Abstract
Psychoeducation (PE) is defined as an intervention with systematic, structured, and didactic knowledge transfer for an illness and its treatment, integrating emotional and motivational aspects to enable patients to cope with the illness and to improve its treatment adherence and efficacy. PE is considered an important component of treatment in both medical and psychiatric disorders, especially for mental health disorders associated with lack of insight, such as alcohol and substance use disorders (ASUDs). New advancements in neuroscience have shed light on how various aspects of ASUDs may relate to neural processes. However, the actual impact of neuroscience in the real-life clinical practice of addiction medicine is minimal. In this chapter, we provide a perspective on how PE in addiction medicine can be informed by neuroscience in two dimensions: content (knowledge we transfer in PE) and structure (methods we use to deliver PE). The content of conventional PE targets knowledge about etiology of illness, treatment process, adverse effects of prescribed medications, coping strategies, family education, and life skill training. Adding neuroscience evidence to the content of PE could be helpful in communicating not only the impact of drug use but also the beneficial impact of various treatments (i.e., on brain function), thus enhancing motivation for compliance and further destigmatizing their symptoms. PE can also be optimized in its “structure” by implicitly and explicitly engaging different neurocognitive processes, including salience/attention, memory, and self-awareness. There are many interactions between these two dimensions, structure and content, in the delivery of neuroscience-informed psychoeducation (NIPE). We explore these interactions in the development of a cartoon-based NIPE to promote brain recovery during addiction treatment as a part of the brain awareness for addiction recovery initiative.
Keywords: Psychoeducation, Metacognitive, Self, Cartoon, Addiction, Training, Neuroscience
1 INTRODUCTION
“What type of disorder do I have?,” “What are its symptoms?,” “How can I cope with my symptoms?,” “How can my disorder be treated or controlled?,” “What are the treatment options and which one is the best?,” and “What are the side effects of the treatments?” are some of the initial questions asked by patients after they have been diagnosed with an illness (Shuyler and Knight, 2003; Yoonessi and Ekhtiari, 2013). When treatment providers offer very little, or poorly construed education, patients may seek answers from less accurate resources, acquire misperceptions about their illness, and lose trust in their providers. This can subsequently lead to decreased compliance with treatments and poor outcomes (Bäuml et al., 2006; Swaminath, 2009). If treatment providers offer clear and accurate information through a two-way collaborative discussion, they can help simplify the complexities of the illness and provide patients with enriched insight and motivation for treatment (Swaminath, 2009). When the patient’s family is involved in these educational sessions, they can also assist the patient in their understanding and support their progress through treatment. With optimal education, patients can become an expert of their own illness, helping them to make appropriate decisions about their treatment and become more compliant (Swaminath, 2009).
Educating patients and their relatives about mental health conditions are a critical therapeutic intervention termed “psychoeducation (PE),” a term first used in 1980 by Anderson et al. when describing such an intervention for schizophrenic patients (Bäuml et al., 2006). PE provides systematic, structured, didactic information about the disorder, and its treatment to the patient and/or their family and caregivers, and integrates emotional aspects to enable patients to cope with the illness (Bossema et al., 2011). PE has been reported to be effective in reducing rehospitalization rates, symptom burden, and the likelihood of relapse, as well as improving patient’s compliance with treatment (Pitschel-Walz et al., 2006; Sv et al., 2012). Owing to its promising effects, PE quickly became a popular aspect of treatment for disorders other than Schizophrenia, including depression (Donker et al., 2009), anxiety (Donker et al., 2009), multiple sclerosis (Mcguire and Stojanovic-radic, 2015), and even nonmental health conditions such as cancer (Northouse et al., 2014). People with alcohol and substance use disorders (ASUDs) have been considered an important target for PEs due to their lack of insight and knowledge about their disorder (Chandiramani, 1993). PE can be employed as a standalone intervention or as a part of a treatment package for ASUDs, from before the early phase of abstinence and through to the later stages of recovery. Different PEs have been effective at improving various treatment outcomes (e.g., length of abstinence and rate of consumption). Table 1 presents few sample studies using PE for ASUDs.
Table 1.
Examples of Published Studies With Different Types of Psychoeducation (PE) Among People With Alcohol and Substance Use Disorders
| Study | Sample | Types of PE | Main Outcomes |
|---|---|---|---|
|
| |||
| Richmond et al. (1986) | Cigarette smokers | Adverse effects of nicotine presented with pictures and booklets | Length of abstinence |
| Kominars (1997) | Substance users | Psychoeducation treatment groups | Levels of emotional arousal, self-efficacy, and coping resources |
| Sobell et al. (2002) | Alcohol users | Information about the effects of alcohol given with pamphlets | Rate of drinking |
| Hulse and Tait (2002) | Alcohol users | Information package to reduce alcohol consumption | Rate of drinking |
| Davis et al. (2002) | Alcohol users | Education movies about the effects of alcohol | Reduction in amount of alcohol consumed, length of abstinence |
| Fals-stewart et al. (2006) | Alcohol users | Lectures about substance abuse (epidemiology, etiology, effects on the body, effects on the brain) | Drinking frequency |
| Bowen and Marlatt (2009) | Cigarette smokers | Psychoeducation instructions about coping strategies | Smoking-related urges and smoking behavior |
| De Maricourt et al. (2016) | Benzodiazepine users | Group sessions focused on understanding the illness and coping skills | Benzodiazepine intake |
| Bjelland et al. (2017) | Substance users | Mindfulness-based psychoeducational film | Patients’ perspectives |
Neuroscience has made tremendous progress in terms of understanding the basic mechanisms of ASUDs (Ekhtiari and Paulus, 2016). Hundreds and thousands of papers have described mechanisms involved in drug-induced toxicity, tolerance, withdrawal, dependency, craving, stress, allostasis, relapse, psychiatric and medical comorbidities, as well as abstinence and recovery (Courtney and Ray, 2016; Ekhtiari et al., 2016; Lemieux and Absi, 2016; Mohammad Ahmadi Soleimani et al., 2016). For example, a simple search for the terms “addiction +neuroscience/brain” yields over 14,000 results in PubMed (as of July, 2017). Currently, there is a large gap between the content of conventional PEs and this newly generated knowledge base of addiction neuroscience. We have not found any published study of PE that uses neuroscience-informed psychoeducation (NIPE) to answer potential questions among patients and families. This may be due to the many challenges for offering neuroscience-based PE, including (1) how to identify aspects of the neuroscience literature that would be most helpful to use in PEs, (2) how to translate the complex and massive neuroscience literature into verbiage that is simple and clear enough to communicate meaningfully to patients, families, and other providers, and (3) how to determine that the addition of neuroscience-based information can improve the efficacy of PE. To address these challenges, we need to bring together researchers who are knowledgeable about the neuroscientific literature with researchers and clinicians who are knowledgeable about the practical considerations of delivering PE.
In addition to the potential for NIPE to incorporate new content, we can also leverage knowledge gained from neuroscience and cognitive/affective psychology to optimize the methods we use to deliver PEs. This can involve structuring PE in a way that enhances engagement of networks involved in cognitive–affective processes that will support its efficacy, including attention, memory, executive control, salience processing, and social and metacognition. This could include, among other things, choosing specific presentation mediums (written vs visual; specific artistic styles), contexts (i.e., group vs family vs individual sessions), and timing (i.e., immediately after diagnosis vs delayed; duration and frequency of sessions). These insights have been recently embedded into specific therapeutic programs, such as cognitive rehabilitation and therapy programs for individuals with traumatic brain injury (Jak et al., 2015).
In this opinion paper, we review opportunities for neuroscience evidence to be added to the content of PEs and then explore the potential for PE structure to be informed by cognitive–affective neuroscience/psychology. In the context of structure, we explore cartoons as a medium to convey neuroscience-based educational information in a way that engages attentional and affective processes. As an example, we introduce our experience in the development of a cartooned brain-based PE program for alcohol/substance users in which both content and structure are informed by a neurocognitive perspective. Finally, we will introduce future directions and challenges for NIPE in addiction medicine.
2 NEUROCOGNITIVE APPROACH TO PE: CONTENT
In NIPE, the main focus is on conveying neuroscientific knowledge about the disorder and related treatments. In other words, complex evidence from neuroscience research should be simplified and communicated during PE in verbiages that can be easily understood by patients (Maarefvand et al., 2013). We address below the possible main points that can be presented in NIPEs designed for alcohol/substance users. Notably, there remains uncertainty regarding some of these topics in the addiction neuroscience literature—which can pose challenges to clearly communicating these topics in NIPE. However, the simple act of developing and examining NIPE interventions can help to identify these points of uncertainty and provide further motivation for neuroscientific research to tackle these questions. In other words, NIPE opens up further channels for research to inform clinical work as well as for clinical work to inform research.
