Abstract
Background
The overuse of antibiotics has been identified as a major challenge in regard to the rational prescription of medicines in low and middle income countries. Extensive studies on the effectiveness of persuasive interventions, such as guidelines have been undertaken. There is a dearth of research pertaining to the effects of restrictive interventions. This study aimed to evaluate the impacts of prescription restrictions in relation to types and administration routes of antibiotics on antibiotic procurement in primary care settings in China.
Methods
Data were drawn from the monthly procurement records of medicines for primary care institutions in Hubei province over a 31-month period from May 2011 to November 2013. We analyzed the monthly procurement volume and costs of antibiotics. Interrupted time series analyses with a difference-in-difference approach were performed to evaluate the effect of the restrictive intervention (started in August 2012) on antibiotic procurement in comparison with those for cardiovascular conditions. Sensitivity tests were performed by replacing outliers using a simple linear interpolation technique.
Results
Over the entire study period, antibiotics accounted for 33.65% of the total costs of medicines procured for primary care institutions: mostly non-restricted antibiotics (86.03%) and antibiotics administered through parenteral routes (79.59%). On average, 17.14 million defined daily doses (DDDs) of antibiotics were procured per month, with the majority (93.09%) for non-restricted antibiotics and over half (52.38%) for parenteral administered antibiotics. The restrictive intervention was associated with a decline in the secular trend of costs for non-restricted oral antibiotics (− 0.36 million Yuan per month, p = 0.029), and for parenteral administered restricted antibiotics (− 0.28 million Yuan per month, p = 0.019), as well as a decline in the secular trend of procurement volume for parenteral administered non-restricted antibiotics (− 0.038 million DDDs per month, p = 0.05).
Conclusions
Restrictive interventions are effective in reducing the procurement of antibiotics. However, the effect size is relatively small and antibiotic consumptions remain high, especially parenteral administered antibiotics.
Keywords: Administrative regulation, Restrictive intervention, Antibiotics, Primary care, China
Background
The overuse of antibiotics has been identified as a major global challenge, especially in low and middle income countries (LMIC) [1, 2]. It has been proved to be associated with the development and spread of antimicrobial resistance (AMR). Rising AMR levels, in combination with a lack of new effective antibiotics, increases the morbidity and mortality of infectious diseases [3]. The overuse of antibiotics also drives inflation related to healthcare costs.
Empirical evidence shows that irrational antibiotic prescriptions are most prevalent in primary care settings [4]. The overuse of antibiotics for upper respiratory tract infections in primary care, for instance, was observed worldwide [5]. Antibiotic abuse has also been identified as a serious problem in China [6]. The primary health network in China includes community/township health centres and outreach stations/clinics. They provide essential medical care services (covering outpatient, inpatient and rehabilitation care in line with the Essential Medicines List policy) and essential public health services (including personal health records, health education, planned immunization, child (0–6 years) health care, maternity care, aged care, management of chronic conditions (hypertension and diabetes), management of severe mental disorders, management of tuberculosis, use of Chinese medicines for health promotion, reporting and emergency response to infectious diseases, supporting health inspection activities, free supply of contraception, and improving the health literacy of consumers) [7]. A growing body of literature has revealed a very high level of use of antibiotics in primary care settings in China [8]. The direct cost associated with the overuse of antibiotics in China is estimated to be around 2.91–13.93 billion yuan ($0.42–2.02 billion USD) per year [9]. A recent national survey shows that 52.9% of patients visiting primary care institutions in China were prescribed antibiotics, but only 39.4% of those who received antibiotics needed them based on their clinical condition [10].
Increased AMR triggered a surge of interventions on antibiotic prescribing practices [11]. Clinical guidelines are perhaps the most commonly used instrument for promoting rational prescriptions. Guidelines alone may play a limited role in changing prescribing practices [12, 13]. In a systematic review, Ivers and colleagues found that audit and feedback can bring about 70% compliance with prescription guidelines, leading to a 16% reduction in antibiotic prescriptions [14]. Nonetheless, the current intervention strategies have been heavily biased towards persuasive measures (such as guidelines), and restrictive interventions (such as administrative rules on prescribers) are rare both in practice and in research [15]. Some researchers argue that restrictive interventions may have great potential for curbing antibiotic abuse [14, 16].
In China, both persuasive and restrictive measures have been used to address antibiotic over-prescriptions [17]. In the recent round of health system reform launched in 2009, access to antibiotics in primary care facilities has been restricted to medicines listed in the Essential Medicines List (EML) and these medicines have to be sold with zero-markup [18]. Unfortunately, limited effects on antibiotic prescribing practices have been observed after a few years of implementing these policies [19–21]. In 2012, the Chinese government issued ‘‘administrative rules for the clinical use of antibiotics’’, which are considered the most rigid regulatory control over antibiotic prescriptions to date [22, 23].
This study aimed to evaluate the effects of the “administrative rules” on antibiotic prescriptions in primary care in Hubei province. Hubei’s “administrative rules for clinical use of antibiotics” involve three major components [24]: (1) antibiotics are categorized into three groups—non-restricted, restricted and controlled. The EML for primary care contains non-restricted and restricted antibiotics only; (2) administrative restrictions are imposed on health facilities and medical practitioners in relation to prescriptions of restricted and controlled antibiotics, as well as intravenous infusion of antibiotics; (3) penalties apply to those who violate the rules (Box 1).
