Abstract
Substantial challenges exist for investigating the cannabinoid receptor type 1 (CB1)-mediated discriminative stimulus effects of the endocannabinoids, 2-arachidonoylglycerol (2-AG) and N-arach-idonoylethanolamine (anandamide; AEA), compared with exogenous CB1 receptor agonists, such as Δ9-tetrahydrocannabinol (THC) and the synthetic cannabinoid CP55,940. Specifically, each endocannabinoid is rapidly degraded by the respective hydrolytic enzymes, monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH). Whereas MAGL inhibitors partially substitute for THC and fully substitute for CP55,940, FAAH inhibitors do not substitute for either drug. Interestingly, combined FAAH-MAGL inhibition results in full THC substitution, and the dual FAAH-MAGL inhibitor SA-57 serves as its own discriminative training stimulus. Because MAGL inhibitors fully substitute for SA-57, we tested whether the selective MAGL inhibitor MJN110 would serve as a training stimulus. Twelve of 13 C57BL/6J mice learned to discriminate MJN110 from vehicle, and the CB1 receptor antagonist rimonabant dose-dependently blocked its discriminative stimulus. CP55,940, SA-57, and another MAGL inhibitor JZL184, fully substituted for MJN110. In contrast, the FAAH inhibitor PF-3845 failed to substitute for the MJN110 discriminative stimulus, but produced a 1.6 (1.1–2.2; 95% confidence interval) leftward shift in the MJN110 dose-response curve. Inhibitors of other relevant enzymes (i.e., ABHD6, COX-2) and nicotine did not engender substitution. Diazepam partially substituted for MJN110, but rimonabant failed to block this partial effect. These findings suggest that MAGL normally throttles 2-AG stimulation of CB1 receptors to a magnitude insufficient to produce cannabimimetic subjective effects. Accordingly, inhibitors of this enzyme may release this endogenous brake producing effects akin to those produced by exogenously administered cannabinoids.
Keywords: 2-Arachidonoylglycerol (2-AG), Cannabinoid-1 (CB1) receptor, Fatty acid amide hydrolase (FAAH), Monoacylglycerol lipase (MAGL), Alpha/beta-hydrolase domain 6 (ABHD6), N-arachidonoylethanolamine (anandamide AEA), Endogenous cannabinoids, Discriminative stimulus, Drug discrimination
1. Introduction
More than four decades have elapsed since Jarbe and Henriksson established that Δ9-tetrahydrocannabinol (THC), the predominant psychoactive constituent of Cannabis sativa (Gaoni and Mechoulam, 1964), elicits subjective effects in laboratory animals with a high degree of sensitivity and selectivity (Henriksson and Järbe, 1972; Järbe and Henriksson, 1974,1973; Järbe et al., 1977). The findings that CB1 receptor antagonists block the subjective effects of THC (Järbe et al., 2001; McMahon, 2009; Wiley et al., 1995b) as well as the potent cannabinoid receptor agonist CP55,940 (Owens et al., 2016; Wiley et al., 1995a) establish a receptor mechanism for the discriminative stimulus produced by the naturally occurring and synthetic cannabinoids. Given the challenges associated with investigating the rewarding properties of THC and other cannabinoids in traditional preclinical behavioral assays of abuse liability, the drug discrimination paradigm offers utility to investigate psychoactive properties of this class of drugs (Tanda, 2016). In contrast to THC and synthetic cannabinoid receptor agonists, which elicit long-lasting and robust pharmacological effects, in vivo investigation of the endogenous cannabinoids, N-arachidonoylethanol-amine (anandamide; AEA; Devane et al., 1992) and 2-arachidonoylglycerol (2-AG; Mechoulam et al., 1995; Sugiura et al., 1995), pose further challenges because of rapid degradation by their chief respective hydrolytic enzymes fatty acid amide hydrolase (FAAH) (Cravatt et al., 2001, 1996) and monoacylglycerol lipase (MAGL) (Di Marzo et al., 1999; Dinh et al., 2002). Although AEA substitutes for THC and CP55,940 in rats, it does so at high doses that profoundly disrupt response rates (Wiley et al., 1995). Pharmacological inhibitors of FAAH and MAGL that elevate brain levels of endogenous cannabinoids serve as useful investigative tools to reveal the pharmacological properties of endocannabinoids. Notably, FAAH inhibitors do not substitute for the THC discriminative stimulus (Gobbi et al., 2005; Owens et al., 2016; Solinas et al., 2007; Wiley et al., 2014), but increases substitution of exogenously administered AEA (Solinas et al., 2007; Stewart and McMahon, 2011; Vann et al., 2012; Wiley et al., 2014). Moreover, FAAH (−/−) mice learn to discriminate AEA from vehicle, an effect that was blocked by the CB1 receptor antagonist rimonabant (Walentiny et al., 2011). Whereas FAAH inhibitors lack intrinsic cannabimimetic subjective effects, MAGL inhibitors partially substitute for THC (Long et al., 2009a,b; Wiley et al., 2014; Walentiny et al., 2011), and fully substitute for the CP55,940 discriminative stimulus (Ignatowska-Jankowska et al., 2015).
