Abstract
Aim:
Aim of the present study was to carry out the partial purification and biochemical characterization of glutathione S-transferase (GST) from the somatic tissue of ruminal amphistome parasite, Gastrothylax crumenifer (Gc) infecting Indian water buffalo (Bubalus bubalis).
Materials and Methods:
The crude somatic homogenate of Gc was subjected to progressive ammonium sulfate precipitation followed by size exclusion chromatography in a Sephacryl S 100-HR column. The partially purified GST was assayed spectrophotometrically, and the corresponding enzyme activity was also recorded in polyacrylamide gel. GST isolated from the amphistome parasite was also exposed to variable changes in temperature and the pH gradient of the assay mixture.
Results:
The precipitated amphistome GST molecules showed maximum activity in the sixth elution fraction. The GST subunit appeared as a single band in the reducing polyacrylamide gel electrophoresis with an apparent molecular weight of 26 kDa. The GST proteins were found to be fairly stable up to 37°C, beyond this the activity got heavily impaired. Further, the GST obtained showed a pH optima of 7.5.
Conclusion:
Present findings showed that GST from Gc could be conveniently purified using gel filtration chromatography. The purified enzyme showed maximum stability and activity at 4°C.
Keywords: Bubalus bubalis, Gastrothylax crumenifer, glutathione S-transferase, purification, somatic tissue
Introduction
Amphistomes are digenetic trematodes comprising a characteristic posteriorly located muscular acetabulum. There are more than 70 species of amphistome parasites all over the world [1], particularly in the hot and humid land stretches. They are known to parasitize a wide variety of small and large ruminants and are responsible for substantial economic loss. In India, reports on the prevalence of these flukes have been archived from all the major provinces. Gastrothylax crumenifer (Gc) is a commonly found amphistome parasite infecting the rumen of the Indian Water buffalo, Bubalus bubalis in this part of north India. In general, the adult forms of amphistome parasites manifest diminished pathogenicity, but the immature rumen flukes during their course of migration lead to severe pathological disturbances including hemorrhagic inflammation in the wall of alimentary tract [2]. In India, several outbreaks of acute amphistomosis associated with high mortality among young sheep, goats, cattle, and buffaloes have been recorded [3-6]. In the absence of commercial vaccine, chemotherapy is the only way to deal with these rampant and neglected epidemic infections, but reports on emerging anthelmintic resistance have necessitated the quest to find new drug targets and promising vaccine candidates.
Glutathione S-transferases (GSTs) are a diverse family of multifunctional proteins that find widespread distribution in the aerobic organisms. GSTs mediate the covalent addition of the tripeptide glutathione (GSH) to a structurally diverse set of electrophiles [7]. GST enzymes are involved in the active detoxification of xenobiotics by conjugation to GSH. They are also known to neutralize endogenous secondary metabolite formed during oxidative stress [8]. These enzymes occur in multiple forms and catalyze a multitude of reactions involving electrophilic functional groups [9]. The GST has got a principal role in the process of inactivating a wide range of exogenous/endogenous toxic molecules and to turn them into water-soluble compounds. GSTs gained vital significance in parasites as the main detoxification system due to the lack of cytochrome P450 (CYP450) activity [10]. It has been postulated that GSH mediated antioxidant system is responsible for the prolong survival of helminth parasites in the mammalian definitive host. Occurrence of GSTs in helminth parasites protect them from the reactive oxygen species (ROS) generated during normal metabolism and by the immune effector cells of the invaded host [11,12]. The inherent capacity of helminth GSTs to neutralize cytotoxic components furnished by ROS of host origin on cell membrane strengthens the potential of GSTs as a protective tool against the host immune response. The inhibition of helminth GSTs dismantles the parasite defense against mounting oxidative stress and immunogenic attack of the host [13,14]. These facts place them as targets for the development of vaccines or chemotherapeutic agents [15,16]. The discovery and exploration of biochemical attributes of GSTs in helminth parasites have resulted into elaborate testing of this enzyme form as a target immunoprotective antigen.
There are many reports available on these vaccination experiments [17-20]. Therefore, to cater to that need, preliminary studies were designed and executed to purify and characterize GST from Gc to generate a baseline data which could further be exploited for immune and chemotherapeutic assessments to check amphistome infections in domesticated ruminants.
Materials and Methods
Ethical approval
Ethical approval is not necessary to pursue this type of study.
Collection of parasites
Mature and active Gc amphistome parasites were collected from the rumen of the Indian water buffalo (B. bubalis) slaughtered at Aligarh abattoirs. The parasites were thoroughly washed in phosphate buffered saline (100 mM, pH 7.4) and then briefly rinsed in the same washing buffer containing 0.01% penicillin-streptomycin (Merck, Germany) solution to remove any possible microbial contamination.