Neurocognitive risk factors for ASUDs: There is evidence to suggest that there are preexisting neurocognitive risk factors, including abnormalities in brain structures and deficits in cognitive functions (e.g., decision-making, learning, and memory), which may increase vulnerability to addiction (Wood et al., 2013). However, it is important in PE to clearly communicate that the presence or absence of these risk factors will not completely determine whether a person develops ASUDs or not. There are many other factors, such as environment, that have perhaps even stronger influences and may interact with neurocognitive risk factors.
How dependency develops in terms of brain processes and then how the dependent brain responds: NIPE can include explanations regarding the neural circuitry that supports homeostasis and reward/stress processing, and the impact of alcohol and other addictive substances on this neural circuitry that supports the development of dependency. Tolerance and withdrawal can be discussed as two common clinical conditions experienced during drug dependency, which relate to specific neuroscientific models (Adinoff, 2004).
Neurocognitive deficits caused by alcohol/substance consumption and their importance: It is well established that long-term use of alcohol and other substances can be associated with alterations in brain function as observed through changes in neural activations and morphology (particularly within the prefrontal cortex) and cognitive performance deficits (including working memory and attention in particular) (Squeglia et al., 2009). Clarifying observable behavioral deficits associated with these neurocognitive impairments can help patients identify the impact that ASUDs have had on their functioning. Notably, these neurocognitive deficits not only disturb daily life activities (e.g., social communication and self-care) but also have a negative impact on treatment (e.g., denial of addiction and need for treatment) (Bates et al., 2013).
Neural circuits involved in stress/cue-induced craving: Specific brain regions, including corticostriatal limbic circuitry important for affective and reward processing, become activated in response to stress and drug cues (Ekhtiari et al., 2016). This process subsequently supports unconscious and automated drug use responses following chronic use (Potenza et al., 2012). However, it would also be important to inform patients of the neural mechanisms to override these automated processes and to teach them strategies which can prevent or modify activation of related neural circuits. For example, prefrontal circuitry is thought to be particularly important for supporting inhibition of automated responses and cognitive reappraisal of emotional/appetitive information (Cutuli, 2014; Hermann et al., 2014). Reducing cues in the environment can also be helpful in preventing activation of craving-related circuitry (Otto et al., 2007; Volkow et al., 2016). NIPE that communicates this information may help patients to prevent and manage drug craving.
The impact of mindfulness and interoceptive awareness on neural circuits of ASUDs: Explaining the neural processes for interoceptive awareness, i.e., in receiving, processing, and integrating body-relevant signals together with external stimuli, during emotions, craving, or withdrawal can be useful content in the NIPE. Approach or avoidance behavior (e.g., toward stimuli such as drug cues) is being motivated as a result of this process (Koob and Volkow, 2010). Promoting interoceptive and exteroceptive awareness, for example through meditation or mindfulness, may help support self-awareness and behavior change (Paulus et al., 2013). There is a growing body of evidence on how mindfulness influences the way the brain responds to salient drug cues. Mindfulness is thought to increase awareness of the present moment, including both sensory cues and interoceptive processes. This in turn can improve awareness and tolerability of craving and decrease the automatic habitual response (i.e., substance consumption) (Witkiewitz and Lustyk, 2013).
The possible neurocognitive effects of commonly prescribed medications: Explaining the potential side effects of prescribed medications on neurocognitive functions as well as their positive results may help patients to be more informed about and compliant with pharmacological treatments (Prosser et al., 2006). For example, methadone, which has been widely prescribed for opioid users, can have both positive and negative outcomes (Rass et al., 2014).
The neurocognitive processes/deficits associated with overdose and lapses: Overdose experiences are known to be toxic and produce a state of anesthesia that leads to oxygen deprivation and potential damage to the brain (Evans, 1980). Communicating the biological basis of why tolerance to drugs can decrease after a period of abstinence and increase the risk of overdose would be another important component of NIPE for ASUDs. Such information could be useful in motivating patients’ efforts to prevent overdose experiences during active ASUDs or abstinence. Other than overdose, there is a growing body of evidence that indicates lapses after a period of abstinence can cause more serious harm to the brain than drug use during active, chronic ASUDs. Natural barriers of the brain resist against the effects of exogenous and endogenous toxins. These barriers become more active during chronic drug use to partially compensate toxic effects of drugs. These natural biological barriers will decline after a period of abstinence and make the brain more vulnerable to the toxic effects of drugs during lapses (Mohammad Ahmadi Soleimani et al., 2016). However, further evidence is needed to identify the most effective strategies for harm reduction in drug-induced toxicities during drug use and relapses.
The length of time needed for brain recovery: There is evidence that the brain recovers gradually during abstinence (Wobrock et al., 2009). While there is no certain answer regarding how long the brain takes to recover after abstinence, we estimate that the process of recovery might take several years (DuPont et al., 2015; Fein et al., 2006). Research has identified factors that facilitate and slow down this process (i.e., as listed in the following items). Communicating this information during PE can help patients identify that their brain is “in recovery” and thus, bring realistic hope and motivation for behavior change.
The effects of treatment on brain functions and structures: The possible neural impact of not only medication, but also cognitive and/or behavioral therapies, could also be included as a part of NIPE (Tabatabaei-Jafari et al., 2014). Discussion of how such treatment may influence brain circuits involved risk–reward decision making (e.g., ventromedial prefrontal cortex), cognitive control (e.g., anterior cingulate cortex), planning and executive functioning (e.g., dorsolateral prefrontal cortex) may be particularly important (Chung et al., 2016; Potenza et al., 2011). Such information could help to enhance motivation for treatment and communicate how even nonpharmacologic based interventions can also help to reverse some of the negative effects of ASUDs and enhance the brain’s ability to initiate and maintain recovery.
The effects of healthy life style on the brain recovery process: Explaining the effects of healthy diets, social interaction, participation in positive leisure activities, physical activities, and sleep quality on brain health (Gomez-pinilla and Kostenkova, 2008) could be an important part of PE.
The supplementary role of cognitive rehabilitation therapy to restore neurocognitive deficits: Recent neuroscientific research supports the potential benefit of structured cognitive rehabilitation therapy for people with ASUDs (Bates et al., 2013; Rezapour et al., 2016). Cognitive rehabilitation strategies consist of either practice-based restorative exercises (i.e., attention, executive function, or memory-based computer games) or strategies to compensate for cognitive deficits (i.e., calendar use, mnemonics, reminders, etc.) (Rezapour et al., 2017). There is evidence for the potential effectiveness of both types of interventions for people with ASUDs (Bates et al., 2013; Bickel et al., 2011), though findings are mixed and further research is needed.
3 NEUROCOGNITIVE APPROACH TO PE: STRUCTURE
Embedding the informational content into a more brain-friendly structure could enhance the efficacy of PE for patients with ASUDs who suffer from various types of cognitive deficits. In particularly, we propose that by applying mediums which enhance attentional and memory processes and promote self-awareness, NIPE can serve to enhance knowledge of one’s disorder and treatments and optimally support motivation for behavior change.
3.1 ATTENTION AND MEMORY
There is a growing body of literature on neurocognitive strategies to enhance learning, which could be useful for enhancing the effects PEs—particularly for populations (such as ASUDs) with cognitive impairments (i.e., attention and memory) creating barriers for information retention (Farley et al., 2013; Rezapour et al., 2016). Strategies to enhance attention to PE materials include (1) use of visually engaging materials, especially those that include facial stimuli, which are salient and thus prioritized for neural processing (Peterson et al., 2010), (2) limit the duration of time spent on presenting information at one time, to keep patients attentive and vigilant (e.g., as it is estimated that the uninterrupted attention span for adults is approximately 20min) (Farley et al., 2013; Lowe, 2011), and (3) limit the speed of information presented (not too slow to be boring and not so fast as to be confusing) (Lowe, 2011). Some strategies identified to enhance memory of learned information include (1) chunking information into meaningful categories, (2) using visual imagery, (3) linking information presented to a mental story or something they already know or that is important to them (Morrison and Chein, 2011), (4) practicing free recall of presented information and spaced retrieval (McDougall, 1998), and (5) presenting information in multiple modalities (visual, auditory, tactile, etc.) (Shams and Seitz, 2008).
