Abstract
Background
Sodium glucose transporter-2 inhibitors are the newest antidiabetic drugs that seem to be cardioprotective and can prevent type 2 diabetes in patients with high cardiovascular risks. Previous clinical trials have shown that these inhibitors can alleviate endothelial dysfunction, but the mechanism of action remains unknown. How SGLT inhibitor influences the release of NO in PA-induced HUVECs has never been reported.
Material/Methods
To explore the potential effects of the endothelial-protective mechanism of phlorizin and its impact on nitric oxide (NO), human umbilical vein endothelial cells (HUVECs) were incubated with palmitic acid (PA) and then treated with phlorizin. Western blotting was performed to assess the phosphorylation of AKT, eNOS, and IRS-1. To further explore potential targets, siRNA transfection was used to demonstrate the role of SGLT1 and SGLT2.
Results
Phlorizin suppressed the expression of SGLT1 and SGLT2, activated the PI3K/AKT/eNOS signaling pathway, increased the output of NO, and promoted the consumption of glucose in PA-induced HUVECs. Through demonstrating siRNA suppression of the expression of SGLT1 and SGLT2 in PA-induced HUVECs, this study provides a new understanding of the mechanism behind SGLT1 and SGLT2.
Conclusions
Our data demonstrate that phlorizin ameliorates the endothelial dysfunction link with the activation of the PI3K/AKT/eNOS signaling pathway and augmentation of the release of NO, partially through suppressing the expression of SGLT1 and SGLT2 in PA-induced HUVECS.
MeSH Keywords: Diabetes Mellitus, Type 2; Endothelial Cells; Lactase-Phlorizin Hydrolase; Nitric Oxide; Sodium-Glucose Transporter 1; Sodium-Glucose Transporter 2
Background
Diabetes mellitus is a major global health and economic problem. Obesity, high blood glucose, and dyslipidemia have long been recognized as risk factors for type 2 diabetes [1]. Improved glycemic control is crucial in delaying the progression of potential metabolic dysfunction in diabetic patients and reducing the risk of diabetic complications, including nephropathy and cardiovascular disease, which is usually achieved by increased endothelial function [1,2]. Type 2 diabetes is a chronic disease with defective micro- and macro-vascular complications that result in excessive morbidity and premature mortality [3].
Phlorizin is a type of flavonoid that can be found on the rind of various fruit trees. It is also regarded as a sodium-glucose co-transporter inhibitor [4]. As a natural product, phlorizin is a relatively nonselective but potent inhibitor of the sodium glucose cotransporters (SGLTs), which regulate renal reabsorption and intestinal absorption of glucose, and it has been demonstrated to induce glycosuria and anti-hyperglycemia in diabetic animal models [5]. Phlorizin obviously inhibits SGLT2 and SGLT1, with a 10-fold higher affinity for the former. Patients who have been diagnosed with type 2 diabetes may have new cure options due to the recent use of SGLT2-selective inhibitors, which effectively reduce renal glucose reabsorption and improve glycosuria [6]. Inhibition of renal reabsorption is prone to reduce the renal threshold for glucose, allowing the excretion of excess glucose into the urine and thus reducing plasma glucose concentrations [7,8]. Because this mechanism of action does not rely on insulin secretion or insulin action to lower plasma glucose levels, it is predicted to be efficacious in a wide variety of diabetic patients [9]. Many studies have convincingly shown that SGLT2 inhibitors not only lower the A1C levels in diabetic individuals with an overall improvement in cardiovascular function, but also induce the development of renal and peripheral insulin sensitivity and β cell function, which means that the presence of SGLT2 inhibitors is of functional significance [10]. More importantly, studies have shown a beneficial decline in several other CV risk factors, cardiovascular morbidity, and mortality, and even all-cause death by using of SGLT2 inhibitors [11].
Dysfunction of endothelial cells mediates abnormal vasomotor function, which is a characteristic of complications and has been demonstrated as the critical initiator [12,13]. NO plays a key role in mediating endothelial function homeostasis [14,15]. NO, which is derived from the endothelium, is a vasodilator with effective and widespread influence and has many different kinds of vasoprotective effects [16]. Pathological changes such as the metabolic alterations and insulin resistance in type 2 diabetes mellitus (T2DM) can result in endothelial dysfunction and relatively low NO levels, which are now regarded as the major mechanisms of macro-vascular complications in T2DM [13,17]. Some antidiabetic agents exert direct protective effects on HUVECs via the PI3K/AKT-eNOS pathway [18]. Regulated by PI3K/AKT/eNOS to elevate NO production, NO has also been suggested to be a crucial mediator in a broad spectrum of functions, including its vasorelaxing role in the cardiovascular system. However, the underlying mechanisms of phlorizin behind these effects have not been sufficiently investigated. The gastrointestinal tract has high expression level of SGLT1, while the kidney is known to be where SGLT2 is expressed. Additionally, 90% of renal glucose reabsorption occurs in the S1 segment of the proximal tubule, where SGLT2 is localized, whereas SGLT1 appears in the more distal S3 segment of the proximal tubule [7,19]. There have been several publications indicating that SGLT1 and SGLT2 expression occurs in endothelial cells including HUVECs [20–22]. However, whether phlorizin offers directly unique benefits independent of its glucose-lowering endothelial function is not fully understood.
