Abstract
Background
This study was designed to investigate the potential anticonvulsant and neuroprotective effects of methylene blue (MB) on self-sustaining status epilepticus (SSSE) induced by prolonged basolateral amygdala stimulation (BLA) in Wistar rats.
Material/Methods
The rats were randomly divided into 4 groups: (1) the Control group (rats without any treatment); (2) the Sham group (rats received electrode implantation but without electrical stimulation); (3) the SSSE group (rats received electrode implantation and additional electrical stimulation); and (4) the SSSE+MB group (rats received 1 mg/kg MB intraperitoneal injection 5 min after SSSE). SSSE models were established by prolonged BLA stimulation. The severities of SSSE were assessed by the number of separate seizures and the accumulated time of seizures. The variations of malondialdehyde/glutathione (MDA/GSH) were assessed 24 h after the establishment of SSSE. Nissl staining was performed to detect the surviving neurons in hippocampal CA1 and CA3 regions, and Western blotting assays were used to detect Caspase-3 (CASP3), B cell lymphoma 2 (BCL2), and BCL2-associated X protein (BAX).
Results
Compared with the SSSE group, treatment with MB (1) markedly reduced the number and accumulated time of seizure activities; (2) significantly attenuated the increase of MDA and the decrease of GSH hippocampal levels; (3) markedly improved the cell morphology and alleviated the neuronal loss in hippocampal CA1 and CA3 regions; (4) significantly attenuated the increase of CASP3 and BAX and the decrease of BCL2 hippocampal levels.
Conclusions
MB has a protective effect in the SSSE model and may be useful as an adjuvant for preventing or treating epilepsy in humans.
MeSH Keywords: Apoptosis, Methylene Blue, Neuroprotective Agents, Oxidative Stress, Status Epilepticus
Background
Epilepsy is one of the most prevalent neurological disorders. Temporal lobe epilepsy (TLE), the most common form of epilepsy among adults, is characterized by recurrent seizures deriving from the medial structures of the temporal lobe, including the hippocampus and amygdala [1]. Status epilepticus (SE) is defined as seizures lasting longer than 30 min or failing to regain consciousness within that interval [2]. SE is a serious threat to human health due to its high morbidity and mortality rates, and it carries a high risk of progression to epilepsy or other neurological diseases (such as depression and cognitive impairment). Although the existing medications can symptomatically suppress seizure activities to a certain degree, they lose effectiveness or produce adverse effects in about one-third of epileptic patients [3]. Additional, the present antiepileptic drugs (AEDs) are often related to adverse effects, such as cognitive injury or psychological problems [4]. Therefore, it is imperative to speed up studies of new antiepileptic drugs to control refractory epilepsy and to prevent the development of epileptogenesis.
A growing number of studies suggest that oxidative stress underlies the pathogenesis of several neurological conditions, including Alzheimer disease, Parkinson disease, and epilepsy [5–7]. Oxidative stress can induce excessive accumulation of free radicals, which are involved in the development of TLE and SE [8]. Furthermore, oxidative stress is implicated in neuronal injury and is a potential mechanism of epileptic activity [9,10]. Based on these facts, it is meaningful to search for more powerful neuroprotective agents that can mitigate the oxidative stress injury during epileptic seizure.
In addition to the above-mentioned oxidative stress damage, SE can cause obvious brain damage characterized by neuronal cell loss, which preferentially occurs in the hippocampus in human and animal models [11,12]. Animal models of epilepsy have shown that the pattern of SE-related neuronal cell death includes apoptosis and necrosis [13–15]. The neuronal cell death during SE is caused by overactivation of glutamate receptors, which subsequently leads to calcium influx, and ultimately causes activation of proteolytic enzymes and collapse of intracellular organelles. The neuronal cell death, in turn, contributes to the development of epileptogenesis [16]. Thus, neuroprotective strategies are increasingly seen as a promising therapy to prevent and/or manage epileptic conditions.
Methylene blue (MB) is a water-soluble thiophenazine compound widely used in clinical treatment of multitudinous diseases. Previous studies have demonstrated that MB exerts diverse pharmacological effects such as anti-inflammatory, antioxidant, and anti-apoptosis effects [17–19]. Therefore, in recent years, the role of MB in the treatment of various central nervous system diseases has generated widespread interest. It has already been confirmed that MB produces a nerve-protective effect in ischemic brain damage, Leber optic neuropathy, Alzheimer disease (AD), Parkinson disease, and other neurodegenerative diseases [20–22]. MB interferes with the assembly of tau protein and effectively prevents its accumulation in the brain in AD patients, and it is believed to be the most advanced tau aggregation inhibitor in clinical development for the treatment of AD [23–24]. Some researchers have reported that antioxidants may provide partial neuroprotection [8,9,25]. Thus, due to the powerful antioxidant property of MB, it is plausible to hypothesize that MB has a neuroprotective role in epilepsy.