Box 1 Administrative rules for the clinical use of antibiotics
1. Antibiotic categorization
Antibiotics are categorized into three groups: non-restricted, restricted, and controlled. Non-restricted antibiotics can be used for the prevention and treatment of mild infections. Restricted antibiotics can be used for severe infections, infections in patients with immune dysfunction, and infected pathogens that are sensitive only to restricted antibiotics. Controlled antibiotics can only be used in special circumstances. Detailed guidelines were issued by the government in relation to the type of antibiotics that applies to various clinical conditions and evidence that is required to justify antibiotic prescriptions.
2. Prescribing authorization
Prescribing privileges are conditional to qualification, professional title, and training of prescribers. Only doctors with a middle or senior professional title are allowed to prescribe controlled antibiotics. Medical practitioners without a professional title (such as assistant doctors) are not allowed to prescribe restricted antibiotics. Health workers in village clinics can only prescribe non-restricted antibiotics. The intravenous infusion of antibiotics in village/community clinics is subject to approval from county health bureaux.
3. Pharmaceutical management committee
Secondary and tertiary hospitals are required to establish a pharmaceutical management committee, consisting of representatives of physicians, pharmacists, microbiologists, and managers. Primary care institutions are required to establish an antimicrobial working group.
4. Monitoring and evaluation of antibiotic prescriptions
Antibiotic prescriptions should be audited on a regular basis in line with the prescribing guidelines.
5. Penalty
Institutions that violate the rules are subject to penalties imposed by the health authorities, which include downgraded accreditation and dismissal of managers. Medical practitioners involved may lose permission to prescribe antibiotics, have their medical registration revoked, or prosecuted.
Methods
Setting
This study was conducted in Hubei province. Hubei is located in central China, with a population of over 61 million across a geographic area of 185,900 km2. The annual average income per capita in Hubei ranks in the middle range of all provinces: 6898 yuan ($1000 USD) for rural residents and 18,374 yuan ($2659 USD) for urban residents (2012).
Data used in this study were extracted from the procurement database for urban community health centers and rural township health centers. There are 1430 community/township health centers in Hubei. The procurement of medicines for the 1235 state-owned community/township health centers is made through a provincial tendering system managed by the Hubei Medical Procurement Administrative Agency (HMPA). Primary care institutions are only allowed to stock and dispense medicines listed in the EML at zero markup. The procurement system covers all medicines listed in the EML, including 32 generic non-restricted antibiotics and 4 generic restricted antibiotics.
Study design
The procurement system recorded volumes and prices of medicines delivered to community/township health centers. The medicines were coded using the Anatomical Therapeutic Chemical (ATC) coding system. We compared the changes in volumes and costs of antibiotics for systemic use (ATC code, J01) with those of medicines used for the cardiovascular system (ATC code, C).
We adopted a controlled interrupted time series design with a difference-in-difference approach. An interrupted time series is a strong quasi-experimental design in which data are collected at multiple time points before and after the intervention. The advantage of this design is that it can detect a possible underlying secular trend which occurs after the intervention. By adding a control group, it is possible to separate the intervention effect from other confounding effects that may have occurred at the same time [25–27]. In this study, the ATC “C” group of medicines served as a control group because it contained large volumes of orders and it is not subject to the influence of the restrictive intervention tested in this study.
The design of this study was further strengthened by adopting a difference-in-difference approach, which enables us to estimate the effect size of the restrictive intervention, adjusting for pre-existing differences and the confounding influence of other factors.
Data collection and management
Data were extracted from the HMPA procurement system, which contained monthly records in relation to the unit strength, pack size, price, procurement volume, and total cost of each delivered medicine. Procured medicines in the ATC “J01” group included 32 products classified as non-restricted antibiotics and 4 products classified as restricted antibiotics, compared with 39 products in the ATC “C” group.
The procurement records over a 31-month period (from May 2011 to November 2013) were collected. The administrative restrictive rules on antibiotic prescriptions were introduced in August 2012. This resulted in a final sample of 15 months of pre-intervention records and 16 months of post-intervention records. We discontinued the data collection in December 2013 because a new version of EML was introduced at that time.
Statistical analysis
Two indicators were used to examine the outcomes of the restrictive intervention: volume and costs of procured medicines. The cost of the procured medicines was calculated based on the price and volume of each product, without adjustment for present values. The volume of procured medicines was measured using defined daily dose (DDD, the average maintenance dose per day for a drug used for its main indication in adults), a measurement developed by the World Health Organization (WHO) to compare drug consumptions. According to the WHO Collaborative Centre for Drug Statistics Methodology [28], DDD equivalence per package (DPP) of medicines was calculated in DDD units [DPP = (unit strength × pack size/DDD)]. The total volume for each group of procured medicines (DDDs) was estimated as the summed DPPs of all-inclusive products [29].
Ni represents the number of packages of certain product (i) delivered to the community/township health centers.