Moreover, it has been shown that palmitic acid (PA), one of the fatty acids most commonly present in the Western diet, can decrease the release of NO and the activation of AKT and eNOS phosphorylation, resulting in increased insulin resistance (IR) [16,23]. In the present research, we investigated the advantage of the phlorizin on endothelial function impaired by PA in HUVECs and explored the underlying molecular mechanisms, including the PI3K/AKT/eNOS signaling pathway, and endothelial function-related molecules involving NO. To further this study, we used siRNA transfection to suppress the expression of SGLT1 and SGLT2 to examine this underlying mechanism.
Our results improve understanding of the mechanism by which phlorizin exerts cardiovascular protective effects. We first examined the expression of SGLT1 and SGLT2 in HUVECs. Our findings are the first to demonstrate that phlorizin ameliorates endothelial dysfunction in PA-induced HUVECs via the PI3K/AKT/eNOS signaling pathway.
Material and Methods
Reagents
Phlorizin was obtained from Sigma-Aldrich (St Louis, MO). HUVECs were obtained from Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). LY294002 was purchased from Calbiochem (CA, USA). Antibodies to anti-phosphor-AKT (S473), anti-phosphor-eNOS (S1177), and anti-phosphor-IRS-1 were purchased from Cell Signaling Technology (Danvers, MA). Anti-SGLT1 was purchased from Affinity (Cincinnati, OH), and anti-SGLT2 was purchased from Abcam (Cambridge, UK). The nitric oxide assay kit was obtained from Cayman Chemical (Ann Arbor, MI).
PA preparation
PA (256 mg) was diluted in 0.1 mol/L sodium hydroxide (10 mL) at 70°C and combined with 3 mL of fatty acid-free BSA (10%) at 55°C. The complex concentration was 2 mM. The stock solution was filter-sterilized and stored at −4°C.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were grown in RPMI-1640 medium (Gibco/BRL, Life Technologies, Eggenstein, Germany) containing 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C in 5% CO2. Phlorizin was dissolved in dimethylsulfoxide (DMSO), and the solvent concentration was identically maintained in the control group. After being incubated overnight under serum starvation conditions, the cells were incubated with or without 300 μM palmitic acid (PA) for 18 h and then treated with phlorizin 50nM for 30 min in the presence or absence of LY294002 (10 μM, 1 h).
Real-time quantitative PCR
Total RNA was extracted and purified from the cell lines using Trizol reagent (Thermo Fisher Scientific, Carlsbad, CA) and the RNeasy Mini Kit (Qiagen Sciences, Germantown, MD), respectively, according to the manufacturer’s instructions. A 2-step M-MLV Platinum SYBR Green qPCR SuperMix-UDG kit (Thermo Fisher) was used to carry out reverse transcription and quantitative PCR in the Eppendorf Mastercycler ep realplex detection system (Eppendorf, Hamburg, Germany). The mRNA expression of target gene level for each of the transcripts was normalized to GAPDH.
Western blot analysis
Western blotting was carried out to quantify the total proteins of SGLT1 and SGLT2 and phosphorylated eNOS, AKT, and IRS-1. The cell lysates were centrifuged (12 000 rpm, 15 min, 4°C), and the supernatant was collected to be resolved through 10% SDS-PAGE and transferred onto a nitrocellulose membrane. The membranes were blocked in TBS-T containing 5% non-fat milk, followed by overnight incubation with specific antibodies. The membranes were thoroughly washed 3 times and subsequently incubated with peroxidase-conjugated goat anti-rabbit IgG (1: 10,000) in TBS-T for 1 h at room temperature. The membranes were thoroughly washed 3 times and the immunoassayed proteins were visualized by ECL.
Measurement of nitric oxide
Nitric oxide release in HUVECs was examined by nitrate (converted to nitrite) reductase method according to the manufacturer’s instructions of the nitrate/nitrite fluorometric assay kit. The cells were treated with PA for 18 h and stimulated by phlorizin for 30 min. The 10–20 μL supernatant collected from the experimental conditions was diluted with assay buffer to adjust the volume to 80 μL in the 96 wells and mixed with 10 μL enzyme cofactors and nitrate reductase. After 30-min incubation at room temperature, we added 10 μL of DAN regent to each well for 10-min incubation. Finally, we added 20 μL NaOH to each well and read the plate in a fluorometer using an excitation wavelength of 360–365 nm and emission wavelength of 430 nm.