The model of basolateral amygdala (BLA) kindling has been well established to explore the basic mechanisms underlying epileptogenesis and the development of therapeutic substitution. After the implantation of bipolar stimulation electrodes in the BLA, a high-intensity (700 μA) stimulus string lasting 25 min can induce self-sustaining status epilepticus (SSSE) [26]. The objective of the present study was to determine the roles of MB in SSSE induced by prolonged BLA stimulation in Wistar rats.
Material and Methods
Animals and reagents
A total of 50 healthy adult male Wistar rats, weighing 180–230 g, were purchased from the Experimental Animal Center of Hebei Medical University. They were housed at a constant temperature (25+2°C), provided a fixed 12-h alternating cycle of light and dark, and allowed free access to food and water. All animals were handled according to the guidelines for experimental animal management issued by the Ministry of Science and Technology of the People’s Republic of China [1988] No. 134. Great efforts were made to minimize the number of animals used and to minimize their pain.
MB was purchased from Qichuan Pharmaceutical Group co., LTD (Jiangsu, China). The malondialdehyde (MDA) and glutathione (GSH) detection kits were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China).
Experimental design and procedure
The rats were randomly divided into 4 groups: (1) Control group (rats without any treatment); (2) Sham group (rats received electrode implantation but without electrical stimulation); (3) SSSE group (rats received electrode implantation and additional electrical stimulation); (4) SSSE+MB group (rats received 1 mg/kg MB intraperitoneal injection 5 min after establishment of SSSE). The Control and Sham groups contained 12 rats each, and the SSSE and SSSE+MB groups contained 13 rats each. MB was diluted in 0.9% saline and the concentration was based on a previous report [27], which showed that 1 mg/kg MB had a better protective effect.
For electrodes implantation, the rats were deeply anesthetized by pentobarbital (45 mg/kg, i.p.) and mounted on a stereotaxic instrument (RWD Life Science, China). Body temperature was maintained at 37°C with a heating pad throughout the surgery. The stimulating electrodes were made of twisted stainless-steel Teflon-coated wires (0.1 mm in diameter; A.M. Systems. Inc., USA). The terminal insulated layer was stripped about 0.5 mm and the bipolar distance was 0.5–0.7 mm. According to the Rat Brain in Stereotaxic Coordinates of Paxinos and Watson (6th edition), the twisted electrodes were implanted into the right BLA (AP=−2.2 mm, ML=−4.7 mm, DV=8.5 mm). One stainless-steel screw was implanted in the right occipital epidural to be used as a ground electrode. Three other screws were properly implanted in order to fix the dental cement. All electrodes were connected to a miniature receptacle and attached to the skull with the screws using acrylic dental cement. After surgery, the animals were housed individually and were allowed to recover for at least 10 days.
Determination of ADT and induction of SSSE
Electrical stimulation was delivered by a constant-current stimulator (Master 8, AMPI, Israel), and electroencephalograms (EEG) at the BLA were recorded with a PowerLab system (AD instruments, Australia). The day before the induction of SSSE, after-discharge threshold (ADT) of each rat was determined with a 1 s stimulus of 60 Hz monophasic square-waves at 1 ms per pulse. The stimulation intensity of ADT was measured from 60 μA, increased by 20 μA per 30 min until at least 5 s of the discharge spike wave was recorded, and the minimum current intensity was designated as ADT for that animal. The rats were grouped based on the ADT value, and we ensured that the average of threshold in each group was at an approximately comparable level. The range of ADT was 80–300 μA. If it exceeded 300 μA, we considered the position of stimulating electrodes was poor, and ruled the animal out of the next experiment. At 24 h after the determination of ADT, the stimulation of the BLA for induction of SSSE was performed as previously described [26]. In short, the stimulus program consisted of 100-ms trains of 1 ms biphasic square wave pulses, and the trains were given at a frequency of 2/s and the intra-train pulse frequency was 50/s. Peak pulse intensity was 700 μA and the stimulation lasted 25 min in total. After the cessation of stimulation, an EEG of amygdala was recorded through the same electrodes. As previously described by Nissinen [28], a prominent feature of SSSE on EEG recording is the appearance of high-amplitude (>2×baseline) and high-frequency (>8 Hz) discharges (HAFDs) that lasted for at least 5 s. The end of the electrographic seizure was characterized by a brief (1–3 s) ‘flat period’ on the EEG (Figure 1). The HAFDs were repeated intermittently for at least 15 min, and we confirmed the successful establishment of SSSE. Five minutes later, the rats in drugs-treated groups received an intraperitoneal injection of MB. Then, the animals were monitored by continuous EEG recordings for 12 h. The severities of SSSE in different groups were assessed by the quantity of separate seizures and the accumulated time (total recording time minus interictal time) of seizures [29], which reflected the effect of MB on controlling acute seizures, as well as its neuroprotective effect.