3.2 SELF-AWARENESS
Self-awareness is a metacognitive function defined as declarative knowledge of one’s ability, thoughts, feelings, and mental states, and implies that one actively identifies, processes, and stores information about the self (Banks, 2008; Bivona et al., 2014). Lack of self-awareness in ASUDs has been well established and often presents itself in denying that addiction is a problem. So, lack of self-awareness can act as both a barrier and a target for PEs. There are various structures (mediums) for delivering information in a way to promote self-focus and self-awareness, including but not limited to (1) reviewing pictures, audio, or video recordings of oneself, (2) writing or storytelling about oneself or one’s life story, (3) imagining one’s personal future or self-related future events, or (4) using materials that include self-relevant images. Initial findings suggest that looking at videos of oneself can be efficacious through manipulation of self-focused attention (Silvia, 2012). Asking healthy subjects to write about how they differ from their family and friends and from people in general has been found to improve self-focused attention and self-awareness (Silvia and Eichstaedt, 2004). Storytelling is another potentially powerful way of enhancing self-awareness. It offers a universal form of communication in which an individual can creatively represent their experiences and the way he/she understands the world. The impact of storytelling-based education has been recently examined in the field of addiction prevention. Raising awareness among young people about addiction and its detrimental impacts on health through narrative methods was found to be effective in reducing adolescents’ risk to drug use (Moghadam et al., 2016). In addition to telling their own stories, listening to others reflect on their own experiences with a disorder and the benefit of particular treatments or coping strategies can help patients recognize their similar struggles and decrease resistance to treatment and behavior change (Gucciardi et al., 2016; Houston et al., 2011). The practice of “episodic future thinking” (EFT), or imagining one’s personal future and projecting the self in a preexperienced event (Atance and O’Neill, 2001) may also be helpful. There are recent evidences that EFT with general positive events can decrease delayed discounting and reduce alcohol consumption among people with alcohol dependence (Snider et al., 2016). More specifically, EFT may also be used in the context of conveying educational materials such as when and how patients can apply coping strategies to produce positive outcomes and help them to depict the roadmap for recovery. Lastly, when individuals feel that they, or people similar to them, are represented in educational materials—they are more likely attend to the information and find it relevant to their own lives (Kircher et al., 2000). One modern example of this approach is found within computerized games, in which users create an avatar that represents their own features (Baylor, 2009). Educational games with self-avatars could be a potential medium for future PEs (Andrade et al., 2016).
Structuring PEs using strategies and mediums to optimize attention and memory and engage self-related processes have high potential to optimize PEs and enhance their efficacy. There are many other neurocognitive dimensions that could be used to inform structures in NIPEs such as decision making, executive processes, and social cognition—which is beyond the breadth of this chapter to discuss. Future research is needed to fully explore how our knowledge of various neurocognitive process can be leveraged to inform the structure of PEs for individuals with ASUDs.
In summary, neuroscience has the potential to contribute to PE from two distinct but interrelated dimensions: content and structure. “Content” concerns what information is actually presented to patients. Unlike conventional PE in which illness-related information is the main focus of education, NIPE emphasizes neuroscience-based research in terms of brain functional and structural resiliency, vulnerability, adaptation, and impairment from the early phase of addiction to lifetime abstinence. “Structure” is related to how we can convey our educational materials using various mediums. Designing the structure to optimally engage processes related to self-awareness, salience/attention, and memory could significantly improve addiction treatment outcome. Integration of these two dimensions in transferring information may lead to added value and facilitation of learning effects. For example, in the case of ASUDs, NIPE can combine both realistic brain images (MRI) and humorous cartoons to show brain alterations as a result of drug use in a way that is self-relevant and engages the attention of the patient. NIDA has recently used such combinations in published educational posters focusing on adverse effects of drug use on the brain and body (https://drugpubs.drugabuse.gov/). This integration may increase the transfer effects of NIPE by taking abstract knowledge and pictorially representing how the information is practically relevant in patients’ daily life activities. Despite the fact that agencies are beginning to utilize such approaches, there has been a lack of research on whether such neuroscience-informed educational materials are beneficial above and beyond conventional PEs. In the second part of this chapter, we illustrate the use of “cartooned brain-based PE” as an example of how NIPE may be developed and empirically examined.
4 CARTOON AS A STRUCTURE FOR PSYCHOEDUCATION: A NEUROCOGNITIVE PERSPECTIVE
Using comics or a cartoon medium for visual illustration of verbal instructions has a long history in the field of education. This includes education related to literacy, science, reading comprehension, ethics, and public health awareness (Sim et al., 2014). Studies suggest that integration of imagery and language improves learning and contributes to longer lasting learning effects (Sim et al., 2014). Thus far, limited studies have used cartoon-based interventions to transfer psychoeducational content (Table 2). However, there are several probable cognitive mechanisms through which cartoon-based illustrations may enhance psychoeducation.
Table 2.
Publisheda Studies and Ongoing Clinical Trials With Cartoon/Animation-Based Psychoeducation (PE)
| Study | Sample | Interventions (Including Cartoons) | Main Findings |
|---|---|---|---|
|
| |||
| Scheeringa et al. (2011) | Children with posttraumatic stress disorder | Cognitive behavioral therapy including PE vs control intervention | Improved PTSD symptoms |
| Ly et al. (2012) | Healthy adults | PE without control condition | Improved valued action and psychological flexibility |
| Williams and Andrews (2013) | Adult with depression | Cognitive behavioral therapy including PE without control condition | Reductions in depressive symptoms |
| Mataix-cols et al. (2014) | Adolescents with obsessive–compulsive disorder | Cognitive behavioral therapy including PE without control condition | Improved clinical outcomes |
| Thurston et al. (2015) | Adults with HIV | PE without control condition | Improved treatment adherence |
| Garrison et al. (2015) | Adult smokers | PE vs control intervention | Completed clinical trial without published results |
| Schmidt et al. (2017) | Adult with cooccurring anxiety psychopathology and active suicidal ideation | PE and interoceptive exposure plus cognitive bias modification vs health information condition plus sham intervention | Reductions in anxiety sensitivity |
| Ongoing clinical trial (NCT02413216) (Clinicaltrials.gov) | Children and adults with Tic disorders | PE vs internet-based resources condition | NA |
These studies are not designed to specifically test whether the cartoons add anything to noncartoon-based PE. They simply test whether the PE with cartoons is helpful or not.
To the best of our knowledge, the table includes all the studies/trials used cartoon/animation-based psychoeducation.
Cartoon as attention grabber: The brain’s attentional and working memory resources are facilitated by bottom-up cognitive processes which are preferentially triggered by salient cues in our environment (Menon and Uddin, 2010). By being visually engaging, they may trigger multiple neurocognitive networks relating to visual perception, attention, salience, and self-relevant processing (Sim et al., 2014). If designed to have a humorous nature, comic materials can trigger an affective response or physiological arousal that further enhances attentional engagement (Schmidt and Schmidt, 2010). Thus, using verbally presented information combined with visually engaging and humorous cartoons can create a more exciting and salient context, helping the reader effectively attend to the information presented.
Cartoon as a memory-friendly medium: A striking characteristic of human memory is that pictures are remembered better than words. According to the dual encoding theory of memory, storing and recalling verbal information increases when also using relevant pictures (Norris, 2012). In particular, pictures presented in colorful and humorous styles are most effective for enhancing memory retention (Dzulkifli and Mustafar, 2013; Schmidt and Schmidt, 2010). Colors may enhance memory by increasing our attention level and arousal. Humor is thought to improve memory due not only to enhancing attentional engagement, but also by increasing higher-order cognitive processes and encouraging mental rehearsal of the information (i.e., as people who enjoy a humorous joke or cartoon tend to rethink about it frequently) (Dzulkifli and Mustafar, 2013; Schmidt and Schmidt, 2010; Summerfelt et al., 2010). It has been well documented that negative or positive affective stimuli are encoded and recalled better than neutral stimuli (Yonelinas and Ritchey, 2016). Cartoons may be useful in depicting information in affectively salient ways that enhance memory recall. It is also possible that visualization of information related to self-relevant conditions (e.g., presence of illness-related symptoms in daily life activities) can create a “light bulb moment” which is a vivid imprinted memory of a particular event that is long lasting (Norris, 2012).