Interestingly, the dual FAAH-MAGL inhibitors JZL195 and SA-57 fully substitute for the THC discriminative stimulus in mice (Long et al., 2009a,b; Hruba et al., 2015). Moreover, mice learn to discriminate SA-57 from vehicle, and rimonabant blocks this discriminative stimulus, implicating CB1 receptor involvement (Owens et al., 2016). CP55,940, as well as the MAGL inhibitors MJN110 and JZL184 fully substitute for SA-57 (Owens et al., 2016). Thus, in the present study we examined whether mice would learn to discriminate a MAGL inhibitor from vehicle. We selected MJN110 as the training drug because of its high potency and increased selectivity compared with other MAGL inhibitors such as JZL184 (Niphakis et al., 2013). We chose 2.5 mg/kg MJN110 as the training dose because this dose fully substitutes for SA-57 2 h following administration without decreasing response rates (Owens et al., 2016). Rimonabant was used to test whether CB1 receptors mediate the discriminative stimulus effects of MJN110. Finally, we conducted a series of substitution studies to explore if additional targets contribute to the MJN110 discriminative stimulus. Specifically, we tested whether CP55,940, SA-57, the MAGL inhibitor JZL184, and the FAAH inhibitor PF-3845 would substitute for the MJN110 discriminative stimulus. Because MJN110 also inhibits ABHD6, another serine hydrolase that degrades 2-AG (Blankman et al., 2007; Niphakis et al., 2013), we examined whether KT182, a selective inhibitor for this enzyme, would substitute for the training drug. Additionally, as MAGL plays a rate-limiting role in the production of arachidonic acid and its metabolites such as the prostaglandins (Nomura et al., 2011), we tested the COX-2 inhibitor valdecoxib for MJN110 substitution. Finally, we tested two noncannabinoid psychoactive drugs, nicotine (de Moura and McMahon, 2017; Shannon and Herling, 1983) and diazepam (Ator and Griffiths, 1989), the latter of which partially substitutes for the discriminative stimuli of THC (Wiley, 1999) and of SA-57 (Owens et al., 2016).
2. Materials and methods
2.1. Subjects
Male C57BL/6J mice (Jackson Laboratories; Bar Harbor, ME) were used as subjects for all studies. The mice, aged 9—11 weeks at the beginning of training, were individually housed in clear plastic cages on ventilated racks in a temperature (20—22 °C) and humidity controlled vivarium in accordance with Virginia Commonwealth University Institutional Animal Care and Use Committee guidelines. The subjects were given ad libitum access to water and were maintained at a target weight that was 85—90% of their free-fed body weight. Target weight was established before the mice began their initial training and then re-established every six months following a two-week period of ad libitum access to food. Mice were maintained at target weight by restricting daily rations of standard rodent chow (supplied by Harlan Labs, Frederick, MD, Rodent diet 7912).