Preparation of somatic extracts
The parasites were homogenized in a chilled mortar-pestle over ice in cold phosphate buffer solution (100 mM, pH 7.4). The sample was centrifuged at 10,000×g for 10 min in a refrigerated centrifuge (Hitachi, Japan) and the supernatants were collected as soluble protein fractions.
Protein estimation
The protein contents were estimated following the dye binding method of Bradford [21] as modified by Spector [22]. Bovine serum albumin was used as a standard.
Ammonium sulfate precipitation
The soluble proteins of somatic extract of Gc were fractionated between 0-20%, 20-40%, 40-60%, 60-80%, and 80-100% ammonium sulfate saturation in a stepwise ascending grade of protein salting out method. After 4 h, the precipitated proteins from each of the fractions were procured by centrifugation at 10,000×g for 30 min at 4°C in a cooling centrifuge (Remi, India).
Dialysis of samples
A small portion of the supernatants from each salting out step was saved and the precipitate was dissolved in a minimum volume of 100 mM sodium phosphate buffer (pH 7.4). The supernatant and the precipitate obtained from each salting out step were dialyzed separately thrice against 1200 ml of the same buffer overnight at 4°C to remove ammonium sulfate content from the protein samples.
Protein estimation of each fraction
The protein content of the dialyzed supernatant and precipitated samples were estimated as mentioned earlier.
Assay of GST enzyme
Following protein estimation, all the supernatant and ammonium sulfate precipitated samples of Gc were assayed spectrophotometrically to determine the activity of GST enzyme. The precipitated fractions with maximum GST specific activity were selected and processed for gel filtration chromatography.
Gel filtration chromatography
A Sephacryl S 100-HR column (Sigma-Aldrich, USA) was prepared as recommended by Peterson and Sober [23] with necessary adjustments and modifications. Pre-swollen gel suspended in ethanol was soaked in sufficient amount of double distilled water and washed at least thrice. The finer resin fragments were removed by suspending the gel in two- to four-fold excess of 100 mM sodium phosphate buffer, pH 7.4 and the gel was allowed to settle down. A glass column (70 cm×2 cm) was mounted on a sturdy vertical support after introducing the glass wool on its opening near the bottom end which was fitted with rubber tubing. Following the clamping of rubber tubing, the column was filled to one-third of its length with operating buffer to check leaks and flush air bubbles from the dead space. The de-aerated gel slurry was poured with the help of a glass rod into the column with care to avoid generating air bubbles. The column was left to stand overnight. Flow rate was increased gradually, and after achieving a constant flow rate (higher than that required for final elution), the column was adjusted to the required flow rate. The packed column was thoroughly washed with two-bed volumes of operating buffer (100 mM sodium phosphate buffer, pH 7.4). To check uniform packing and to determine void volume of the column, 2% (w/v) solution of blue dextran in 100 mM sodium phosphate buffer (pH 7.4) was passed through the column. The volume of the blue dextran and protein solution applied was not more than 2-3% of the total bed volume. The dialyzed sample was subjected to gel filtration chromatography on Sephacryl S-100-HR column equilibrated with 100 mM sodium phosphate buffer, pH 7.4 at 4°C. The flow rate of the column was set at15 ml/h during the process of filtration. Fractions of 5 ml were collected and assayed for protein content and GST activity. Homogeneity of the purification was analyzed by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Collection of eluents
The eluents containing protein samples were collected into 14 subsequent fractions (5 ml) and were assayed for protein content and GST activity.
GST activity in each eluent
The eluted fractions showing detectable amount of protein content were assayed to determine GST enzyme activity in a UV-visible spectrophotometer (Taurus Scientific, U.S.A). GST activity was determined by the method of Habig et al. [24] with minor modifications. The assay was performed in a total volume of 3.0 ml reaction mixture containing 300 µl of 1.0 mM reduced GSH, 10 µl of 1.0 mM 1-chloro-2,4-dinitrobenzene (CDNB), and 50 µl of protein samples. The remaining volume was adjusted with 0.1 M sodium phosphate buffer (pH 6.5). CDNB and GSH were dissolved in ethanol and 0.1 M sodium phosphate buffer (pH 6.5), respectively. The control assay mixture did not have any protein (enzyme) sample. The assay was carried out at least in three replicates. The change in absorbance at 340 nm was recorded for 3 min. The change in absorbance was calculated and used for determining the enzyme specific activity. The GST activity is defined as the amount of enzyme that catalyzes the formation of 1.0 µmol of S-(2,4-dinitrophenyl) GSH/min/mg protein under the standard assay conditions. The unit of enzyme activity is expressed as nmoles/mg protein/minute.
Molecular weight determination of GST enriched fraction by SDS-PAGE
The eluted fractions showing highest activity for GST were subjected to SDS-PAGE to assess the homogeneity of purification as well as relative percentage of GST protein in the GST enriched fractions. All the samples and buffers used in this study were filtered through 0.22 μm Millipore filters to remove small particles and the gel solutions were additionally degassed before gel polymerization.