Glucose absorption
Glucose absorption in HUVECs was examined by using a commercial kit (Nanjing Jiancheng, Jiangsu, China). The cells were treated with PA for 18 h and stimulated by phlorizin or insulin for 30 min. The supernatant was collected for measurement in a 550-nm enzyme immunoassay.
siRNA-induced gene silencing
Gene silencing in cells was performed using specific siRNA sequences. SGLT1 and SGLT2 siRNAs were purchased from Gene Pharma Co. Ltd. (Shanghai, China). Transfection of HUVECs with siRNA was carried out using the Lipofect AMINETM 2000 (Thermo Fisher, Carlsbad, CA) reagent for 12 h. Cells were then washed and cultured in fresh medium with PA for 18 h. Gene knockdown was confirmed by qPCR and Western blot analysis.
Statistical analysis
Statistical analysis was performed using GraphPad Pro (GraphPad, San Diego, CA). All data are represented as average ±S.E. The significant differences between groups were compared using the t test or ANOVA for multiple comparisons. P<0.05 was considered statistically significant.
Results
Effects of PA and phlorizin on the expression of SGLT1 and SGLT2 in HUVECs
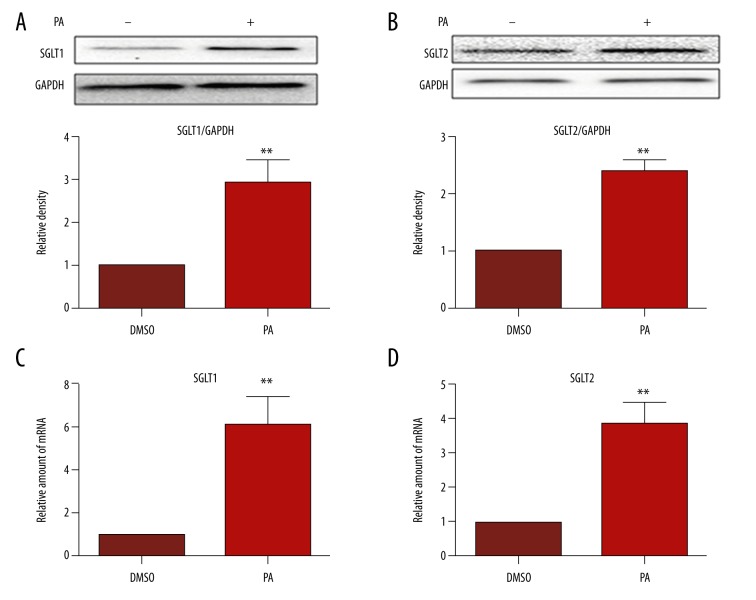
To investigate the effects of PA in endothelial cells, HUVECs were treated with PA (300 μM) for 18 h. As shown in Figure 1, PA upregulated the expression of SGLT1 and SGLT2 remarkably compared to control (Figure 1A, 1B). In addition, qRT-PCR demonstrated that the SGLT1 and SGLT2 mRNA levels were higher than those of the control (Figure 1C, 1D). These results demonstrated that PA, which leads to insulin resistance in T2DM, tended to significantly increase the expression of SGLT1 and SGLT2 in HUVECs. However, phlorizin significantly suppressed the expression level caused by PA (Figure 2B–2D).
Figure 1.
PA stimulates expression of SGLT1 and SGLT2 in HUVECs. Cells were incubated for 18 h in the presence of PA (300 μM). (A, B) SGLT1 and SGLT2 proteins in the samples were analyzed by Western blotting. Bar plots show the summarized data of the relative density after being normalized to GAPDH and are expressed as average percentage of control ±SEM. (C, D) Quantitative PCR analysis of mRNA expression of SGLT1 and SGLT2, normalized to GAPDH mRNA. ** P<0.01.
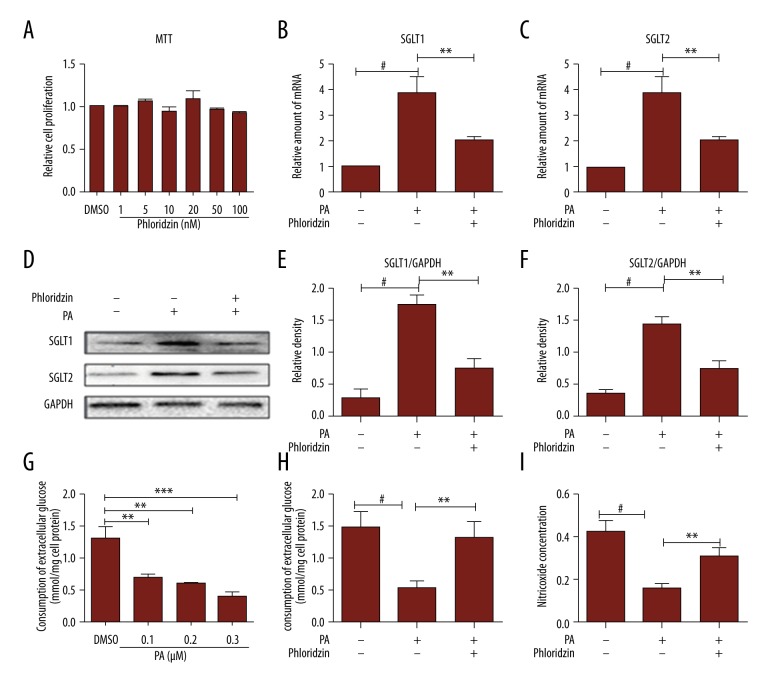
Figure 2.