2.2. Drugs
SA-57, MJN110, and KT182 were synthesized in the Cravatt laboratory at The Scripps Research Institute (La Jolla, California, USA) as previously described (Long et al., 2009a; Niphakis et al., 2012, 2013; Hsu et al., 2013). CP55,940, JZL184, PF-3845, diazepam, and rimonabant (SR144528), were supplied by the National Institute on Drug Abuse (NIDA) (Rockville, MD). Nicotine was obtained commercially (Sigma-RBI; Natick, MA). Valdecoxib was supplied by Ironwood Pharmaceuticals, Inc. (Cambridge, MA). Each compound was dissolved in a vehicle consisting of ethanol, castor oil ethoxylate (30) (Solvay S.A., Brussels, Belgium), and saline (0.9%) in a ratio of 1:1:18. All injections were given in a volume of 1 ml per 100 g of body mass with the exception of 100 mg/kg JZL184, which was given at an injection volume of 2 ml per 100 g of body mass. The increased volume of vehicle did not affect rates of operant responding. All injections were administered via the intraperitoneal (i.p.) route of administration with the exception of CP55,940 and its vehicle control which were administered via the subcutaneous (s.c.) route of administration. MJN110, JZL184, PF-3845, SA-57, and KT182 were administered 120 min before drug discrimination testing. CP55,940, diazepam, nicotine, and valdecoxib were administered 30 min before testing. In the CB1 receptor antagonist experiments, mice received rimonabant 10–15 min before the test compound.
2.3. Apparatus
The drug discrimination assay was conducted in eight standard mice operant conditioning chambers (18 × 18 × 18 cm) that were sound and light-attenuated (MED Associates, St. Albans, VT). Each operant chamber was equipped with a house light, two nose poke apertures (left and right), and a recessed well centered between the two apertures. Sweetened 14-mg pellet reinforcements were delivered to the recessed well when nose poking satisfied scheduled contingencies. Houselights were illuminated during all operant sessions. Computer software (MED-PC® IV, MED Associates, St. Albans, VT) was used to record data and control pellet delivery.
3. Drug discrimination paradigm
3.1. Training and testing
Thirteen male C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) were trained to discriminate MJN110 (2.5 mg/kg) from vehicle. In addition, twelve male C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) trained to discriminate CP55,940 (0.1 mg/kg) from vehicle were used for the cross substitution time course study. Alternate poke apertures were assigned as the drug-associated side. Treatments were administered daily (Monday — Friday) with a pretreatment time of 120 min before each 15 min session in a double alternation sequence (e.g., vehicle, vehicle, drug, drug). During each session, both nose poke apertures were available, but only responses into the correct treatment-associated side resulted in delivery of food reinforcement after 10 nose pokes (FR10).
Subjects were considered to have acquired the task when they met the following three criteria on nine of the previous ten consecutive training sessions: 1) correct completion of the first FR10 (i.e., first 10 consecutive responses into the appropriate aperture); 2) ≥ 80% correct responding; and 3) average response rates ≥ 10 responses/min. During test sessions, 10 responses in either aperture resulted in food reinforcement. Once drug testing commenced, subjects were required to satisfy the criteria listed above on the most recent drug and vehicle training sessions that took place between each test session. A maximum of two test sessions were scheduled each week, with a minimum of 72 h between test days. Before conducting substitution tests, a series of dose-response tests with the training drugs were conducted to characterize a generalization gradient to the discriminative stimulus dose of each training drug. The number of days required to achieve MJN110 acquisition were tracked and the MJN110 dose-response generalization curve was evaluated in all MJN110 trained mice. These mice were split into two groups and assigned substitution test treatments in a randomized sequence. The mice trained to discriminate CP55,940 were assessed for the time course of MJN110 (2.5 mg/kg) substitution.
3.2. Data analysis
The percentage of drug appropriate responses and response rates (responses/min) were recorded for each experiment. Full substitution was defined as greater than or equal to 80% nose pokes that occurred into the aperture associated with the training drug. Partial substitution was defined as greater than or equal to 20% and less than 80% nose pokes in the training drug-paired aperture. Less than 20% nose pokes on the drug-paired aperture was defined as no substitution (Solinas et al., 2006). ED50 values (and 95% confidence intervals) for generalization or substitution were calculated using least squares linear regression analysis. AD50 values were calculated using a transformation to percent effect then using least squares linear regression analysis. Potency ratios (and 95% confidence intervals) were calculated using linear regression (Colquhoun, 1971). All data are depicted as mean ± S.E.M. and were analyzed using one-way or two-way ANOVA. Dunnett's tests or Bonferroni post hoc analyses were used following a significant ANOVA for the response rate data. GraphPad Prism 6.0 statistical software (Graph Pad Software, Inc., La Jolla, CA) was used for data analysis.