Figure 1.
Representative electrographic recordings from the right basolateral amygdala during self-sustaining status epilepticus (SSSE). A prominent feature of beginning of SSSE on EEG recording was the appearance of high-amplitude (>2×baseline) and high-frequency (>8 Hz) discharges (HAFDs, indicated with solid arrows). The end of HAFD was typically followed by brief (1–3 s) “flat periods” on the EEG (indicated by a star). They were recorded 10 and 30 min (A and B, respectively) after 25-min amygdala stimulation.
Tissue collection
At 24 h after MB treatment, the animals were anesthetized with 10% chloral hydrate (4.0 ml/kg, i.p.). For biochemical and Western blotting assays, the rats were killed by decapitation. The bilateral hippocampus tissues in the brain were rapidly stripped off on ice and stored separately in freezing tubes. Then, the tissues were frozen in liquid nitrogen and stored at −80°C for subsequent detection. For Nissl staining, at 24 h after MB treatment, the rats were transcardially perfused with 0.9% saline solution followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer solution (pH=7.3). Then, the brains were removed, post-fixed in the same solution for 24 h at 4°C, and soaked in 30% sucrose solutions until they sank to the bottom. Later, they were embedded with O.C.T. compound, frozen in liquid nitrogen, and kept at −80°C until cut.
Assessment of oxidative stress-related markers
The left hippocampus was assigned to detect the content of oxidative stress markers (n=6 for each group). For the experimental procedures, the hippocampus tissue was prepared as a 10% tissue homogenate in ice-cold 0.9% saline solution. After centrifugation (3500 rpm, 4°C, 15 min), the supernatant fraction of homogenates was collected to detect the levels of MDA and GSH. MDA, used as a marker of lipid peroxidation, was determined by testing thiobarbituric acid reacting substances (TBARS) in the supernatant, according to a previous study [30]. GSH was measured according to the manufacturer’s instructions, which are based on the spectrophotometric method as described before [31].
Nissl staining
Coronal sections were cut on a freezing microtome (Leica, Germany) at a thickness of 20 μm for Nissl staining with 1% thionine for 5 min. For every fifth section (n=6 for each group, 6 sections per animal), the number of surviving intact pyramidal neurons per 1-mm length of both CA1 and CA3 subfields of the hippocampus were counted using a light microscope (Olympus, USA). The sections were observed by 2 observers blinded to the treatment history.
Western blotting assays for apoptosis-related markers
The right hippocampus was assigned to detect the protein expression of Caspase-3 (CASP3), B-cell lymphoma 2 (BCL2), and BCL2-associated X protein (BAX) using Western blotting assays (n=6 for each group). For protein extraction, tissue was collected and added to RIPA buffer with 1% PMSF and then homogenized in lysis buffer on ice. Total proteins were extracted following the manufacturer’s instructions (Applygen Technologies, Inc., China). After determining protein concentrations, samples (30–50 μg) were separated on 10% sodium dodecyl sulfate-polyacrylamide gels and subsequently transferred to immobile polyvinylidene difluoride membranes. Nonspecific bindings were blocked with 5% nonfat milk in TBST buffer (pH=7.4) for 1 h, then the membranes were incubated with the following primary antibodies overnight at 4°C: anti-CASP3 (1: 500; Bioworld, USA), BCL2 (1: 500; Bioworld, USA), BAX (1: 500; Bioworld, USA), and anti-β-actin (1: 1000; Santa Cruz, CA, USA). Subsequently, after washing with 0.1% TBST, membranes were incubated with secondary antibody (1: 4000; Rockland, Gilbertsville) for 1 h at room temperature and washed in 0.1% TBST solution. Bands were detected using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA) and the relative density of bands was estimated using Image J analysis software.
