Abstract
Background
Gremlin has been reported to be up-regulated in glomerular mesangial cells in diabetic nephropathy (DN). However, the regulation of gremlin in podocytes is still rarely reported. This study aimed to investigate the underlying mechanisms by which gremlin mediates the pathogenesis of DN via transforming growth factor-β (TGF-β) signaling pathways.
Material/Methods
Lentiviral and RNAi transfection were performed to increase and decrease gremlin expression in high-glucose conditions. Expression at the mRNA and protein level was detected by RT-qPCR and Western blotting.
Results
The expression of gremlin was significantly higher in high-glucose (HG, 30 mM) than normal-glucose (NG, 5.5 mM) conditions. The gremlin overexpression significantly suppressed the expression of nephrin and synaptopodin. The phosphorylation of canonical TGF-β signaling pathway components, including Smad2/3 and MKK, was increased in the gremlin-overexpressing group. In addition, the expression levels of Bax and cleaved caspase-3 were also higher in the gremlin-overexpressing group. TGF-β pathway inhibitor (SB505124) significantly inhibited TGF-β pathway activity and enhanced the expression of nephrin and synaptopodin.
Conclusions
These results indicate that gremlin can aggravate podocyte lesions through the TGF-β signaling pathway, providing a novel therapeutic target for DN.
MeSH Keywords: Diabetic Nephropathies, Podocytes, Transforming Growth Factor beta1
Background
Diabetic nephropathy (DN) is a serious advanced complication of diabetes that has few effective treatments and is pathologically characterized by glomerular hypertrophy, albuminuria, and accumulation of glomerular matrix, ultimately culminating in tubulointerstitial fibrosis, glomerulosclerosis, and progressive kidney failure [1,2]. Studies have proved that high glucose is a major factor in the development of DN, and podocyte lesions play an important role in the development and progression of DN. The main consequences of podocyte lesions are cell detachment and decreased podocyte number, which result in glomerular basement membrane (GBM) bareness and glomerulosclerosis [3]. Podocytes are highly differentiated cells with poor proliferative ability. Podocyte-related proteins (nephrin and podocin) leak from the slit diaphragm (SD) when podocytes are damaged. Generally, SD damage due to any reason will induce proteinuria [4].
Gremlin is a secretory protein that is 20.7 kDa and composed of 184 amino acids. It is highly conserved and acts as a member of the bone morphogenetic protein (BMP) antagonist family [5]. Gremlin modulates BMP activity to regulate the morphological development of the kidney during embryonic development, then gradually disappears after kidney maturity [6]. However, gremlin expression can be induced when podocytes repair the damage caused by high glucose and excessive force, which conversely cause new damage to the kidneys [7]. In different stages of DN, gremlin is expressed sequentially in podocytes, mesangial cells, and the tubulointerstitial area [8].
It is widely known that the TGF-β signaling pathway is an essential factor in the physiopathology of DN [9]. Li et al. (2013) found that inhibiting canonical TGF-β signaling, including Smad2/3, could inhibit gremlin-induced podocyte injury and that activating endogenous canonical TGF-β1/Smad signaling induced podocyte injury [10]. Thus, the extensive role of the TGF-β signaling pathway makes it attractive for exploring the mechanism underlying DN damage.
In the present study, we evaluated whether gremlin can regulate podocyte lesions induced by high-glucose conditions through the TGF-β signaling pathway.
Material and Methods
Cell culture
Conditionally immortalized mouse podocytes were purchased from the Institute of Basic Medical Sciences of the Chinese Academy of Medical Sciences (Cell Resource Center of Peking Union Medical College, Beijing, China). The cell lines were acquired from the glomeruli of H-2Kb-tsA58 transgenic mice and express SV40-T antigen. The cells were cultured in RPMI 1640 medium (containing 10% FCS and 100 U/ml penicillin–streptomycin). Then, 100 U/ml recombinant mouse γ-interferon was added to induce the H-2Kb promoter and synthesize the conditionally immortalizing tsA58 T antigen (TAg). Five days later, the cells were digested using trypsin and filtered with a 33-mm-pore sieve. Culture dishes were coated with 0.1 mg/ml type-I collagen to promote the adherence of podocytes. The undifferentiated cells were cultured in DMEM-F12 culture medium at permissive conditions (containing 10% FBS, 10 U/ml γ-interferon, 100 U/ml penicillin, 100 μg/ml streptomycin, 33°C, and 5% CO2). After 10–14 days of culture, the cells were induced to differentiate along podocyte lines at non-permissive conditions without γ-interferon (containing 10% FBS, 10 U/ml γ-interferon, 100 U/ml penicillin, 100 μg/ml streptomycin, 37°C, and 5% CO2).
