Abstract
Background
Over 100 million Americans are living with chronic pain, and pain is the most common reason that patients seek medical attention. Despite the prevalence of pain, the practice of pain management and the scientific discipline of pain research are relatively new fields compared to the rest of medicine – contributing to a twenty-first century dilemma for health care providers asked to relieve suffering in the “Fifth Vital Sign” era.
Methods
This manuscript provides a narrative review of the basic mechanisms of chronic pain and history of chronic pain management in the United States – including the various regulatory, health system and provider factors that contributed to the decline of multidisciplinary pain treatment in favor of the predominant opioid treatment strategy seen today. Multiple non-opioid pain treatment strategies are then outlined. The manuscript concludes with three key questions to help guide future research at the intersection of pain and addiction.
Conclusions
The assessment and treatment of chronic pain will continue to be one of the most common functions of a health care provider. To move beyond an over reliance on opioid medications, the addiction and pain research communities must unite with chronic pain patients to increase the evidence base supporting non-opioid analgesic strategies.
Keywords: Opioids, Chronic pain, Chronic pain management, Multidisciplinary pain treatment, John J. Bonica
1. Introduction
Although pain is one of the most common human experiences, the scientific discipline of pain research and the medical subspecialty of pain management are relatively new fields. Prior to the 1800s, pain was largely viewed as an existential experience and accepted as a consequence of aging (Meldrum, 2003), but the twentieth century saw the medicalization of pain management with a growth in the knowledge of pain pathophysiology and in the variety of pain treatment strategies. This paper will focus on chronic pain, as this is an emerging field with high public health importance, and will provide (1) a review of the etiology of chronic pain, (2) an overview of the history of chronic pain management – including reasons behind the dramatic rise in opioids for the treatment of most chronic pain disorders, (3) an overview of non-opioid analgesic treatment strategies, and (4) guidance for future pain research needs.
To ensure consistency throughout this paper, it is important to first define chronic pain. Pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (International Association for the Study of Pain, 1986). In this definition, pain is understood not just as a function of neuronal activity, but highlights the importance of higher-level cognitive processes that help interpret and define the pain experience for individuals. Pain is understood as an inherently subjective experience that does not require identifiable tissue damage to be clinically significant. Chronic pain is defined as pain that has lasted beyond the normal healing time for a given injury, operationalized as pain lasting >3 months (International Association for the Study of Pain, 1986). For treatment purposes, chronic pain is further divided as associated with or not associated with a terminal illness (usually cancer).
2. The epidemic of chronic pain in the United States
2.1. Differences between acute and chronic pain
A comprehensive review of the pain signaling pathway is beyond the scope of this review, but readers can reference reviews by Clark and Treisman (2004) and Leknes and Tracey (2008) for more information. Acute pain has a clear evolutionary and life-sustaining purpose – to bring attention to the occurrence of actual or potential tissue damage and to motivate the organism to remove itself from the cause of pain. Nociceptors are peripheral neurons whose main purpose is to detect painful stimuli, and can be stimulated by extremes in temperature (heat or cold), pressure, and/or chemicals most often released in the inflammatory response (Clark and Treisman, 2004). For example, only when a temperature stimulus reaches a physiologically determined point (usually 43 °C in healthy individuals) does a cutaneous heat nociceptor fire an action potential to indicate pain (Walk and Poliak-Tunis, 2016). Nociceptors transmit action potentials to the spinal cord or brainstem, and then to the cerebral cortex and thalamus. Direct injury to nerves through trauma, surgery, or chronic illnesses like diabetes mellitus or alcohol use disorder can also result in pain through spontaneous nociceptor transmission without painful stimuli, enhanced pain facilitation or pathologic neuroplasticity (Costigan et al., 2009).
The rate of nociceptor firing (interpreted as pain intensity) can be influenced by the level of painful stimulus but also by peripheral and central sensitization. Peripherally, nociceptors can be changed via inflammatory mediators or repeated stimulation to fire at a lower intensity of stimuli that would not normally be painful (e.g., temperatures below 43 °C) (Petho and Reeh, 2012). Centrally, second-order neurons that transmit nociceptor input to the cortex and thalamus can also be sensitized to increase rate of firing by direct injury or ongoing inflammation (Cheng, 2010; Stemkowski and Smith, 2012). Lastly, neurons that originate from the periaqueductal gray and rostroventral medulla can amplify or decrease pain signaling (Ossipov et al., 2014).
Acute pain resolves after tissue healing in most individuals. However, certain persons progress from acute to chronic pain, known as pain chronification. Although the processes underlying chronification are not yet well understood, central nervous system changes to pain facilitation and inhibition (such as those mentioned above) are thought to play a role (Ossipov et al., 2014).
The rates of pain chronification vary based upon the type of acute pain (e.g., low back, post-surgical, diabetic neuropathy) (Ossipov et al., 2014). However, the most consistent predictors of chronification across most types of acute pain are social and psychological factors. For example, Chou and Shekelle (Chou and Shekelle, 2010) found that maladaptive pain coping behaviors and co-occurring psychiatric illnesses were two of the strongest baseline predictors of chronic back pain. Pain catastrophizing, a validated set of negative emotional/cognitive processes involving pessimism, perceptions of helplessness, and magnification of pain-related symptoms, is associated with pain chronification after surgery (Edwards et al., 2009; Theunissen et al., 2012). Once developed, chronic pain may be considered a separate disease process that will need tailored interventions other than repair of the original injury to improve function. In that cure is rarely possible, a chronic disease approach is helpful in conceptualizing care of patients with chronic pain.
2.2. The prevalence of chronic pain
Approximately 100 million adults in the United States are affected by chronic pain at any given time, with chronic low back pain and headaches the most commonly diagnosed conditions (Gaskin and Richard, 2012). There are known demographic factors that predispose a person to develop chronic pain. For instance, women are more likely to report chronic pain than men (34.3% vs. 26.7% in a nationally representative sample in the US) (Johannes et al., 2010). In addition, the prevalence of chronic pain increases with age (Rustoen et al., 2005; Tsang et al., 2008; Shmagel et al., 2016). Persons with lower annual household income have greater odds of reporting chronic pain compared to persons with higher annual income (Shmagel et al., 2016). Lastly, persons with mental illness (McWilliams et al., 2003) have greater odds of chronic pain compared to the general population without these disorders. Irrespective of these differences in chronic pain prevalence, the total health care costs for chronic pain treatment are estimated to range between $560 to 635 billion per year in the United States, eclipsing the annual costs of heart disease, diabetes and cancer (Gaskin and Richard, 2012).
3. A history of chronic pain management: follow the money
3.1. The beginning: multidisciplinary in nature
John J. Bonica, an anesthesiogist, is widely considered to be the father of modern pain management. He was trained in an era of the “specificity theory of pain” that stated pain resulted from an identifiable injury. According to this perspective, correction of that injury (through surgery or rehabilitation) or blockade of the nociceptors in that area (“regional anesthesia”) should provide complete pain relief. Dissatisfied with the results of his own management of chronic pain in soldiers using regional anesthesia during WWII, John Bonica began consulting with clinicians from different disciplines (e.g., neurology, psychiatry, and orthopedics) on difficult patients to improve analgesic response and functional outcomes. As he noticed an improvement in pain relief and function in this consultation practice, he developed the first multidisciplinary pain clinic based upon these experiences at Tacoma General Hospital in the 1950s. This clinic attempted to increase the efficiency of consultative practice and decrease patient burden by co-locating all staff members in the same space. He transferred the clinic to the University of Washington in Seattle in the 1960s (Benedetti and Chapman, 2005) when he became chair of the Department of Anesthesia. Dr. Bonica’s original clinic had inpatient and outpatient components.
The subsequent addition of operant conditioning methods from the psychologist Wilbert Fordyce allowed the clinic to provide marked improvements in patient self-management of pain (Fordyce et al., 1973). These methods included rewarding patient behaviors associated with improvement through positive staff feedback (e.g., improved exercise tolerance, increases in work and social activities) and extinction of “pain behaviors” (e.g., verbal or facial pain complaints, reliance on medications for pain management, and stopping exercise to sit) by administering medication on a schedule (as opposed to whenever a patient reported increased pain) and providing rest only after exercise regimens are completed (as opposed to when pain increases).