Statistical analysis
The experiment results are presented as the mean ±SEM. All statistical analyses were conducted using SPSS 22.0 Statistics Software for Windows. Means of the number and accumulated time of seizure activities between SSSE and SSSE+MB was compared using the unpaired t test. Means among multiple groups were compared by one-way ANOVA followed by least significant difference (LSD) post hoc tests for different pair-wise comparisons. A difference was considered statistically significant if P<0.05.
Results
MB treatment reduced the number and accumulated time of seizure activities of SSSE
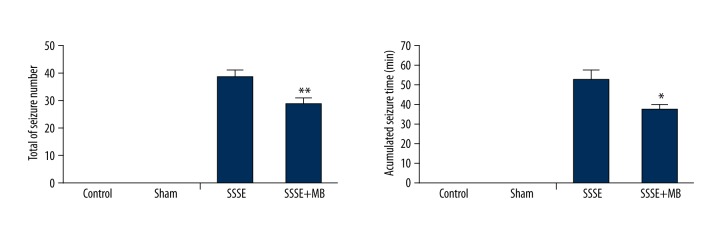
After 25 min of prolonged stimulus at BLA, 1 rat in the SSSE+MB group failed to induce SSSE and 1 rat in the SSSE group died during the stimulus process. These rats were excluded from the follow-up experiments. At 12 h after MB treatment, we calculated the final number of seizure activities and the cumulative time from the EEG data. As shown in Figure 2, there were 24–50 (38.92±2.41) seizure activities in the SSSE group and the cumulative time was 25–86 min (52.58±4.96). However, the eventual number (28.92±1.99) and cumulative time (37.25±3.18) of seizure activities in the SSSE+MB group were significantly decreased compared to the SSSE group (P<0.01; P<0.05, respectively). In the Control and Sham groups, there were no seizure activities.
Figure 2.
Effect of MB treatment on the number and cumulative time of seizure activities of SSSE. Values are expressed as mean ±SEM. (n=12 per group) (unpaired T test) * P<0.05 vs. SSSE group; ** P<0.01 vs. SSSE group.
Effects of MB on MDA and GSH in SSSE rats
At 24 hours after establishment of SSSE, the SSSE group showed a marked increase in MDA content (P<0.01) and reduction of GSH (P<0.01) vs. the Control group. However, treatment with MB significant lowered MDA (P<0.01) and raised the activity of GSH (P<0.01) compared with the SSSE group. There were no significant differences between the Sham group and the Control group regarding the 2 parameters (P>0.05) (Figure 3). Those results suggest that MB markedly changed the detrimental trends of oxidative stress markers during SSSE.
Figure 3.
Effect of MB treatment on the levels of MDA and GSH in left hippocampus 24 h after establishment of SSSE. Values are expressed as mean ±SEM. (n=6 per group) (one-way ANOVA) ** P<0.01 vs. Control group; ## P<0.01 vs. SSSE group.
Effects of MB on the loss of neurons in hippocampus during SSSE
Nissl staining showed the surviving neurons in hippocampal CA1 and CA3 regions at 24 h after establishment of SSSE. As shown in Figure 4, neurons of hippocampal CA1 and CA3 in the Control group displayed integrative and well-maintained morphology. However, in the SSSE group, the surviving pyramidal neurons in both CA1 and CA3 appeared sparse and shrunken, and their number significantly decreased compared with the Control group (P<0.01). Treatment with MB markedly improved the cell morphology and alleviated the neuronal loss compared with the SSSE group (P<0.01). There was no significant difference in the number of surviving neurons between the Sham group and the Control group (P>0.05).
Figure 4.
Representative photo of Nissl staining of the hippocampal CA1 (A–D) and CA3 (E–H) pyramidal neurons at 24 h after establishment of SSSE (400×). (A, E) Control group, showing normal pyramidal neurons. (B, F) Sham group, showing no significant changes compare with Control group. (C, G) SSSE group, showing loss of pyramidal neurons. (D, H) SSSE+MB group, showing the effect of MB on neuronal loss. (I) Quantitative representation of the expression of remaining pyramidal neurons in CA1. (J) Quantitative representation of the expression of remaining pyramidal neurons in CA3. Results are expressed as means ±SEM. (n=6 per group) (one-way ANOVA) ** P<0.01 vs. Control group; ## P<0.01 vs. SSSE group.