To estimate the effect of the intervention on the outcome variables, the following segmented linear regression model was developed [25]:
In this model, Yt is the outcome indicator in month t; Tt is a continuous variable indicating the months passed at month t since the start of the observation period; It represents the two periods before (value = 0) and after (value = 1) the intervention; T is a continuous variable indicating months passed since the intervention (time prior to the intervention is coded 0); G represents the two groups (0 for the control group and 1 for antibiotic group); β6 estimates the mean difference in pre-post (intervention) changes between the two groups in relation to the outcome indicators (the effect size of the intervention); β7 estimates the difference in secular trend changes in time series between the two groups (the change in trend due to the intervention). β8 and β9 were used to correct for a potential seasonality effect [30]. To correct for dependency of time series data, New-West standard errors were calculated in these models [31].
To better understand the changing pattern of prescribing practices, we also estimated the effects of the intervention on the consumption of non-restricted antibiotics, restricted antibiotics, oral antibiotics, and antibiotics administered through the parenteral route, respectively.
We observed two obvious outliners (at time point 21 and 22) which were caused by the holiday season (Chinese New Year). Sensitivity tests were performed by replacing the outliers using a simple linear interpolation technique [32].
All of the analyses were performed using Stata 15.0 (Stata Corp LP, College Station, TX, USA).
Results
Overall, antibiotics accounted for 33.65% of the total procurement costs for all drugs (36.10% before intervention and 32.11% after intervention). On average, ¥47.97 million yuan per month were spent on antibiotics (¥44.31 million per month before intervention and ¥51.41 million after intervention). The percentage increase in costs over time was smaller for antibiotics than for cardiovascular medicines (Fig. 1).
Fig. 1.

Time trend of monthly costs for procured medicines: antibiotics vs medicines for cardiovascular system
Non-restricted antibiotics accounted for 86.03% of the total cost of antibiotics (17.43% oral and 68.6% parenteral), while restricted antibiotics accounted for 13.97% of the total cost of antibiotics (2.98% oral and 10.99% parenteral). As a group, parenteral antibiotics accounted for almost 80% (79.59%) of the total cost of antibiotics (Fig. 2).
Fig. 2.

Time trend of monthly costs for antibiotic subgroup
On average, 16.79 million DDDs of antibiotics were procured per month (13.60 million before intervention and 19.78 million after intervention), compared with 10.01 million DDDs of medicines for the cardiovascular system (8.24 million before intervention and 11.66 million after intervention). The percentage increase in DDDs over time was smaller for antibiotics than for cardiovascular medicines (Fig. 3).
Fig. 3.
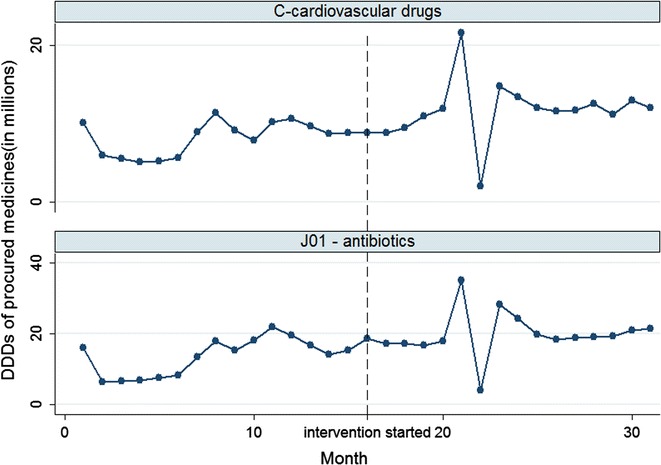
Time trend of monthly DDDs for procured medicines: antibiotics vs medicines for the cardiovascular system
Non-restricted antibiotics accounted for 93.09% of the DDDs for antibiotics (46.11% oral and 46.98% parenteral), while restricted antibiotics accounted for 6.91% of the total cost of antibiotics (1.52% oral and 5.39% parenteral). As a group, parenteral antibiotics accounted for more than half (52.38%) of the DDDs for antibiotics (Fig. 4), despite their much higher contribution to the cost of antibiotics.
Fig. 4.
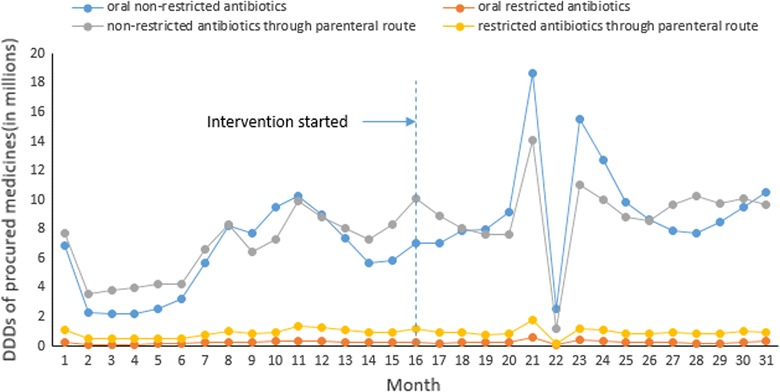
Time trend of monthly antibiotic DDDs by subgroups
The segmented linear regression models revealed that the intervention was associated with a decline in the secular trend of costs for non-restricted oral antibiotics (− 0.36 million Yuan per month, p = 0.029) compared to the control. Prior to the intervention, there was an increasing secular trend (average increase per month 0.25 million Yuan, p = 0.021). The intervention was associated with a 27.84% reduction in the cost of non-restricted oral antibiotics. The intervention was also associated with a decline in the secular trend of costs for restricted antibiotics administered through the parenteral route (− 0.28 million Yuan per month, p = 0.019) compared to the control. Prior to the intervention, there was not a significant increasing secular trend (p = 0.114). The interventions were associated with a 33.64% reduction in the cost of restricted antibiotics administered through the parenteral route. No significant trend changes were observed for the costs of non-restricted antibiotics administered through the parenteral route or oral restricted antibiotics. Despite the secular trend changes, the mean differences in the magnitude of changes remained statistically insignificant (Table 1).