Overexpression and knockdown of gremlin
Gremlin knockdown expression lentiviral plasmid: The lentiviral vector was constructed with the Block-IT™ Lentiviral RNAi Expression System (Invitrogen). The sequence was as follows: forward: 5′-CAC CGCACTATCATC AATCGCTTCTCG AAAGAAGCGATTGAT GATAGT GC-3′; reverse: 5′-AAA AGCACTATC ATCAATCGCTTC TTTCGA GAAGCG ATTGA GATAGT GC-3′. The sequence of a scrambled control construct was as follows: forward: 5′-CAC CGACTACCATTA CCATTGCTTCCGAAG AAGCAATGGTAA TGGTAG TC-3′; reverse: 5′-AAA AGACTACCATTA CCATTGCTTCTT CGGAAGCAATGGTAA TGGTAG TC-3′. The above primer sequences were inserted into pLenti6/Block-IT vectors [11]. The annealing reaction system included 10 μl of 100 nmol/μl forward primer and 10 μl of 100 nmol/μl reverse primer. The reaction was held at 95°C in a water bath for 2 min and cooled naturally to room temperature before being held on ice for 3 min. RNA was extracted and cDNA was generated through reverse transcription. The gremlin gene was amplified using the above primers, and the fragments were separated using gel electrophoresis. In addition, the fragments were purified using gel electrophoresis. The subsequent steps included a coupled reaction, conversion, plasmid extraction, and enzyme identification. Stable transfected cells were screened for drug resistance. Recombinant gremlin growth factor was utilized to establish overexpression. The optimal concentration of sterile gremlin recombinant growth factor was 0.75 μg/ml. The subsequent steps were similar to those described for the above experiments.
Transfection of lentivirus plasmid
Podocytes were inoculated into 6-well plates at a concentration of 5×104 cells/well and incubated at 37°C and 5% CO2 for 24 h. When cell confluence reached 30% to 45%, the lentiviral plasmid was mixed with polybrene to a concentration of 4.8 μg/ml and then added into the plates. Twelve hours later, the culture medium on the cells was replaced with normal culture medium. Forty-eight hours later, the cell culture medium was changed to 21 μg/ml puromycin medium to select for drug-resistant cells.
Western blot analysis
Total protein was extracted from podocytes and quantified using the Coomassie brilliant blue method. Eighty-microgram protein samples were denatured in 100°C water for 3 min and rapidly centrifuged for 15 s. A 10% SDS polyacrylamide gel was fixed in an electrophoresis tank, and protein samples were slowly loaded into the wells. Gel electrophoresis was completed at a constant voltage of 90 V. Proteins were transferred to a PVDF membrane by electrophoresis in low-temperature conditions. The PVDF membrane was blocked in TBST with 5% fat-free milk for 2 h. The diluted gremlin antibody (1: 300) and β-actin antibody (1: 1000) were added and incubated with the membrane at 4°C overnight. The PVDF membrane was washed 3 times for 10 min each time. Then, a secondary antibody, IgG (rabbit, 1: 1000) tagged with horseradish peroxidase, was added and incubated with the membrane at 4°C overnight. The bands on the PVDF membrane were visualized using the Odyssey FC imaging system. The Western blot bands were quantitatively analyzed with Lab Works 4.5 software (UVP, US). Results are expressed as the ratio of the optical density of the target and β-actin bands.
Real-time quantitative PCR
Total RNA was extracted from podocytes. The purity and concentration of RNA were determined using an ultraviolet spectrophotometer at 260–280 nm. The OD260/OD280 ratio was calculated to estimate the purity of RNA. It indicated that the purity of the RNA samples met the requirements and was free from residual protein when the ratio was higher than 1.8. A 20-μl reaction volume (containing 2 μl total RNA) was used to synthesize cDNA. All the primers were synthesized by Shanghai Sangon Biotech Company. The primer pairs used for PCR amplification were: GAPDH forward: 5′-CCCACTAACATCAAATGGGG-3′; reverse: 5′-ATCCACAGTCTTCTGGGTGG-3′ and gremlin forward: 5′-AAGCGAGACTGGTGCAAAAC-3′; reverse: 5′-CTTGCAGAAGGAGCAGGACT-3′. The reaction conditions were: a pre-denaturation step was completed at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, 60°C for 60 s, and a final elongation step at 72°C for 10 min. The relative levels of gene expression were normalized to GAPDH and calculated using the 2−ΔΔCt method.