Effects of MB on expression of protein contents of CASP3, BCL2, and BAX in the hippocampus during SSSE
As shown in Figure 5, normal levels of CASP3, BCL2, and BAX in hippocampus were observed in the Control and Sham groups, and there was no significant difference between these 2 groups. Compared with the Control group, the level of CASP3 in the SSSE group was remarkably increased 24 h after establishment of SSSE (P<0.05), whereas treatment with MB significantly weakened the increase of CASP3 during SSSE (P<0.05). Unlike CASP3, BCL2 activity was notably decreased compared with the Control group (P<0.05), and treatment with MB inhibited the declining trend of BCL2 activity (P<0.05). The variable trend of BAX shared the same pattern with CASP3, which decreased during SSSE, and treatment with MB prevented this decreasing trend (P<0.05).
Figure 5.
Effects of MB on expression of protein contents of CASP3, BCL2, and BAX in right hippocampus. (A) Western blotting protein bands. (B) The relative level of CASP3 of the hippocampus in the 4 groups. (C) The relative level of BCL2 of the hippocampus in the 4 groups. (D) The relative level of BAX of the hippocampus in the 4 groups. Results are expressed as means ±SEM. (n=6 per group) (one-way ANOVA) * P < 0.05 vs. Control group; # P<0.05 vs. SSSE group.
Discussion
In our study, we found that treatment with MB: (1) markedly reduced the number and accumulated time of seizure activities; (2) significantly attenuated the increase of MDA and the decrease of GSH hippocampal levels; (3) markedly improved the cell morphology and alleviated the neuronal loss in hippocampal CA1 and CA3 regions; and (4) significantly attenuated the increase of CASP3 and BAX and the decrease of BCL2 hippocampal levels. The above findings clearly show that MB exerts anticonvulsant and neuroprotective effects on SSSE induced by prolonged BLA stimulation.
The electric kindling model is considered an ideal model to mimic the onset of human temporal lobe epilepsy [32]. Compared with the chemical convulsants (e.g., Kainic acid) usually used to build an epileptic model, it has a natural advantage in exclusion of the direct neurotoxic effects of the convulsant on the nervous system [33]. Thus, the potential interpretative difficulties associated with these drugs are avoided in the electric kindling model. Previous studies have shown that prior kindling is not necessary for the induction of SSSE [26], so we performed the direct stimulus program in non-kindled Wistar rats in the present study, saving time and labor costs. Soon after 25 min of prolonged stimulus at BLA, SSSE was induced successfully in nearly all animals. After the cessation of stimulation, the number and accumulated time of seizure activities of SSSE were calculated to measure the anticonvulsant potency of MB.
MB displays diverse biological activities, including memory-enhancing, antioxidant, anti-inflammatory, and anti-tumor effects [17–19,34]. Its strong lipophilic property make it rapidly cross the blood-brain barrier and easily accumulate in the brain tissue to exert a direct neuroprotective effect [20,35,36]. MB plays a neuroprotective role in ischemic stroke by restoring mitochondrial function, enhancing autophagy, and inhibiting neuronal apoptosis [37,38]. Our results showed that MB significantly reduced the number and duration of seizure activities, indicating that MB had an anticonvulsant effect in the SSSE model.
Oxidative stress starts when an imbalance between the endogenous reactive oxygen species (ROS) and antioxidant enzyme occurs [39]. Accumulating evidence suggests that an epileptic seizure induces excessive production of ROS, which takes part in the mechanism leading to neuron death [40]. MDA is the final product of lipid peroxidation, indicating that the state of free radicals and GSH belongs to the cellular antioxidant defense system, which can reduce oxidative stress injury [41,42]. In our study, there was an increase in lipid peroxidation and a decrease in GSH level during SSSE, which was in line with a previous study [29]. The imbalance between oxidant and antioxidant defense systems may be at least partially attributed to the epileptic activities. Previous research has showed that MB remarkably suppresses the increase in MDA in a streptozotocin-induced Alzheimer rat model [38]. In the present study, administration of MB partially prevented the increased level of lipid peroxidation and decreased level of GSH, which supports the powerful antioxidant function of MB [43].