Table 1.
Effects of the intervention on the cost of antibiotics in comparison with controls: findings from the segmented linear regression models
| Outcome indicators | Original models | Models with replaced outliers | ||
|---|---|---|---|---|
| Coefficient [95% CI] | p | Coefficient [95% CI] | p | |
| Total cost for antibiotics (vs controls) | ||||
| Mean difference in pre-post changes (million yuan) | − 9.02 [− 26.74, 8.69] | 0.312 | − 8.31 [− 18.68, 2.06] | 0.114 |
| Difference in secular trend changes (million yuan per month) | − 1.58 [− 3.20, 0.03] | 0.054 | − 1.56 [− 2.96, − 0.15] | 0.030 |
| Cost for non-restricted antibiotics (vs controls) | ||||
| Oral antibiotics (vs controls) | ||||
| Mean difference in pre-post changes (million yuan) | − 0.80 [− 5.05, 3.44] | 0.706 | − 0.76 [− 3.56, 2.03] | 0.585 |
| Difference in secular trend changes (million yuan per month) | − 0.36 [− 0.68, − 0.04] | 0.029 | − 0.35 [− 0.61, − 0.079] | 0.012 |
| Antibiotics administered through parenteral route (vs controls) | ||||
| Mean difference in pre-post changes (million yuan) | − 7.35 [− 18.71, 4.00] | 0.200 | − 6.61 [− 13.64, 0.42] | 0.065 |
| Difference in secular trend changes (million yuan per month) | − 0.85 [− 1.93, 0.22] | 0.118 | − 0.85 [− 1.80, 0.11] | 0.080 |
| Cost for restricted antibiotics (vs controls) | ||||
| Oral antibiotics (vs controls) | ||||
| Mean difference in pre-post changes (million yuan) | − 0.74 [− 2.74, 1.26] | 0.463 | − 0.77 [− 1.55, 0.01] | 0.053 |
| Difference in secular trend changes (million yuan per month) | − 0.032 [− .19, 0.121] | 0.675 | − 0.036 [− 0.15, 0.08] | 0.529 |
| Antibiotics administered through parenteral route (vs controls) | ||||
| Mean difference in pre-post changes (million yuan) | − 0.63 [− 0.29, 1.63] | 0.577 | − 0.69 [− 2.28, 0.90] | 0.388 |
| Difference in secular trend changes (million yuan per month) | − 0.28 [− 0.52 , − 0.05] | 0.019 | − 0.27 [− 0.49, − 0.06] | 0.015 |
Italic values indicate significance of p value (p < 0.05)
The intervention was associated with a decline in the secular trend of the procurement volume of restricted antibiotics administered through the parenteral route (− 0.038 million DDDs per month, p = 0.05) compared to the control. Prior to the intervention, there was not a significant increasing secular trend (p = 0.128). The interventions were associated with a 26.82% reduction in the volume of restricted antibiotics administered through the parenteral route. No significant trend changes were observed for the volumes of non-restricted antibiotics, oral restricted antibiotics, and oral restricted antibiotics administered through the parenteral route. Again, the mean differences in the magnitude of volume changes remained statistically insignificant (Table 2).
Table 2.
Effects of the intervention on procurement volumes of antibiotics in comparison with controls: findings from the segmented linear regression models
| Outcome indicators | Original models | Models with replaced outliers | ||
|---|---|---|---|---|
| Coefficient [95% CI] | p | Coefficient [95% CI] | p | |
| Total DDDs for antibiotics (vs controls) | ||||
| Mean difference in pre-post changes (DDDs in millions) | − 0.59 [− 7.96, 6.79] | 0.874 | − 0.32 [− 3.92, 3.27] | 0.857 |
| Difference in secular trend changes (DDDs in millions per month) | − 0.51 [− 1.09, 0.080] | 0.089 | − 0.51 [− 0.97, − 0.05] | 0.031 |
| DDDs for non-restricted antibiotics (vs controls) | ||||
| Oral antibiotics (vs controls) | ||||
| Mean difference in pre-post changes (DDDs in millions) | 0.11 [− 4.74, 4.96] | 0.964 | 0.18 [− 1.77, 2.14] | 0.852 |
| Difference in secular trend changes (DDDs in millions per month) | − 0.22 [− 0.56, 0.13] | 0.213 | − 0.22 [− 0.47,0.02] | 0.073 |
| Antibiotics administered through parenteral route (vs controls) | ||||
| Mean difference in pre-post changes (DDDs in millions) | − 0.40 [− 4.27, 3.47] | 0.837 | − 1.43 [− 4.60, 1.74] | 0.370 |
| Difference in secular trend changes (DDDs in millions per month) | 0.03 [− 0.27, 0.33] | 0.849 | 0.08 [− 0.21, 0.37] | 0.595 |
| DDDs for restricted antibiotics (vs controls) | ||||
| Oral antibiotics (vs controls) | ||||
| Mean difference in pre-post changes (DDDs in millions) | − 0.24 [− 4.06, 3.58] | 0.899 | − 0.33 [− 2.08, 1.42] | 0.704 |
| Difference in secular trend changes (DDDs in millions per month) | 0.066 [− 0.23, 0.37] | 0.660 | 0.055 [− 0.18, 0.29] | 0.642 |
| Antibiotics administered through parenteral route (vs controls) | ||||
| Mean difference in pre-post changes (DDDs in millions) | − 0.16 [− .54, 0.229] | 0.420 | − 0.18 [− 0.44, 0.086] | 0.183 |
| Difference in secular trend changes (DDDs in millions per month) | − 0.038 [− 0.075, 0.00] | 0.050 | − 0.035 [− 0.069, 0.001] | 0.043 |
Italic values indicate significance of p value (p < 0.05)
The results of the regression models using data with and without replacing outliers were consistent, with similar coefficients for the change in the secular trend of antibiotic prescriptions: − 1.58 vs − 1.56 for total cost; − 0.51 vs − 0.51 for total DDDs.