Statistical analysis
Experiments were carried out at least in triplicate. Quantitative data are expressed as a mean ± standard deviation (SD). Differences were analyzed using the t test and one-way ANOVA. A p value<0.05 was considered statistically significant.
Results
The expression of gremlin in podocytes
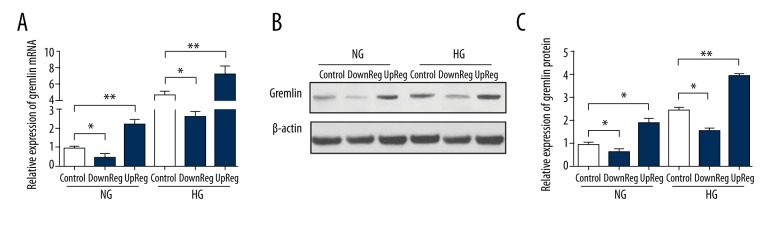
In this study, we constructed stable cell lines with increased or decreased gremlin expression using a lentivirus. The expression of gremlin protein and mRNA was detected by Western blotting and RT-qPCR. Figure 1 shows that lentiviral or siRNA transfection effectively decreased or increased the expression levels of gremlin mRNA and protein in HG conditions (30 mM) or NG condition (5.5 mM). These results indicate that the gremlin-overexpressing and knockdown models were successfully constructed.
Figure 1.
Lentiviral-mediated gremlin expression in podocytes. (A) The expression of gremlin mRNA was detected by RT-qPCR. (B, C) The expression of gremlin protein was analyzed by Western blotting. The data represent the means ±SD of 3 experiments. * P<0.05 compared with the control group. DownReg represents knockdown, upReg represents overexpression.
Gremlin regulates podocyte-associated proteins
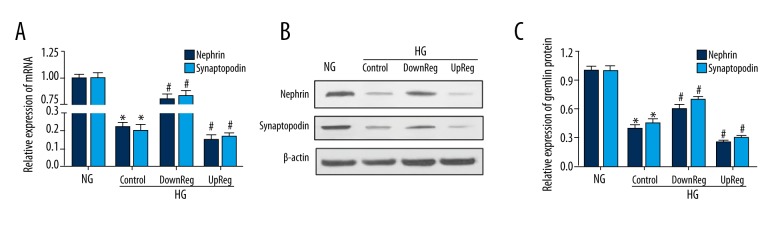
Gremlin rescued the expression of nephrin and synaptopodin at the protein levels in podocytes treated with high glucose. Western blotting and RT-qPCR identified the expression of nephrin and synaptopodin in podocytes under HG conditions. Figure 2A shows the mRNA expression of nephrin and synaptopodin. Figure 2B shows the protein expression of nephrin and synaptopodin. The Western blotting and RT-qPCR results show that the expression of nephrin and synaptopodin was inhibited by high glucose. Moreover, overexpression of gremlin inhibited the expression of these proteins more clearly, while knockdown of gremlin partly reversed this inhibition.
Figure 2.
The expression of nephrin and synaptopodin was detected by Western blotting and RT-qPCR. (A) The expression of nephrin and synaptopodin mRNA was detected by RT-qPCR. (B, C) The expression of nephrin and synaptopodin was analyzed by Western blotting. * P<0.05 compared with the NG group.
The effects of gremlin regulation on corresponding TGF-β signaling pathways
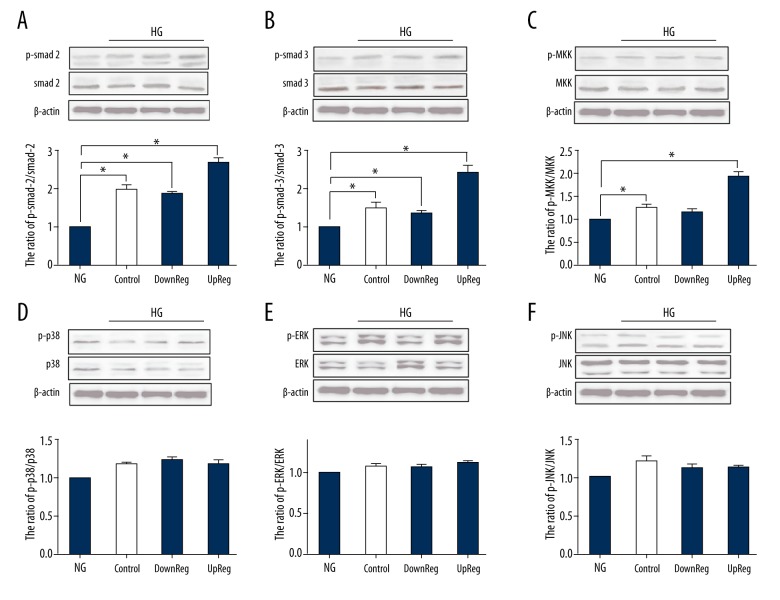
The phosphorylation of Smad2/3, p38, MKK, JNK and ERK1/2 was detected by Western blotting. Figure 3 shows that phosphorylation of the canonical TGF-β signaling pathway, including Smad2/3 and MKK, was increased in the gremlin-overexpressing group. Reduced expression of gremlin suppressed the activation of these proteins relative to that in the control group in HG. Other components of the TGF-β signaling pathway, such as p38, JNK, and ERK, were not affected by changes in gremlin expression.