4. Results
As shown in Fig. 1 and 12 of 13 test subjects learned to discriminate MJN110 by day 126, with an average of 61.5 days. For comparison, 12 of 12 mice learned to discriminate CP55,940 and 23 of 24 mice to learn to discriminate SA-57 by day 37 in a previous study (Owens et al., 2016). The rates of acquisition in mice trained to discriminate SA-57 or CP55,940 in C57BL/6J mice were comparable to one another with an average of 25.4 and 27.4 days, respectively, and achieved criteria with significantly fewer training days than the mice trained to discriminate MJN110 [p < 0.0001]. In order to assess the time course of action of MJN110, we used a separate cohort of mice trained to discriminate CP55,940 that underwent MJN110 (2.5 mg/kg) substitution tests. MJN110 produced full substitution at 1 h, partial substitution at 2 h, and by 24 h all mice responded on the vehicle-paired aperture (Supplementary Fig. 1A). Rates of responding for each time point did not significantly differ compared to CP55,940 and vehicle control measures in the time course study (Supplementary Fig. 1B).
Fig. 1.
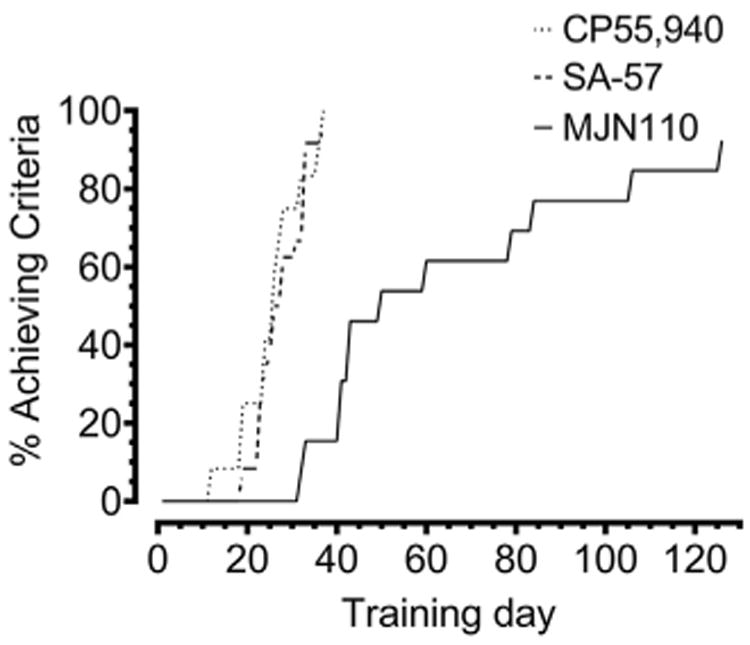
Comparison of acquisition of MJN110 (2.5 mg/kg) to previously reported acquisition rates (Owens et al., 2016) of CP55,940 (0.1 mg/kg) and SA-57 (10 mg/kg) in C57BL/6J mice trained in the drug discrimination paradigm. Values represent percentage of mice that achieved criteria across days; n = 24 mice for SA-57, 12 mice for CP55,940, and 13 mice for MJN110. The SA-57 and CP55,940 acquisition were previously published (Owens et al., 2016) and are reproduced with permission from the Journal of Pharmacology and Experimental Therapeutics.