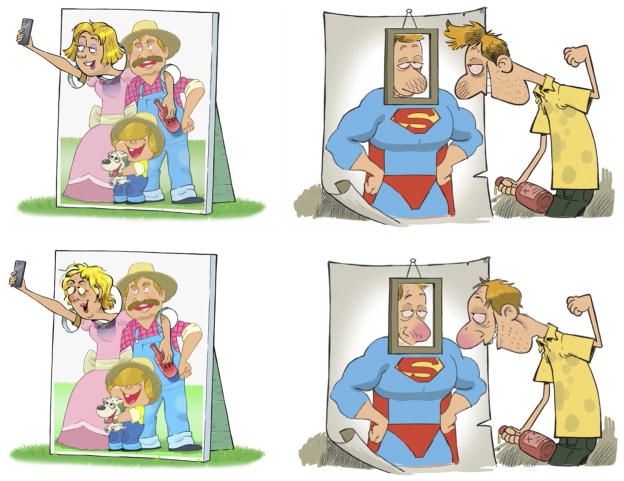
Cartoon as a method to enhance self-awareness: Cartoons used with an educational purpose can depict conditions that relate to past events, current situations, or future experiences that are commonly experienced by the target group (Norris, 2012). Self-relevant stimuli preferentially activate salience and attentional networks (Kircher et al., 2000). It therefore may be important for NIPE to include cartoons that depict individuals similar in diversity in terms of age, ethnicity, gender, etc. (Fig. 1) and depicting the variety of experiences common (e.g., drug craving in the case of ASUDs) to the patient population.
Cartoon as a visual channel for self to travel through time: In 2001, Atance and O’Neill first introduced a term called “episodic future thinking (EFT)” as a cognitive function related to episodic memory. They described EFT as a projection of the self into the future to preexperience an event (Atance and O’Neill, 2001). It encompasses the multimodal reconstruction of past experiences and projects them into the future (Kretschmer-trendowicz et al., 2016). EFT-related interventions have been shown effective in reducing impulsivity in tempting situations (Snider et al., 2016). In these interventions, a person is audio-recorded while talking about personal future goals. The audio recording is then used as a cue to help the person focus more on future health goals instead of momentary pleasures derived from impulsive decisions (e.g., high calories foods or substance consumption) (O’Neill et al., 2016). Thus, providing cues that relate to future conditions (i.e., future negative ramifications of continued drug use vs future goal-relevant states) may positively impact a person’s choice and behaviors. Cartoons can be used as a medium in which future events can be vividly depicted for people and preexpose them to probable consequences of their decisions.
Cartoon as a self-affirmation booster: Patients can often have two different behavioral responses to psychoeducational material: (1) they accept the messages or (2) they ignore and even reject them. From the point of self-affirmation theory, people have their own self-image that often includes personal positive characteristics (being intelligent, independent, autonomous, and good team member). People often try to restore this global self-worth through defensive reactions (e.g., denying that the information is relevant for them) when exposed to information that may have negative implications on their self-image (e.g., when the material is showing negative consequences of drug use and they are a drug user). For example, depicting negative health effects of smoking one packet of cigarettes for smokers with low affirmation may lead to defensive responses in terms of ignoring the message. Individuals with high self-affirmation are more likely to accept contradictory information, incorporate it into their self-concept, and begin the process of changing their thoughts or behavior (Dibello et al., 2015; Kessels et al., 2016). Cartoons using characters with positive characteristics (good looking, intelligent, autonomous, active learner, etc.) as the representative of the patient population may promote self-affirmation. In other words, patients may recognize that they can still identify with positive characteristics while simultaneously recognizing the negative consequences of some of their behaviors. Promoting self-affirmation of the audiences in the cartoons can support the successful transfer of information/knowledge with the material (Fig. 1).
Cartoon as a tool to reframe emotional or appetitive events: Cognitive reappraisal is defined as the reframing of an event or situation (cue) in order to change one’s emotional or appetitive response. This is an important emotion regulation and craving management strategy used for patients with ASUDs (Wu et al., 2015). Using this cognitive strategy, people learn in what situations they experience certain negative emotions and drug craving, and how to modify their thought processes during these experiences to impact the intensity of the emotional/appetitive experience (Steinberger et al., 2011). Cartoons can depict certain situations, such as exposure to drug cues, and provide pictorially an alternative appraisal of that situation. Such an image may become paired with the actual situation in their memory. Thus, during exposure to the situation/cue in real-life, the patient may recall the cartoon as well. This can provide a competing “cue” to reappraise one’s thoughts to the situation, thus modifying the emotional and/or appetitive experience and hopefully result in a different behavioral responses (Ekhtiari et al., 2016).
Cartoon as heuristic tool to speed up decision making: Heuristic devices are simple cues applied in conditions requiring rapid decision making. Appearance of objects, familiar pictures, shapes, sizes, logos, brands, and prices are sample of heuristic assistive tools that help individuals find efficient, though often imperfect, answers to difficult questions (Cohen and Babey, 2013). Cartoons of desired behaviors stored in memory may serve as a heuristic cue when needing to make rapid decisions in real-life situations (i.e., to remove oneself from a situation involving drug cues).
FIG. 1.
Cartoons to visualize self-awareness impairment among drug users. Gender and ethnicity will play a role in the content and structure of the cartoons as self-engagement is an important target for NIPE. Patients should be able to see themselves from the lens of the cartoons. While the use of stereotypes in cartoons can often support the humorous nature, images that are disrespectful, or overly stereotypical might interfere in this engagement. These cartoons are selected from our explorations in the brain awareness for addiction recovery initiative (BARI) to develop self-engaging cartoons in different genders and with different levels of stereotypy. Feedback from treatment providers and patients regarding the impact of the images will be crucial to informing further development of PE materials.
5 NIPE EXAMPLE: BRAIN AWARENESS FOR ADDICTION RECOVERY INITIATIVE
As a part of a new psychoeducational intervention, coined brain awareness for addiction recovery initiative (BARI), we developed (HE, TR, Brad Collins, and MP) a new standalone, cartooned, brain-based PE. BARI incorporates neuroscience content, using the NIMH Research Domain Criteria (RDoC) as the basic framework. RDoC dimensions include negative valence (e.g., anxiety and loss), positive valence (e.g., reward), cognitive systems (e.g., attention, executive control, and working memory), social processes (e.g., affiliation), and arousal/modulatory systems (e.g., sleep–wake) (Insel et al., 2010). There are previous experiences in using the RDoC framework to develop interventions for people with depression (Henje Blom et al., 2014) or to organize neuropsychological rehabilitation for people with psychiatric disorders (Rezapour et al., 2017). BARI uses cartoons and affiliated text to incorporate neurocognitive informed structure, targeting the seven cognitive mechanisms introduced in the previous section (Fig. 2). In terms of content, we divided the package into three main parts as follows:
FIG. 2.
Cartoons for three main parts of the brain awareness for addiction recovery initiative (BARI) presented as three educational posters.
Part 1: Cartoons for neurocognitive symptoms associated with ASUDs: In the RDoC framework, we introduce 10 main neurocognitive impairments among people with ASUDs (Table 3). We explain these complicated symptoms with simple and short sentences and from the first-person perspective. For example, with the cartoons in Fig. 1 related to “lack of insight,” we use “Despite what some of my friends, relatives, and co-workers might say, I do not see myself as someone who is sick, needs medical care or other treatment. I only drink and use drugs recreationally and can stop anytime I want to. I simply don’t want to. I am tempted to use because it feels good when I use. I like the effects produced by alcohol and/or other drugs, cannot see, in the using brain state, how they hurt me; therefore, I do not see the benefit of abstaining or asking for help.” The latter is meant to further encourage self-relevant processing identifying with the character’s experience. Fig. 2, left panel, illustrates the first poster.
Part 2: Cartoons for the messages promoting brain recovery: Following the first part aimed at promoting self-awareness for neurocognitive impairments and their associated observable behavioral deficits, useful and practical life style messages are introduced in terms of coping strategies, stress-management strategies, emotion regulation techniques, self-awareness interventions, and life style modifications (including sleep hygiene nutrition, physical activity, social activities, and cognitive exercises). Fig. 2, middle panel, illustrates this part.
Part 3: Cartoons for brain exercises that might promote recovery: There are different strategies to promote recovery targeting 10 major neurocognitive dysfunction associated with ASUDs, as introduced in Part 1. This includes specific cognitive rehabilitation strategies as well as behavioral and life style changes. In Part 3, we propose practical exercises and strategies that may be useful in helping to promote recovery and improve patients’ cognitive functions, awareness and insight, sleep, etc. Word exercises, writing in a daily journal, mindfulness exercises, and monitoring one’s own posture are some examples discussed within this part. Fig. 2, right panel, illustrates this part.
Table 3.