Figure 3.
The activity of the corresponding TGF-β signaling pathway was detected by Western blotting. (A) The expression of gremlin protein was analyzed by Western blotting. (B–G) The ratio of p-Smad2/Smad2, p-Smad3/Smad3, p-MKK/MKK, p-p38/p38, p-ERK/ERK, and p-JNK/JNK. * P<0.05 compared with the NG group.
A TGF-β receptor inhibitor (SB505124) up-regulates nephrin and synaptopodin expression
A TGF-β receptor inhibitor (SB505124) was used to confirm the effect of gremlin on the TGF-β signaling pathways. SB505124 was added to the gremlin-overexpressing and knockdown groups. Figure 4 shows that SB505124 up-regulated the expression of nephrin and synaptopodin in the gremlin-overexpressing and knockdown groups. The above results revealed that SB505124 increased the expression of nephrin and synaptopodin in HG-treated podocytes.
Figure 4.
The effect of SB505124 on nephrin and synaptopodin expression. (A) nephrin and synaptopodin protein expression levels were detected by Western blotting. (B, C) Quantitated protein levels of nephrin and synaptopodin. * P<0.05 vs. the control group.
Gremlin regulates the apoptosis of podocytes
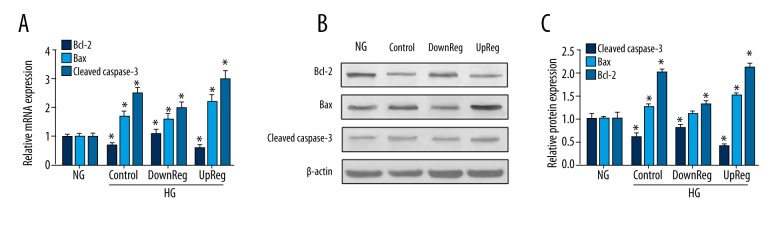
To examine podocyte apoptosis, the expression levels of Bcl-2, Bax, and cleaved caspase-3 were detected by Western blotting and RT-PCR. Figure 5 shows that HG increased cleaved caspase-3 and Bax expression and decreased Bcl-2 expression. In the gremlin knockdown group, the expression of cleaved caspase-3 and Bax decreased significantly. In the gremlin-overexpressing group, the expression of Bax and cleaved caspase-3 increased, while the expression of Bcl-2 decreased. Based on these results, we conclude that gremlin overexpression accelerates the apoptosis of podocytes.
Figure 5.
The effect of gremlin regulation on podocyte apoptosis. (A) mRNA expression levels were detected by RT-qPCR analysis in different groups. (B, C) The protein levels of Bcl-2 and Bax were quantified by Western blotting. * P<0.05 vs. the NG group.
Discussion
Gremlin, a member of the BMP antagonist family, is up-regulated in chronic kidney disease, and gremlin expression is correlated with TGF-β pathway activity [12]. In vitro and in vivo experiments have demonstrated that gremlin inhibition is beneficial in treating DN [13,14]. Podocytes are critical in the pathogenesis of chronic kidney disease and DN [15,16]. However, in vitro studies on the relationship between gremlin and podocyte injury are still rare. Building on existing studies, our study aimed to explore how the regulation of gremlin affects podocyte injury through the TGF-β signaling pathways.