Over time, the pain community came together to provide guidelines to what constitutes a multidisciplinary pain treatment program. First, the clinic should be staffed with clinicians trained in pain management from various disciplines: at least two physicians, a pain psychologist, a physical therapist, and additional health care providers as needed to address unique patient populations served in the clinic (e.g., occupational therapist with knowledge about return to work evaluations) (Gatchel et al., 2014). Second, there should be regular meetings with all staff members present to discuss ongoing patient care issues. Third, assessment and treatment options should be comprehensive and include (at a minimum): physical exam, medication management, biopsychosocial evaluation, cognitive behavioral treatment for chronic pain, physical therapy, occupational therapy, and ability to refer to specialists not offered by the team. Fourth, the clinic team should be co-located in the same space and share a common philosophy of pain rehabilitation.
Due to improvement in overall patient functioning, reductions in health care expenditures and an increase in rate of patients returned to employment, the multidisciplinary pain clinic was deemed a success (Flor et al., 1992; Kamper et al., 2015). In these published meta-analyses, multidisciplinary pain clinics were compared to single discipline treatment (e.g., physical therapy, medication management), usual medical care, and/or no treatment. Impressively, treatment gains remained evident for up to 13 years (Roberts et al., 1993; Patrick et al., 2004). Analyses of patient outcomes revealed that it wasn’t any single component of the clinic treatment that led to improvements in pain outcomes, but rather an effect of the concerted biopsychosocial team approach (Linssen and Spinhoven, 1992; Schatman, 2010). Subsequently, the number of pain clinics and accredited pain fellowship programs flourished, most of which used opioid therapy sparingly due to concerns of addiction and poor outcomes (Meldrum, 2003). These early clinics were multidisciplinary using similar methods to Bonica’s original model, or clinics focused on a single pain syndrome (e.g., migraine headaches) or single treatment modality (e.g., providing nerve blockade or physical therapy) (Bonica, 1990).
3.2. The peculiar fall of multidisciplinary pain clinics: money matters
Several economic factors have been attributed to a decrease in the number of multidisciplinary pain clinics since the 1990s and an increase in the single modality, interventional and opioid-focused clinics. First, market forces emerged to reduce reimbursement rates for multidisciplinary clinics. Use of the American Medical Association’s current procedural terminology (CPT) codes became necessary for reimbursement of most health services in the early 1980s. As CPT codes were originally designed to be used in medical charting to describe an intervention (e.g., an appendectomy), these codes inherently emphasized fee-for-service model of health care delivery, and ran counter to the multidisciplinary pain clinic model of providing a package of services – all of which were necessary for optimal patient outcomes. Unless individual clinics contracted with payers (e.g., worker’s compensation carriers, insurance carriers) to provide bundled services for enrollees, clinics had to bill for each service separately. Denials of claims became commonplace and threatened clinic solvency due to lengthy appeals processes.
The growth of managed care in the late 1980s and early 1990s led to “carving out” specific services in the multidisciplinary pain clinic, e.g., physical therapy would not be reimbursable if performed by therapists in the multidisciplinary pain clinic but needed to be performed by an alternate clinic across town. Subsequent studies showed worse pain treatment and decreased overall cost savings outcomes with this approach (Gatchel et al., 2001; Robbins et al., 2003). For example, patients at one multidisciplinary pain treatment center who were required by insurance companies to attend off-site physical therapy had no significant improvements in overall pain level, pain related disability, or physical/mental functioning, compared to similar contemporaneous patients completing the full multidisciplinary treatment program on-site who achieved significant improvements in all of those realms.
Second, the academic medical centers that housed and often subsidized many of the multidisciplinary pain clinics became increasingly concerned with profits. Finite clinic space began to be allocated to programs with the highest profit margins (e.g., plastic surgery, orthopedics, or cardiology), at the expense of multidisciplinary pain treatment programs, which brought in significantly less revenue after the advent of managed care (Schatman, 2010).
Third, as pain fellowship physician training programs developed, they came to be recognized as a subspecialty under anesthesiology, thus training and accreditation bodies placed more emphasis on proficiency in procedure-based care (e.g., nerve blocks, ablations, and insertions of spinal cord stimulators – please see below) than being a member of a multidisciplinary care team. This is reflected by the training curriculums and certifying examinations (Accreditation Council for Graduate Medical Education, 2016). As a result, increasing numbers of pain fellowship graduates were prepared for and sought employment performing procedures in often highly lucrative modality specific pain clinics. As a result of all these forces, many of the multidisciplinary pain treatment clinics established in the 1970s and 80 s closed due to financial concerns – leaving the majority of chronic pain care in the hands of primary care providers and modality specific chronic pain treatment clinics.
3.3. Pain as the “Fifth Vital Sign”
Since the passage of the Harrison Narcotic Act in 1914, physicians and patients alike had been afraid of developing addiction if placed on morphine or other opioids (Meldrum, 2003). Therefore, opioids were used sparingly in the treatment of chronic pain; in addition to addiction, concerns of tolerance limiting analgesic efficacy contraindicated their use for treating chronic pain. However, there was increased recognition that many terminal patients lived out their last days in agonizing pain. Specialists in palliative care, such as Kathleen Foley and her fellow, Russell Portenoy, at Memorial Sloan Kettering recognized the relief that opioids brought to dying cancer patients through extensive clinical experience in the 1970s and 80 s (Foley, 1985; Portenoy and Foley, 1986). Concurrently, the World Health Organization developed cancer pain treatment guidelines that included opioids for the first time and recognized the treatment of pain as a universal right (World Health Organization, 1986; Lohman et al., 2010).
Shortly thereafter, the American Pain Society (APS) initiated an influential campaign, “Pain, The Fifth Vital Sign,” to raise awareness among health professionals of pain assessment and management. Although opioids were described as just one possible treatment option, the initiative did advocate a change in philosophy around use of opioids for chronic pain. Opioids were promoted as a way to improve quality at end of life. The Veteran’s Health Administration (VHA), the largest government run health-care system in the US, adopted pain as the 5th vital sign initiative in 1999–giving strong credibility to the campaign (Mularski et al., 2006).
By the late 1990s, it was generally accepted that all patients are entitled to the assessment and treatment of pain, resulting in influential regulatory bodies such as the Joint Commission on the Accreditation of Healthcare Organizations (JCAHO) mandating pain assessment and treatment of all patients in accredited health care settings by 2001 in order to receive federal health care dollars (Manchikanti et al., 2010a,b; Ahmedani et al., 2014). The Federation of State Medical Boards made a clear statement in 1998 that physicians would not receive excessive regulatory scrutiny if prescribing notable amounts of opioids – a fear that had previously reduced the willingness of physicians to prescribe opioids for chronic pain (Joranson et al., 2002). The Drug Enforcement Agency (DEA) in 2001 also agreed to follow a “balanced policy” in examining prescribing practices that would encourage use of opioids to relieve pain and reduce oversight of physicians that had high rates of opioid prescribing.
Although the APS campaign led to increases in pain research, education, and an important focus on pain relief, there have been unintended consequences resulting in an overreliance on opioids to treat chronic non-malignant pain. Health care providers accredited by the JCAHO were mandated to implement adequate pain assessment and treatment methods for all patients in a relatively short period of time (2 years). Although specific analgesic treatments (non-pharmacologic and pharmacologic) were left up to the individual physician or provider, these health care facilities developed policies that liberalized the use of opioids in an attempt to meet JCAHO standards. In addition, as patient satisfaction (including pain relief) became an increasingly valued health care outcome, clinicians in these facilities were further encouraged to use opioids to control pain quickly and as completely as possible. Although higher opioid use remains a theoretical risk of rewarding patient satisfaction scores (Zgierska et al., 2012), a national study showed health care facilities that have highest patient satisfaction scores reported greater expenditures on prescription drugs then those with lowest satisfaction scores (Fenton et al., 2012).
