of the retargeted virus (MV-CKPint) and the parental Edmonston strain of virus (MV-GFP) in U87MG human glioblastoma cells infected with virus at an MOI of 0.1 for 3 hr at 37°C. The titer of the infectious virus particles was determined by TCID50 quantitation assay. Average of 3 replicates. Error bars show SEM.
Infection of MV-CKPint Is Dependent on the Integrin Binding of the CKP
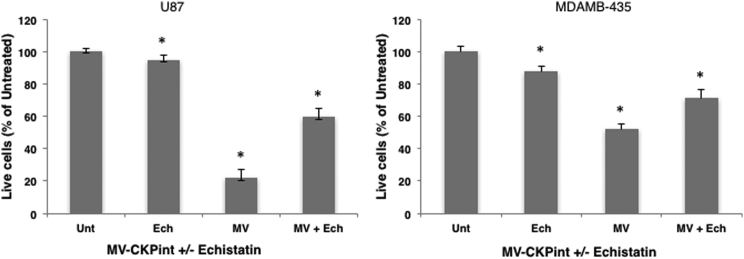
If the integrin-binding motif of CKP is responsible for cell binding and entry of the retargeted virus, the presence of a soluble antagonist of target integrins should inhibit the interaction of virus with cells, thereby blocking infection. To verify the requirement for integrin binding, we assessed infection and cell killing by MV-CKPint in the presence of echistatin, a potent competitive binder of αvβ3 and α5β1 integrins.12 Human glioblastoma and melanoma cells were infected with MV-CKPint virus at an MOI of 1 in the absence and presence of 1 μM soluble echistatin. As shown in Figure 5, echistatin significantly decreased killing of both cell lines by the MV-CKPint virus as a result of inhibition of cellular infection. However, inhibition was not complete. Because echistatin and CKP in the virus compete to bind the same target integrins, a partial infection of cells with the virus may not be completely eliminated. In addition, echistatin has been shown to strongly bind and antagonize αvβ3 and α5β1 integrins,13, 14 but its interaction with αvβ5 integrin, which is also a target of the CKP used in the MV-CKPint virus, is not well-known. This suggests that the retargeted virus utilizes CKP-mediated binding with target integrins for cellular entry.
Figure 5.
Echistatin Competitively Inhibits Infection by MV-CKPint Virus
Human glioblastoma and melanoma cells were incubated with the virus in the absence and presence of 1 μM soluble Echistatin (Ech) for 3 hr at 37°C. Number of live cells was quantified 96 hr post-infection using the trypan blue exclusion method. The data represent three independent experiments. Error bars indicate SEM. *p < 0.05.
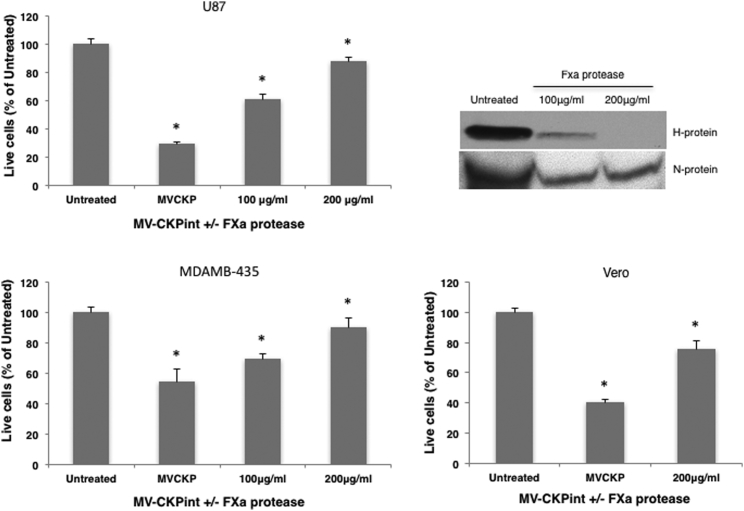
To confirm integrin-binding-mediated cellular entry of the virus via CKP, we removed the CKP motif from the viral H protein using the activated factor X (FXa) protease cleavage site present right before the CKP sequence in the viral H protein (Figure 1A). Pre-incubation of virus with FXa protease markedly abrogated cell killing in dose-dependent manner (Figure 6). Proteolytic removal of the CKP moiety resulted in a more than 4-fold decrease in virus infectivity, thereby cell killing in U87 and MDAMB-435 cells, and a more than 2-fold decrease in Vero cells at 200 μg/mL FXa. Inhibition of viral infection was clearly demonstrated but was incomplete. This could be because CKP moiety was not completely removed by FXa even at 200 μg/mL. These results confirm that the integrin-binding CKP motif is required for cell binding and entry of the MV-CKPint virus.