The protective effects of phlorizin on PA-induced endothelial dysfunction in HUVECs. (A) The cell viability was determined by MTT assay of HUVECs treated with phlorizin in different concentrations for 30 min. Representative immunofluorescence microscopy (magnification 200, scale bar: 100 mm) showed extracellular HS expression. (G) PA reduced the glucose uptake in endothelial cells in a dose-dependent manner. (B, C) The relative amounts of mRNA of SGLT1 and SGLT2 were analyzed by qPCR. (D–F) Proteins of SGLT1 and SGLT2 were analyzed by Western blotting. (H) The course of phlorizin (50 nM)-induced consumption of extracellular glucose. (I) NO production was detected by the nitric oxide assay kit. # P<0.01 vs. controls, * P<0.05, ** P<0.01, *** P<0.001 for a chance difference compared with the relevant group.
Phlorizin attenuated endothelial dysfunction caused by PA
To investigate whether increased SGLT levels are associated with improving endothelial function, we used PA to determine whether impaired endothelial function was attenuated. PA reduced glucose uptake in the endothelial cells in a dose-dependent manner (Figure 2G). The glucose oxidase peroxidase enzymatic method was completed to determine the concentrations of glucose in the culture medium and then we evaluated whether the insulin resistance models of the cells were successfully prepared. According to the glucose absorption test, we found that PA in 300 μM was the proper concentration to take shape of IR. In addition, the release of NO catalyzed by the nitric oxide assay kit was also reduced because of the presence of PA (300 μM, 18 h) in HUVECs (Figure 2I). Treatment with phlorizin significantly ameliorated the impaired endothelial function in the release of NO (Figure 2I) and the glucose uptake (Figure 2H), compared with the PA-induced HUVECs. These data indicate that some of the dysfunctions caused by PA can be reversed by cultivating phlorizin, and phlorizin attenuated insulin resistance, thus improving endothelial function.
Phlorizin activated the PI3K/AKT/eNOS signaling pathway in PA-induced HUVECs
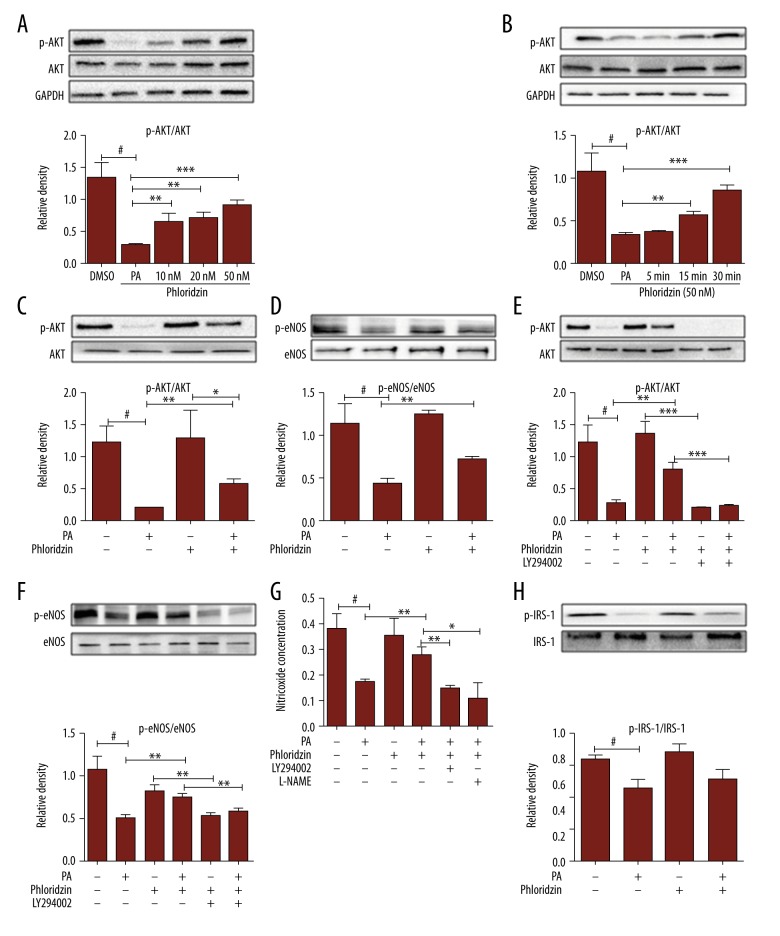
We further investigated the molecular mechanism by which phlorizin attenuated PA-induced endothelium function impairment. HUVECs were incubated with PA (300 μM) in the presence or absence of phlorizin for 18 h. Our results suggested that phlorizin increased the levels of the phosphorylation of AKT in a dose- (Figure 3A) and time- (Figure 3B) dependent manner. PA decreased nitric oxide (NO) release and AKT/eNOS phosphorylation compared with controls. Phlorizin reversed these effects and further increased the AKT (Figure 3C)/eNOS (Figure 3D) phosphorylation. Phlorizin augmented AKT and eNOS phosphorylation in HUVECs, with the effect of AKT phosphorylation (Figure 3E) completely abolished and the phosphorylation of eNOS (Figure 3F) attenuated after exposure to LY294002 (10 μM, 1 h), which is a special inhibitor of PI3K. We found that phlorizin elevated the output of NO by activating PI3K/AKT/eNOS, which disappeared when combined with the LY294002 or L-NAME (a special inhibitor of eNOS, 100 nM, 1 h) pretreatment (Figure 3G). These results suggest that phlorizin reversed the PA-decreased NO levels in a PI3K/AKT/eNOS-dependent manner, and the NO output partially relies on the activation of eNOS. Phlorizin had no effect on the phosphorylation of IRS-1 (Figure 3H). This implies that IRS-1 does not participate in the mechanism of phlorizin-improved endothelial function in HUVECs induced by PA.