Fig. 2 shows the dose-response relationship for MJN110 generalization, as well as the CP55,940, SA-57, JZL184 and MJN110 substitution tests in mice trained to discriminate MJN110 (2.5 mg/kg) from vehicle. The ED50 value (95% CL) for the MJN110 discriminative stimulus was 0.46 (0.30–0.70) mg/kg (Fig. 2A). CP55,940, SA-57, and JZL184 dose-dependently substituted for MJN110 (Fig. 2A), with respective ED50 (95% CL) values of 0.087 (0.056–0.13) mg/kg, 2.2 (1.8–2.8) mg/kg, and 14.6 (3.9–55.1) mg/kg. The discriminative stimulus effects of MJN110 were 33.5 (19.4–63.2) fold more potent (95% CL) than those of JZL184. MJN110 [p = 0.1], CP55,940 [p = 0.3] and JZL184 [p = 0.085] did not affect response rates relative to vehicle levels (Fig. 2B).
Fig. 2.
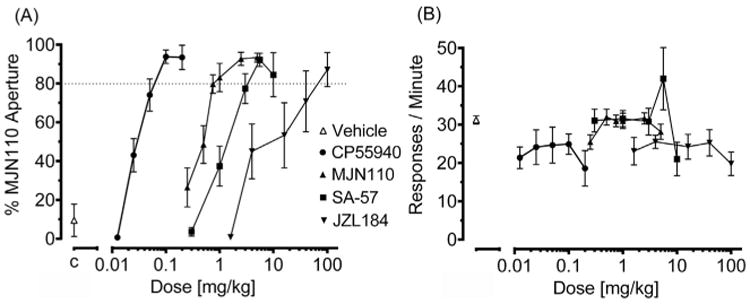
Generalization curve of MJN110 (0.25–5 mg/kg) and substitution of CP55,940 (0.0125–0.2 mg/kg), SA-57 (0.3–10 mg/kg), and JZL184 (1.6–100 mg/kg) on percentage of responses in the MJN110-paired aperture. A) Dose-dependent generalization of MJN110 and dose-dependent substitution of CP55,940, SA-57, and JZL184 for the MJN110 discriminative stimulus. B) CP55,940, MJN110, SA-57 and JZL184 did not decrease rates of responding compared to vehicle. Values represent mean ± SEM; n = 7–12 mice/group.
As shown in Fig. 3, diazepam partially substituted for MJN110 and significantly reduced response rates [F (5,38) = 6.91, p < 0.05]. In contrast, mice administered the ABHD6 inhibitor KT182 (1 and 2 mg/kg), the selective cyclooxygenase-1 inhibitor valdecoxib (10 mg/kg), or nicotine (1.5 mg/kg) selected the vehicle aperture, and none of these drugs affected response rates (Table 1).
Fig. 3.
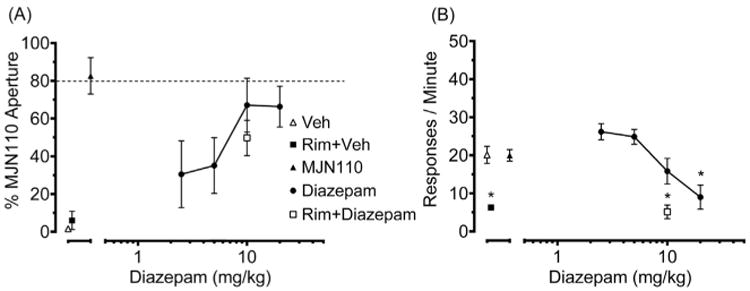
Diazepam (2.5–20 mg/kg) partially substitutes for MJN110 (2.5 mg/kg). Substitution of diazepam (10 mg/kg) alone and in combination with rimonabant (3 mg/kg) on percentage of responses in the MJN110-paired aperture and response rates. A) Dose-related partial substitution of diazepam for the MJN110 discriminative stimulus. Rimonabant had no significant effects alone or in combination with diazepam on responses in the MJN110-paired aperture. B) Diazepam dose-dependently decreased rates of responding. Rimonabant in combination with vehicle or diazepam decreased rates of responding compared to vehicle, MJN110, and diazepam alone. Asterisks indicate significant difference (p < 0.05) versus vehicle. Values represent mean ± SEM; n = 6–9 mice/group.
Table 1.