We established an in vitro podocyte diabetic nephropathy cell model induced by high glucose (30 mM). The expression of gremlin in podocytes transfected with lentivirus and siRNAs was significantly higher or lower than that in the control group (Figure 1). The results indicated that gremlin overexpression suppresses the expression of nephrin and synaptopodin (Figure 2). Nephrin and synaptopodin are vital molecules that play important roles in the establishment and maintenance of the cytoskeleton [17,18]. They are crucial for maintaining podocyte morphology and function. Recovery of the expression and distribution of nephrin and synaptopodin reduces podocyte apoptosis, and these proteins play a vital role in podocyte protection [19–21]. The SD of podocytes functions as a barrier in the glomerular capillary wall to retrieve plasma proteins [22]. Nephrin is located at the outer layer of the plasma membranes of the SD. Saleem et al. (2002) demonstrated that the relationship between nephrin/podocin and filamentous actin is closely intertwined, and the disruption of nephrin/podocin could be a final common pathway leading to foot process effacement in DN [23].
It is widely known that the TGF-β signaling pathway is an essential factor in the physiopathology of DN [9,24]. Our results show that the phosphorylation of Smad2, Smad3, and MKK was clearly higher in the gremlin-overexpressing group. In contrast, the phosphorylation of p38, JNK, and ERK exhibited less variation (Figure 3). Li et al. (2013) found that inhibiting canonical TGF-β signaling (primarily Smad2/3, not JNK or p38) inhibited the gremlin-mediated injury of podocytes and likely activated endogenous canonical TGF-β1/Smad signaling to induce podocyte injury (10). We used a TGF-β receptor inhibitor (SB505124) to investigate the effect of gremlin on the TGF-β signaling pathways. In the gremlin knockdown and gremlin-overexpressing groups, SB505124 reduced the inhibition of gremlin on nephrin and synaptopodin relative to that observed in the control group (Figure 4). This indicates that the inhibition of TGF-β by SB505124 induced the distribution of nephrin and synaptopodin to return to physiological status. Rodrigues et al. (2013) reported that gremlin increased TGF-β production and directly activated Smad signaling through a TGF-β-independent process [25].
Podocyte apoptosis is correlated with the early stages of DN. Combined with previous results from other researchers, our laboratory has demonstrated an important role of gremlin in the regulation of podocyte apoptosis. Cell apoptosis-related proteins (Bcl-2 and Bax) are activated in the pathophysiological processes underlying DN. The Bcl-2 mRNA and protein levels in the gremlin-overexpressing group were lower than those in the gremlin knockdown group (Figure 5). Comparison of the expression of Bcl-2, Bax, and cleaved caspase-3 revealed that gremlin overexpression aggravates podocyte apoptosis. Lee et al. (2009) found that cleaved caspase-3 and Bax expression was significantly increased, whereas Bcl-2 expression was significantly decreased in HG-stimulated DN podocytes [26].
Conclusion
Our results indicate that gremlin regulates glomerular podocyte lesions through the TGF-β signaling pathway. The above findings provide a novel understanding of the function of gremlin in podocyte lesions and represent a significant contribution to a therapy for DN.
Footnotes
Conflicts of interest
None.
Source of support: None
References
- 1.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. New Engl J Med. 2008;358:580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 2.Haneda M, Utsunomiya K, Koya D, et al. A new classification of Diabetic Nephropathy 2014: A report from Joint Committee on Diabetic Nephropathy. Clin Exp Nephrol. 2015;19:1–5. doi: 10.1007/s10157-014-1057-z. [DOI] [PubMed] [Google Scholar]
- 3.Weil EJ, Lemley KV, Yee B, et al. Podocyte detachment in type 2 diabetic nephropathy. Am J Nephrol. 2011;33:21–24. doi: 10.1159/000327047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pichaiwong W, Hudkins KL, Wietecha T, et al. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol. 2013;24:1088–102. doi: 10.1681/ASN.2012050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topol LZ, Bardot B, Zhang Q, et al. Biosynthesis, post-translation modification, and functional characterization of Drm/Gremlin. J Biol Chem. 2000;275:8785–93. doi: 10.1074/jbc.275.12.8785. [DOI] [PubMed] [Google Scholar]