Figure 6.
Proteolytic Removal of the CKP Prevents Infection by MV-CKPint Virus
MV-CKPint was treated with FXa protease at 100 and 200 μg/mL for 2 hr. Untreated and protease-treated virus was used to infect human glioblastoma (U87), melanoma (MDAMB-435) and vero cells for 3 hr at 37°C. The number of live cells was quantified 96 hr post-infection using the trypan blue exclusion method (bar graphs). The experiments were performed in three independent repeats. Error bars represent SEM. *p < 0.05. The degree of CKP removal by the protease was determined by western blotting of the MV-CKPint lysate using antibody against the 6xHis-tag, which is expressed after the CKP motif. MV nucleoprotein (N protein) was used as the control for loading.
MV-CKPint Reaches Tumor Sites and Forms Syncytia after Intravenous Delivery
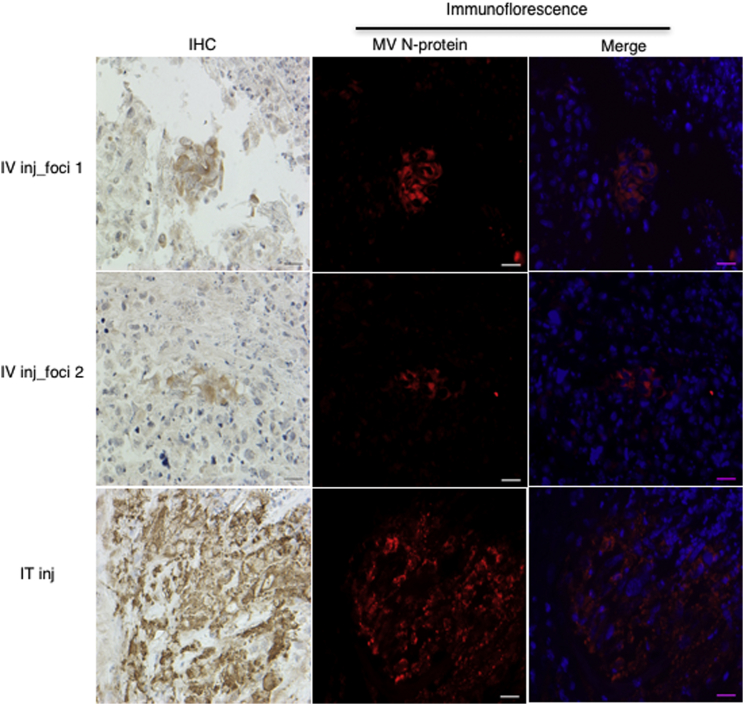
We have established that MV-CKPint infects, replicates in, and kills U87-MG glioblastoma tumor cells in vitro. To investigate intravenous (i.v.) delivery of this modified virus, we injected mice bearing subcutaneous U87-MG glioblastoma tumors with a single dose of MV-CKPint (0.5 × 106 TCID50 [50% tissue culture infective dose]/100 μL) or an equal volume of PBS (control) via the tail vein. Additional control mice were injected directly into the tumor with the same dose of MV-CKPint. Eight days post-injection, tumors were harvested and analyzed for the presence of MV by immunohistochemistry. In tumors from mice receiving i.v. injection of MV-CKPint, multiple scattered small syncytia were noticed; two representative regions of infection (foci 1 and 2) are shown in Figure 7. The syncytia stained positive for MV nucleoprotein, indicating for the presence of MV. For intratumoral injection, a large but localized region of MV nucleoprotein-positive syncytia was observed at the injection site. Additional sections from same tumors used for immunohistochemistry were also examined by immunofluorescence staining to confirm the presence of MV (Figure 7). This indicates that MV-CKPint virus successfully reached the tumor bed after i.v. injection.
Figure 7.