The ABHD6 inhibitor KT182, the COX-2 inhibitor valdecoxib, and nicotine do not substitute for the MJN110 (2.5 mg/kg) discriminative stimulus. Values represent mean ± SEM. n = 6–8 mice/group.
| Drug (mg/kg) | % MJN110 Aperture | Responses/min |
|---|---|---|
| Vehicle | 4.2 ± 1.9 | 26.7 ± 5.2 |
| KT182 (1) | 1.5 ± 0.8 | 30.6 ± 5.3 |
| KT182 (2) | 2.3 ± 1.0 | 27.1 ± 6.0 |
|
| ||
| Vehical | 1.4 ± 1.0 | 19.4 ± 2.1 |
| Valdecoxib (10) | 0.6 ± 0.3 | 17.8 ± 1.6 |
|
| ||
| Vehicle | 0.4 ± 0.4 | 26.4 ± 5.7 |
| Nicotine (1.5) | 2.7 ± 1.4 | 22.7 ± 3.8 |
To assess whether CB1 receptors mediated discriminative performance, mice were administered rimonabant before injection of the training dose of MJN110 (2.5 mg/kg) or diazepam. As shown in Fig. 4A, rimonabant (0.03–1 mg/kg) dose-dependently reduced the discriminative stimulus of MJN110, with an AD50 (95% CL) of 0.18 (0.11–0.31) mg/kg. In addition, 0.3 and 1.0 mg/kg rimonabant reduced response rates compared with the vehicle and MJN110 alone test conditions (Fig. 4B). In contrast, rimonabant (3 mg/kg) failed to alter the partial substitution produced by diazepam (Fig. 3A), though it reduced response rates compared with diazepam, vehicle, and MJN110 alone test conditions [F (4,30) = 13.37, p < 0.05; Fig. 3B].
Fig. 4.
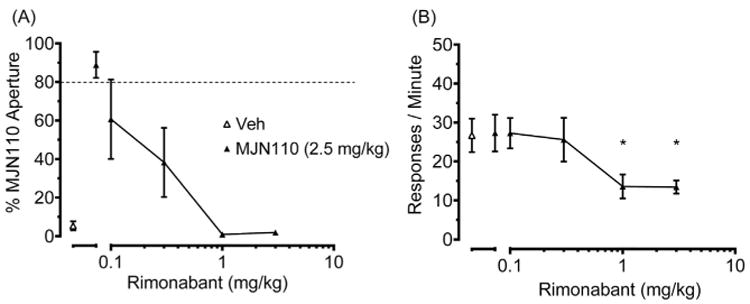
The MJN110 discriminative stimulus requires CB1 receptor activation. A) Rimonabant (0.3–1 mg/kg) attenuated responding in the MJN110 aperture. B) Rimonabant (0.3, and 1 mg/kg) reduced response rates. Asterisks indicate significant difference (p < 0.05) versus vehicle. Values represent mean ± SEM; n ¼ 4–5 mice/group.
Although the FAAH inhibitor PF3845 (10 mg/kg) did not substitute for MJN110, it produced a 1.6 (1.1–2.2; 95% CL) leftward shift in the MJN110 dose response curve (Fig. 5A). The respective ED50 values and 95% CL for the combination treatments of MJN110 + Vehicle and MJN110+ PF-3845 were 0.51 (0.40–0.65) mg/kg and 0.33 (0.28–0.38) mg/kg. The response rates following administration of either MJN110 + Vehicle [p = 0.085] or MJN110+PF-3845 [p = 0.5] did not differ from the Vehicle alone condition (Fig. 5B).
Fig. 5.
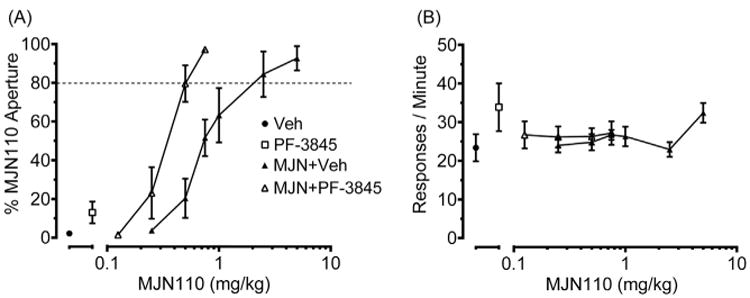
PF-3845 alone and in combination with MJN110. A) The FAAH inhibitor PF-3845 (10 mg/kg) did not substitute for MJN110, but produced a leftward shift in the MJN110 generalization dose-response curve. B) PF-3845 alone or in combination with MJN110 did not decrease response rates. Values represent mean ± SEM; n = 7–8 mice/group.