Protein loading and electrophoresis
Eluted fractions containing highest GST activity in Gc were separately mixed with the SDS sample buffer containing β-mercaptoethanol in the ratio of 1:2 (Sample: Sample buffer) and then the mixture was heated for 3-5 min at 95°C in water bath. Samples were loaded in different wells along with the standard protein markers (Precision Plus Protein™ Dual Color Standard; BIO-RAD) in a separate lane. After that, the gels were placed in a Benchtop Mini-Boat gel electrophoresis assembly and then running buffer was poured into the buffer tank. Electrophoresis [25] was carried out at 100 V for 60 min at RT. The gels were then carefully removed and thoroughly washed twice with distilled water before incubation in CBBR-250 solution overnight at RT. The over stained gels were destained, photographed, and analyzed.
Native-PAGE for GST activity determination
Gel preparation
To resolve the isozyme pattern and activity of GST enzyme molecules, the polyacrylamide gels were cast between glass cassettes in an electrophoresis assembly. A 12% separating gel and stacking gel of 5% concentration were prepared.
Protein loading and electrophoresis
Samples (desired eluted fractions of Gc) were mixed with the non-reducing sample buffer in the ratio of 1:2 (sample: sample buffer) and then the mixture was held at 4°C for 2-4 h. The prepared samples were loaded and electrophoresis was done at 100 V for 90 min.
Activity of GST in native polyacrylamide gel
The activity of GST enzyme in gel was performed according to the protocol of Ricci et al. [26]. The electrophoresed gels were then incubated in a series of reagents to develop achromatic bands depicting GST isozyme.
Photography of stained gels
The stained gels were scanned on a computer-driven laser scanner for analysis and all the images were saved for further analysis as well as to maintain digital record in the laboratory.
Effect of pH on partially purified GST
Variation in the enzyme activity of partially purified GST obtained from Gc was examined at various pH values (5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, and 9.0). Purified GST enzymes (1 mg) from the amphistome parasites were incubated separately in 1 ml of 100 mM sodium phosphate buffered solution having different pH at a constant temperature of 4°C for 30 min. Following incubation, each sample was assayed for GST activity as mentioned above.
Effect of temperature on partially purified GST
The variation in specific activity of GST from Gc was also investigated as a function of five different temperature (4, 25, 37, 60, and 80)°C. Purified GST enzymes (1 mg) were incubated separately in 1 ml of sodium phosphate buffered solution (100 mM, pH 7.4) pre-maintained at specific temperatures, for 30 min. Following incubation, each sample was assayed for GST activity according to the methods mentioned earlier.
Results
The present work deals with the partial purification of GST protein from the somatic tissues of amphistome parasites, Gc. The methodology involved two steps isolation process using tissue homogenates. The aqueous protein extracts were subjected to ammonium sulfate precipitation (40-60%) for Gc. The precipitated protein fractions from the flukes were passed through a Sephacryl S-100 HR column. Different steps of typical purification process of GST proteins, their specific enzyme activity, fold purification, and percent yield are summarized in Table-1. Fractionation of the soluble proteins with ammonium sulfate decreased the total amount of protein obtained in the subsequent precipitate. The ammonium sulfate precipitation (40-60%) revealed 47.74% yield of the total activity in crude protein but the GST protein showed 2.78-fold purification as compared to the crude homogenate of Gc.
Table-1.
Different steps involved in the purification of GST proteins obtained from the somatic tissues of Gastrothylax crumenifer (GcGST).
| Steps of purification | Total volume (ml) | Total protein (mg) | Total activity (n moles) | Specific activity (nmoles/mg protein/min) | Fold purification | Percent (%) yield |
|---|---|---|---|---|---|---|
| Crude homogenate | 65 | 1187.355 | 1315.300 | 1.108 | 1 | 100 |
| (40-60)% Ammonium sulfate precipitate | 10 | 203.510 | 628.000 | 3.086 | 2.785 | 47.746 |
| Gel filtration (Sephacryl S-100 HR) | 6 | 9.920 | 244.800 | 24.677 | 22.272 | 18.612 |
GST=Glutathione S-transferase
Gel filtration chromatography
The ammonium sulfate precipitated proteins (40-60%) obtained were chromatographed on a Sephacryl S-100 HR column pre-equilibrated with 100 mM sodium phosphate buffer (pH 7.4). Single peak showing highest GST specific enzyme activity (24.677 nmoles/mg protein/min) was obtained for the rumen flukes as shown in Figure-1. The sixth eluted fraction corresponding to the peak was used for further analyses. It was observed that the size exclusion chromatography could purify the GST protein by 22.27-folds from crude homogenates of Gc. The percent yields of GST protein after size exclusion chromatography in Gc were found to be 18.61 as compared to their corresponding crude forms (Table-1).