Discussion
The restrictive intervention on antibiotic prescriptions is associated with some positive changes. This study demonstrated that the restrictive intervention resulted in a 26.82% reduction in the procurement volume of parenteral administered restricted antibiotics, a 33.64% reduction in the cost of parenteral administered restricted antibiotics, and a 27.84% reduction in the procurement cost of non-restricted oral antibiotics. This is understandable because the administrative rules set up a very strong control over the use of restricted antibiotics (details in Box 1). Although there is a paucity in the literature documenting the effectiveness of restrictive measures on prescribing practices [15], a recent study shows that a financial penalty for violating the existing guidelines can decrease subsequent antibiotic prescriptions and associated costs [33]. There was no change in the price of medicines over the study period. Medicines for primary care facilities were procured through a provincial tendering system. The price of the procured medicines was fixed until the next round of the tendering process. Over the study period, there was no new round of tendering. Therefore, the decline in cost reflects a reduction in the volume of prescriptions, not a reduction in price.
The cost-saving effect of the restrictive interventions on the use of antibiotics in primary care settings should not be interpreted as an effect on the entire health delivery system for several reasons. First, the restrictive intervention may encourage more referrals from primary care workers, increasing the cost associated with doctors’ time. Second, the restrictive intervention involves additional administrative costs. Third, it is also likely that some patients may bypass primary care and seek more expensive care from hospitals. In China, primary care is delivered in both primary care facilities and hospitals, and patients enjoy the freedom to choose their preferred primary care providers [34]. In 2011, China established a medication review system for antibiotic prescriptions, where a medication review team involving physicians and pharmacists is required to provide advice on the rational use of antibiotics [35]. But there is a lack of mechanisms for action. The auditing and penalty strategies were supposed to serve as an instrument for the medication review team to introduce actions. No additional investment is required.
Restrictive intervention strategies may have the potential to complement persuasive intervention strategies. A few studies reported the limited effects of guidelines on antibiotic prescribing practices. For instance, a national guideline recommended no initial antibiotic therapy on acute otitis media and adult sinusitis. But the rate of encounters at which no antibiotics was prescribed for these clinical conditions had not changed since the publication of the guideline [12, 13, 36]. However, an audit and feedback plus the distribution of a pocket version of the guidelines increased the prescribing compliance in a Norwegian hospital in terms of the right choice of empirical antibiotics, appropriate treatment duration, and decreased use of high-dose benzyl penicillin [37]. Public reporting may also help enhance the effects of practice guidelines, albeit in a small effect size [38, 39].
The intervention strategies tested in this study comprise multifaceted measures, including prescribing guidelines, audit and feedback, administrative rules and penalties, The multifaceted approach is particularly important in a health system where prescribers have varied qualifications and financial incentives are not always well aligned with the quality of care [33], as is often the case in low and middle income countries.
No significant impact on the overall cost or volume of antibiotics was detected in this study, although the results were numerically lower in all cases except the change in volume of non-restricted orals and the trend in the volume of restricted orals. Clearly, the overconsumption of antibiotics remains a significant challenge in China, despite the enormous efforts made by the government. Empirical evidence shows that economic motivation plays a crucial role in physicians’ prescribing practices [40]. The Chinese government has tried to decouple the link between the income of physicians and the sale of prescribed medicines through its EML and zero markup policies. However, it has resulted in a substantial loss of revenue for primary care facilities [41, 42] due to a shortage of government subsidies. These facilities have to turn their attention to other avenues to compensate for the loss, including user charges for the parenteral administration of medicines [38]. The regional centralized procurement arrangement does not forfeit the autonomy of health facilities to decide what and how much they can spend on medicines [43, 44]. In such a system context, persuasive measures alone barely have any significant impact on prescribing practices. The Antibacterial Use in Clinical Practice (2004) was proved to be fragmented and incomplete and has made only limited progress in containing antibiotic resistance [3, 45]. A coordinated systems approach may further tackle the issue of the irrational use of antibiotics.