In our study, MB significantly prevented neuron death caused by acute seizure in the CA1/CA3 field of the hippocampus. This nerve-protective effect was at least to some extent related to the regulating effect of MB in the oxidative stress injury during SSSE. Additional, the protective effect also might be related to the anti-apoptosis property of MB. Neuronal cell death, which was induced in the initial status epilepticus rather than in the subsequent spontaneous seizures, was found both in vitro and in vivo [44–46], indicating the crucial importance of suppressing SE as soon as possible to prevent a wide range of cell loss. The pattern of neuronal cell death includes apoptosis and necrosis [13–15], and neuronal apoptosis has been demonstrated to contribute to neuronal loss in the sclerotic hippocampus [47,48]. Another recent study showed that 24 h after the establishment of SSSE induced by prolonged stimulus at BLA, the apoptosis-related neuron death was obviously increased, which is in agreement with our results [49]. CASP3 is a crucial mediator during the process of apoptosis. The apoptotic signals, which derive from the Fas-mediated pathway and mitochondrion-mediated pathway, trigger activity of CASP3, and subsequently lead to apoptosis. The BCL2 family is well known for regulation of cell apoptosis in the mitochondrion-mediated apoptosis pathway. BCL2 is localized on the membrane of mitochondria and its activation inhibits apoptosis via stabilizing the membrane of mitochondria [50]. BAX is a pro-apoptotic protein in the BCL-2 family, and it can inhibit the anti-apoptotic function of BCL-2 [51]. Our study revealed that the protein levels of CASP3 and BAX were elevated, and the expression of BCL2 declined. However, these biochemical alterations were significantly reversed by MB application. Therefore, this finding indicates that MB also exerts anti-apoptotic effects on hippocampal neurons. The overproduction of ROS in neuronal cells can result in the collapse of cellular components, such as lipids, proteins, and DNA, which can ultimately lead to cell death by apoptosis and necrosis [52]. Therefore, we can speculate that the anti-apoptotic properties of MB on SSSE are partially due to its antioxidant effect.
One of the potential limitations in our study may be the absence of measurement of MB in either brain or plasma of the rats. It would be meaningful in the subsequent studies to perform this measurement to correlate in vitro and in vivo activities of MB. In addition, a growing body of evidence in animal models indicates that inflammation in the brain during SE may play a determinant role in epileptogenesis [53]. Unfortunately, we did not measure markers of neuroinflammation, neither in terms of activated microglia or reactive astrocytes nor in terms of cytokine brain levels. This may be considered in future studies.
Conclusions
Our results suggest that MB has a protective effect in the SSSE model induced by prolonged stimulus at BLA, and may be an adjuvant in epilepsy for preventing and/or treating epilepsy in humans. Nevertheless, further molecular studies are necessary to fully understand the mechanisms underlying the effect of MB during epileptogenesis and status epilepticus.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Chang BS, Lowenstein DH. Epilepsy. New Engl J Med. 2003;349:1257–66. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 2.Delorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. Humana Press; 2004. [PubMed] [Google Scholar]
- 3.Löscher W. Molecular mechanisms of drug resistance in status epilepticus. Epilepsia. 2009;50:74–77. doi: 10.1111/j.1528-1167.2009.02367.x. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz B. Effects of antiepileptic drugs on mood and behavior. Epilepsia. 2006;47:28–33. doi: 10.1111/j.1528-1167.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 5.Rowley S, Patel M. Mitochondrial oxidative stress and epilepsy. Springer; Berlin Heidelberg: 2014. [Google Scholar]
- 6.Migliore L, Fontana I, Colognato R, et al. Searching for the role and the most suitable biomarkers of oxidative stress in Alzheimer’s disease and in other neurodegenerative diseases. Neurobiol Aging. 2005;26:587–95. doi: 10.1016/j.neurobiolaging.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Perry G, Nunomura A, Hirai K, et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic Biol Med. 2002;33:1475–79. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 8.Sedaghat R, Taab Y, Kiasalari Z, et al. Berberine ameliorates intrahippocampal kainate-induced status epilepticus and consequent epileptogenic process in the rat: Underlying mechanisms. Biomed Pharmacother. 2017;87:200–8. doi: 10.1016/j.biopha.2016.12.109. [DOI] [PubMed] [Google Scholar]