Figure-1.
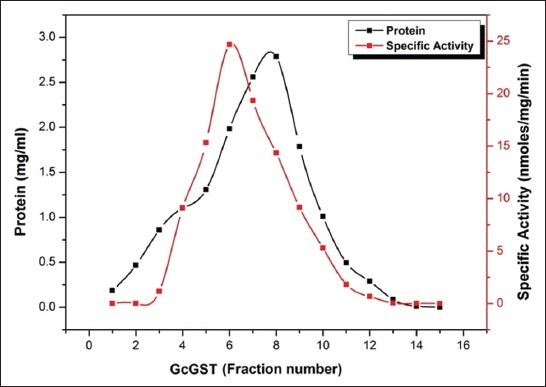
Elution profile of 40-60% ammonium sulfate precipitated protein fraction of Gastrothylax crumenifer as obtained by gel filtration chromatography on Sephacryl S-100 HR gel matrix.
Homogeneity of the purified GST proteins
Eluted GST proteins from Gc showed a single peak corresponding to highest enzyme specific activity suggested a homogenous soluble extract preparation (Figure-1). In addition, the preparations had minimum interference with the ions of sodium and phosphates as are evident from the symmetric peaks and patterns of eluting fractions in terms of protein content and GST specific activity. Physical evidence for homogeneity was further provided by PAGE.
Reducing SDS-PAGE
Partially purified GST proteins from Gc (Eluted fraction-6) were analyzed on PAGE under reducing and non-reducing (in absence of SDS and β-mercaptoethanol) conditions. The PAGE (12%) profile under reducing conditions showed that the GST migrated as a single prominent band in the parasites under study (Figure-2a). The specific polypeptides corresponding to GST monomeric proteins in the rumen amphistomes were observed to be lying in the low molecular weight range.
Figure-2.
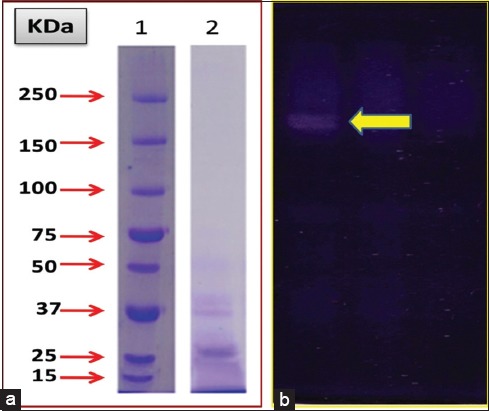
(a) Sodium dodecyl sulfate polyacrylamide gel electrophoresis (PAGE) analysis of purified glutathione S-transferase (GST) from Gastrothylax crumenifer (Gc) in a 12% gel. Lane 1 - standard protein marker (SPM), Lane 2 - purified GST from Gc (GcGST). (b) In-gel activity of purified GST from Gc in 10% native PAGE gel. The activity band appears as an achromatic zone on a blue insoluble formazan background.
Non-reducing (native) PAGE
Partially purified GST proteins from the eluted fraction number 6 for Gc (GcGST) were analyzed on native PAGE under non-reducing conditions. Subsequent staining of the gel matrix for in-gel activity of GST revealed a single achromatic band over a blue insoluble formazan dark background (Figure-2b).
Properties of the partially purified GST proteins
Molecular weight determination
Analysis of the resolved polypeptide on a 12% SDS polyacrylamide gel in a Bio Rad Gel Documentation system revealed a single band of 26 kDa polypeptide corresponding to GST monomer (Figure-2a).
Effect of pH on partially purified GST
Effect of pH on the specific activity of partially purified GST was examined at various pH values ranging from 5.0 to 9.0 (Figure-3). The GST activities in the parasitic flukes were seen to be drastically affected at both the extreme of the test range. The GST proteins were found to have considerable enzymatic activities in a pH range of 6.5-8.5 in the parasite under study. Maximum stability of the catalytic properties of GST proteins was observed at pH 7.5 (Figure-3). A rise in pH from 7.5 to 8.0 resulted in an evident decrease in the enzyme activity. This suggests that the GST enzyme in this amphistome parasite has specific pH optima.
Figure-3.
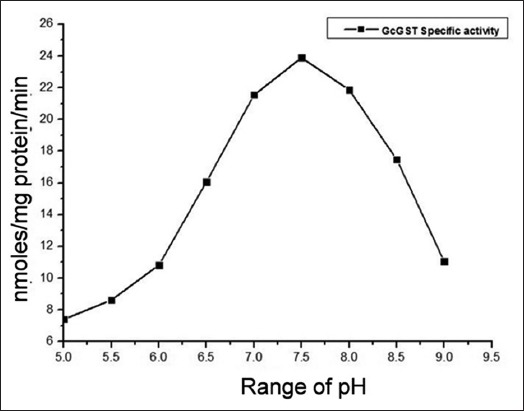
The pH stability of the purified glutathione S-transferase (GST) proteins from Gastrothylax crumenifer in 100 mM sodium phosphate buffer having different pH (5.0-9.0). Purified GSTs (1 mg) were incubated in 1 ml of phosphate buffer solution at 4°C for 30 min.