MV-CKPint Virus Infects Subcutaneous U87 Glioblastoma Tumor after Intravenous Delivery
Mice containing flank tumor were given 5 × 105 TCID50 MV-CKPint virus intravenously (i.v.) via tail vein injection. Intratumoral injection of the same dose was used as the positive control of infection. After 8 days, tumors were harvested and processed to detect measles virus nucleoprotein by immunohistochemistry (IHC) and immunofluorescence (red channel). Two representative foci of infection from i.v.-treated tumors (foci 1 and 2) and one for the IT injection are shown. Formation of syncytia and positive MV nucleoprotein reactivity in perinuclear cytoplasmic space (merge panel) was detected in both routes of delivery. Original magnification 400×. Scale bars, 20 μm.
Discussion
In this paper, we have described a novel approach to manufacture a retargeted MV. We used a CKP that specifically and tightly binds to the relatively tumor-specific integrins αvβ3, αvβ5, and α5β1 to direct virus binding to tumor cells.11 We used a well-described “double-blind” virus system that abrogates binding by the virus H protein to its normal receptors to assure that the CKP was, in fact, responsible for binding of the virus to the cell. We show that cell killing by this retargeted MV-CKPint virus was comparable to that of the parental CD46-targeting Edmonston strain of MV on five different cell lines. Also, the retargeted virus efficiently replicated in U87 cells, suggesting that conjugation with a CKP does not have an adverse effect on the virus. We have demonstrated that echistatin, a competitive binder of the integrins, abrogates infection by the virus, and that proteolytic cleavage of the CKP motif from the viral H protein also prevents infection and killing of cells. Thus, we have shown in two different ways that MV-CKPint uses the specific binding of the CKP for cell binding and entry.
In the treatment of solid tumors, MV has required intratumoral injection for efficacy.5 Indeed, both the GBM and the medulloblastoma phase 1 trials for solid tumor recurrence use direct injection of the tumor resection bed for virus delivery. Because the αvβ3, αvβ5, and α5β1 integrins targeted by the CKP used here have been shown to be expressed by tumor vascular endothelium, the use of this CKP may allow for effective therapy with i.v. injection of virus.15, 16 To examine this possibility, we injected animals bearing flank tumors with a single dose of 5 × 105 TCID50 of MV-CKPint i.v. and harvested tumors for analysis at 8 days after injection. A localized large area of syncytia was observed in intratumoral-treated tumors compared to multiple small foci of infection with intravascular virus delivery. The successful delivery of MV-CKPint in xenograft tumors upon i.v. injection raises the possibility that the virus is infecting and replicating in tumor vascular endothelium, and the resultant virus is then spreading to the tumor cells. Further experimentation to compare the efficacy of treatment of solid tumors both in the flank and in the brain will be needed to determine the method of viral spread and overall efficacy of i.v. therapy.
An oncolytic adenovirus (Ad5) and MV have been engineered to express integrin-binding RGD motif on its glycoproteins.17, 18, 19 The modified virus demonstrated localization in vascular endothelial cells and slightly improved antitumor activity.20, 21
One of the advantages of MV in this regard is a relative receptor threshold effect. The more surface receptor present on a cell, the more efficacious the MV infection.22 Indeed, there is a threshold below which productive infection does not occur. This effect is important with regard to integrins. While the αvβ3, αvβ5, and α5β1 integrins have been described as tumor specific, low levels of expression on normal cell types such as dendritic cells, T lymphocytes, vascular smooth muscle cells, and bone have been reported.23, 24, 25, 26, 27 However, this may have no effect of MV toxicity, as the receptor expression of these normal cells may be too low to support productive infection. This is analogous to the situation with the CD46 receptor used by current oncolytic MVs. This protein, an inhibitor of complement-mediated lysis, is expressed at below MV threshold levels by many normal cell types, but is highly overexpressed, for obvious reasons, by most tumor cell types.28, 29
CKPs have been used to detect and monitor tumors of various types. In fact, the CKP used here has been labeled with radio-iodine and shown to specifically label medulloblastoma cells in a murine model.30 Similarly, chlorotoxin, a CKP isolated from scorpion venom, has been conjugated to a fluorescent marker to label tumors in vivo.31 This conjugate, called Tumor Paint, may allow for pre-resection labeling of tumor and intra-operative detection of small amounts of residual tumor, leading to a more complete tumor removal.32
Indeed, the simplicity of CKP structure lends itself to the creation of CKP libraries. Such libraries could then be screened to identify a CKP that binds with high affinity to a target of choice. The selected CKP could then easily be incorporated into MV to direct infection by the virus.33
One issue with the use of CKP for virus targeting is the requirement for correct peptide folding and correct formation of the disulfide bonds in the CPK for binding activity. It appears that passage of the viral protein containing the CKP through an infected cell’s endoplasmic reticulum provides the proper environment to assure proper folding and bond formation of the CKP.34, 35 Passage of the viral protein through the endoplasmic reticulum (ER) occurs with most enveloped viruses. Currently, many enveloped viruses, including herpes simplex virus, Newcastle disease virus, and vesicular stomatitis virus, are used both for oncolysis and as gene therapy vectors.36 The results presented here provide proof of principle for the use of a CKP of interest to retarget any enveloped virus for both oncolytic and gene therapy purposes.