- 9.Khamse S, Sadr SS, Roghani M, et al. Rosmarinic acid exerts a neuroprotective effect in the kainate rat model of temporal lobe epilepsy: Underlying mechanisms. Pharm Biol. 2015;53(12):1818–25. doi: 10.3109/13880209.2015.1010738. [DOI] [PubMed] [Google Scholar]
- 10.Asuntha G, Raju YP, Sundaresan CR, et al. Effect of Argemone mexicana (L.) against lithium-pilocarpine induced status epilepticus and oxidative stress in Wistar rats. Indian J Exp Biol. 2015;53:31–35. [PubMed] [Google Scholar]
- 11.Degiorgio CM, Tomiyasu U, Gott PS, Treiman DM. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33:23–27. doi: 10.1111/j.1528-1157.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 12.Mikati MA, Abi-Habib RJ, El Sabban ME, et al. Hippocampal programmed cell death after status epilepticus: evidence for NMDA-receptor and ceramide-mediated mechanisms. Epilepsia. 2003;44:282–91. doi: 10.1046/j.1528-1157.2003.22502.x. [DOI] [PubMed] [Google Scholar]
- 13.Pollard H, Charriaut-Marlangue C, Cantagrel S, et al. Kainate-induced apoptotic cell death in hippocampal neurons. Neuroscience. 1994;63:7–18. doi: 10.1016/0306-4522(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 14.Sloviter RS, Dean E, Sollas AL, Goodman JH. Apoptosis and necrosis induced in different hippocampal neuron populations by repetitive perforant path stimulation in the rat. J Comp Neurol. 1996;366:516–33. doi: 10.1002/(SICI)1096-9861(19960311)366:3<516::AID-CNE10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 15.Fujikawa DG, Shinmei SS, Cai B. Seizure-induced neuronal necrosis: Implications for programmed cell death mechanisms. Epilepsia. 2010;41:S9–13. doi: 10.1111/j.1528-1157.2000.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 16.Henshall DC, Murphy BM. Modulators of neuronal cell death in epilepsy. Curr Opin Pharmacol. 2008;8(1):75–81. doi: 10.1016/j.coph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Dinc S, Caydere M, Akgul G, et al. Methylene Blue inhibits the inflammatory process of the acetic acid-induced colitis in the rat colonic mucosa. Int Surgery. 2015 doi: 10.9738/INTSURG-D-15-00118.1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol. 2012;96:32–45. doi: 10.1016/j.pneurobio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Culo F, Sabolović D, Somogyi L, et al. Anti-tumoral and anti-inflammatory effects of biological stains. Agents Actions. 1991;34:424–28. doi: 10.1007/BF01988739. [DOI] [PubMed] [Google Scholar]
- 20.Mori T, Koyama N, Segawa T, et al. Methylene blue modulates beta-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice. J Biol Chem. 2014;289:30303–17. doi: 10.1074/jbc.M114.568212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Q, Du F, Huang S, et al. Neuroprotective efficacy of methylene blue in ischemic stroke: An MRI study. PLoS One. 2013;8:e79833. doi: 10.1371/journal.pone.0079833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojas JC, John JM, Lee J, Gonzalez-Lima F. Methylene blue provides behavioral and metabolic neuroprotection against optic neuropathy. Neurotox Res. 2009;15:260–73. doi: 10.1007/s12640-009-9027-z. [DOI] [PubMed] [Google Scholar]
- 23.Panza F, Solfrizzi V, Seripa D, et al. Tau-centric targets and drugs in clinical development for the treatment of Alzheimer’s disease. Biomed Res Int. 2016;2016:3245935. doi: 10.1155/2016/3245935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seripa D, Solfrizzi V, Imbimbo BP, et al. Tau-directed approaches for the treatment of Alzheimer’s disease: Focus on leuco-methylthioninium. Exp Rev Neurother. 2016;16:259–77. doi: 10.1586/14737175.2016.1140039. [DOI] [PubMed] [Google Scholar]
- 25.Mohd Sairazi NS, KNSS, Asari MA, et al. Effect of tualang honey against KA-induced oxidative stress and neurodegeneration in the cortex of rats. BMC Complement Altern Med. 2017;17(1):31. doi: 10.1186/s12906-016-1534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandt C, Glien M, Potschka H, et al. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res. 2003;55:83–103. doi: 10.1016/s0920-1211(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 27.Visarius TM, Stucki JW, Lauterburg BH. Inhibition and stimulation of long-chain fatty acid oxidation by chloroacetaldehyde and methylene blue in rats. J Pharmacol Exp Ther. 1999;289(2):820–24. [PubMed] [Google Scholar]