- 6.Michos O, Panman L, Vintersten K, et al. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development (Cambridge, England) 2004;131:3401–10. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- 7.Lappin DW, McMahon R, Murphy M, Brady HR. Gremlin: An example of the re-emergence of developmental programmes in diabetic nephropathy. Nephrol Dial Transplant. 2002;17(Suppl 9):65–67. doi: 10.1093/ndt/17.suppl_9.65. [DOI] [PubMed] [Google Scholar]
- 8.Dolan V, Murphy M, Sadlier D, et al. Expression of gremlin, a bone morphogenetic protein antagonist, in human diabetic nephropathy. Am J Kidney Dis. 2005;45:1034–39. doi: 10.1053/j.ajkd.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Wang WN, Zhang WL, Zhou GY, et al. Prediction of the molecular mechanisms and potential therapeutic targets for diabetic nephropathy by bioinformatics methods. Int j Mol Med. 2016;37:1181–88. doi: 10.3892/ijmm.2016.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G, Li Y, Liu S, et al. Gremlin aggravates hyperglycemia-induced podocyte injury by a TGFbeta/smad dependent signaling pathway. J Cell Biochem. 2013;114:2101–13. doi: 10.1002/jcb.24559. [DOI] [PubMed] [Google Scholar]
- 11.Maciel TT, Melo RS, Campos AH. The bone morphogenetic protein antagonist gremlin promotes vascular smooth muscle cell apoptosis. J Vasc Res. 2009;46:325–32. doi: 10.1159/000189793. [DOI] [PubMed] [Google Scholar]
- 12.Shankland SJ. The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–47. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 13.Roxburgh SA, Kattla JJ, Curran SP, et al. Allelic depletion of grem1 attenuates diabetic kidney disease. Diabetes. 2009;58:1641–50. doi: 10.2337/db08-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Shi Y, Wada J, et al. In vivo delivery of Gremlin siRNA plasmid reveals therapeutic potential against diabetic nephropathy by recovering bone morphogenetic protein-7. PLoS One. 2010;5:e11709. doi: 10.1371/journal.pone.0011709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Nino MD, Benito-Martin A, Ortiz A. New paradigms in cell death in human diabetic nephropathy. Kidney Int. 2010;78:737–44. doi: 10.1038/ki.2010.270. [DOI] [PubMed] [Google Scholar]
- 16.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–84. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 17.Rezende GM, Viana VS, Malheiros DM, et al. Podocyte injury in pure membranous and proliferative lupus nephritis: Distinct underlying mechanisms of proteinuria? Lupus. 2014;23:255–62. doi: 10.1177/0961203313517152. [DOI] [PubMed] [Google Scholar]
- 18.Lioudaki E, Stylianou KG, Petrakis I, et al. Increased urinary excretion of podocyte markers in normoalbuminuric patients with diabetes. Nephron. 2015;131:34–42. doi: 10.1159/000438493. [DOI] [PubMed] [Google Scholar]
- 19.Szeto CC, Wang G, Chow KM, et al. Podocyte mRNA in the urinary sediment of minimal change nephropathy and focal segmental glomerulosclerosis. Clin Nephrol. 2015;84:198–205. doi: 10.5414/CN108607. [DOI] [PubMed] [Google Scholar]
- 20.Patrie KM, Drescher AJ, Welihinda A, et al. Interaction of two actin-binding proteins, synaptopodin and alpha-actinin-4, with the tight junction protein MAGI-1. J Biol Chem. 2002;277:30183–90. doi: 10.1074/jbc.M203072200. [DOI] [PubMed] [Google Scholar]
- 21.Asanuma K, Kim K, Oh J, et al. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest. 2005;115:1188–98. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawachi H, Miyauchi N, Suzuki K, et al. Role of podocyte slit diaphragm as a filtration barrier. Nephrology (Carlton, Vic) 2006;11:274–81. doi: 10.1111/j.1440-1797.2006.00583.x. [DOI] [PubMed] [Google Scholar]
- 23.Saleem MA, Ni L, Witherden I, et al. Co-localization of nephrin, podocin, and the actin cytoskeleton: Evidence for a role in podocyte foot process formation. The Am J Pathol. 2002;161:1459–66. doi: 10.1016/S0002-9440(10)64421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiffer M, Schiffer LE, Gupta A, et al. Inhibitory smads and tgf-Beta signaling in glomerular cells. J Am Soc Nephrol. 2002;13:2657–66. doi: 10.1097/01.asn.0000033276.06451.50. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues-Diez R, Rodrigues-Diez RR, Lavoz C, et al. Gremlin activates the Smad pathway linked to epithelial mesenchymal transdifferentiation in cultured tubular epithelial cells. Biomed Res Int. 2014;2014:802841. doi: 10.1155/2014/802841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SH, Yoo TH, Nam BY, et al. Activation of local aldosterone system within podocytes is involved in apoptosis under diabetic conditions. Am J Physiol Renal Physiol. 2009;297:F1381–90. doi: 10.1152/ajprenal.00101.2009. [DOI] [PubMed] [Google Scholar]