Materials and Methods
Cell Culture
The human embryonic kidney cells (HEK293T), green monkey kidney cells (Vero), human glioblastoma (U87MG), diffuse intrinsic pontine glioma (DIPG-SF8628), melanoma (MDAMB-435), and medulloblastoma (DAOY, D283-med) cell lines were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 2 mM L-glutamine and maintained at 37°C in a humidified incubator set at 5% CO2. The authentication of all human cell lines was confirmed by short tandem repeat (STR) DNA profiling technique (DNA sequencing facility, UC Berkeley, Berkeley, CA, USA).
Preparation and Rescue of MV-CKPint
The cDNA of the integrin-specific CKP (EETI 2.5F) was commercially synthesized at Genscript (Piscataway, NJ, USA) using the amino acid sequence 5′-GCPRPRGDNPPLTCKQDSDCLAGCVCGPNGFCG-3′.11 The sequence was flanked with 5′-Sfi1 and 3′-Not1 restriction sites, and the enzyme-digested fragment was inserted in-frame at the C terminus of the MV H gene in the shuttle vector pTNH6aa.37 The H gene in this vector contains two point mutations resulting in the substitutions Y481A and R533A, which eliminates binding of the H protein to its normal receptors, CD46 and SLAM, respectively. After cloning the CKP, the entire H gene was isolated from pTNH6aa after digesting with Pac1 and Spe1 restriction enzymes, and ligated into the corresponding sites in the p(+)MV-EGFP vector that codes for all six genes of MV. The viral plasmid was amplified by transforming One Shot Top10 competent cells (Life Technologies, Carlsbad, CA, USA) and purified using the plasmid maxi prep kit from QIAGEN (Valencia, CA, USA). The resulting MV-CKPint virus was prepared by transfecting HEK293T cells with the recombinant p(+)MV-EGFP-CKP vector along with packaging plasmids using the FuGENE HD transfection reagent (Promega, Madison, WI, USA). For robust preparation of MV-CKPint stocks, we used Vero-αHis cells. These cells are modified to express membrane-bound single-chain antibodies for anti-hexahistidine that allows cellular entry of this blind virus due to the 6xHis-tag attached to the H protein after the CKP sequence, as described before.37 Cells were scraped from plates in minimal volume of Opti-MEM media, lysed to release virus by two cycles of freeze-thaw in liquid nitrogen, and centrifuged at 10,000 × g for 5 min to clarify virus stock from cellular debris. The aliquots of supernatant-containing virus were stored at −80°C, and the titer was determined as TCID50 by infecting αHis Vero cells in serial dilutions as described previously.38
In Vitro Cell Killing, Cytopathic Effect, and Virus Replication
One hundred thousand tumor cells per well were seeded in 12-well plates. The next day, cells were infected with MV-CKPint at different MOIs in 0.5 mL of Opti-MEM media for 3 hr at 37°C. Control wells were incubated in Opti-MEM only. The virus was removed and cells were maintained in the growth medium. After 96 hr, the number of live cells was determined by the trypan blue exclusion method and counted using Countess, the automated cell counting instrument (Thermo Fisher Scientific, Carlsbad, CA, USA). The percent survival from each treatment was calculated by dividing the number of viable cells in the infected well by the average of viable cells in control wells. The MV-CKPint virus expresses the EGFP, hence the infection of cells and formation of syncytia were confirmed by fluorescence microscopy. To examine the replication efficiency of the virus, we seeded 2 × 105 U87 and MDAMB-435 cells per well in a six-well format plate and infected them with MV-CKPint virus at an MOI of 0.2 for 3 hr on rocker at 37°C. To compare the replication of retargeted MV-CKPint virus with the parental Edmonston vaccine strain of MV (MV-GFP), we infected U87 cells (2 × 105 per well) in six-well plates in parallel with MV-CKPint virus and MV-GFP virus at an MOI of 0.1 for 3 hr on rocker at 37°C. The incubator temperature was kept at 37°C for the first 48 hr and then decreased to 32°C until 96 hr post-infection, to avoid rupturing of syncytia to maximize collection of cell-associated virus. At 24, 48, 72, and 96 hr post-infection, supernatant was removed, and cells were scraped from the dish and lysed with two rounds of freeze-thaw in liquid nitrogen to harvest cell-associated virus particles. After centrifugation at 5,000 × g for 5 min to remove cell debris, the supernatant was immediately used in the serial dilution assay on αHis Vero cells or Vero cells to quantify the titer of MV-CKP-int and MV-GFP virus, respectively.