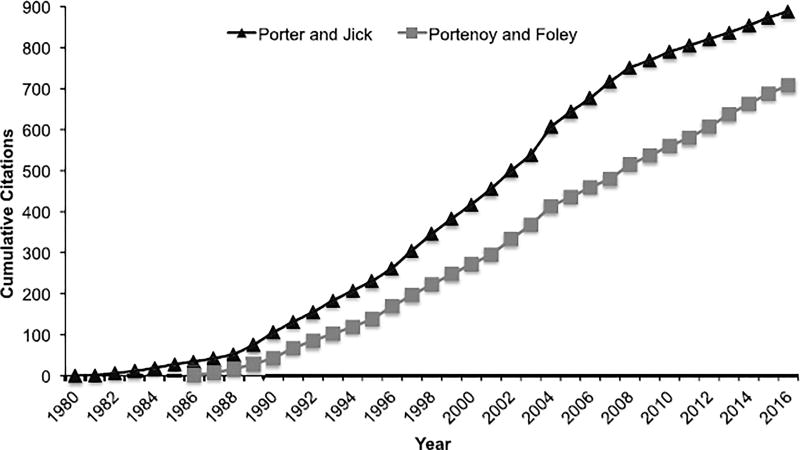
During the 1990s and early 2000s, provider and patient fears of addiction to prescribed opioids were minimized due to an overemphasis on the findings of two small retrospective studies which suggested that patients rarely develop opioid use disorder when opioids were prescribed for the treatment of pain (Porter and Jick, 1980; Portenoy and Foley, 1986). The first was a letter that suggested very low (0.03%) addiction rates in hospitalized patients provided doses of opioids for acute, non-recurrent pain (Porter and Jick, 1980). The second reported on 38 patients from one practice prescribed opioids for chronic non-malignant pain (Portenoy and Foley, 1986), and showed only 2 patients (5.3%), both with history of substance use disorder, developed management problems (dose escalation and diversion, respectively). These reports became heavily cited in peer-reviewed and non-peer reviewed literature (Fig. 1); the reports were also incorporated into training of new physicians, nurses, dentists and other health care providers. However, these early claims of low addiction risk when generalized to chronic pain management were based upon insufficient evidence as history has shown.
Fig. 1.
Cumulative citations of two influential articles on the “low risk” of addiction with opioid use. A letter published in 1980 by Porter and Jick that dealt with opioids for acute pain as well as a report by Portenoy and Foley in 1986 on 38 cases of persons treated with opioids for chronic non-malignant pain were used hundreds of times as evidence to demonstrate that opioids had low risk for addiction. Cumulative citations for each article were obtained from Google Scholar.
3.4. Opioid prescribing skyrockets
Related to the historical shifts outlined above, the treatment of chronic non-cancer pain became a new and growing indication for an opioid prescription. Most famously, Purdue Pharmaceuticals introduced Oxycontin® (oxycodone extended release) in 1996, which was marketed aggressively with FDA-approved labeling to claim that iatrogenic addiction was “very rare” and “delayed absorption of OxyContin was believed to reduce the abuse liability of the drug” (Van Zee, 2009). This resulted in an exponential increase in the number of Oxycontin prescriptions from 670,000 in 1997 to about 6.2 million in 2002, when the label was changed to drop the above language. Despite the change in label and a lawsuit judgment for over $630 million for continuing to claim falsely that OxyContin was less addictive than other opioids, US sales of Oxycontin continued to be close to 6.5 million prescriptions annually until August 2010 when a reformulated “abuse deterrent” Oxycontin was brought to market (Hwang et al., 2015). As with Oxycontin, prescriptions for all opioids increased dramatically throughout the late 1990s and 2000s. Opioid prescriptions were frequently written by practitioners without specialty training (including dentists), and in a few rare cases, by providers who focused on writing opioid prescriptions for profit.
3.5. Does chronic opioid therapy improve treatment outcomes?
At present, there is no conclusive answer to the above question. A systematic review was completed in preparation for the 2016 CDC guidelines on the use of opioids for the treatment of chronic non-malignant pain in primary care. The review inclusion criteria specified studies of opioid analgesic effectiveness must have >12 months of follow-up and be compared to a non-opioid control, but studies of opioid harms could be uncontrolled and have any length of follow-up. As a result, no controlled studies on opioid effectiveness met inclusion criteria but 19 studies (mostly uncontrolled studies using claims databases) were included that examined harms. The review concluded that there were no well-controlled long-term studies indicating that opioid treatment for pain beyond twelve weeks effectively relieves pain or improves function (Chou et al., 2015). These clinical trials focused mainly on pain relief and did not typically report on other pain related outcomes, including quality of life, reduction in disability, or return to work. In contrast, there are multiple reports in the peer-reviewed literature from single-arm observational studies, open label designs and case reports that include hundreds of individuals maintained on chronic opioid therapy (>12 months) for a variety of pain diagnoses that saw sustained improvement in pain levels (Zenz et al., 1992; Milligan et al., 2001; Allan et al., 2005; Chao, 2005; McIlwain and Ahdieh, 2005; Portenoy et al., 2007). Thus, despite the lack of controlled long-term studies >12 weeks, there is clinical consensus from pain management practitioners that some patients do well on chronic opioids (Chou et al., 2009). Given the discrepancy between systematic reviews of controlled studies and clinical consensus, there is no clear answer on whether chronic opioid therapy can improve pain level, pain-related disability, or quality of life in patients. In addition, it is important to include in a balanced perspective that chronic opioid therapy is associated with overdose death, development of substance use disorder, fractures, and sexual dysfunction (Chou et al., 2015).
4. Effective pain management strategies: beyond prescription opioids
An unfortunate consequence of the recent focus on prescription opioids to treat chronic pain has been a lack of research on, and clinical attention to the efficacy of a wide variety of non-opioid chronic pain management strategies. The primary goals of chronic pain management are discovering a cause, alleviating suffering, and restoring function. Biologic, psychological, and social factors all play a role in the perception and chronification of pain and each should be assessed and managed as needed. As previously reviewed, strong consensus supported by meta-analytic reviews affirms that a multidisciplinary rehabilitation approach to the treatment of chronic pain is more effective compared to single modality/single practitioner treatment options (Flor et al., 1992; Kamper et al., 2015; Semrau et al., 2015) However, this treatment option is not widely available. Therefore, the following section outlines various treatment approaches to better assist the reader in understanding the wide array of other chronic pain treatment options. Included are opioid and non-opioid pharmacotherapies, physical therapy, psychological and behavioral therapies, complementary and alternative medicine strategies, peripheral procedures, spinal procedures, and surgery (Table 1). As with opioid clinical trials, controlled trials of non-opioid analgesic strategies are typically short (< 12 weeks) and so long-term effectiveness data for chronic pain using these strategies is limited (Tayeb et al., 2016).
Table 1.
Common non-opioid chronic pain management strategies.