Effect of temperature on partially purified GST
Alteration in the specific activity of GST from Gc was also investigated as a function of temperature in a range from 4 to 80°C for 30 min. The GST protein remained considerably active within the temperature of 4-37°C for the parasite species (Figure-4). However, a rise in temperature from 25 to 30°C resulted into sharp decline in the GST specific activity profile in the parasites under study (Figure-4). When the temperature was raised from 4 to 25°C, the abrupt slump in the activities of GST proteins was recorded. This suggested that GST from Gc is appreciably robust in terms of minor thermal fluctuations at lower temperature ranges.
Figure-4.
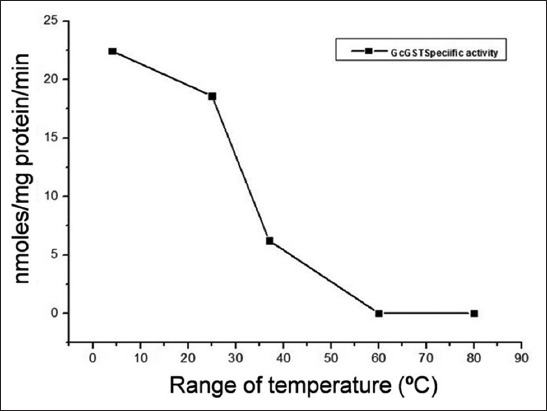
Thermal stability of the purified glutathione S-transferase (GST) proteins from Gastrothylax crumenifer in 100 mM sodium phosphate buffer (pH 7.4) pre-maintained at five different temperatures (4, 25, 37, 60, and 80°C). Purified GSTs (1 mg) were incubated in 1 ml of phosphate buffer solution having different temperatures for 30 min.
Discussion
GSTs exist in all living organisms and they are involved in various detoxification pathways and antioxidant processes. Since, helminth parasites lack p-450 monoxygenases, the Phase-II biotransformations are carried out by GST isoforms and hence are of cardinal importance [27]. GSTs perform several important functions in the body and are associated with several pathological conditions including cancer [28], rheumatoid arthritis [29], osteoporosis [30], renal dysfunctions [31], cardiovascular diseases [32,33], and Alzheimer’s disease [34]. GSTs have been purified and characterized from various helminth parasites including nematodes, trematodes, and cestodes such as Clonorchis sinensis [35], Setaria digitata [36], Schistosoma mansoni [37], Schistosoma japonicum [38], Fasciola hepatica [39], Necator americanus [40], and Echinococcus granulosus [41]. Due to lack of information on GSTs from a neglected group of digenetic trematodes, the amphistome parasites, present work has been carried out. To the best of our knowledge, this is the first description about the purification and properties of GST enzymes obtained from the amphistome parasites, Gc. It was envisaged that a thorough study on GST from the ruminal amphistomes will shed some light toward better understanding of GSTs of helminth origin. This will be useful in generating a base line data so that the salient enzymatic properties can be compared with other known GST from different parasitic fauna. In the present work, GST was purified from Gc by the method as described in the materials and method section. The two step procedure involved ammonium sulfate fractionation and gel filtration chromatography. The simple isolation procedure provided a percent yield of 18.61- and 22.27-fold purification for Gc. Purification of the GST isozymes from a variety of helminth parasites has been reported using a combination of several methods including affinity chromatography, chromatofocusing, gel filtration, and ion exchange chromatography [12,14,42-44]. However, the procedure used in this study is simple and cost-effective which give appreciable yield and fold purification as compared to the values reported in literature for other helminth parasites [36]. The partially purified GST obtained from the amphistome parasites was found to be homogenous on the basis of charge as shown by native-PAGE. In SDS-PAGE under reducing conditions, GST from Gc gave a single band which could be of dimeric nature (native state) with two identical subunits.
GSTs obtained from amphistome parasites were partially purified on a Sephacryl S 100-HR resin matrix. The molecular mass of the subunits comprising the native GST molecule was found to be made up of two equal sub-units of 26 kDa in Gc following denaturing PAGE technique in the presence of β-mercaptoethanol. Subunits of GST with comparable molecular weights (26 kDa) have also been reported in a wide variety of helminths such as S. mansoni [37], S. japonicum [45], F. hepatica [46], Taenia solium [42], and Setaria cervi [43]. In general, cytosolic GSTs have monomers of 23-28 kDa with an average of 220 amino acids in their sequences. They all share the same tertiary and quaternary dimeric structural features. The dimer may have identical subunits (homodimer) or different subunits (heterodimer) of the same class [47]. In the present study, appreciable stability of the partially purified GSTs from Gc was observed in the broad range of pH (6.5-8.5) as well as temperature (4-37°C). Similar kinds of observations were made with GST purified from S. digitata which showed good enzyme activity between 0°C and 40°C, while a sharp decline in activity was observed beyond 40°C with complete loss of activity at 80°C [36]. A similar trend in the GST enzyme activity was reported earlier with temperature optima of around 40°C [42,43] and loss of activity at higher temperatures. The enzyme activity of GSTs from Gc was seen to vary with change in pH. The optimum GST activities for this amphistome were observed at pH 7.5; a similar finding was also reported in GST purified from S. digitata [36].