Flow Cytometry
The expression of αvβ3, αvβ5, and α5β1 integrin dimers on cell surface was analyzed by fluorescence-activated cell sorting (FACS) analysis on live cells. Cells were removed from the dish with TrypLE (GIBCO, Grand Island, NY, USA), centrifuged in cold media, and the pellet was washed with cold flow cytometry (FC) buffer (PBS containing 0.1% sodium azide and 2 mM EDTA). Cells were incubated with phycoerythrin-conjugated primary antibodies for αvβ3 (EMD Millipore, Temecula, CA, USA), αvβ5 (R&D, Minneapolis, MN, USA), and the IgG-isotype control (eBioscience, San Diego, CA, USA). Unconjugated antibody was used for staining α5β1 integrin (EMD Millipore). All primary antibodies were incubated for 1 hr at 4°C. Alexa Fluor 594-conjugated secondary antibody for 30 min at 4°C was used for α5β1 integrin. After antibody incubations, cells were washed three times with FC buffer, and live cells were analyzed on the BD FACSCalibur using the Cell Quest software.
Competitive Binding Assay and FXa Cleavage of MV-CKPint
U87MG glioblastoma and MDAMB-435 melanoma cells were seeded at 105 cells per well in 12-well plates. Cells were washed with PBS and incubated with MV-CKPint at an MOI of 1 in the absence and presence of 1 μM echistatin in Opti-MEM medium for 3 hr on rocker at 37°C. The supernatant containing unbound virus and echistatin was removed, and cells were grown in 10% FBS media. For the FXa proteolysis assay, prior to cellular infection, the number of MV-CKPint virus equivalent to an MOI of 1 was incubated with FXa protease (New England Biolabs, Ipswich, MA, USA) at final concentrations of 100 and 200 μg/mL in Opti-MEM media. The virus was incubated with FXa at room temperature for 1 hr and then for an additional 1 hr at 37°C in the water bath. The untreated and FXa-treated virus was added to cells for 3 hr at 37°C on a rocker in the 5% CO2 incubator. The unbound virus was removed, and cells were grown in 10% FBS media. At 96 hr post-infection, the number of live cells was counted with the Countess using the trypan blue method.
In Vivo Model and Immunohistochemistry
All animal procedures were approved and performed according to the University of California, San Francisco (UCSF) Institutional Animal Care and Use Committee (IACUC) guidelines. Human U87 glioblastoma cells (1 × 106 in 100 μL) were injected subcutaneously in the right flank of 6-week-old NOD-SCID IL2Rγnull (NSG)-immunodeficient mice. At an approximate tumor diameter of 1 cm, mice were injected i.v. with 5 × 105 TCID50 of MV-CKPint virus diluted in 100 μL of PBS by the tail vein. Two mice were also injected with the virus by intratumoral injection and used as the positive control for immunohistochemistry analysis. After 8 days of virus injection, tumors were harvested after cardiac perfusion of animals with PBS and fixed in 10% neutral-buffered formalin for 24 hr at 4°C followed by storage at 4°C in 70% ethanol. Tissues were paraffin-embedded; then 5 μm sections were affixed on charged slides. For immunohistochemistry, sections were deparaffinized in xylene and then hydrated by running through graded ethanol (100%, 90%, 70%, and 50%) solutions. Sections were subjected to heat-induced antigen retrieval in sodium citrate buffer (pH 6.0) by cooking in a pressure cooker for 35 min. After gradual cooling at room temperature for 20 min, slides were washed with distilled water. Incubation in 3% H2O2 solution was performed for 20 min to block the endogenous peroxidase activity. After washing with TBST (Tris-buffered saline solution containing 0.2% Tween 20), tissue sections were encircled with an immunohistochemistry PAP pen (Enzo Life Sciences, NY, USA) and incubated in a protein super block solution for 15 min (ScyTek Laboratories, UT, USA) at room temperature. Slides were incubated with biotin-blocking solutions A and B for 15 min each (ScyTek Laboratories). After washing with TBST, tissue sections were