| Strategy | Examples | Usual Dose Range | FDA Approved Chronic Pain Indications |
Significant Side Effects | Notes | |
|---|---|---|---|---|---|---|
| Multidisciplinary pain treatment | Johns Hopkins Hospital Pain Treatment Program | 3–4 weeks of intensive 5–7 days/week attendance at clinic | Not subject to FDA approval. Has been shown to improve any type of chronic pain, especially with no identifiable cause or where other approaches have failed. | None | Patient should be willing to decrease/stop opioid medications. Insurance coverage may not be available or require significant prior authorization process. | |
| Non-opioid medications | NSAID | aspirin | 325 to 650 mg every 4 h (Max 4 g/day) | Disorders of joint of spine, generalized pain, headaches, OA, RA | Bleeding, gastric ulcer, tinnitus, bronchospasm, and Reye’s syndrome | Should not take OTC aspirin for more than 10 days at a time without instruction from physician. |
| ibuprofen | 200 to 800 mg every 4–6 h (Max 3.2 g/day) | Generalized pain, headaches, OA, RA | Congestive heart failure, myocardial infarction, stroke, Stevens-Johnson syndrome, and hearing loss | Should not take OTC ibuprofen for more than 10 days at a time without instruction from physician. | ||
| naproxen | 250 to 500 mg every 12 h (Max 1 g/day) | Ankylosing spondylitis, bursitis, generalized pain, OA, RA | Congestive heart failure, myocardial infarction, stroke, Stevens-Johnson syndrome, bleeding, and renal failure | OTC naproxen can be taken for 6 months without instruction from physician. | ||
| Anti-depressants | duloxetine | 30 to 60 mg daily | Diabetic peripheral neuropathy, fibromyalgia, chronic musculoskeletal pain | May be fatal in overdose, suicidal ideation, Stevens-Johnson syndrome, myocardial infarction, liver failure, and serotonin syndrome | Cannot be stopped suddenly or may have withdrawal syndrome. Pain relief may take 4–6 weeks after achieving an effective dose. | |
| venlafaxine | 75 to 225 mg daily | None. | Hyponatremia, bleeding, hepatitis, seizure, suicidal thoughts, and serotonin syndrome | RCTs have demonstrated efficacy for neuropathic pain and prophylaxis of migraine/tension type headaches. Pain relief may take 4–6 weeks after achieving an effective dose. | ||
| nortriptyline | 50 to 100 mg daily | None. | Sudden cardiac death, SIADH, hepatic failure, stroke, suicidal thoughts, dizziness, and falls | Dosing should be guided by plasma blood level. RCTs have shown efficacy for neuropathic pain. Pain relief may take 4–6 weeks after achieving an effective dose. | ||
| Anti-epileptic drugs | gabapentin | 300 to 600 mg every 8 h | Post-herpetic neuralgia | Stevens-Johnson syndrome, hypoglycemia, sedation, suicidal thoughts, dizziness, and falls. Misuse and/or abuse of gabapentin has also been reported. | RCTs have also shown efficacy for diabetic peripheral neuropathy, and fibromyalgia. Pain relief may take 4–6 weeks after achieving an effective dose. | |
| pregabalin | 75 to 150 mg every 12 h (Max 450 mg/day) | Diabetic peripheral neuropathy, fibromyalgia, neuropathic pain from a spinal cord injury, post-herpetic neuralgia | Jaundice, suicidal thoughts, and acute renal insufficiency | Pain relief may take 4–6 weeks after achieving an effective dose. | ||
| Physical therapy (PT) | Exercise therapy | 8–12 PT sessions over 4–6 weeks | Not subject to FDA approval. Used to treat back and neck pain, arthritis, fibromyalgia | Worsening pain, new injury, myocardial infarction, and sudden death | Referral is needed. Patient needs to practice skills at home. Exercises are widely variable by therapists. | |
| Psychological therapies | CBT | Weekly hour-long individual sessions for 12 weeks | Not subject to FDA approval. Used for all types of pain. | None | Referral may be needed. Patients need to complete homework. Pain relief is only short-term. Not all therapists trained in pain CBT. | |
| MBSR | Weekly 2-h long group sessions for 8 weeks | Not subject to FDA approval. Used for all types of pain. | None | Optional 6-h retreat. Instructors for MBSR are not widely available. Pain improvement is short-term but can improve physical functioning for up to 26 weeks. | ||
| CAM | Acupuncture | 6–12 weekly sessions over 6–12 weeks | Not subject to FDA approval. Used to treat OA, chronic pelvic pain, chronic prostatitis, chronic neck pain, chronic back pain | Nerve injury causing worse pain and infection | Not always covered by insurance or available; no widely accepted protocol of acupuncture delivery; most studies performed outside USA | |
| Peripheral procedures | Trigger point injections | Lidocaine, corticosteroid, or “dry needling” | Single injection by physician. May be repeated. | Not subject to FDA approval. Used to treat chronic neck pain, headaches, iliac crest syndrome, and myofascial pain. | Nerve injury causing worse pain, infection, pneumothorax, seizure, and local tissue necrosis | Need to palpate location of maximum tenderness. Few long-term studies. Should be done with PT. |
| Intra-articular Injection | Sodium hyaluronate, corticosteroid | Single Injection by physician. May be repeated. | Not subject to FDA approval. Used to treat OA, RA, hip arthritis, low back pain, shoulder pain, TMJ, de Quervain’s tenosynovitis | Nerve injury causing worse pain and infection | 1–2 office visits. No clear evidence that these injections provide greater pain relief compared to sham procedures. | |
| Spinal procedures | Epidural steroid injection | Corticosteroid +/− local anesthetic (see nerve block for examples) | 1–3 injections separated by at least a month (no more than 3 injections in 12 months) | None. | Infection, bleeding, vertebral fracture (after multiple injections), paralysis, stroke, loss of vision and death. | Best results use fluoroscopic guidance. Multiple different procedures to enter epidural space. No clear evidence that these injections provide greater pain relief compared to sham procedures. |
| Nerve block | 0.25%-0.5% bupivacaine, 2% lignocaine, or 1% lidocaine | One diagnostic block and then second long-term nerve block (3–6 months pain relief). | Chronic radiculopathy, cancer- related, facet join degeneration | Nerve injury causing worse pain, infection, paralysis, and seizure | Outpatient surgical procedure. May repeat every 6–12 months as needed | |
| Radiofrequency denervation | 1–2 diagnostic nerve blocks followed by fluoroscopic guided destruction of nerve | Facet joint pain, low back pain with disc herniation, sacroiliac pain. | Nerve injury causing worse pain, infection, and paralysis | Outpatient surgery. May be repeated. Systematic reviews of trials have not shown significant benefit over sham. | ||
| Spinal cord stimulator | Insertion of temporary stimulator to determine efficacy and then insertion of permanent device | Chronic intractable pain of the trunk and/or limbs, pain associated with failed back surgery syndrome, complex regional pain syndrome | Nerve injury causing worse pain, infection, and paralysis | Pre-op psychological evaluation, at least two outpatient surgery visits, regular follow-up. Revisions may be necessary. | ||
| Surgery | Discectomy, spinal fusion | Back pain with nerve injury (e.g. disc herniation), spinal stenosis, | Failed back surgery syndrome, death, paralysis, and infection | Pre-op evaluation, surgery, +/− post-op hospital stay, usually 6 weeks off work, at least 3 month healing time, +/− PT |
NSAID = non-steroidal anti-inflammatory drugs; FDA = Food and Drug Administration; OTC = over the counter; CAM = complementary and alternative medicine; RCT = randomized clinical trials; CBT = cognitive behavioral therapy; MBSR = mindfulness based stress reduction; OA = osteoarthritis; RA = rheumatoid arthritis; SIADH = syndrome of inappropriate antidiuretic hormone secretion. These strategies are not mutually exclusive and can be combined as directed by a health care provider. Unfortunately, little information is known about the comparative effectiveness of these strategies.
4.1. Non-opioid pharmacotherapies
There are numerous medications used to treat chronic non-malignant pain that target the mechanisms of peripheral and central sensitization, the proposed mechanisms contributing to pain chronification, e.g., sodium and calcium channel upregulation, spinal hyperexcitability, descending modulation and loss of inhibitory interneurons. Aspirin, acetaminophen, and nonsteroidal anti-inflammatory drugs (NSAIDS; e.g., ibuprofen or naproxen) are the first choice in the pharmacological management of acute pain (Blondell et al., 2013), but can also be useful in the management of mild to moderate chronic pain as single agents or as a component of multimodal pain control in severe chronic pain (Enthoven et al., 2016). NSAIDs are more effective in the treatment of chronic musculoskeletal pain (e.g., osteoarthritis, chronic non-specific low back pain) compared to neuropathic pain. In addition, naproxen has been FDA approved for pain associated with ankylosing spondylitis, an inflammatory autoimmune disorder that causes the vertebrae of the spine to fuse together. Significant side effects of NSAIDs include bleeding, Stevens-Johnson syndrome, and renal failure. Long-term use is not recommended unless under the care of a physician or other prescriber.
Antidepressants and anticonvulsants are frequently used in the treatment of chronic neuropathic pain (McCleane, 2008; Attal and Bouhassira, 2015; Finnerup et al., 2015). The analgesic effects of antidepressants are thought to be independent of their antidepressant effects, as persons without current major depressive disorder receive significant analgesic benefit (Lynch and Watson, 2006). Duloxetine is the only antidepressant to have FDA approval for the treatment of chronic pain (diabetic peripheral neuropathy, fibromyalgia, and chronic musculoskeletal pain), although antidepressants with dual serotonin and norepinephrine reuptake inhibition are used off-label to provide pain relief (Table 1). Prescribers should watch for the development of new suicidal thoughts, hyponatremia, serotonin syndrome, and hepatitis/liver failure while using anti-depressants, and patients should be counseled that pain relief often occurs slowly over time (usually 4–6 weeks after reaching an effective dose). Anticonvulsants reduce pain by inhibiting excessive neuronal firing, including nociceptors. First and second generation anticonvulsants (e.g., gabapentin and pregabalin) are FDA approved for the treatment of a variety of neuropathic conditions including diabetic neuropathy, trigeminal neuralgia, and post-herpetic neuralgia (Lynch and Watson, 2006). As with anti-depressants, patients should be counseled that pain relief may take 4–6 weeks to occur and monitored for the development of new-onset suicidal ideation, sedation leading to falls, hypoglycemia, and acute renal insufficiency (Table 1). There is also increasing evidence that patients may misuse or abuse gabapentin (Chiappini and Schifano, 2016; Smith et al., 2016).