In most of the helminth parasites, CYP450 mediated biotransformation has not been reported. Hence, the GST enzyme system plays an indispensible role in carrying out the Phase II detoxification pathways. Due to this, purified GSTs from various helminth parasites have been screened for immune and chemotherapeutic leads and targets to design and develop a better and reliable parasite control measure. The present study was an attempt to purify GST enzyme from an amphistome parasite to determine and obtain the basic biochemical attributes which could help in the formulation of new drug to control the amphistomosis in our farm animals.
Conclusion
The present study is an attempt to purify and conduct basic biochemical characterization of GST from the rumen infecting amphistome Gc using size exclusion chromatography.
Authors’ Contributions
SA and AS prepared the study design and carried out the research under the supervision of MKS. SK and SHK isolated the parasites and analyzed the data. The manuscript was drafted and revised by SA and SK under the guidance of MKS. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to the Chairman, Department of Zoology, Aligarh Muslim University, Aligarh, for providing necessary laboratory facilities. The first author is also thankful to the University Grants Commission (UGC), Government of India, for financial assistance in the form of MAN-JR and MAN-SR fellowships (Grant number: F1-17.1/2010/MANF-MUS-JHA-2014/(SA-III).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Toledo R, Fried B. Advances in Experimental Medicine and Biology 766 (Digenetic Trematodes) New York: Springer Publications, Springer Science+Business Media; 2014. pp. 365–392. [Google Scholar]
- 2.Saifullah M.K, Ahmad G, Abidi S.M.A. Immunodetection of coproantigens for the diagnosis of amphistomosis in naturally infected Indian Water Buffalo Bubalus bubalis. Vet. Parasitol. 2013;191:66–72. doi: 10.1016/j.vetpar.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan V.D, Patel P.V, Hasnani J.J, Pandya S.S, Pandey S, Pansuriya D.V, Choudhary V. Study on hematological alterations induced by amphistomosis in buffaloes. Vet. World. 2015;8(3):417–420. doi: 10.14202/vetworld.2015.417-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar B, Maharana B.R, Prasad A, Joseph J.P, Patel B, Patel J.S. Seasonal incidence of parasitic diseases in bovines of south western Gujarat (Junagadh), India. J. Parasit. Dis. 2016;40(4):1342–1346. doi: 10.1007/s12639-015-0686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixit A.K, Das G, Baghel R.P.S. Gastrointestinal helminthosis:Prevalence and associated determinants in goats of Jabalpur, India. J. Parasit. Dis. 2017;41(2):414–416. doi: 10.1007/s12639-016-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorathiya L.M, Fulsoundar A.B, Rao T.K.S, Kumar N. Prevalence and risk factors for gastrointestinal parasitism in traditionally maintained goat flocks of South Gujarat. J. Parasit. Dis. 2017;41(1):137–141. doi: 10.1007/s12639-016-0766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheehan D, Meade G, Foley V.M, Dowd C.A. Structure, function and evolution of glutathione transferases:Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Mannervik B. The isoenzymes of glutathione transferases. Adv. Enzymol. Relat. Areas Mol. Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- 10.Callahan H.L, Crouch R.K, James E.R. Helminth anti-oxidant enzymes:A protective mechanism against host oxidants? Parasitol. Today. 1988;4(8):218–225. doi: 10.1016/0169-4758(88)90162-7. [DOI] [PubMed] [Google Scholar]
- 11.Brophy P.M, Pritchard D.I. Immunity to helminths:Ready to tip the biochemical balance? Parasitol. Today. 1992;8(12):419–422. doi: 10.1016/0169-4758(92)90195-8. [DOI] [PubMed] [Google Scholar]
- 12.Brophy P.M, Barret J. Glutathione-S-transferase in helminthes. Parasitology. 2009;100:345–349. doi: 10.1017/s0031182000061369. [DOI] [PubMed] [Google Scholar]
- 13.Brophy P.M, Pritchard D.I. Parasitic helminth glutathione S-transferases:Update on their potential for immuno and chemotherapy. Exp. Parasitol. 1994;79(1):89–96. doi: 10.1006/expr.1994.1067. [DOI] [PubMed] [Google Scholar]