Figure 3.
The effect of phlorizin on phospho-eNOS, phospho-AKT, and NO release in HUVECs treated with PA. (A) The effect of dose-dependent stimulation of phlorizin on AKT phosphorylation after 30-min incubation in PA-induced HUVECs. (B) Time course of effect of phlorizin (50 nM) on AKT phosphorylation. (C, D, H) Phosphorylation of AKT, eNOS, and IRS-1 were analyzed by Western blotting. (H) Phlorizin did not induce phosphorylation of IRS-1. (E, F) Effects of phlorizin (50 nM) on the levels of p-AKT and p-eNOS, co-treated with LY294002 (LY; a PI3K inhibitor; 10 μM, 1 h). (G) The nitric oxide assay kit for detecting the release of NO in the presence or absence of the specific PI3K inhibitor, LY294002 (10 μM, 1 h) or L-NAME (A special eNOS inhibitor; 100 nM, 1h). # P<0.01 vs. the control group, * P<0.05, ** P<0.01, *** P<0.001 for a chance difference compared with the relevant group.
These data indicate that phlorizin reversed PA-induced endothelial dysfunction by increasing AKT and eNOS phosphorylation levels and upregulating NO production. This effect may be mediated by both SGLT-dependent and SGLT-independent mechanisms. These data suggest that phlorizin can attenuate insulin resistance and endothelial function through endothelial nitric oxide synthase (eNOS) activation and increase NO release in endothelial cells.
Confirmation that blocking SGLT1 and SGLT2 in the endothelial cells was functional
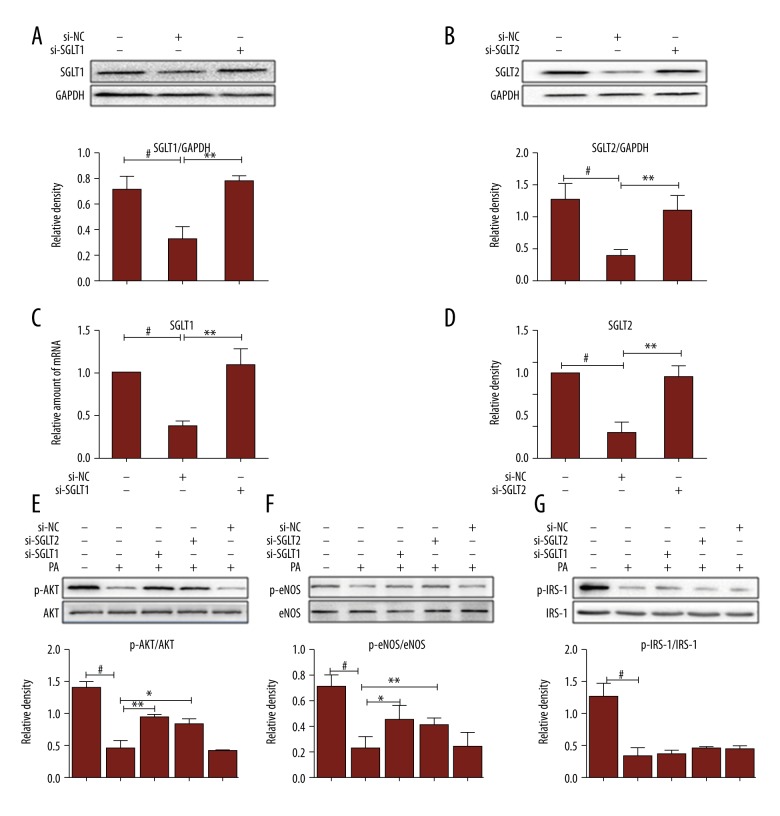
A scrambled sequence for the SGLT siRNA was taken to be the negative control (si-NC). SGLT1 and SGLT2 were markedly inhibited through special siRNA. qPCR (Figure 4C, 4D) and Western blot (Figure 4A, 4B) also demonstrated that siRNA can suppress their gene translations and protein expression of SGLT1 and SGLT2 compared with the si-NC. As expected, exposure of endothelial cells to siRNA markedly increases AKT (Figure 4E) and eNOS (Figure 4F) phosphorylation levels. Thus, the expression of SGLT1 and SGLT2 may play an important role in regulating NO-mediated endothelial function.