An RDoC-Oriented Frameworka for the Cartooned Brain-Based Psychoeducation Development to Promote Brain Recovery During Abstinence Among People With Alcohol and Substance Use Disorders
| RDoC Domains | RDoC Constructs | Neurocognitive Symptoms | Examples of Strategies and Exercises |
|---|---|---|---|
|
| |||
| Negative valence system | Acute threat Potential threat |
Anxious feeling Anhedonia Attentional bias to threat |
Exposure to avoided situations Reappraisal of negative cognitions Reducing environmental cues that activate negative emotions |
| Positive valence system | Reward valuation Willingness to work Initial responsiveness to reward attainment |
Hypervaluation of the substance- related stimuli Reluctance to do previously enjoyable activities |
Engage in pleasant or reinforcing activities Remind oneself of previous positive memories Savor current positive experiences Engage in altruistic behaviors |
| Cognitive system | Attention Memory Movement and speech Decision making and control |
Attentional bias to substance-related stimuli Difficulties in multitasking Impairment of shifting attention Memory deficits in recalling recent information Difficulties in finding right words Impairment in coordination and dexterity Making impulsive decisions Mood instability Interoceptive impairment |
Use compensatory strategies (calendar use, mnemonic strategies, paraphrase) Stay cognitively active (through doing brain games, taking classes to learn new information) Practice mindfulness |
| Social processes | Perception of self Perception of others Social communications |
Lack of self-awareness Denial of disease Refusing to receive treatment Overestimation of one’s power to control consumption Difficulties in self-expression Low ability to interpret social cues Difficulties in making friends |
Engage in social activities Utilize one’s social support Practicing communication skills Reappraise negative cognitions about the self or others Practice compassion for others |
| Arousal and regulatory system | Sleep–wakefulness Arousal |
Sleep–wake dysregulation Sleep-time hyperarousal Day-time hypoarousal |
Sleep hygiene practices Staying consistent with activities of daily living (e.g., showering) Practicing relaxation techniques (progressive muscle relaxation, spa activities) |
The content presented in posters 1 and 3 (Fig. 2, right and left panels) is specifically designed based on RDoC model, while poster 2 includes general health strategies for early abstinence.
6 FUTURE DIRECTIONS FOR NIPE
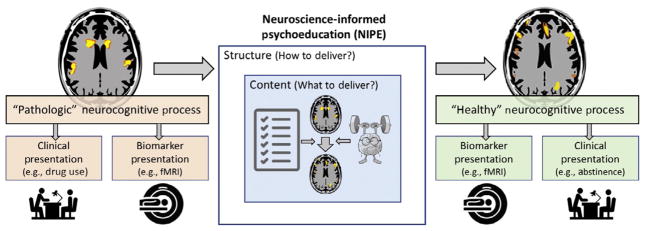
The presented approach offers a framework for bringing neuroscience-informed structure and content to conventional PEs. Notably, PEs are often developed when an institution or individual treatment providers see a need to provide this education as a service to patients or society at large. Such materials are often used without conducting research to validate that the structure and content will achieve the goals of the intervention. We propose that empiricism should not only motivate the development of NIPE, but should be used to continually validate and inform NIPE (also see Fig. 3). Some of the challenges for NIPE in terms of development, validation, and dissemination are discussed below.
FIG. 3.
Our expectations from the neuroscience-informed psychoeducation (NIPE). We hope the NIPE would target a specific set of neurocognitive processes that correspond main core clinical presentations of the disease. Content of the NIPE will be designed to change these processes of interests (POIs). Structure of the NIPE will also be informed with the POIs and other neurocognitive disorders associated with the disease. We also hope to have a reliable and valid biomarker like a neuroimaging paradigm to predict and/or monitor effects of the NIPE. We still have a long road ahead of us to realize these hopes.
Development of neuroscience-informed (NI) content for PE among people with ASUD: There is a serious gap in educational materials extracted from neuroscience-based evidences for people with ASUDs and their families. The first step in developing NIPE would be to extract and simplify such information to present within PE materials. Presenting such information within self-engaging mediums may result in an effective PE with a high level of transfer effects. However, research needs to be undertaken to identify the optimal ways in which complex neuroscientific information can be communicated to patients and their families. In addition, research needs to be undertaken to identify whether the inclusion of such information enhances the impact of PE and contributes to positive outcomes for patients.
Development of NI structures for PE among people with ASUD: The second step to developing NIPE is to identify PE structures to effectively overcome the impairments in sustained attention, memory, and self-related processes associated with chronic drug use. Using the combination of various mediums in both computerized and noncomputerized interventions might be helpful to enrich the effects of education. Research can be done during the development of such materials to get feedback from patients regarding their impressions of the material, how engaging they find the material, and whether or not they are viewed as relevant to their experiences. Empirical studies need to be completed to then examine if such PE structures enhance the impact of PE and contribute to positive outcomes for patients.
Validation of the developed NIPEs using neuroscience research: During the development of NIPE materials, it will also be important to validate that the materials used within NIPE engage the neural processes being targeted (Fig. 3). Neuroimaging studies, utilizing functional magnetic resonance imaging (fMRI) or electroencephalography (EEG), may be particularly useful for identifying brain circuits engaged by PE and NIPE. Few studies have made attempts in this field. For example, Favre and his colleagues found increased activity of inferior frontal gyri and decreased activity of the right hippocampus and the parahippocampal gyrus in euthymic bipolar patients after they received psychoeducation (including information about disease, symptoms, pharmacological treatment, and stress management) (Favre et al., 2013). Perhaps even more relevant are fMRI studies conducted to examine the neural response of individuals when viewing antismoking ads (Wang et al., 2013). However, in addition to using fMRI to understand brain responses pre/post-PE or in response to final versions of NIPE materials, we can utilize such neurocognitive methods to help inform the development, refinement, and modification of such materials. For example, one could develop different versions of NIPE visual materials and use eye tracking to identify the optimal way to structure the material so that attention is drawn toward the most important aspects and use fMRI to identify which versions most robustly activate salience networks. We hope future studies will address neural circuits that can be targeted and modulated with NIPEs, particularly for individuals with ASUDs. Notably, while such research will be helpful for informing the development of NIPE materials, efficacy in terms of treatment outcomes (e.g., severity of drug use or length of abstinence) should always be confirmed in the real setting of clinical practice.
Dissemination of NIPEs into clinical environments: Complexity in neuroscience-based content could present an obstacle for treatment providers to deliver such interventions. This is particularly true for treatment providers with no neuroscience background. The development of materials that organize the information using simple wording and meaningful images will hopefully reduce this complexity and increase adherence and efficacy of NIPEs. However, inclusion of real-world treatment providers in the development and validation of materials (for example, to provide feedback on the utility and clarity of the materials) is important for ensuring that the materials are ultimately able to impact clinical care and patient outcomes.
7 CONCLUSION
Psychoeducation is an important aspect of the treatment process by informing individuals about their symptoms, providing motivation for change, and offering treatment options. PE may be particularly important for patients with ASUDs who often lack insight into their symptoms, negative consequences of behaviors, and need for treatment. We currently have a wealth of neuroscientific and neurocognitive knowledge related to ASUDs and methods for presenting information in ways that optimize attention, memory, and self-awareness. However, this knowledge has yet to make demonstrable changes to real-life clinical care. NIPE offers a framework through which we can immediately leverage such knowledge to inform the content and optimize the structure of psychoeducational materials. By doing so, we may have a more powerful and long-lasting impact on patients’ knowledge, insight, treatment compliance, and outcomes.
Acknowledgments
The authors would like to thank Seyed Naeim Tadayon Nabavi Fadafan (illustrator) and Mohsen Farhadi (graphic designer) for their contribution in creating cartoons and designing posters. We should thank Brad Collins, 12&12 Addiction Treatment Center, for his leadership in the BARI project and his insightful comments and suggestions for this manuscript. Educational materials developed for the BARI are copyright protected. But, authors are happy to distribute them for “non-for-profit” use in academic and clinical settings. Authors would also like to thank Prof. Tae-Yoon Hwang, Korean National Center for Mental Health (NCMH) and Prof. Felix Kessler, Brazilian Center for Drug and Alcohol Research (CPAD), for development of the Korean and Portuguese versions of the materials. Authors welcome any other collaborations to develop BARI materials in different languages and cultural environments.