incubated with the anti-measles nucleoprotein primary antibody (NB100-1856; Novus Biologicals, CO, USA) at a 1:200 dilution for 2 hr at room temperature. To assure specific binding of the anti-measles antibody, we used an IgG-isotype control side by side from the same species as the primary antibody. Then, slides were incubated with the ready-to-use UltraTek Anti-Polyvalent Biotinylated secondary antibody (ScyTek Laboratories) for 30 min. Then, sections were incubated in UltraTek streptavidin-conjugated HRP solution (ScyTek Laboratories) for 15 min. Then, TBST-washed slides were incubated with the diaminobenzidine (DAB) substrate (ScyTek Laboratories) for 2 min and washed with tap water. Sections were counterstained with hematoxylin for 5 min and blued using Scott’s water. Slides were washed with running tap water and dehydrated in increasing ethanol concentrations followed by rinsing in xylene. A coverslip was mounted over tissue sections using the Cytoseal-60 mounting medium (Thermo Fisher Scientific, MI, USA). One section from each mouse was used for H&E staining. For immunofluorescence, after antigen retrieval, tumor sections were treated for 5 min with 0.5% Triton X-100 and then blocked for 60 min in buffer containing 5% normal goat serum, 2% bovine serum albumin, and 0.1% Tween 20 dissolved in PBS. Slides were incubated with the primary antibody for 2 hr followed by 1 hr incubation with the secondary antibody at room temperature. After three washes with TBST, a coverslip was mounted with ProLong Gold antifade media containing DAPI (Molecular Probes, Thermo Fisher Scientific, OR, USA). The immunohistochemistry and immunofluorescence staining images were captured with the monochrome camera (400× magnification) using the Zeiss Axio Imager.A2 microscope. The merge channel images in Figure 7 were created using the ImageJ software from the NIH.
Statistical Analysis
Experiments were performed at least three times with three replicates per group of treatment. Statistical analysis was performed with Microsoft Office Excel using Student’s paired t test, and the level of significance was set at p < 0.05.
Author Contributions
Overall Conception and Design: S.L. and C.R.; Experimental Methodology and Acquisition of Data: S.L.; Analysis and Interpretation of Data: S.L. and C.R.; Manuscript Writing and Revisions: S.L. and C.R.
Conflicts of Interest
The authors do not have any conflict to disclose.
Acknowledgments
This project was supported by a startup grant from the UCSF Department of Neurological Surgery and Specialized Program of Research Excellence (SPORE) grant P50 CA097257, awarded to C.R., from the Brain Tumor Research Center at UCSF.
References
- 1.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Johnson D.L., McCabe M.A., Nicholson H.S., Joseph A.L., Getson P.R., Byrne J., Brasseux C., Packer R.J., Reaman G. Quality of long-term survival in young children with medulloblastoma. J. Neurosurg. 1994;80:1004–1010. doi: 10.3171/jns.1994.80.6.1004. [DOI] [PubMed] [Google Scholar]
- 3.Maddrey A.M., Bergeron J.A., Lombardo E.R., McDonald N.K., Mulne A.F., Barenberg P.D., Bowers D.C. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J. Neurooncol. 2005;72:245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- 4.Studebaker A.W., Hutzen B., Pierson C.R., Russell S.J., Galanis E., Raffel C. Oncolytic measles virus prolongs survival in a murine model of cerebral spinal fluid-disseminated medulloblastoma. Neuro-oncol. 2012;14:459–470. doi: 10.1093/neuonc/nor231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Studebaker A.W., Hutzen B., Pierson C.R., Shaffer T.A., Raffel C., Jackson E.M. Oncolytic measles virus efficacy in murine xenograft models of atypical teratoid rhabdoid tumors. Neuro-oncol. 2015;17:1568–1577. doi: 10.1093/neuonc/nov058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Studebaker A.W., Kreofsky C.R., Pierson C.R., Russell S.J., Galanis E., Raffel C. Treatment of medulloblastoma with a modified measles virus. Neuro-oncol. 