Figure 4.
AKT and eNOS activity is stimulated in siRNA transfected HUVECs. (A, B) A scrambled sequence for the SGLT siRNA was taken to be the negative control (si-NC). The siRNA-treated HUVECs for 12 h were collected to be analyzed by Western blotting. Band intensities normalized to GAPDH. (C, D) The SGLT1 and SGLT2 mRNA levels determined by real-time RT-PCR and normalized to GAPDH mRNA. (E–G) Then, transfected HUVECs were collected for SDS-PAGE and immunoblotted with phospho-AKT, phospho-eNOS, and phospho-IRS-1 specific antibodies. Values were normalized using GAPDH controls. Representative of three experiments with similar results. # P<0.01 vs. the control group, * P<0.05, ** P<0.01 compared with relevant control.
Discussion
A growing number of studies have illustrated that SGLT2 inhibitors reduce the CV risk factors in T2DM and demonstrated that the function of attenuating cardiovascular dysfunction was not a significant contributor to the metabolic effects [11,24,25]. Ipragliflozin improved hyperglycemia, ameliorated the impaired p-AKT and p-eNOS, and prevented the development of endothelial dysfunction in STZ-induced diabetes mice, at least partially by attenuation of oxidative stress [26]. Our study may offer one of the potential mechanisms for these effects. In this study, we demonstrated that phlorizin ameliorated the endothelial dysfunction and improved the insulin resistance by activation of the PI3K/AKT/eNOS signaling pathway and augmentation of the release of NO, partially through suppressing the expression of SGLT1 and SGLT2 in PA-induced HUVECs.
SGLT1and 2 are the co-transporters of glucose sodium, which is found on the cell surface, mediating the transfer of extracellular and intracellular glucose [7]. SGLT1 is mainly expressed in the small intestine and also in human hearts [27]; however, SGLT2 appears in the kidney and contributes to renal glucose reabsorption. Consistent with results reported by other researchers, we found that SGLT1 and 2 are also expressed in HUVECs [20,22]. In our study, we focused on the effect of phlorizin on endothelial function associated with SGLT1 and SGLT2 and the release of NO, elucidating the underlying molecular mechanisms. As a type of FFA, palmitic acid can be used to simulate the environment of high FFA in T2DM. There was no toxic effect of phlorizin in HUVECs based on MTT (Figure 2A). We have discovered that PA upregulates the expression of SGLT1and SGLT2, and inhibited glucose utilization and the NO output; however, phlorizin can change the detrimental influences of PA, indicating that the potentially direct beneficial effects of phlorizin are linked to SGLT1 and SGLT2 in the vascular endothelium. Additionally, animal study showed that SGLT2 inhibitors improved vascular function in SGLT2-knocked mice [28]. As Figure 4 shows, HUVECs blocked the expression of SGLT1 and SGLT2 through the special siRNA incubated with PA for 18 h, and the phosphorylation of AKT and eNOS were at higher levels compared with the si-NC group. All results taken together, we demonstrated the AKT/eNOS signaling pathway is been correlated with SGLT1 and SGLT2, supporting a potential direct protection mechanism of phlorizin on PA-induced HUVECs.
We suggest that when PA exists, the function of SGLT1 and SGLT2 is damaged, and negative feedback to increase its effect and create a high-sugar extracellular environment, leading AGEs to promote the production of ROS. High AGEs, on the one hand, hinder IRS tyrosine phosphorylation, leading to loss of the combination of IRS with insulin receptor, further inducing insulin resistance. On the other hand, PA or high AGEs inhibited the activation of the AKT/eNOS signaling pathway. AKT, one of the PI3K signaling pathway downstream molecules, is an important protein kinase, mediating cell proliferation, migration, and metabolic response, and eNOS mediates endogenous NO output [29]. As Figure 3 shows, phlorizin increased the levels of p-AKT in a dose- and time-dependent manner. Phlorizin elevated the release of NO and the phosphorylation of AKT/eNOS compared with controls, in spite of the presence of PA. Addition of the PI3K inhibitor LY294002 to the HUVECs completely diminished the effects of phlorizin on NO production, AKT, and eNOS phosphorylation, respectively. We maintain that phlorizin may have a beneficial cardiovascular effect by increasing p-AKT and p-eNOS by the upper molecular PI3K. These effects were regulated primarily through activation of the PI3K/AKT pathway and subsequent improvement of eNOS. The results of the present study demonstrate the PI3K plays a critical role in which phlorizin protects against endothelial dysfunction in type 2 diabetes.