The findings of this study have significant policy implications: administrative restrictive measures have the potential to lower antibiotic consumptions. However, it is important to note that the effect size of the tested intervention is rather small and antibiotic consumption remains high, especially parenteral administered antibiotics. Overall, antibiotics accounted for 33.65% of the total cost of procured medicines for primary care institutions in Hubei, much higher than the average level (11–18%) across all healthcare settings in Shanghai [46] or children’s hospitals (17.1%) in the US [47]. Antibiotics administered through the parenteral route still comprise over half of DDDs of antibiotics, contributing to almost 80% of the total cost of antibiotics. Since the 2009 health system reform, primary care institutions in China are no longer able to make a profit from dispensing medicines. However, they are allowed to charge a fee for services (injections) and consumables (syringes). This has added complications to the efforts to curb the overuse of injections. There is a need to organize a coordinated systems approach to tackle this issue.
This study has several strengths. The SMPA dataset includes the procurement records for almost all the primary care institutions in Hubei, covering all essential medicines. The institutionalization of longitudinal data reporting and a control group in this study avoids much of the bias of sampling. The use of cardiovascular medicines was unlikely to involve any significant changes over the study period, because primary care facilities were only allowed to dispense medicines listed in the EML which remained unchanged over the study period and the age structure and disease pattern of the population had limited, if any, changes in such a small time window (31 months). We also used more sophisticated methods, “interrupted time series”, which can make a more precise estimation of the policy impact compared with a simple pre-post comparison [48]. The sensitivity tests further enhanced the reliability of the results.
There are several limitations in this study. First, the data used in this study were drawn from procurement records, which do not directly reflect the actual use of medicines. We were unable to evaluate the appropriateness of antibiotic usage at the individual patient level. Second, prescriptions in private primary care facilities and the use of non-prescribed antibiotics (e.g. over-the-counter and leftover antibiotics at home) were excluded in this study. However, the impact of such exclusion is anticipated to be minimal. In 2012, primary care institutions received 68.51% of total outpatient visits in Hubei province. The participating community/township health centers in this study covered 86.36% of all primary care institutions in Hubei. Patients who visit health facilities usually fill their prescriptions at the same facility [44, 49]. We used multiple models and multiple tests to ensure the robustness of the study findings. However, some significant differences might arise due to chance where multiple tests were performed.
Conclusions
Administrative restrictive regulations on antibiotic prescriptions are effective in reducing both the cost and volume of parenteral administered restricted antibiotics (26.82% reduction in volume and 33.64% reduction in cost). However, the restrictive interventions have failed to have a significant impact on overall cost and volume of antibiotic procurement. It is also important to note that costs may shift to other professionals and providers as a result of restrictions on primary care. Antibiotic usage remains high in Hubei, China, especially parenteral administered antibiotics. A coordinated systems approach is needed to further tackle the issue of the irrational use of antibiotics.
Authors’ contributions
YT made contributions to the study design, acquisition, analysis and interpretation of data, and drafted the manuscript. CL made significant contributions to the analysis and interpretation of data and writing of the manuscript. XZ made substantial contributions to the project design, acquisition, and interpretation of data. ZZ participated in the acquisition and interpretation of data. All authors agreed to be accountable for the accuracy and integrity of the work. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Liping Ye for her hard work in data cleansing and data analysis.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data from which these findings were drawn is available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This study was funded by the Fundamental Research Funds for the Central Universities of Ministry of Education of China (No. 2017WKYXQY003). The funding body plays no roles in study design, collection, analysis, and interpretation of data, writing of the manuscript, or the decision to submit the manuscript for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- LMIC
low and middle income countries
- AMR
antimicrobial resistance
- EML
Essential Medicines List
- ATC
Anatomical Therapeutic Chemical
- HMPA
Hubei Medical Procurement Administrative Agency
- DDD
defined daily dose
- WHO
World Health Organization
- DPP
defined daily dose equivalence per package
Contributor Information
Yuqing Tang, Email: dr_tyq@163.com.
Chaojie Liu, Email: c.liu@latrobe.edu.au.
Zinan Zhang, Email: zinan19880609@126.com.
Xinping Zhang, Email: xpzhang602@hust.edu.cn.