4.2. Physical therapy (PT)
Reductions in pain related to medications and invasive procedures can enable patients to fully participate with PT, which has an important role in pain relief and restoration of function in chronic pain patients (Krismer et al., 2007). PT should strongly be considered for the management of chronic pain to gradually increase flexibility and strength, for example in knee osteoarthritis where there is demonstrated benefit (Fransen et al., 2015). A referral from a physiatrist, primary care physician or nurse practitioner is often required before initiating PT. Although initially therapist-directed, PT can become self-directed over time. A course of PT usually requires an intake assessment and 8–12 PT sessions over the course of 4–6 weeks. Techniques include stretching exercises, manipulations, hot or cold applications, traction, transcutaneous electrical nerve stimulation (TENS), and massage. Risks of PT include myocardial infarction leading to sudden death, as well as worsening pain (especially at beginning of treatment).
4.3. Psychological and behavioral therapies
Many psychological and behavioral therapies have been used in the treatment of pain and its associated disability and distress – irrespective of the presence or absence of mental illness (Eccleston et al., 2009). Cognitive-behavioral therapy (CBT) is an effective approach based on the theory that beliefs, attitudes, and expectations affect emotional and behavioral reactions to life experiences, including pain. Patients are taught to be active participants in the management of their pain with the goals of increasing activity, independence, and resourcefulness. Persons with high levels of pain catastrophizing may especially benefit from psychological and behavioral therapies (Smeets et al., 2006). CBT usually requires 12 weeks of treatment to see maximum benefit, with hour-long weekly individual sessions with a therapist and homework to be completed outside of sessions. A referral is often needed, and the availability of pain-informed CBT therapists is a problem for many patients. Unfortunately, a systematic review found that pain relief that occurred after CBT does not persist long-term (Williams et al., 2012).
A recent randomized clinical trial has also shown the promise of mindfulness based stress reduction (MBSR) as a treatment for chronic pain (Cherkin et al., 2016). MBSR is usually provided in group sessions lasting 2 h weekly for up to 8 weeks. Patients are also asked to practice MBSR techniques at home in between sessions. Pain relief tends to be short-term but improvements in physical functioning have been maintained up to 26 weeks of follow-up (Cherkin et al., 2016). MBSR therapists do not require masters or doctoral level training in counseling/psychology. However, specialized training is required, and insurance does not typically cover MBSR.
4.4. Complementary and alternative medicine (CAM)
Increasingly, patients are turning to pain interventions that are not commonly taught in medical training and are less often covered by insurance payers. CAM treatments include acupuncture, manipulation, vitamins and supplements (fish oil, capsaicin, glucosamine), yoga, music therapy, biofeedback, and hypnosis. Other than acupuncture and spinal manipulation, these strategies have been less rigorously studied in clinical trials and little is known about the long-term benefits or harms (Murthy et al., 2015). Acupuncture is a part of traditional Chinese Medicine that involves the stimulation of specific parts of the body through the use of needles. Acupuncture has been used for pain management for two thousand years in China. Recently, electrical currents have been added to further stimulate the needles in electroacupuncture, although manual acupuncture without electrical current is the most commonly used method. The exact analgesic mechanism for acupuncture is not known, although growing evidence points towards acupuncture needles stimulating mechanoreceptors, which triggers a release of endorphins and increased activity of the descending inhibitory pain pathways (Leung, 2012). Although acupuncture has been used to treat a wide variety of pain syndromes (e.g., osteoarthritis, chronic pelvic pain, fibromyalgia, chronic low back pain), systematic reviews revealed mostly short-term benefits. In patients with osteoarthritis, acupuncture can improve physical functioning that is maintained over follow-up but does not provide significantly greater pain relief compared to sham acupuncture in long-term follow-up (Lin et al., 2016). In addition, no long-term follow up studies have demonstrated that acupuncture provides greater pain relief for chronic neck pain or chronic nonspecific low back pain compared to sham acupuncture but short-term pain relief is superior comparing active to sham acupuncture (Yuan et al., 2015; Trinh et al., 2016). Acupuncture is superior to sham acupuncture for reducing pain from chronic prostatitis/chronic pelvic pain syndrome, although long-term follow-up studies are lacking. (Liu et al., 2016).
Spinal manipulation are treatments that “use high velocity/low amplitude to move a joint that is exhibiting somatic dysfunction through its restrictive barrier” (Ruddock et al., 2016). Typically, chiropractors perform spinal manipulations although doctors of osteopathic medicine are also trained in these treatments. There is no consensus on the exact procedure for spinal manipulation, and insurance does not often cover them. In a recent meta-analysis of sham controlled RCTs, there was evidence that spinal manipulation improved pain ratings post-treatment and in 1 month follow-up compared to sham in patients with chronic non-specific low back pain (Ruddock et al., 2016). However, there have been no well-controlled long-term follow-up studies to demonstrate if these improvements are lasting. In addition, adverse events are not always reported in RCTs of spinal manipulation, so weighing risks versus benefits is challenging (Gorrell et al., 2016).
4.5. Invasive pain management interventions: peripheral procedures, spinal procedures, and surgery
Invasive treatments that are used for the management of chronic pain include injections of local anesthetics and steroids, peripheral procedures, electrical stimulation, and surgery (Manchikanti et al., 2010a,b), which are typically provided by anesthesiologists, neurosurgeons, physiatrists, and other physicians trained in pain medicine (Table 1). The growth of interventional pain medicine (as described in Section 3) has shown dramatic growth in utilization of invasive pain management interventions. (Manchikanti et al., 2012), although, as described below, evidence of efficacy is often lacking.
Peripheral procedures include trigger point injections and intraarticular injections. Trigger point injections involve dry needling into the muscle tissue to induce a localized twitch response and subsequent ending of contracture (Gerwin et al., 2004). A recent systematic review showed that dry needling decreases pain immediately post-treatment compared to sham, and may result in pain relief lasting 3–6 months (Boyles et al., 2015). Injection of lidocaine or corticosteroid is also performed in some trigger point injections, although medication injection is not thought to improve upon dry needling (Cummings and White, 2001; Boyles et al., 2015). For arthritic pain related to cartilage loss, intra-articular injections are performed with either a corticosteroid to reduce inflammation or sodium hyaluronate to form a viscoelastic solution that serves as a protective buffer between joints. Despite the lack of evidence from systematic reviews (Arrich et al., 2005; Machado et al., 2013; Juni et al., 2015; Witteveen et al., 2015; McCabe et al., 2016), intraarticular injections continue to be performed (often repeatedly) in at least a third of Medicare patients with knee osteoarthritis (Koenig et al., 2016).
Spinal procedures are intended to interrupt the pain signaling pathway and include epidural injection of anesthetic (nerve blocks) +/− steroids, radiofrequency denervation, and insertion of spinal cord stimulators. Systematic reviews have not shown significant benefit of epidural steroid injections over standard treatment in chronic low back pain from any cause (Staal et al., 2009). Nerve blocks (involving epidural injections of lidocaine without steroids), however, do provide significant pain relief for lumbar radicular pain or pain from spinal stenosis compared to steroid injections (Manchikanti et al., 2016). Radiofrequency ablation uses electrical heat to produce a lesion in a pain-transmitting nerve, thereby blocking pain transmission and providing pain relief (Shealy, 1975). Systematic reviews have not demonstrated significant long-term pain relief with ablation compared to sham treatments, but may provide short-term pain relief for facet joint related pain (Maas et al., 2015). Electrical stimulation of the spinal cord occurs through an implanted device using low voltage electrical impulses to block pain transmission (Song et al., 2014), consistent with the gate control theory of pain (Melzack and Wall, 1965). Although pain relief can be significant in patients with few other options, there is a relatively high complication rate from this procedure (35% in a recent retrospective study (Hayek et al., 2015)) and ongoing device management is required.
Surgical interventions are usually reserved for patients that have failed to respond to more conservative measures or when there would be irreversible neurological damage without emergent surgical intervention (e.g., disc herniation, spinal stenosis) (Deyo et al., 2004). Scar tissue and adhesions can worsen the pain over time, and re-injury can occur (Chan and Peng, 2011). Therefore, a lengthy recovery time is recommended to ensure re-injury does not occur.