References
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12(6):305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade AD, Idrees T, Karanam C, Anam R, Ruiz JG. Effects of an avatar-based anti-smoking game on smoking cessation intent. Stud Health Technol Inform. 2016;220:15–18. [PubMed] [Google Scholar]
- Atance CM, O’Neill D. Episodic future thinking. Trends Cogn Sci. 2001;5(12):533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Banks SJ. Self-awareness and self-monitoring of cognitive and behavioral deficits in behavioral variant frontotemporal dementia, primary progressive aphasia and probable Alzheimer’s disease. Brain Cogn. 2008;67(1):58–68. doi: 10.1016/j.bandc.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Buckman JF, Nguyen TT. A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol Rev. 2013;23(1):27–47. doi: 10.1007/s11065-013-9228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäuml J, Froböse T, Kraemer S, Rentrop M, Pitschel-Walz G. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32:1–9. doi: 10.1093/schbul/sbl017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor AL. Promoting motivation with virtual agents and avatars: role of visual presence and appearance. Philos Trans R Soc, B Biol Sci. 2009;364(1535):3559–3565. doi: 10.1098/rstb.2009.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Christensen DR, Marsch LA. A review of computer-based interventions used in the assessment, treatment, and research of drug addiction. Subst Use Misuse. 2011;46(1):4–9. doi: 10.3109/10826084.2011.521066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelland IE, Johansen A, Darnell F, Brendryen H. Using a film intervention in early addiction treatment: a qualitative analysis of process. Int J Behav Med. 2017 doi: 10.1007/s12529-017-9654-3. [DOI] [PubMed] [Google Scholar]
- Bivona U, Riccio A, Ciurli P, Carlesimo GA, Donne VD, Pizzonia E, … Costa A. Low self-awareness of individuals with severe traumatic brain injury can lead to reduced ability to take another person’s perspective. J Head Trauma Rehabil. 2014;29(2):157–171. doi: 10.1097/HTR.0b013e3182864f0b. [DOI] [PubMed] [Google Scholar]
- Bossema ER, de Haar CA, Westerhuis W, Beenackers BP, Blom BC, Appels MC, van OC. Psychoeducation for patients with a psychotic disorder: effects on knowledge and coping. Prim Care Companion CNS Disord. 2011;13(4):213–223. doi: 10.4088/PCC.10m01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Marlatt A. Surfing the urge: brief mindfulness-based intervention for college student smokers. Psychol Addict Behav. 2009;23(4):666–671. doi: 10.1037/a0017127. [DOI] [PubMed] [Google Scholar]
- Chandiramani K. Psychoeducational group therapy for alcohol and drug dependence recovery. Indian J Psychiatry. 1993;35(3):169–172. [PMC free article] [PubMed] [Google Scholar]
- Chung T, Noronha A, Carroll KM, Potenza MN, Hutchison K, Calhoun VD, … Feldstein Ewing SW. Brain mechanisms of change in addictions treatment: models, methods, and emerging findings. Curr Addict Rep. 2016;3(3):332–342. doi: 10.1007/s40429-016-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Babey SH. Contextual influences on eating behaviors: heuristic processing and dietary choices. Obes Rev. 2013;13(9):766–779. doi: 10.1111/j.1467-789X.2012.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ray LA. Clinical neuroscience of amphetamine-type stimulants: from basic science to treatment development. Prog Brain Res. 2016;223:295–310. doi: 10.1016/bs.pbr.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Cutuli D. Cognitive reappraisal and expressive suppression strategies role in the emotion regulation: an overview on their modulatory effects and neural correlates. Front Syst Neurosci. 2014;8(175):1–6. doi: 10.3389/fnsys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WT, Campbell L, Tax J, Lieber CS. A trial of “standard” outpatient alcoholism treatment vs. a minimal treatment control. J Subst Abuse Treat. 2002;23(1):9–19. doi: 10.1016/s0740-5472(02)00227-1. [DOI] [PubMed] [Google Scholar]
- De Maricourt P, Gorwood P, Hergueta T, Galinowski A, Salamon R, Diallo A, Vaugeois C, Lépine JP, Olié JP, Dubois O. Balneotherapy together with a psychoeducation program for benzodiazepine withdrawal: a feasibility study. Evid Based Complement Alternat Med. 2016;2016:8961709. doi: 10.1155/2016/8961709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibello AM, Neighbors C, Ammar J. Addictive behaviors self-affirmation theory and cigarette smoking warning images. Addict Behav. 2015;41:87–96. doi: 10.1016/j.addbeh.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Donker T, Griffiths KM, Cuijpers P, Christensen H. Psychoeducation for depression, anxiety and psychological distress: a meta-analysis. Database Abstr Rev Effects. 2009;9:1–9. doi: 10.1186/1741-7015-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont RL, Compton WM, McLellan AT. Five-year recovery: a new standard for assessing effectiveness of substance use disorder treatment. J Subst Abuse Treat. 2015;58:1–5. doi: 10.1016/j.jsat.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Dzulkifli MA, Mustafar MF. The influence of colour on memory performance: a review. Malays J Med Sci. 2013;20(2):3–9. [PMC free article] [PubMed] [Google Scholar]
- Ekhtiari H, Paulus M. Neuroscience for addiction medicine: from prevention to rehabilitation—constructs and drugs. In: Ekhtiari H, Paulus M, editors. Progress in Brain Research. Vol. 223. Elsevier; Amsterdam: 2016. [DOI] [PubMed] [Google Scholar]
- Ekhtiari H, Nasseri P, Yavari F, Mokri A, Monterosso J. Neuroscience of drug craving for addiction medicine: from circuits to therapies. Prog Brain Res. 2016;223:115–141. doi: 10.1016/bs.pbr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Evans L. Psychological effects caused by drugs in overdose. Drugs. 1980;19(3):220–242. doi: 10.2165/00003495-198019030-00005. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, Birchler GR, Kelley ML. Learning sobriety together: a randomized clinical trial examining behavioral couples therapy with alcoholic female patients. J Consult Clin Psychol. 2006;74(3):579–591. doi: 10.1037/0022-006X.74.3.579. [DOI] [PubMed] [Google Scholar]
- Farley J, Risko EF, Kingstone A. Everyday attention and lecture retention: the effects of time, fidgeting, and mind wandering. Front Psychol. 2013;4:619. doi: 10.3389/fpsyg.2013.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre P, Baciu M, Pichat C, De Pourtalès M, Fredembach B, Garçon S, … Polosan M. Modulation of fronto-limbic activity by the psychoeducation in euthymic bipolar patients A functional MRI study. Psychiatry Res Neuroimaging. 2013;214:285–295. doi: 10.1016/j.pscychresns.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2006;30(9):1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Pal P, Rojiani R, Dallery J, Malley SSO, Brewer JA. A randomized controlled trial of smartphone-based mindfulness training for smoking cessation: a study protocol. BMC Psychiatry. 2015;15(83):1–7. doi: 10.1186/s12888-015-0468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-pinilla F, Kostenkova K. The influence of diet and physical activity on brain repair and neurosurgical outcome. Surg Neurol. 2008;70(4):333–336. doi: 10.1016/j.surneu.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gucciardi E, Jean-Pierre N, Karam G, Sidani S. Designing and delivering facilitated storytelling interventions for chronic disease self-management: a scoping review. BMC Health Serv Res. 2016;16:249. doi: 10.1186/s12913-016-1474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henje Blom E, Duncan LG, Ho TC, Connolly CG, LeWinn KZ, Chesney M, … Yang TT. The development of an RDoC-based treatment program for adolescent depression: training for awareness, resilience, and action; (TARA) Front Hum Neurosci. 2014;8:1–19. doi: 10.3389/fnhum.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Bieber A, Keck T, Vaitl D, Stark R. Brain structural basis of cognitive reappraisal and expressive suppression. Soc Cogn Affect Neurosci. 2014;9(9):1435–1442. doi: 10.1093/scan/nst130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston TK, Allison JJ, Sussman M, Horn W, Holt CL, Trobaugh J, Salas M, Pisu M, Cuffee YL, Larkin D, Person SD, Barton B, Kiefe CI, Hullett S. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med. 2011;154(2):77–84. doi: 10.7326/0003-4819-154-2-201101180-00004. [DOI] [PubMed] [Google Scholar]