References
- 1.Dijk KH, Van L. The World medicines situation 2011: rational use of medicines. Geneva: WHO; 2011. [Google Scholar]
- 2.Shankar PR. Medicines use in primary care in developing and transitional countries: fact book summarizing results from studies reported between 1990 and 2006. Bull World Health Organ. 2009;87:804–805. doi: 10.2471/BLT.09.070417. [DOI] [Google Scholar]
- 3.Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 1057;2013:13. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 4.Goossens H. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)70799-6. [DOI] [PubMed] [Google Scholar]
- 5.Hurley R. Can doctors reduce harmful medical overuse worldwide? BMJ Clin Res. 2014;28:g4289. doi: 10.1136/bmj.g4289. [DOI] [PubMed] [Google Scholar]
- 6.Heddini A, Cars O, Qiang S, Tomson G. Antibiotic resistance in China—a major future challenge. Lancet. 2009;373:30. doi: 10.1016/S0140-6736(08)61956-X. [DOI] [PubMed] [Google Scholar]
- 7.Su M, Zhang Q, Lu J, Li X, Tian N, Wang Y, Yip W, Cheng KK, Mensah GA, Horwitz RI, et al. Protocol for a nationwide survey of primary health care in China: the China PEACE (Patient-centered Evaluative Assessment of Cardiac Events) MPP (Million Persons Project) Primary Health Care Survey. BMJ Open. 2017;7:e016195. doi: 10.1136/bmjopen-2017-016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin X, Song F, Gong Y, Tu X, Wang Y, Cao S, Liu J, Lu Z. A systematic review of antibiotic utilization in China. J Antimicrob Chemother. 2013;68:2445–2452. doi: 10.1093/jac/dkt223. [DOI] [PubMed] [Google Scholar]
- 9.Xiao YH, Hou F, Wang J, Yan Q, Sun ZY, Lv XJ. An investigation into socio-economic impact of adverse drug reactions of antibacterial agent irrational use. Chin Health Econ. 2010;29:94–96. [Google Scholar]
- 10.Wang J, Wang P, Wang X, Zheng Y, Xiao Y. Use and prescription of antibiotics in primary health care settings in china. Jama Intern Med. 1914;2014:174. doi: 10.1001/jamainternmed.2014.5214. [DOI] [PubMed] [Google Scholar]
- 11.Aw VDV, Pijpers EJ, Kuyvenhoven MM, Tonkincrine SK, Little P, Verheij TJ. Effectiveness of physician-targeted interventions to improve antibiotic use for respiratory tract infections. Br J Gen Pract. 2012;62:801–807. doi: 10.3399/bjgp12X659268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharyya N, Kepnes LJ. Patterns of care before and after the adult sinusitis clinical practice guideline. Laryngoscope. 2013;123:1588–1591. doi: 10.1002/lary.23980. [DOI] [PubMed] [Google Scholar]
- 13.Coco A, Vernacchio L, Horst M, Anderson A. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics. 2010;125:214–220. doi: 10.1542/peds.2009-1115. [DOI] [PubMed] [Google Scholar]
- 14.Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, O’Brien MA, Johansen M, Grimshaw J, Oxman AD. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Libr. 2012;13:CD000259. doi: 10.1002/14651858.CD000259.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyar OJ, Beovic B, Vlahovic-Palcevski V, Verheij T, Pulcini C. How can we improve antibiotic prescribing in primary care? Exp Rev Anti-infect Ther. 2016;23:9–10. doi: 10.1586/14787210.2016.1151353. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Pulcini C, Rabaud C, Boivin JM, Birgé J. Inventory of antibiotic stewardship programs in general practice in France and abroad. Mã©decine Et Maladies Infectieuses. 2015;45:111. doi: 10.1016/j.medmal.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Zhang X, Liang X, Bloom G. Addressing antimicrobial resistance in China: policy implementation in a complex context. Global Health. 2016;12:30. doi: 10.1186/s12992-016-0167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan X, Liang H, Xue Y, Shi L. An analysis of China’s national essential medicines policy. J Public Health Policy. 2011;32:305–319. doi: 10.1057/jphp.2011.34. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Wang L, Chen W, Zhang L, Jiang H, Mao W. Does economic incentive matter for rational use of medicine? China’s experience from the essential medicines program. Pharmacoeconomics. 2014;32:245–255. doi: 10.1007/s40273-013-0068-z. [DOI] [PubMed] [Google Scholar]
- 20.Gong Y, Yang C, Yin X, Zhu M, Yang H, Wang Y, Li Y, Liu L, Dong X, Cao S, Lu Z. The effect of essential medicines programme on rational use of medicines in China. Health Policy Plan. 2016;31:21–27. doi: 10.1093/heapol/czv008. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Liu C, Ferrier JA, Zhou W, Zhang X. The impact of the National Essential Medicines Policy on prescribing behaviours in primary care facilities in Hubei province of China. Health Policy Plan. 2013;28:750–760. doi: 10.1093/heapol/czs116. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Y, Li L. Legislation of clinical antibiotic use in China. Lancet Infect Dis. 2013;13:189–191. doi: 10.1016/S1473-3099(13)70011-2. [DOI] [PubMed] [Google Scholar]
- 23.The measures for the management of the clinical application of antibacterial drugs. http://www.moh.gov.cn/mohyzs/s3584/201205/54645.shtml. Accessed 2 Oct 2017.
- 24.The Measures for the clinical management of clinical application of antimicrobial agents in medical institutions in Hubei Province. http://www.hbjycg.com/Upload/2012-07-30-10-10-34.pdf. Accessed 2 Oct 2017.
- 25.Koskinen H, Mikkola H, Saastamoinen LK, Ahola E, Martikainen JE. Time series analysis on the impact of generic substitution and reference pricing on antipsychotic costs in Finland. Value Health. 2015;18:1105–1112. doi: 10.1016/j.jval.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care. 2003;19:613. doi: 10.1017/S0266462303000576. [DOI] [PubMed] [Google Scholar]
- 27.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 28.The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD). http://www.who.int/classifications/atcddd/en/. Accessed 1 Aug 2017.