5. The provider’s dilemma: how to best treat chronic pain
A health care provider must often make clinical decisions based upon insufficient evidence, despite the call to evidence-based practice exclusively. In the case of chronic pain treatment, there are risks and benefits associated with each treatment option but little long-term (>12 months) follow-up data. In addition, patients must consider, the practicalities of time required for each treatment, whether or not insurance covers the particular modality, and whether there are available providers who are licensed and taking new patients in the area. The importance of effectively treating pain is underscored by the increased odds of suicide, major depressive disorder, substance use and substance use disorders seen in chronic pain patients compared to persons without chronic pain (Barry et al., 2013; Gerrits et al., 2014; Hassett et al., 2014; Alford et al., 2016). In addition, there is evidence that untreated pain can also lead to relapse in patients with prior history of substance use disorders (Weiss et al., 2014).
In the face of moral, ethical, legal and regulatory pressures to relieve suffering, writing a prescription, especially for opioids, has become the quickest and least expensive (to the patient) option in the US health care system for individuals seeking traditional medical treatment for chronic pain. Any physician, nurse practitioner, or physician assistant can prescribe opioids as long as he or she has the appropriate DEA and state practice license (exact guidelines differ between each state), and prescription opioids are almost universally covered by insurance. However, guidelines from various professional societies and governmental agencies provide conflicting recommendations on the use of opioids for chronic non-cancer pain (Chou et al., 2009; Dowell et al., 2016), so the provider is left with a difficult decision of whether or not to prescribe them.
If opioids are no longer the preferred chronic pain management strategy and there is a lack of multidisciplinary pain treatment centers, the primary care provider must overcome many hurdles to develop a multimodal treatment approach for each patient. Physical, psychological and behavioral therapies can be burdensome to the patient, requiring frequent (usually weekly or more) visits, as well as individual practice using techniques/exercise away from the clinic. Although usually covered by insurance, these often have higher out-of-pocket expenses compared to prescription opioids, and limits on number of yearly visits – especially with psychological interventions. Pain relief usually takes time to manifest with PT, psychological and behavioral therapies, unlike immediate relief as after prescription opioid administration. Experience of the practitioner can vary, such that not every therapist is trained in pain or participates with an individual’s insurance plan. Other system barriers include need of referral, availability of licensed providers (including health behaviorists), time off from work, lack of childcare, and availability of transportation. In addition, patients may be hesitant to agree to psychological interventions due to perceived stigma or religious/cultural biases (Jimenez et al., 2013).
Lastly, although single modality pain treatment centers are very frequent, there are geographic variations with rural areas having fewer practitioners. Additionally, practitioners may not have the expertise to perform the more complex procedures, such as insertion of spinal cord stimulator. These procedures also require insurance prior-authorization, and spinal cord stimulator insertion requires a psychological evaluation prior to procedure. In the current US health care system and with a limited evidence base, there are no easy solutions for providers to effectively treat chronic pain patients using a multimodal approach.
6. Future directions
As the use of opioids for the treatment of chronic pain undergoes increasing scrutiny, the next generation of pain treatment strategies is needed to take their place. It is doubtful, however, if the approximately 5–8 million individuals currently prescribed opioids for chronic pain are going to suddenly stop or taper easily (Reuben et al., 2015). Although calls for responsible opioid prescribing will grow, there will certainly be opioid prescribing at high levels for years to come. In addition, then, to the existing data showing efficacy for multidisciplinary pain treatment approaches absent opioid pharmacotherapy, there is a new set of questions that will need to be answered to inform pain treatment of the future.
What is the safety and efficacy of opioid medications used for the treatment of chronic non-cancer pain for periods >1 year? Data on longer-term outcomes from studies on the efficacy and risks of opioid therapy for chronic non-malignant pain are lacking. It is important to understand the conditions under which long-term benefits of pain relief might be observed and how the pain relief may change over time. It is also important to understand whether the risks of chronic exposure, especially addiction and physical dependence, are modulated by any long-term benefits of pain relief. An understanding of patient pain syndrome and medication-specific risk factors for both good and poor outcomes is also needed. It would be very helpful to enroll both persons with and without substance use disorders into future long-term studies.
What are the system specific needs and barriers for the utility of non-opioid therapies for the treatment of chronic pain? Noted throughout this review is that access to potentially effective non-medication interventions is often limited by current models of health service reimbursement. New research strategies such as comparative efficacy and factorial design trials could provide useful guidance on the best and most cost-effective approaches to pain management in individuals with different characteristics and types of chronic pain. Funding for these studies should come from government and industry partnerships.
What is the role of opioid pharmacotherapy in combination with non-opioid therapies in chronic pain management? If research shows that long-term use of opioids is useful at least for some types of pain patients, then this approach will need to be examined in interaction with other non-opioid therapies to determine optimal combination strategies.
This is an ambitious research agenda. However, given the debilitating effects of chronic pain, the numbers of individuals involved and the cost to society, it would appear to be a timely and useful undertaking. Clinicians continue to have an ethical and legal obligation to assess and treat pain. Therefore, research is urgently needed to increase the evidence base of chronic pain management so that we can begin the post-“opioid-opioid-opioid” era in pain management while improving outcomes and functionality for patients.
Acknowledgments
DAT has received medication supplies from Indivior, Inc. (formerly Reckitt-Benckiser Pharmaceuticals) for an investigator-initiated research protocol; consulted with Astra-Zeneca and Theravance; and is site principal investigator for a multisite clinical trial funded by Alkermes.
Role of funding source
Salary support for DAT while preparing this manuscript was provided by NIDA (K23 DA029609). Although not playing a role in the preparation of this manuscript, a percentage of salary support for PC is provided by NIDA (U01 DA029580).
Footnotes
Conflict of interest
JGH and PC have no conflicts of interest relevant to the contents of this manuscript.
Contributions
DAT conceived of the topic for the manuscript. DAT, JGH and PC contributed to writing the first draft and substantial editing of the manuscript. All authors have approved the final article.
References
- Accreditation Council for Graduate Medical Education (ACGME) ACGME Program Requirements for Graduate Medical Education in Pain Medicine (Anesthesiology, Neurology, Or Physical Medicine and Rehabilitation) [Downloaded October 5 2016];2016 Available at https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/530_pain_medicine_2016_1-YR.pdf.
- Ahmedani BK, Peterson EL, Wells KE, Lanfear DE, Williams LK. Policies and events affecting prescription opioid use for non-cancer pain among an insured patient population. Pain Physician. 2014;17:205–216. [PMC free article] [PubMed] [Google Scholar]
- Alford DP, German JS, Samet JH, Cheng DM, Lloyd-Travaglini CA, Saitz R. Primary care patients with drug use report chronic pain and self-medicate with alcohol and other drugs. J. Gen. Intern. Med. 2016;31:486–491. doi: 10.1007/s11606-016-3586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan L, Richarz U, Simpson K, Slappendel R. Transdermal fentanyl versus sustained release oral morphine in strong-opioid naive patients with chronic low back pain. Spine. 2005;30:2484–2490. doi: 10.1097/01.brs.0000186860.23078.a8. [DOI] [PubMed] [Google Scholar]
- Arrich J, Piribauer F, Mad P, Schmid D, Klaushofer K, Mullner M. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ. 2005;172:1039–1043. doi: 10.1503/cmaj.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal N, Bouhassira D. Pharmacotherapy of neuropathic pain: which drugs, which treatment algorithms? Pain. 2015;156(Suppl. 1):S104–14. doi: 10.1097/01.j.pain.0000460358.01998.15. [DOI] [PubMed] [Google Scholar]