- Hulse GK, Tait RJ. Six-month outcomes associated with a brief alcohol intervention for adult in-patients with psychiatric disorders. Drug Alcohol Rev. 2002;21(2):105–112. doi: 10.1080/09595230220138993a. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Aupperle R, Rodgers CS, Lang AJ, Schiehser DM, Norman SB, Twamley EW. Evaluation of a hybrid treatment for veterans with comorbid traumatic brain injury and posttraumatic stress disorder: study protocol for a randomized controlled trial. Contemp Clin Trials. 2015;45(Pt. B):210–216. doi: 10.1016/j.cct.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Kessels LTE, Harris PR, Ruiter RAC, Klein WMP. Attentional effects of self-affirmation in response to graphic antismoking images. Health Psychol. 2016;35(8):891–897. doi: 10.1037/hea0000366. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, Simmons A, Williams SC, Bartels M, David AS. Towards a functional neuroanatomy of self processing: effects of faces and words. Brain Res Cogn Brain Res. 2000;10(1–2):133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Kominars KD. A study of visualization and addiction treatment. J Subst Abuse Treat. 1997;14(3):213–223. doi: 10.1016/s0740-5472(96)00068-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer-trendowicz A, Ellis JA, Altgassen M. Effects of episodic future thinking and self-projection on children’s prospective memory performance. PLoS One. 2016;11(6):1–16. doi: 10.1371/journal.pone.0158366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux A, Absi M. Stress psychobiology in the context of addiction medicine: from drugs of abuse to behavioral addictions. Prog Brain Res. 2016;223:43–62. doi: 10.1016/bs.pbr.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Lowe W. Is the sun setting on lecture-based education? Int J Ther Massage Bodywork. 2011;4(4):7–9. doi: 10.3822/ijtmb.v4i4.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly KH, Dahl J, Carlbring P, Andersson G. Development and Initial Evaluation of a Smartphone Application Based on Acceptance and Commitment Therapy. SpringerPlus; London, UK: 2012. p. 1.p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarefvand M, Ghiasvand HR, Ekhtiari H. Drug craving terminology among opiate dependents; a mixed method study. Iranian J Psychiatry. 2013;8(2):97–103. [PMC free article] [PubMed] [Google Scholar]
- Mataix-cols D, Thulin U, Lenhard F, Vigerland S, Andersson E, Ru C, Serlachius E. Internet-delivered cognitive behavior therapy for adolescents with obsessive-compulsive disorder: an open trial. PLoS One. 2014;9(6):1–11. doi: 10.1371/journal.pone.0100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ. Increasing memory self-efficacy and strategy use in Hispanic elders. Clin Gerontol. 1998;19(2):57–76. doi: 10.1300/j018v19n02_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcguire KB, Stojanovic-radic J. Development and effectiveness of a psychoeducational wellness program for people with multiple sclerosis. Int J MS Care. 2015;17(1):1–8. doi: 10.7224/1537-2073.2013-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam MP, Sari M, Balouchi A, Madarshahian F, Moghadam K. Effects of storytelling-based education in the prevention of drug abuse among adolescents in Iran based on a readiness to addiction index. J Clin Diagn Res. 2016;10(11):IC06–IC09. doi: 10.7860/JCDR/2016/23170.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad Ahmadi Soleimani S, Ekhtiari H, Cadet JL. Drug-induced neurotoxicity in addiction medicine: from prevention to harm reduction. Prog Brain Res. 2016;223:19–41. doi: 10.1016/bs.pbr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon Bull Rev. 2011;18(1):46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- Norris EM. The constructive use of images in medical teaching: a literature review. JRSM Short Rep. 2012;3(5):33. doi: 10.1258/shorts.2012.011158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northouse L, Schafenacker A, Barr KLC, Katapodi M, Yoon H, Brittain K, … An L. A tailored web-based psycho-educational intervention for cancer patients and their family caregivers. Cancer Nurs. 2014;37(5):321–330. doi: 10.1097/NCC.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Daniel TO, Epstein LH. Episodic future thinking reduces eating in a food court. Eat Behav. 2016;20:9–13. doi: 10.1016/j.eatbeh.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, O’Cleirigh CM, Pollack MH. Attending to emotional cues for drug abuse: bridging the gap between clinic and home behaviors. Sci Pract Perspect. 2007;3(2):48–55. doi: 10.1151/spp073248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL, Haase L. Treatment approaches for interoceptive dysfunctions in drug addiction. Front Psych. 2013;4:137. doi: 10.3389/fpsyt.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EB, Thomsen S, Lindsay G, John K. Adolescents’ attention to traditional and graphic tobacco warning labels: an eye-tracking approach. J Drug Educ. 2010;40(3):227–244. doi: 10.2190/DE.40.3.b. [DOI] [PubMed] [Google Scholar]
- Pitschel-Walz G, Bäuml J, Bender W, Engel RR, Wagner M, Kissling W. Psychoeducation and compliance in the treatment of schizophrenia: results of the Munich psychosis information project study. J Clin Psychiatry. 2006;67(3):443–452. doi: 10.4088/jcp.v67n0316. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69(4):695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong K, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169(4):406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser J, Cohen LJ, Steinfeld M, Eisenberg D, London ED, Galynker II. Neuropsychological functioning in opiate-dependent subjects receiving and following methadone maintenance treatment. Drug Alcohol Depend. 2006;84(3):240–247. doi: 10.1016/j.drugalcdep.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Kleykamp BA, Vandrey RG, et al. Cognitive performance in methadone maintenance patients: effects of time relative to dosing and maintenance dose level. Exp Clin Psychopharmacol. 2014;22(3):248–256. doi: 10.1037/a0035712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezapour T, DeVito EE, Sofuoglu M, Ekhtiari H. Perspectives on neurocognitive rehabilitation as an adjunct treatment for addictive disorders: from cognitive improvement to relapse prevention. Prog Brain Res. 2016;224:345–369. doi: 10.1016/bs.pbr.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Rezapour T, Wurfel B, Simblett SK, Ekhtiari H. Neuropsychological rehabilitation of psychiatric disorders. In: Wilson BA, Winegardner J, van Heugten CM, Ownsworth T, editors. Neuropsychological Rehabilitation: The International Handbook. Routledge; New York: 2017. [Google Scholar]
- Richmond RL, Austin A, Webster IW. Three year evaluation of a programme by general practitioners to help patients to stop smoking. Br Med J (Clin Res Ed) 1986;292(6523):803–806. doi: 10.1136/bmj.292.6523.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa MS, Weems CF, Cohen JA, Amaya-jackson L, Guthrie D. Trauma-focused cognitive-behavioral therapy for posttraumatic stress disorder in three through six year-old children: a randomized clinical trial. J Child Psychol Psychiatry. 2011;52(8):853–860. doi: 10.1111/j.1469-7610.2010.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SR, Schmidt SR. The humour effect: differential processing and privileged retrieval the humour effect: differential processing and privileged retrieval. Memory. 2010;10(2):127–138. doi: 10.1080/09658210143000263. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Norr AM, Allan NP, Raines AM, Capron D. A randomized clinical trial targeting anxiety sensitivity for patients with suicidal ideation. J Consult Clin Psychol. 2017;85(6):596–610. doi: 10.1037/ccp0000195. [DOI] [PubMed] [Google Scholar]
- Shams L, Seitz AR. Benefits of multisensory learning. Trends Cogn Sci. 2008;12(11):411–417. doi: 10.1016/j.tics.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24. doi: 10.2196/jmir.5.4.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ. Mirrors, masks, and motivation: implicit and explicit self-focused attention influence effort-related cardiovascular reactivity. Biol Psychol. 2012;90(3):192–201. doi: 10.1016/j.biopsycho.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Eichstaedt J. A self-novelty manipulation of self-focused attention for internet and laboratory experiments. Behav Res Methods Instrum Comput. 2004;36(2):325–330. doi: 10.3758/bf03195578. [DOI] [PubMed] [Google Scholar]
- Sim MG, Mcevoy AC, Wain TD, Khong EL. Improving health professional’s knowledge of hepatitis B using cartoon based learning tools: a retrospective analysis of pre and post tests. BMC Med Educ. 2014;14:1–8. doi: 10.1186/s12909-014-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, LaConte SM, Bickel WK. Episodic future thinking: expansion of the temporal window in individuals with alcohol dependence. Alcohol Clin Exp Res. 2016;40(7):1558–1566. doi: 10.1111/acer.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Agrawal S, Johnson-Young L, Cunningham JA. Promoting self-change with alcohol abusers: a community-level mail intervention based on natural recovery studies. Alcohol Clin Exp Res. 2002;26(6):936–948. [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert S. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40(1):31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger A, Payne JD, Kensinger EA. The effect of cognitive reappraisal on the emotional memory trade-off. Cognit Emot. 2011;25(7):1237–1245. doi: 10.1080/02699931.2010.538373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfelt H, Lippman L, Hyman I., Jr The effect of humor on memory: constrained by the pun. J Gen Psychol. 2010;137(4):376–394. doi: 10.1080/00221309.2010.499398. [DOI] [PubMed] [Google Scholar]