NO, an endothelium-derived vasodilator, plays a chief role in regulating endothelial function homeostasis [30]. Loss of NO bioavailability is a key feature of endothelial dysfunction in preceding and deteriorating atherosclerosis. Elevating the NO levels has also been considered as a major regulator in a broad spectrum of functions involving a vasodilation effect in the cardiovascular system [30–32]. Nitric oxide is generated by the endothelial and inducible NO synthases (eNOS and iNOS, respectively) [33]. We used L-NAME, a special eNOS inhibitor, to interdict NO, which is produced by endothelial synthases. On the basis of NO concentrations, L-NAME (100 nM, 1 h) eliminated the function of phlorizin on NO, which was partially dependent on p-eNOS. To the best of our knowledge, this is the first study to investigate the role of phlorizin therapy on cardiovascular endothelial function via elevation of NO levels by activating p-eNOS, which may be a potential treatment for heart failure.
We found that phlorizin in reduction of p-IRS-1 induced by PA was not significantly increased, and it may not be able to function through IRS-1. Under normal circumstances, the IRS is an important signaling protein in insulin signal transduction pathways, with activating the downstream of the IRS-1/PI3K/AKT and regulating the cell growth and proliferation. However, the IRS, which has many subtypes, should be assessed in further experiments to detect the possible role of phlorizin. There are some limitations to our study. First, it did not distinguish the roles of SGLT1 and SGLT2. Further studies are needed to elucidate these effects of SGLT1 and SGLT2, which present the domain status in protecting the endothelial cells. Second, in vitro trials are relatively restricted, and animal trials would help to more convincingly clarify its cardiovascular protective effects and underlying mechanisms. Finally, our study did not discover how phlorizin stimulated the PI3K to elevate the NO levels in HUVECs.
Conclusions
Our results provide new insights into the beneficial effects of phlorizin, which were associated with attenuation of endothelial dysfunction, and into the underlying mechanism, which at least in part is an elevation of the NO levels. Taken together with the results of previous studies, our results show that phlorizin has direct protective effects. These non-glycemic properties of phlorizin may provide attractive therapeutic options for type 2 diabetic patients, in whom endothelial dysfunction and coronary artery disease adversely affect survival.
Abbreviations
- PI3K
phosphoinositide-3-kinase
- eNOS
endothelial nitric oxide synthase
- NO
nitric oxide
- PA
palmitic acid
- HUVECs
human umbilical vein endothelial cells
- AKT
serine/threonine kinase
- SGLT
sodium glucose cotransporter
- IRS-1
insulin receptor substrate 1
- siRNA
small interfering RNA
Footnotes
Conflict of interest
None.
Source of support: This study was supported by National Natural Science Foundation of China (No. 81670777), Key Medical Science and Technology Plan of Zhejiang Province (WKJ-ZJ-1625), Wenzhou Science and Technology Bureau Public Welfare Science and Technology Projects(H20150001)
References
- 1.Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, et al. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength. World J Diabetes. 2014;5(4):444–470. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimbrone MA, Jr, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–36. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang CC, Reusch JE. Diabetes and cardiovascular disease: Changing the focus from glycemic control to improving long-term survival. Am J Cardio. 2012;110(9 Suppl):58B–68B. doi: 10.1016/j.amjcard.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenkranz JR, Lewis NG, Kahn CR, et al. Phlorizin: A review. Diabetes Metab Res Rev. 2005;21(1):31–38. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 5.Zhang SY, Li BY, Li XL, et al. Effects of phlorizin on diabetic retinopathy according to isobaric tags for relative and absolute quantification-based proteomics in db/db mice. Mol Vis. 2013;19:812–21. [PMC free article] [PubMed] [Google Scholar]
- 6.Chao EC, Henry RR. SGLT2 inhibition – a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9(7):551–59. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 7.Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255–70. doi: 10.1146/annurev-med-051013-110046. [DOI] [PubMed] [Google Scholar]
- 8.Thomas MC, Jandeleit-Dahm K, Bonnet F. Beyond Glycosuria: Exploring the intrarenal effects of SGLT-2 inhibition in diabetes. Diabetes Metab. 2014;40:S17–22. doi: 10.1016/S1262-3636(14)72691-6. [DOI] [PubMed] [Google Scholar]
- 9.Dardi I, Kouvatsos T, Jabbour SA. SGLT2 inhibitors. Biochem Pharmacol. 2016;101:27–39. doi: 10.1016/j.bcp.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Dziuba J, Alperin P, Racketa J, et al. Modeling effects of SGLT-2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes Metab. 2014;16(7):628–35. doi: 10.1111/dom.12261. [DOI] [PubMed] [Google Scholar]