4.1. Discussionis
Previous studies have reported that MAGL inhibitors fully substitute for CP55,940 and for the dual FAAH-MAGL inhibitor SA-57, though they only partially substitute for THC (Hruba et al., 2015; Ignatowska-Jankowska et al., 2014; Long et al., 2009b; Owens et al., 2016; Vann et al., 2012; Wiley et al., 2016, 2011). The present results extend these findings to show that the selective MAGL inhibitor MJN110 serves as a training drug in the drug discrimination paradigm. Full substitution for MJN110 by the high efficacy CB1 receptor agonist CP55,940, combined with dose-dependent blockade of MJN110's discriminative stimulus effects by the CB1 receptor antagonist rimonabant, support the inference that CB1 receptors mediate the MJN110 discriminative stimulus. Furthermore, MAGL substitution exhibits selectivity, as the MAGL inhibitor JZL184 and the dual FAAH-MAGL inhibitor SA-57 fully substitute and cross-substitute (Hruba et al., 2015; Owens et al., 2016; Wiley et al., 2016, 2011), whereas the FAAH inhibitor PF-3845, the ABHD6 inhibitor KT182, and three noncannabinoid drugs (i.e., valdecoxib, nicotine, and diazepam) do not elicit full substitution for MJN110.
Although the FAAH inhibitor PF-3845 did not engender MJN110 substitution, full FAAH inhibition produced a significant leftward shift in the MJN110 generalization curve. These findings are consistent with previous reports demonstrating that simultaneous inhibition of FAAH and MAGL produces more robust subjective, motor, and antinociceptive effects (Hruba et al., 2015; Long et al., 2009b; Owens et al., 2016; Anderson et al., 2014; Ghosh et al., 2015; Wilkerson et al., 2017) than those produced by selective inhibition of either enzyme alone. The observations that 2-AG levels are approximately three orders of magnitude higher than AEA levels in wild-type mouse brains (Ahn et al., 2009; Long et al., 2009a) and 2-AG possesses higher CB1 receptor efficacy than AEA (Sugiura et al., 2002) are consistent with our findings that MAGL inhibitors produced a CB1 receptor-mediated subjective stimulus, but PF-3845 lacked efficacy. Alternatively, the possibility that these endocannabinoids activate distinct CB1 receptor-mediated neuronal pathways offers a more parsimonious explanation than explanations of mass action and differences in efficacy for the finding that PF-3845 increased the potency of MJN110 in producing a discriminative stimulus. Accordingly, 2-AG may activate a predominant CB1 receptor-mediated circuit that mediates the discriminative stimulus, while AEA may activate a minor CB1 receptor-mediated circuit that serves to amplify the 2-AG-medi-ated pathway, but lacks sufficiency to elicit a discriminative stimulus on its own. The differential patterns of expression between these enzymes (i.e., predominant FAAH expression on post-synaptic terminals (Gulyas et al., 2004), while primary expression of MAGL on pre-synaptic terminals (Dinh et al., 2002)) are consistent with the possibility of major and minor CB1 receptor-mediated neuronal pathways subserving cannabimimetic subjective effects.
We also tested whether inhibiting ABHD6, an off-target of MJN110 (Niphakis et al., 2013) or COX-2 would engender responding on the MJN110 aperture. The observation that doses of KT182 that selectively and fully block ABHD6 (Hsu et al., 2013) did not substitute for MJN110 suggests that ABHD6 does not play a necessary role in the discriminative stimulus effects of this training drug. Because MAGL serves as a rate-limiting biosynthetic enzyme in arachidonic acid production (Nomura et al., 2011), we tested whether inhibition of COX-2, an enzyme known to be a major metabolizer of arachidonic acid (Marnett et al., 1999), would substitute for MJN110. The selective COX-2 inhibitor valdecoxib failed to produce substitution or affect response rates in the mice, indicating that inhibiting production of COX-2-regulated metabolites do not play a necessary role in the discriminative effects of MJN110. Of course, COX-2-mediated lipid signaling molecules may play a modulatory role in the pharmacological effects resulting from 2-AG stimulation of CB1 receptors, or other metabolites of arachidonic acid or arachidonic acid itself may contribute to the MJN110 discriminative stimulus.