2010;12:1034–1042. doi: 10.1093/neuonc/noq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phuong L.K., Allen C., Peng K.W., Giannini C., Greiner S., TenEyck C.J., Mishra P.K., Macura S.I., Russell S.J., Galanis E.C. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 8.Lal S., Peng K.W., Steele M.B., Jenks N., Ma H., Kohanbash G., Phillips J.J., Raffel C. Safety study: intraventricular injection of a modified oncolytic measles virus into measles-immune, hCD46-transgenic, IFNαRko mice. Hum. Gene Ther. Clin. Dev. 2016;27:145–151. doi: 10.1089/humc.2016.062. [DOI] [PubMed] [Google Scholar]
- 9.Myers R., Harvey M., Kaufmann T.J., Greiner S.M., Krempski J.W., Raffel C., Shelton S.E., Soeffker D., Zollman P., Federspiel M.J. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum. Gene Ther. 2008;19:690–698. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackerman S.E., Currier N.V., Bergen J.M., Cochran J.R. Cystine-knot peptides: emerging tools for cancer imaging and therapy. Expert Rev. Proteomics. 2014;11:561–572. doi: 10.1586/14789450.2014.932251. [DOI] [PubMed] [Google Scholar]
- 11.Kimura R.H., Levin A.M., Cochran F.V., Cochran J.R. Engineered cystine knot peptides that bind alphavbeta3, alphavbeta5, and alpha5beta1 integrins with low-nanomolar affinity. Proteins. 2009;77:359–369. doi: 10.1002/prot.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLane M.A., Vijay-Kumar S., Marcinkiewicz C., Calvete J.J., Niewiarowski S. Importance of the structure of the RGD-containing loop in the disintegrins echistatin and eristostatin for recognition of alpha IIb beta 3 and alpha v beta 3 integrins. FEBS Lett. 1996;391:139–143. doi: 10.1016/0014-5793(96)00716-8. [DOI] [PubMed] [Google Scholar]
- 13.Kumar C.C., Nie H., Rogers C.P., Malkowski M., Maxwell E., Catino J.J., Armstrong L. Biochemical characterization of the binding of echistatin to integrin alphavbeta3 receptor. J. Pharmacol. Exp. Ther. 1997;283:843–853. [PubMed] [Google Scholar]
- 14.Wierzbicka-Patynowski I., Niewiarowski S., Marcinkiewicz C., Calvete J.J., Marcinkiewicz M.M., McLane M.A. Structural requirements of echistatin for the recognition of alpha(v)beta(3) and alpha(5)beta(1) integrins. J. Biol. Chem. 1999;274:37809–37814. doi: 10.1074/jbc.274.53.37809. [DOI] [PubMed] [Google Scholar]
- 15.Jin H., Varner J. Integrins: roles in cancer development and as treatment targets. Br. J. Cancer. 2004;90:561–565. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arap W., Pasqualini R., Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Koziol J., Deisseroth A., Borgstrom P. Methods for delivery of adenoviral vectors to tumor vasculature. Hum. Gene Ther. 2007;18:151–160. doi: 10.1089/hum.2006.135. [DOI] [PubMed] [Google Scholar]
- 18.Hallak L.K., Merchan J.R., Storgard C.M., Loftus J.C., Russell S.J. Targeted measles virus vector displaying echistatin infects endothelial cells via alpha(v)beta3 and leads to tumor regression. Cancer Res. 2005;65:5292–5300. doi: 10.1158/0008-5472.CAN-04-2879. [DOI] [PubMed] [Google Scholar]
- 19.Wesseling J.G., Bosma P.J., Krasnykh V., Kashentseva E.A., Blackwell J.L., Reynolds P.N., Li H., Parameshwar M., Vickers S.M., Jaffee E.M. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969–976. doi: 10.1038/sj.gt.3301473. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H., Clise-Dwyer K., Ruisaard K.E., Fan X., Tian W., Gumin J., Lamfers M.L., Kleijn A., Lang F.F., Yung W.K. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE. 2014;9:e97407. doi: 10.1371/journal.pone.0097407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong H.T., Trejo T.R., Pham L.D., Oberg A.L., Russell S.J., Peng K.W. Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol. Ther. 2009;17:1012–1021. doi: 10.1038/mt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson B.D., Nakamura T., Russell S.J., Peng K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 23.Elizondo D.M., Andargie T.E., Marshall K.M., Zariwala A.M., Lipscomb M.W. Dendritic cell expression of ADAM23 governs T cell proliferation and cytokine production through the α(v)β(3) integrin receptor. J. Leukoc. Biol. 2016;100:855–864. doi: 10.1189/jlb.2HI1115-525R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harui A., Roth M.D., Vira D., Sanghvi M., Mizuguchi H., Basak S.K. Adenoviral-encoded antigens are presented efficiently by a subset of dendritic cells expressing high levels of alpha(v)beta3 integrins. J. Leukoc. Biol. 2006;79:1271–1278. doi: 10.1189/jlb.1105694. [DOI] [PubMed] [Google Scholar]
- 25.Huang S., Endo R.I., Nemerow G.R. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J. Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krom Y.D., Gras J.C., Frants R.R., Havekes L.M., van Berkel T.J., Biessen E.A., van Dijk K.W. Efficient targeting of adenoviral vectors to integrin positive vascular cells utilizing a CAR-cyclic RGD linker protein. Biochem. Biophys. Res. Commun. 2005;338:847–854. doi: 10.1016/j.bbrc.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura I., Pilkington M.F., Lakkakorpi P.T., Lipfert L., Sims S.M., Dixon S.J., Rodan G.A., Duong L.T. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J. Cell Sci. 1999;112:3985–3993. doi: 10.1242/jcs.112.22.3985. [DOI] [PubMed] [Google Scholar]
- 28.Bajzer Z., Carr T., Josić K., Russell S.J., Dingli D. Modeling of cancer virotherapy with recombinant measles viruses. J. Theor. Biol. 2008;252:109–122. doi: 10.1016/j.jtbi.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Shang Y., Chai N., Gu Y., Ding L., Yang Y., Zhou J., Ren G., Hao X., Fan D., Wu K., Nie Y. Systematic immunohistochemical analysis of the expression of CD46, CD55, and CD59 in colon cancer. Arch. Pathol. Lab. Med. 2014;138:910–919. doi: 10.5858/arpa.2013-0064-OA. [DOI] [PubMed] [Google Scholar]
- 30.Moore S.J., Hayden Gephart M.G., Bergen J.M., Su Y.S., Rayburn H., Scott M.P., Cochran J.R. Engineered knottin peptide enables noninvasive optical imaging of intracranial medulloblastoma. Proc. Natl. Acad. Sci. USA. 2013;110:14598–14603. doi: 10.1073/pnas.1311333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daly N.L., Craik D.J. Bioactive cystine knot proteins. Curr. Opin. Chem. Biol. 2011;15:362–368. doi: 10.1016/j.cbpa.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Fidel J., Kennedy K.C., Dernell W.S., Hansen S., Wiss V., Stroud M.R., Molho J.I., Knoblaugh S.E., Meganck J., Olson J.M. Preclinical calidation of the utility of BLZ-100 in providing fluorescence contrast for imaging spontaneous solid tumors. Cancer Res. 2015;75:4283–4291. doi: 10.1158/0008-5472.CAN-15-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fielding A.K. Measles as a potential oncolytic virus. Rev. Med. Virol. 2005;15:135–142. doi: 10.1002/rmv.455. [DOI] [PubMed] [Google Scholar]
- 34.Braakman I., van Anken E. Folding of viral envelope glycoproteins in the endoplasmic reticulum. Traffic. 2000;1:533–539. doi: 10.1034/j.1600-0854.2000.010702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daly N.L., Clark R.J., Craik D.J. Disulfide folding pathways of cystine knot proteins. Tying the knot within the circular backbone of the cyclotides. J. Biol. Chem. 2003;278:6314–6322. doi: 10.1074/jbc.M210492200. [DOI] [PubMed] [Google Scholar]
- 36.Parato K.A., Senger D., Forsyth P.A., Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T., Peng K.W., Harvey M., Greiner S., Lorimer I.A., James C.D., Russell S.J. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 38.Hutzen B., Pierson C.R., Russell S.J., Galanis E., Raffel C., Studebaker A.W. Treatment of medulloblastoma using an oncolytic measles virus encoding the thyroidal sodium iodide symporter shows enhanced efficacy with radioiodine. BMC Cancer. 2012;12:508. doi: 10.1186/1471-2407-12-508. [DOI] [PMC free article] [PubMed] [Google Scholar]