- 11.Younk LM, Lamos EM, Davis SN. Cardiovascular effects of anti-diabetes drugs. Expert Opin Drug Saf. 2016;15(9):1239–57. doi: 10.1080/14740338.2016.1195368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oudegeest-Sander MH, Olde Rikkert MG, Smits P, et al. The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: A randomized factorial design trial. Exp Gerontol. 2013;48(12):1509–17. doi: 10.1016/j.exger.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno G, Mangione CM. Management of cardiovascular disease risk factors in older adults with type 2 diabetes mellitus: 2002–2012 literature review. J Am Geriatr Soc. 2013;61(11):2027–37. doi: 10.1111/jgs.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi M, Kotha SR, Malireddy S, et al. Conundrum of pathogenesis of diabetic cardiomyopathy: Role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Mol Cell Biochem. 2014;386(1–2):233–49. doi: 10.1007/s11010-013-1861-x. [DOI] [PubMed] [Google Scholar]
- 15.Schlegel F, Appler M, Halling M, et al. Reprogramming bone marrow stem cells to functional endothelial cells in a mini pig animal model. Med Sci Monit Basic Res. 2017;23:285–94. doi: 10.12659/MSMBR.905081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25(5):989–94. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 17.Low Wang CC, Hess CN, Hiatt WR, et al. Clinical update: Cardiovascular disease in diabetes mellitus: Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus – mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, Zhang J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin. 2012;33(1):75–81. doi: 10.1038/aps.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–97. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 20.Chen ML, Yi L, Jin X, et al. Absorption of resveratrol by vascular endothelial cells through passive diffusion and an SGLT1-mediated pathway. J Nutr Biochem. 2013;24(11):1823–29. doi: 10.1016/j.jnutbio.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Vrhovac I, Balen Eror D, Klessen D, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467(9):1881–98. doi: 10.1007/s00424-014-1619-7. [DOI] [PubMed] [Google Scholar]
- 22.Elfeber K, Stumpel F, Gorboulev V, et al. Na(+)-D-glucose cotransporter in muscle capillaries increases glucose permeability. Biochem Biophys Res Commun. 2004;314(2):301–5. doi: 10.1016/j.bbrc.2003.12.090. [DOI] [PubMed] [Google Scholar]
- 23.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103(2):253–59. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson PL, Davis TME. Cardiovascular effects of glucose-lowering therapies for type 2 diabetes: New drugs in perspective. Clin Ther. 2017;39(5):1012–25. doi: 10.1016/j.clinthera.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Monami M, Dicembrini I, Mannucci E. Effects of SGLT-2 inhibitors on mortality and cardiovascular events: A comprehensive meta-analysis of randomized controlled trials. Acta Diabetol. 2017;54(1):19–36. doi: 10.1007/s00592-016-0892-7. [DOI] [PubMed] [Google Scholar]
- 26.Salim HM, Fukuda D, Yagi S, et al. Glycemic control with Ipragliflozin, a novel selective SGLT2 inhibitor, ameliorated endothelial dysfunction in streptozotocin-induced diabetic mouse. Front Cardiovasc Med. 2016;3:43. doi: 10.3389/fcvm.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashiwagi Y, Nagoshi T, Yoshino T, et al. Expression of SGLT1 in human hearts and impairment of cardiac glucose uptake by phlorizin during ischemia-reperfusion injury in mice. PLoS One. 2015;10(6):e0130605. doi: 10.1371/journal.pone.0130605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leng W, Ouyang X, Lei X, et al. The SGLT-2 inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE−/− mice. Mediators Inflamm. 2016;2016:6305735. doi: 10.1155/2016/6305735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeno Y, Li Q, Park K, et al. Inhibition of insulin signaling in endothelial cells by protein kinase C-induced phosphorylation of p85 subunit of phosphatidylinositol 3-kinase (PI3K) J Biol Chem. 2012;287(7):4518–30. doi: 10.1074/jbc.M111.286591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanhoutte PM, Zhao Y, Xu A, et al. Thirty years of saying NO: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res. 2016;119(2):375–96. doi: 10.1161/CIRCRESAHA.116.306531. [DOI] [PubMed] [Google Scholar]
- 31.Chinnathambi V, Balakrishnan M, Ramadoss J, et al. Testosterone alters maternal vascular adaptations: role of the endothelial NO system. Hypertension. 2013;61(3):647–54. doi: 10.1161/HYPERTENSIONAHA.111.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kream RM. Nitric oxide regulation of mitochondrial processes: Commonality in medical disorders. Med Sci Monit Basic Res. 2015;20:402–7. doi: 10.12659/AOT.894289. [DOI] [PubMed] [Google Scholar]
- 33.Trajanovska S, Donald JA. Endothelial nitric oxide synthase in the amphibian, Xenopus tropicalis. Comp Biochem Physiol B Biochem Mol Biol. 2011;158:274–81. doi: 10.1016/j.cbpb.2010.12.008. [DOI] [PubMed] [Google Scholar]