- Barry DT, Pilver CE, Hoff RA, Potenza MN. Pain interference and incident mood, anxiety, and substance-use disorders: findings from a representative sample of men and women in the general population. J. Psychiatr. Res. 2013;47:1658–1664. doi: 10.1016/j.jpsychires.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti C, Chapman CR. John J. Bonica. A biography. Minerva Anestesiol. 2005;71:391–396. [PubMed] [Google Scholar]
- Blondell RD, Azadfard M, Wisniewski AM. Pharmacologic therapy for acute pain. Am. Fam. Physician. 2013;87:766–772. [PubMed] [Google Scholar]
- Bonica JJ. Evolution and current status of pain programs. J. Pain Symptom Manage. 1990;5:368–374. doi: 10.1016/0885-3924(90)90032-f. [DOI] [PubMed] [Google Scholar]
- Boyles R, Fowler R, Ramsey D, Burrows E. Effectiveness of trigger point dry needling for multiple body regions: a systematic review. J. Man. Manip. Ther. 2015;23:276–293. doi: 10.1179/2042618615Y.0000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CW, Peng P. Failed back surgery syndrome. Pain Med. 2011;12:577–606. doi: 10.1111/j.1526-4637.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- Chao J. Retrospective analysis of Kadian (morphine sulfate sustained-release capsules) in patients with chronic, nonmalignant pain. Pain Med. 2005;6:262–265. doi: 10.1111/j.1526-4637.2005.05033.x. [DOI] [PubMed] [Google Scholar]
- Cheng HT. Spinal cord mechanisms of chronic pain and clinical implications. Curr. Pain Headache Rep. 2010;14:213–220. doi: 10.1007/s11916-010-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkin DC, Sherman KJ, Balderson BH, Cook AJ, Anderson ML, Hawkes RJ, Hansen KE, Turner JA. Effect of mindfulness-based stress reduction vs. cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240–1249. doi: 10.1001/jama.2016.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the european medicines agency’s ‘Suspected adverse drug reactions’ database. CNS Drugs. 2016;30:647–654. doi: 10.1007/s40263-016-0359-y. [DOI] [PubMed] [Google Scholar]
- Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 2010;303:1295–1302. doi: 10.1001/jama.2010.344. [DOI] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J. Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 2015;162:276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- Clark MR, Treisman GJ. Neurobiology of pain. Adv. Psychosom. Med. 2004;25:78–88. doi: 10.1159/000079059. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch. Phys. Med. Rehabil. 2001;82:986–992. doi: 10.1053/apmr.2001.24023. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery – the case for restraint. N. Engl. J. Med. 2004;350:722–726. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2009;2:CD007407. doi: 10.1002/14651858.CD007407.pub2. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res. Manage. 2009;14:307–311. doi: 10.1155/2009/273783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enthoven WT, Roelofs PD, Deyo RA, van Tulder MW, Koes BW. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst. Rev. 2016;2:CD012087. doi: 10.1002/14651858.CD012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton JJ, Jerant AF, Bertakis KD, Franks P. The cost of satisfaction A national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch. Int. Med. 2012;172:405–411. doi: 10.1001/archinternmed.2011.1662. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H, Fydrich T, Turk DC. Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. Pain. 1992;49:221–230. doi: 10.1016/0304-3959(92)90145-2. [DOI] [PubMed] [Google Scholar]
- Foley KM. The treatment of cancer pain. N. Engl. J. Med. 1985;313:84–95. doi: 10.1056/NEJM198507113130205. [DOI] [PubMed] [Google Scholar]
- Fordyce WE, Fowler RS, Jr, Lehmann JF, Delateur BJ, Sand PL, Trieschmann RB. Operant conditioning in the treatment of chronic pain. Arch. Phys. Med. Rehabil. 1973;54:399–408. [PubMed] [Google Scholar]
- Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br. J. Sports Med. 2015;49:1554–1557. doi: 10.1136/bjsports-2015-095424. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P. The economic costs of pain in the United States. J. Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Noe CE, Garaj NM, Vakharia AS, Polatin PB, Dreschner M, Pulliam C. Treatment carve-out practices: their effect on managing pain at an interdisciplinary pain center. J. Work. Comp. 2001;10:50–63. [Google Scholar]
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am. Psychol. 2014;69:119–130. doi: 10.1037/a0035514. [DOI] [PubMed] [Google Scholar]
- Gerrits MM, van Oppen P, van Marwijk HW, Penninx BW, van der Horst HE. Pain and the onset of depressive and anxiety disorders. Pain. 2014;155:53–59. doi: 10.1016/j.pain.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons’ integrated hypothesis of trigger point formation. Curr. Pain Headache Rep. 2004;8:468–475. doi: 10.1007/s11916-004-0069-x. [DOI] [PubMed] [Google Scholar]
- Gorrell LM, Engel RM, Brown B, Lystad RP. The reporting of adverse events following spinal manipulation in randomized clinical trials-a systematic review. Spine J. 2016;16:1143–1151. doi: 10.1016/j.spinee.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Hassett AL, Aquino JK, Ilgen MA. The risk of suicide mortality in chronic pain patients. Curr. Pain Headache Rep. 2014;18:436. doi: 10.1007/s11916-014-0436-1. [DOI] [PubMed] [Google Scholar]
- Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18:603–608. doi: 10.1111/ner.12312. [DOI] [PubMed] [Google Scholar]
- Hwang CS, Chang HY, Alexander GC. Impact of abuse-deterrent OxyContin on prescription opioid utilization. Pharmacoepidemiol. Drug Saf. 2015;24:197–204. doi: 10.1002/pds.3723. [DOI] [PubMed] [Google Scholar]
- International Association for the Study of Pain. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain. 1986:S1–226. [PubMed] [Google Scholar]
- Jimenez DE, Bartels SJ, Cardenas V, Alegria M. Stigmatizing attitudes toward mental illness among racial/ethnic older adults in primary care. Int. J. Geriatr. Psychiatry. 2013;28:1061–1068. doi: 10.1002/gps.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J. Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Joranson DE, Gilson AM, Dahl JL, Haddox JD. Pain management, controlled substances, and state medical board policy: a decade of change. J. Pain Symptom Manage. 2002;23:138–147. doi: 10.1016/s0885-3924(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Juni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, da Costa BR. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015;10:CD005328. doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RW, Guzman J, van Tulder MW. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi: 10.1136/bmj.h444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig KM, Ong KL, Lau EC, Vail TP, Berry DJ, Rubash HE, Kurtz S, Bozic KJ. The use of hyaluronic acid and corticosteroid injections among Medicare patients with knee osteoarthritis. J. Arthroplasty. 2016;31:351–355. doi: 10.1016/j.arth.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Krismer M, van Tulder M Low Back Pain Group of the Bone and Joint Health Strategies for Europe Project. Strategies for prevention and management of musculoskeletal conditions. Low back pain (non-specific) Best Pract. Res. Clin. Rheumatol. 2007;21:77–91. doi: 10.1016/j.berh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Leung L. Neurophysiological basis of acupuncture-induced analgesia—An updated review. J. Acupunct. Meridian Stud. 2012;5:261–270. doi: 10.1016/j.jams.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Lin X, Huang K, Zhu G, Huang Z, Qin A, Fan S. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J. Bone Joint Surg. Am. 2016;98:1578–1585. doi: 10.2106/JBJS.15.00620. [DOI] [PubMed] [Google Scholar]
- Linssen AC, Spinhoven P. Multimodal treatment programmes for chronic pain: a quantitative analysis of existing research data. J. Psychosom. Res. 1992;36:275–286. doi: 10.1016/0022-3999(92)90092-g. [DOI] [PubMed] [Google Scholar]
- Liu BP, Wang YT, Chen SD. Effect of acupuncture on clinical symptoms and laboratory indicators for chronic prostatitis/chronic pelvic pain syndrome: a systematic review and meta-analysis. Int. Urol. Nephrol. 2016;48:1977–1991. doi: 10.1007/s11255-016-1403-z. [DOI] [PubMed] [Google Scholar]
- Lohman D, Schleifer R, Amon JJ. Access to pain treatment as a human right. BMC Med. 2010;8:8. doi: 10.1186/1741-7015-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Watson CP. The pharmacotherapy of chronic pain. A review. Pain Res. Manag. 2006;11:11–38. doi: 10.1155/2006/642568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas ET, Ostelo RW, Niemisto L, Jousimaa J, Hurri H, Malmivaara A, van Tulder MW. Radiofrequency denervation for chronic low back pain. Cochrane Database Syst. Rev. 2015;10:CD008572. doi: 10.1002/14651858.CD008572.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado E, Bonotto D, Cunali PA. Intra-articular injections with corticosteroids and sodium hyaluronate for treating temporomandibular joint disorders: a systematic review. Dental Press J. Orthod. 2013;18:128–133. doi: 10.1590/s2176-94512013000500021. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Datta S, Gupta S, Munglani R, Bryce DA, Ward SP, Benyamin RM, Sharma ML, Helm S, 2nd, Fellows B, Hirsch JA. A critical review of the American Pain Society clinical practice guidelines for interventional techniques: part 2: Therapeutic interventions. Pain Physician. 2010a;13:E215–64. [PubMed] [Google Scholar]
- Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010b;13:401–435. [PubMed] [Google Scholar]
- Manchikanti L, Falco FJ, Singh V, Pampati V, Parr AT, Benyamin RM, Fellows B, Hirsch JA. Utilization of interventional techniques in managing chronic pain in the Medicare population: analysis of growth patterns from 2000 to 2011. Pain Physician. 2012;15:E969–82. [PubMed] [Google Scholar]
- Manchikanti L, Knezevic NN, Boswell MV, Kaye AD, Hirsch JA. Epidural injections for lumbar radiculopathy and spinal stenosis: a comparative systematic review and meta-analysis. Pain Physician. 2016;19:E365–410. [PubMed] [Google Scholar]
- McCabe PS, Maricar N, Parkes MJ, Felson DT, O’Neill TW. The efficacy of intra-articular steroids in hip osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2016;24:1509–1517. doi: 10.1016/j.joca.2016.04.018. [DOI] [PubMed] [Google Scholar]
- McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22:139–156. doi: 10.2165/00023210-200822020-00005. [DOI] [PubMed] [Google Scholar]
- McIlwain H, Ahdieh H. Safety, tolerability, and effectiveness of oxymorphone extended release for moderate to severe osteoarthritis pain: a one-year study. Am. J. Ther. 2005;12:106–112. doi: 10.1097/01.mjt.0000139442.65914.f9. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- Meldrum ML. A capsule history of pain management. JAMA. 2003;290:2470–2475. doi: 10.1001/jama.290.18.2470. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Milligan K, Lanteri-Minet M, Borchert K, Helmers H, Donald R, Kress HG, Adriaensen H, Moulin D, Jarvimaki V, Haazen L. Evaluation of long-term efficacy and safety of transdermal fentanyl in the treatment of chronic noncancer pain. J. Pain. 2001;2:197–204. doi: 10.1054/jpai.2001.25352. [DOI] [PubMed] [Google Scholar]
- Mularski RA, White-Chu F, Overbay D, Miller L, Asch SM, Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. J. Gen. Intern. Med. 2006;21:607–612. doi: 10.1111/j.1525-1497.2006.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V, Sibbritt DW, Adams J. An integrative review of complementary and alternative medicine use for back pain A focus on prevalence, reasons for use, influential factors, self-perceived effectiveness, and communication. Spine J. 2015;15:1870–1883. doi: 10.1016/j.spinee.2015.04.049. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care. 2014;8:143–151. doi: 10.1097/SPC.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick LE, Altmaier EM, Found EM. Long-term outcomes in multidisciplinary treatment of chronic low back pain: results of a 13-year follow-up. Spine. 2004;29:850–855. doi: 10.1097/00007632-200404150-00006. [DOI] [PubMed] [Google Scholar]
- Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol. Rev. 2012;92:1699–1775. doi: 10.1152/physrev.00048.2010. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171–186. doi: 10.1016/0304-3959(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Farrar JT, Backonja MM, Cleeland CS, Yang K, Friedman M, Colucci SV, Richards P. Long-term use of controlled-release oxycodone for noncancer pain: results of a 3-year registry study. Clin. J. Pain. 2007;23:287–299. doi: 10.1097/AJP.0b013e31802b582f. [DOI] [PubMed] [Google Scholar]
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N. Engl. J. Med. 1980;302:123. doi: 10.1056/nejm198001103020221. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Alvanzo AA, Ashikaga T, Bogat GA, Callahan CM, Ruffing V, Steffens DC. National Institutes of Health Pathways to Prevention Workshop: the role of opioids in the treatment of chronic pain. Ann. Intern. Med. 2015;162:295–300. doi: 10.7326/M14-2775. [DOI] [PubMed] [Google Scholar]
- Robbins H, Gatchel RJ, Noe C, Gajraj N, Polatin P, Deschner M, Vakharia A, Adams L. A prospective one-year outcome study of interdisciplinary chronic pain management: compromising its efficacy by managed care policies. Anesth. Analg. 2003;97:156–162. doi: 10.1213/01.ane.0000058886.87431.32. [DOI] [PubMed] [Google Scholar]
- Roberts AH, Sternbach RA, Polich J. Behavioral management of chronic pain and excess disability: long-term follow-up of an outpatient program. Clin. J. Pain. 1993;9:41–48. doi: 10.1097/00002508-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Ruddock JK, Sallis H, Ness A, Perry RE. Spinal manipulation vs. sham manipulation for nonspecific low back pain: a systematic review and meta-analysis. J. Chiropr. Med. 2016;15:165–183. doi: 10.1016/j.jcm.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustoen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain Differences in health and quality of life among younger, middle-aged, and older adults. Clin. J. Pain. 2005;21:513–523. doi: 10.1097/01.ajp.0000146217.31780.ef. [DOI] [PubMed] [Google Scholar]
- Schatman ME. Interdisciplinary chronic pain management: perspectives on history, current status, and future viability. In: Fishman SM, Ballantyne J, Rathmell J, editors. Bonica’s Management of Pain. Lippincott Williams & Wilkins; Baltimore, MD: 2010. pp. 1523–1532. [Google Scholar]
- Semrau J, Hentschke C, Buchmann J, Meng K, Vogel H, Faller H, Bork H, Pfeifer K. Long-term effects of interprofessional biopsychosocial rehabilitation for adults with chronic non-specific low back pain: a multicentre, quasi-experimental study. PLoS One. 2015;10:e0118609. doi: 10.1371/journal.pone.0118609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shealy CN. Percutaneous radiofrequency denervation of spinal facets. Treatment for chronic back pain and sciatica. J. Neurosurg. 1975;43:448–451. doi: 10.3171/jns.1975.43.4.0448. [DOI] [PubMed] [Google Scholar]
- Shmagel A, Foley R, Ibrahim H. Epidemiology of chronic low back pain in US adults: national health and nutrition examination survey 2009–2010. Arthritis Care Res. 2016;68:1688–1694. doi: 10.1002/acr.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets RJEM, Vlaeyen JWS, Kester ADM, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J. Pain. 2006;7:261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111:1160–1174. doi: 10.1111/add.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Popescu A, Bell RL. Present and potential use of spinal cord stimulation to control chronic pain. Pain Physician. 2014;17:235–246. [PubMed] [Google Scholar]
- Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34:49–59. doi: 10.1097/BRS.0b013e3181909558. [DOI] [PubMed] [Google Scholar]
- Stemkowski PL, Smith PA. Sensory neurons, ion channels, inflammation and the onset of neuropathic pain. Can. J. Neurol. Sci. 2012;39:416–435. doi: 10.1017/s0317167100013937. [DOI] [PubMed] [Google Scholar]
- Tayeb BO, Barreiro AE, Bradshaw YS, Chui KK, Carr DB. Durations of opioid, nonopioid drug, and behavioral clinical trials for chronic pain: adequate or inadequate? Pain Med. 2016;17:2036–2046. doi: 10.1093/pm/pnw245. [DOI] [PubMed] [Google Scholar]
- Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin. J. Pain. 2012;28:819–841. doi: 10.1097/AJP.0b013e31824549d6. [DOI] [PubMed] [Google Scholar]
- Trinh K, Graham N, Irnich D, Cameron ID, Forget M. Acupuncture for neck disorders. Cochrane Database Syst. Rev. 2016;5:CD004870. doi: 10.1002/14651858.CD004870.pub4. [DOI] [PubMed] [Google Scholar]
- Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J. Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Van Zee A. The promotion and marketing of Oxycontin Commercial triumph, public health tragedy. Am. J. Public Health. 2009;99:221–227. doi: 10.2105/AJPH.2007.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk D, Poliak-Tunis M. Chronic pain management An overview of taxonomy, conditions commonly encountered, and assessment. Med. Clin. North Am. 2016;100:1–16. doi: 10.1016/j.mcna.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Griffin ML, McHugh RK, Haller D, Jacobs P, Gardin J, 2nd, Fischer D, Rosen KD. Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain. J. Subst. Abuse Treat. 2014;47:140–145. doi: 10.1016/j.jsat.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst. Rev. 2015;10:CD010643. doi: 10.1002/14651858.CD010643.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Cancer Pain Relief. WHO; Geneva: 1986. [Google Scholar]
- Yuan QL, Guo TM, Liu L, Sun F, Zhang YG. Traditional Chinese medicine for neck pain and low back pain: a systematic review and meta-analysis. PLoS One. 2015;10:e0117146. doi: 10.1371/journal.pone.0117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenz M, Strumpf M, Tryba M. Long-term oral opioid therapy in patients with chronic nonmalignant pain. J. Pain Symptom Manage. 1992;7:69–77. doi: 10.1016/0885-3924(92)90116-y. [DOI] [PubMed] [Google Scholar]
- Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377–1378. doi: 10.1001/jama.2012.419. [DOI] [PMC free article] [PubMed] [Google Scholar]