- 28.Nissinen J, Halonen T, Koivisto E, Pitkanen A. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res. 2000;38:177–205. doi: 10.1016/s0920-1211(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 29.Kan MC, Wang WP, Yao GD, et al. Anticonvulsant effect of dexmedetomidine in a rat model of self-sustaining status epilepticus with prolonged amygdala stimulation. Neurosci Lett. 2013;543:17–21. doi: 10.1016/j.neulet.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–58. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 31.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 32.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 33.Vicedomini JP, Nadler JV. A model of status epilepticus based on electrical stimulation of hippocampal afferent pathways. Exp Neurol. 1987;96:681–91. doi: 10.1016/0014-4886(87)90229-9. [DOI] [PubMed] [Google Scholar]
- 34.Bruchey AK, Gonzalez-Lima F. Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye Methylene Blue. Am J Pharmacol Toxicol. 2008;3:72–79. doi: 10.3844/ajptsp.2008.72.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of Methylene Blue in the nervous system. Med Res Rev. 2011;31:93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Leary JC, 3rd, et al. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol Neurodegener. 2010;5:45. doi: 10.1186/1750-1326-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Z, Watts LT, Huang S, et al. The effects of Methylene Blue on autophagy and apoptosis in MRI-defined normal tissue, ischemic penumbra and ischemic core. PLoS One. 2015;10:e0131929. doi: 10.1371/journal.pone.0131929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Qin L, Lu HL, et al. Methylene blue improves streptozotocin-induced memory deficit by restoring mitochondrial function in rats. Brain Res. 2017;1657:208–14. doi: 10.1016/j.brainres.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Patel MN. Oxidative stress, mitochondrial dysfunction, and epilepsy. Free Radic Res. 2002;36:1139–46. doi: 10.1080/1071576021000016391. [DOI] [PubMed] [Google Scholar]
- 40.Delorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: The calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther. 2005;105:229–66. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Si PP, Zhen JL, Cai YL, et al. Salidroside protects against kainic acid-induced status epilepticus via suppressing oxidative stress. Neurosci Lett. 2016;618:19–24. doi: 10.1016/j.neulet.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 42.Galleano M, Puntarulo S. Role of antioxidants on the erythrocytes resistance to lipid peroxidation after acute iron overload in rats. Biochim Biophys Acta. 1995;1271:321–26. doi: 10.1016/0925-4439(95)00049-a. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Rojas JC, Gonzalez-Lima F. Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox Res. 2006;9:47–57. doi: 10.1007/BF03033307. [DOI] [PubMed] [Google Scholar]
- 44.Mikati M. Neuronal cell death in a rat model of mesial temporal lobe epilepsy is induced by the initial status epilepticus and not by later repeated spontaneous seizures. Epilepsia. 2004;45:296–97. doi: 10.1111/j.0013-9580.2004.58503.x. [DOI] [PubMed] [Google Scholar]
- 45.Deshpande LS, Lou JK, Mian, et al. In vitro status epilepticus but not spontaneous recurrent seizures cause cell death in cultured hippocampal neurons. Epilepsy Res. 2007;75:171–79. doi: 10.1016/j.eplepsyres.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitkänen A, Nissinen J, Nairismägi J, et al. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog Brain Res. 2002;135:67–83. doi: 10.1016/S0079-6123(02)35008-8. [DOI] [PubMed] [Google Scholar]
- 47.Henshall DC, Clark RS, Adelson PD, et al. Alterations in bcl-2 and caspase gene family protein expression in human temporal lobe epilepsy. Neurology. 2000;55:250–57. doi: 10.1212/wnl.55.2.250. [DOI] [PubMed] [Google Scholar]
- 48.Faherty CJ, Xanthoudakis S, Smeyne RJ. Caspase-3-dependent neuronal death in the hippocampus following kainic acid treatment. Brain Res Mol Brain Res. 1999;70:159–63. doi: 10.1016/s0169-328x(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 49.Sun Z, Yu JT, Jiang T, et al. Genome-wide microRNA profiling of rat hippocampus after status epilepticus induced by amygdala stimulation identifies modulators of neuronal apoptosis. PLoS One. 2013;8:e78375. doi: 10.1371/journal.pone.0078375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haeberlein SL. Mitochondrial function in apoptotic neuronal cell death. Neurochem Res. 2004;29:521–30. doi: 10.1023/b:nere.0000014823.74782.b7. [DOI] [PubMed] [Google Scholar]
- 51.Tan KO, Yu VC. MAP-1 is a mitochondrial effector of Bax. Proc Natl Acad Sci USA. 2005;102:14623–28. doi: 10.1073/pnas.0503524102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7:153–63. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 53.Vezzani A, Dingledine R, Rossetti AO. Immunity and inflammation in status epilepticus and its sequelae: Possibilities for therapeutic application. Exp Rev Neurother. 2015;15:1081–92. doi: 10.1586/14737175.2015.1079130. [DOI] [PMC free article] [PubMed] [Google Scholar]