- 29.Lin H, Dyar OJ, Rosales-Klintz S, Zhang J, Tomson G, Hao M, Stalsby Lundborg C. Trends and patterns of antibiotic consumption in Shanghai municipality, China: a 6 year surveillance with sales records, 2009–14. J Antimicrob Chemother. 2016;71:1723–9. [DOI] [PubMed]
- 30.Chandy SJ, Naik GS, Charles R, Jeyaseelan V, Naumova EN, Thomas K, Lundborg CS. The impact of policy guidelines on hospital antibiotic use over a decade: a segmented time series analysis. PLoS ONE. 2014;9:e92206. doi: 10.1371/journal.pone.0092206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newey WK, West KD. Hypothesis testing with efficient method of moments estimation. Int Econ Rev. 1987;28:777–787. doi: 10.2307/2526578. [DOI] [Google Scholar]
- 32.Pourahmadi M. ESTIMATION and interpolation of missing values of a stationary time series. J Time. 2008;10:149–169. doi: 10.1111/j.1467-9892.1989.tb00021.x. [DOI] [Google Scholar]
- 33.Jurij F, Milan IM, Jana M, Damjan K, Stephen C, Samuel C, Gustafsson LL, Luka F, Brian G. The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: findings and implications. Exp Rev Anti-infect Ther. 2015;13:279–289. doi: 10.1586/14787210.2015.990381. [DOI] [PubMed] [Google Scholar]
- 34.Yu W, Li M, Nong X, Ding T, Ye F, Liu J, Dai Z, Zhang L. Practices and attitudes of doctors and patients to downward referral in Shanghai, China. BMJ Open. 2017;7:e012565. doi: 10.1136/bmjopen-2016-012565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Notice regarding national special measure scheme on clinical use of antibiotics in 2011. http://www.nhfpc.gov.cn/. Accessed 2 Oct 2017.
- 36.Bhattacharyya N, Kepnes LJ. Initial impact of the acute otitis externa clinical practice guideline on clinical care. Otolaryngol Head Neck Surg. 2011;145:414–417. doi: 10.1177/0194599811406797. [DOI] [PubMed] [Google Scholar]
- 37.Hogli JU, Garcia BH, Skjold F, Skogen V, Smabrekke L. An audit and feedback intervention study increased adherence to antibiotic prescribing guidelines at a Norwegian hospital. BMC Infect Dis. 2016;16:96. doi: 10.1186/s12879-016-1426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang Y, Liu C, Zhang X. Public reporting as a prescriptions quality improvement measure in primary care settings in China: variations in effects associated with diagnoses. Sci Rep. 2016;6:39361. doi: 10.1038/srep39361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang L, Liu C, Wang L, Yin X, Zhang X. Public reporting improves antibiotic prescribing for upper respiratory tract infections in primary care: a matched-pair cluster-randomized trial in China. Health Res Policy Syst. 2014;12:61. doi: 10.1186/1478-4505-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S, Soumerai SB, Adams AS, Finkelstein JA, Jang S, Rossdegnan D. Antibiotic use following a Korean national policy to prohibit medication dispensing by physicians. Health Policy Plan. 2005;20:302. doi: 10.1093/heapol/czi033. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Ying C, Sufang G, Brant P, Bin L, Hipgrave D. Evaluation, in three provinces, of the introduction and impact of China’s National Essential Medicines Scheme. Bull World Health Organ. 2013;91:184–194. doi: 10.2471/BLT.11.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao Y, Zhao K, Bishai DM, Peters DH. Essential drugs policy in three rural counties in China: what does a complexity lens add? Soc Sci Med. 2013;93:220–228. doi: 10.1016/j.socscimed.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 43.Yang W. How does the pharmaceutical industry influence prescription? A qualitative study of provider payment incentives and drug remunerations in hospitals in Shanghai. Health Econ Policy Law. 2016;11:1–17. doi: 10.1017/S1744133116000086. [DOI] [PubMed] [Google Scholar]
- 44.Yu X, Li C, Shi Y, Yu M. Pharmaceutical supply chain in China: current issues and implications for health system reform. Health Policy. 2010;97:8–15. doi: 10.1016/j.healthpol.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds L, Mckee M. Factors influencing antibiotic prescribing in China: an exploratory analysis. Health Policy. 2008;90:32–6. [DOI] [PubMed]
- 46.Lin H, Dyar OJ, Rosales-Klintz S, Zhang J, Tomson G, Hao M, Stålsby LC. Trends and patterns of antibiotic consumption in Shanghai municipality, China: a 6 year surveillance with sales records, 2009–14. J Antimicrob Chemother. 2016;71:dkw013. doi: 10.1093/jac/dkw013. [DOI] [PubMed] [Google Scholar]
- 47.Ross RK, Hersh AL, Kronman MP, Newland JG, Gerber JS. Cost of antimicrobial therapy across US children’s hospitals. In: IDWeek 2014 meeting of the infectious diseases Society of America; 2014. p. 1242.
- 48.Sun J. Systematic review of interventions on antibiotic prophylaxis in surgery in Chinese hospitals during 2000–2012. J Evid Based Med. 2013;6:126–135. doi: 10.1111/jebm.12048. [DOI] [PubMed] [Google Scholar]
- 49.Xu J, Wang W, Li Y, Zhang J, Pavlova M, Liu H, Yin P, Lu Z. Analysis of factors influencing the outpatient workload at Chinese health centres. BMC Health Serv Res. 2010;10:151. doi: 10.1186/1472-6963-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from which these findings were drawn is available from the corresponding author on reasonable request.