- 14.Liebau E, Wildenburg G, Brophy P.M, Walter R.D, Henkle D.K. Biochemical analysis, gene structure and localization of the 24 kDa glutathione S-transferase from Onchocerca volvulus. Mol. Biochem. Parasitol. 1996;80:27–39. doi: 10.1016/0166-6851(96)02660-6. [DOI] [PubMed] [Google Scholar]
- 15.Brophy P.M, Ben-Smith A, Brown A, Behnke J.M, Pritchard D.I. Glutathione S-transferase from the gastrointestinal nematode Heligmosomoides polygyrus and mammalian liver compared. Comp. Biochem. Physiol. B. 1994;109(4):585–592. doi: 10.1016/0305-0491(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 16.Barrett J. Cytochrome P450 in parasitic protozoa and helminths. Comp. Biochem. Physiol C. 1998;121(1-3):181–183. doi: 10.1016/s0742-8413(98)10039-7. [DOI] [PubMed] [Google Scholar]
- 17.Bourke C.D, Nausch N, Rujeni N, Appleby L.J, Trottein F, Midzi N, Mduluza T, Mutapi F. Cytokine responses to the anti-schistosome vaccine candidate antigen glutathione-S-transferase vary with host age and are boosted by praziquantel treatment. PLoS Negl. Trop. Dis. 2014;8(5):e2846. doi: 10.1371/journal.pntd.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L.G, Wang Z.Q, Liu R.D, Yang X, Liu L.N, Sun G.G, Jiang P, Zhang X, Zhang G.Y, Cui J. Trichinella spiralis:Low vaccine potential of glutathione S-transferase against infections in mice. Acta Trop. 2015;146:25–32. doi: 10.1016/j.actatropica.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Mbanefo E.C, Kumagai T, Kodama Y, Kurosaki T, Furushima-Shimogawara R, Cherif M.S, Mizukami S, Kikuchi M, Huy N.T, Ohta N, Sasaki H, Hirayama K. Immunogenicity and anti-fecundity effect of nanoparticle coated glutathione S-transferase (SjGST) DNA vaccine against murine Schistosoma japonicum infection. Parasitol. Int. 2015;64(4):24–31. doi: 10.1016/j.parint.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Diemert D.J, Freire J, Valentene V, Fraga C.G, Taller F, Grahek S, Campbell D, Amar J, Maria V.P, Martin E, Maria F.G, Maria E.B. Safety and immunogenicity of the Na-GST-1 hookworm vaccine in Brazilian and American adults. PLoS Negl. Trop. Dis. 2017;11(5):e0005574. doi: 10.1371/journal.pntd.0005574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Spector T. Refinement of the coommassie blue method of protein quantitation. Anal. Biochem. 1978;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- 23.Peterson E.A, Sober H.A. In: Methods in Enzymology. 3rd ed. Colowick S.P, Kaplan N.O, editors. Vol. 5. New York: Academic Press Inc; 1962. [Google Scholar]
- 24.Habig W.H, Pabst M.J, Jakoky W.B. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 25.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Ricci G.M, Lo Bello A.M, Caccuri F, Galiazzo F, Federici G. Detection of glutathione transferase activity on polyacrylamide gels. Anal. Biochem. 1984;143(2):226–230. doi: 10.1016/0003-2697(84)90657-2. [DOI] [PubMed] [Google Scholar]
- 27.Precious W.Y, Barrett J. Xenobiotic metabolism in helminths. Parasitol. Today. 1989;5(5):156–160. doi: 10.1016/0169-4758(89)90080-x. [DOI] [PubMed] [Google Scholar]
- 28.Marchewka Z, Piwowar A, Ruzik S, Długosz A. Glutathione S-transferases class Pi and Mi and their significance in oncology. Postepy Hig. Med. Dosw. 2017;71:541–550. doi: 10.5604/01.3001.0010.3835. [DOI] [PubMed] [Google Scholar]
- 29.Mateen S, Moin S, Khan A.Q, Zafar A, Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One. 2016;11(4):e0152925. doi: 10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragab S.M, Badr E.A, Ibrahim A.S. Evaluation of glutathione-S-transferase P1 polymorphism and its relation to bone mineral density in Egyptian children and adolescents with beta-thalassemia major. Mediterr. J. Hematol. Infect. Dis. 2016;8(1):e2016004. doi: 10.4084/MJHID.2016.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shu K.H, Wang C.H, Wu C.H, Huang T.M, Wu P.C, Lai C.H, Tseng L.J, Tsai P.R, Connolly R, Wu V.C. Urinary pi- glutathione S-transferase predicts advanced acute kidney injury following cardiovascular surgery. Nat. Sci. Rep. 2016;6:26335. doi: 10.1038/srep26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balatonyi B, Gasz B, Kovács V, Lantos J, Jancsó G, Marczin N, Rőth E. The role of the inhibition of glutathione-S-transferase in the protective mechanisms of ischemic post conditioning. Can. J. Physiol. Pharmacol. 2013;91(8):625–632. doi: 10.1139/cjpp-2012-0411. [DOI] [PubMed] [Google Scholar]