- Sv P, Zaretsky A, Beaulieu S, Ln Y, Lt Y. A randomized controlled trial of psychoeducation or cognitive-behavioral therapy in bipolar disorder: a Canadian network for mood and anxiety treatments (CANMAT) study [CME] J Clin Psychiatry. 2012;73(6):803–810. doi: 10.4088/JCP.11m07343. [DOI] [PubMed] [Google Scholar]
- Swaminath G. Psychoeducation. Indian J Psychiatry. 2009;51(3):171–172. doi: 10.4103/0019-5545.55082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaei-Jafari H, Ekhtiari H, Ganjgahi H, Hassani-Abharian P, Oghabian MA, Moradi A, Sadighi N, Zarei M. Patterns of brain activation during craving in heroin dependents successfully treated by methadone maintenance and abstinence-based treatments. J Addict Med. 2014;8(2):123–129. doi: 10.1097/ADM.0000000000000022. [DOI] [PubMed] [Google Scholar]
- Thurston IB, Bogart LM, Wachman M, Closson EF. Adaptation of an HIV medication adherence intervention for adolescents and young adults. Cogn Behav Pract. 2015;21(2):191–205. doi: 10.1016/j.cbpra.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of Addiction. N Engl J Med. 2016;374(4):363–371. 28. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Ruparel K, Loughead JW, Strasser AA, Blady SJ, Lynch KG, Romer D, Cappella JN, Lerman C, Langleben D. Content matters: neuroimaging investigation of brain and behavioral impact of televised anti-tobacco public service announcements. J Neurosci. 2013;33(17):7420–7427. doi: 10.1523/JNEUROSCI.3840-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AD, Andrews G. The effectiveness of internet cognitive behavioural therapy (iCBT) for depression in primary care: a quality assurance study. PLoS One. 2013;8(2):1–6. doi: 10.1371/journal.pone.0057447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Lustyk MKBS. Re-training the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351–365. doi: 10.1037/a0029258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobrock T, Falkai P, Schneider-Axmann T, Frommann N, Wölwer W, Gaebel W. Effects of abstinence on brain morphology in alcoholism: a MRI study. Eur Arch Psychiatry Clin Neurosci. 2009;259(3):143–150. doi: 10.1007/s00406-008-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Sage JR, Shuman T, Anagnostaras S. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev. 2013;66(1):193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Winkler MH, Wieser MJ, Andreatta M, Li Y, Pauli P. Emotion regulation in heavy smokers: experiential, expressive and physiological consequences of cognitive reappraisal. Front Psychol. 2015;6:1555. doi: 10.3389/fpsyg.2015.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Ritchey M. The slow forgetting of emotional episodic memories: an emotional binding account. Trends Cogn Sci. 2016;19(5):259–267. doi: 10.1016/j.tics.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoonessi A, Ekhtiari H. Text messages as a tool for assessing public concern about drug problems. Int J Drug Policy. 2013;24(6):624–627. doi: 10.1016/j.drugpo.2013.06.002. [DOI] [PubMed] [Google Scholar]
FURTHER READING
- Boyer EW, Shannon M, Hibberd PL. The internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;15(2):302–305. doi: 10.1542/peds.2004-1199. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. The science of well-being: an integrated approach to mental health and its disorders. Psychiatr Danub. 2006;18(3–4):218–224. [PubMed] [Google Scholar]
- Courtney KE, Ray LA. Clinical neuroscience of amphetamine-type stimulants: from basic science to treatment development. Prog Brain Res. 2016;223:285–310. doi: 10.1016/bs.pbr.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Fraser S, Pienaar K, Dilkes-Frayne E, Moore D, Kokanovic R, Treloar C, Dunlop A. Addiction stigma and the biopolitics of liberal modernity: a qualitative analysis. Int J Drug Policy. 2017;44:192–201. doi: 10.1016/j.drugpo.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Gerretsen P, Menon M, Mamo DC, Fervaha G, Remington G, Pollock BG, Graff-Guerrero A. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: resting state functional connectivity. Schizophr Res. 2015;160:43–50. doi: 10.1016/j.schres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Carter A, Forlini C. The brain disease model of addiction: is it supported by the evidence and has it delivered on its promises? Lancet Psychiatry. 2014;2(1):105–110. doi: 10.1016/S2215-0366(14)00126-6. [DOI] [PubMed] [Google Scholar]
- Ham TE, Bonnelle V, Hellyer P, Jilka S, Robertson IH, Leech R, Sharp DJ. The neural basis of impaired self-awareness after traumatic brain injury. Brain. 2014;137:586–597. doi: 10.1093/brain/awt350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL. Viewing addiction as a brain disease promotes social injustice. Nat Human Behav. 2017;1:1. doi: 10.1038/s41562-017-0216-0. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Addiction: A Disorder of Choice. Harvard University Press; London, UK: 2010. [Google Scholar]
- Hj M, Gazda Gm G, Powell M, Hauser G. Life skill training: psychoeducational training as mental health treatment. J Clin Psychol. 1985;41(3):359–367. doi: 10.1002/1097-4679(198505)41:3<359::aid-jclp2270410308>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Insel TR. Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry. 2009;66(2):128–133. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(Pt. 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Joseph B, Narayanaswamy JC, Venkatasubramanian G. Insight in schizophrenia: relationship to positive, negative and neurocognitive dimensions. Indian J Psychol Med. 2015;37(1):5–11. doi: 10.4103/0253-7176.150797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Gelernter J, Hudziak JJ, Tyrka AR, Coplan JD. The research domain criteria (RDoC) project and studies of risk and resilience in maltreated children. J Am Acad Child Adolesc Psychiatry. 2016;54(8):617–625. doi: 10.1016/j.jaac.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latvala A, Castaneda AE, Perälä J, Saarni SI, Aalto-Setälä T, Lönnqvist J, Kaprio J, Suvisaari J, Tuulio-Henriksson A. Cognitive functioning in substance abuse and dependence: a population-based study of young adults. Addiction. 2009;104(9):1558–1568. doi: 10.1111/j.1360-0443.2009.02656.x. [DOI] [PubMed] [Google Scholar]
- Levy N. Addiction is not a brain disease (and it matters) Front Psychiatry. 2013;4:24, 11. doi: 10.3389/fpsyt.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind SE, Williams DM, Bowler DM, Peel A. Episodic memory and episodic future thinking impairments in high-functioning autism spectrum disorder: an underlying difficulty with scene construction or self-projection? Brown GG, ed Neuropsychology. 2014;28(1):55–67. doi: 10.1037/neu0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus C. Strategies for improving the quality of verbal patient and family education: a review of the literature and creation of the EDUCATE model. Health Psychol Behav Med. 2014;2(1):482–495. doi: 10.1080/21642850.2014.900450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee on Opportunities in Neuroscience for Future Army Applications. Neuroscience and the Army. National Academies Press (US); Washington, DC: 2009. Opportunities in Neuroscience for Future Army Applications; p. 2. [PubMed] [Google Scholar]
- Noël X, Brevers D, Bechara A. A triadic neurocognitive approach to addiction for clinical interventions. Front Psych. 2013;4:1–14. doi: 10.3389/fpsyt.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezapour T, Hatami J, Farhoudian A, Sofuoglu M, Noroozi A, Daneshmand R, … Ekhtiari H. Neuro cognitive rehabilitation for disease of addiction (NECOREDA) program: from development to trial. Basic Clin Neurosci. 2015;6(4):291–298. [PMC free article] [PubMed] [Google Scholar]
- Sadeghi S, Ekhtiari H, Bahrami B, Ahmadabadi MN. Metacognitive deficiency in a perceptual but not a memory task in methadone maintenance patients. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-06707-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvicente-Vieira B, Romani-Sponchiado A, Kluwe-Schiavon B, Brietzke E, Araujo RB, Grassi-Oliveira R. Theory of mind in substance users: a systematic Minireview. Subst Use Misuse. 2017;52(1):127–133. doi: 10.1080/10826084.2016.1212890. [DOI] [PubMed] [Google Scholar]
- Slgo FX, Jameson M. The knowledge—behavior gap in use of health information. Authors, J Assoc Inf Sci Technol. 2000;51(9):858–869. [Google Scholar]
- Stuckey HL, Nobel J. The connection between art, healing, and public health: a review of current literature. Am J Public Health. 2010;100(2):254–263. doi: 10.2105/AJPH.2008.156497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta LS. Enhancing addiction treatment through psychoeducational groups. J Subst Abuse Treat. 1993;10(5):1993. doi: 10.1016/0740-5472(93)90003-k. [DOI] [PubMed] [Google Scholar]