- 33.Phulukdaree A, Khan S, Moodley D, Chuturgoon A.A. GST polymorphisms and early-onset coronary artery disease in young South African Indians. S. Afr. Med. J. 2012;102(7):627–630. doi: 10.7196/samj.5520. [DOI] [PubMed] [Google Scholar]
- 34.de Mendonça E, Salazar-Alcalá E, Fernández-Mestre M. Role of genes GSTM1, GSTT1, and MnSOD in the development of late-onset Alzheimer disease and their relationship with APOE*4. Neurologia. 2014;31(8):535–542. doi: 10.1016/j.nrl.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Kim J.G, Ahn C.S, Kim S.H, Bae Y.A, Kwon N.Y, Kang I, Yang H.J, Sohn W.M, Kong Y. Clonorchis sinensis omega-class glutathione transferases play major roles in the protection of the reproductive system during maturation and the response to oxidative stress. Parasit. Vectors. 2016;9(1):337. doi: 10.1186/s13071-016-1622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan L, Mathew N, Karuna T, Muthuswamy K. Biochemical studies on glutathione S-transferase from the bovine filarial worm Setaria digitata. Parasitol. Res. 2011;109(1):213–219. doi: 10.1007/s00436-010-2227-x. [DOI] [PubMed] [Google Scholar]
- 37.O'Leary K.A, Hathaway K.M, Tracy J.W. Schistosoma mansoni:single-step purification and characterization of glutathione S-transferase isoenzyme 4. Exp. Parasitol. 1992;75(1):47–55. doi: 10.1016/0014-4894(92)90121-p. [DOI] [PubMed] [Google Scholar]
- 38.Smith D.B, Johnson K.S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 39.Panaccio M, Wilson L.R, Crameri S.L, Wijffels G.L, Spithill T.W. Molecular characterization of cDNA sequences encoding glutathione-S-transferases of Fasciola hepatica. Exp. Parasitol. 1992;74(2):232–237. doi: 10.1016/0014-4894(92)90051-b. [DOI] [PubMed] [Google Scholar]
- 40.Asojo O.A, Ceccarelli C. Structure of glutathione S-transferase 1 from the major human hookworm parasite Necator americanus(Na-GST-1) in complex with glutathione. Acta Crystallogr. F. Struct. Biol. Commun. 2014;70:1162–1166. doi: 10.1107/S2053230X1401646X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleitas A.L, Randall L.M, Möller M.N, Denicola A. Purification of a recombinant glutathione transferase from the causative agent of hydatidosis Echinococcus granulosus. Biochem. Mol. Biol. Educ. 2016;44(1):28–33. doi: 10.1002/bmb.20918. [DOI] [PubMed] [Google Scholar]
- 42.Plancarte A, Rendon J.L, Landa A. Purification, characterization and kinetic properties of the Taenia solium glutathione S-transferase isoform 26.5 kDa. Parasitol. Res. 2004;93(2):137–144. doi: 10.1007/s00436-004-1103-y. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Singh A, Yadav M, Singh K, Rathaur S. MALDI mass sequencing and characterization of filarial glutathione-S-transferase. Biochem. Biophys. Res. Commun. 2007;356(2):381–385. doi: 10.1016/j.bbrc.2007.02.147. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad R, Srivastava A.K, Walter R.D. Purification and biochemical characterization of cytosolic glutathione S-transferase from filarial worms Setaria cervi. Comp. Biochem. Physiol. 2008;151(3):237–245. doi: 10.1016/j.cbpb.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Walker J, Crowley P, Moreman A.D, Barrett J. Biochemical properties of cloned glutathione S-transferases from Schistosoma mansoni and Schistosoma japonicum. Mol. Biochem. Parasitol. 1993;61(2):255–264. doi: 10.1016/0166-6851(93)90071-5. [DOI] [PubMed] [Google Scholar]
- 46.Salvatore L, Wijffels G, Sexton J.L, Panaccio M, Mailer S, McCauley I, Spithill T.W. Biochemical analysis of recombinant glutathione-S-transferase of Fasciola hepatica. Mol. Biochem. Parasitol. 1995;69(2):281–288. doi: 10.1016/0166-6851(94)00205-2. [DOI] [PubMed] [Google Scholar]
- 47.Torres-Rivera A, Landa A. Cooperative kinetics of the recombinant glutathione transferase of Taeniasolium and characterization of the enzyme. Arch. Biochem. Biophys. 2008;477(2):372–378. doi: 10.1016/j.abb.2008.07.008. [DOI] [PubMed] [Google Scholar]