An interesting observation in the present study is that diazepam partially substituted for MJN110. Similarly, diazepam partially substitutes for THC in rats (Wiley, 1999) and for SA-57 in mice (Owens et al., 2016). Likewise, diazepam showed some evidence of partial substitution in a human THC discrimination study, though the magnitude of this effect was only 25% (Lile et al., 2014). Diaz-epam may partially substitute for cannabimimetic discriminative stimuli through a common mechanism, such as a GABA component, which is under CB1 receptor modulation (Selley et al., 2004). Nonetheless, it is assumed that a drug must fully substitute for the training drug in order to be considered to elicit similar subjective effects (Harris and Balster, 1971; Overton, 1966).
Although the present study demonstrated that C57BL/6J mice learned to discriminate MJN110 from vehicle, these mice required substantially more training to reach criteria than mice from a previous drug discrimination study that used CP55,940 and SA-57 as training drugs (Owens et al., 2016). With MJN110 as the training drug, mice required a mean of 61.5 days to achieve criteria, with 12 of the 13 subjects demonstrating MJN110 discrimination by day 126. In our previous study, the vast majority of mice (i.e., all 12 mice trained with CP55,940, and 23 of 24 mice trained with SA-57) achieved criteria by day 37 (Owens et al., 2016). A definitive explanation accounting for the disparate rates of achieving discrimination criteria between MJN110 and these other drugs remains to be determined. One possibility is that repeated dosing of these drugs during training led to differential downregulation and/or desensitization of the CB1 receptor. However, the fact that the ED50 values calculated in generalization and substitution experiments did not substantially vary for each respective drug between studies does not support an explanation of functional CB1 receptor tolerance. Another possibility is that elevations of 2-AG alone produce less CB1 receptor activation than that produced by either combined elevation of AEA and 2-AG or CP55,940. For example, CP55,940 and SA-57 produce the full spectrum of cannabimimetic effects in tetrad assay, whereas MAGL inhibition produces a subset of cannabimimetic effects (Grim et al., 2017; Long et al., 2009b; Wilkerson et al., 2017). Finally, the MJN110 time course substitution study showing that MJN110 fully substitutes for CP55,940 at 1 h and only partially substitutes at 2 h (i.e., drug-related responding = 71.4% ± 9.7) suggests that an optimal pretreatment time may not have been used, though previous studies showed that a 2 h pretreatment of 2.5 mg/kg MJN110 fully substitutes for SA57 (Owens et al., 2016) and CP55,940 (Ignatowska-Jankowska et al., 2015).
In conclusion, the results of the present study make a unique observation that has potential clinical implications. It was observed that the selective MAGL inhibitor MJN110 produces a reliable discriminative stimulus, most likely through a 2-AG-mediated stimulation of CB1 receptors. Consequently, MAGL may serve to dampen endocannabinoid-mediated overstimulation of the CB1 receptor, thereby preventing induction of a cannabimimetic subjective state. From a clinical perspective, MAGL inhibitors may have a favorable therapeutic index as analgesics in that the apparent doses required to induce cannabimimetic subjective effects, induce tolerance and dependence, and possibly promote abuse, likely far exceed those required to produce analgesia. For example, 0.5 mg/kg MJN110, which only partially inhibits MAGL (Niphakis et al., 2013), does not engender responding in the aperture associated with cannabimimetic drugs, yet it fully reverses mechanical allodynia in the chronic constriction injury of the sciatic nerve model of neuropathic pain (Ignatowska-Jankowska et al., 2015; Owens et al., 2016). In contrast, the MJN110 training dose used in the present study (i.e., 2.5 mg/kg), which required a notable long time to train suggestive of marginal effectiveness, fully inhibits MAGL (Niphakis et al., 2013) and elicits substantial (i.e., approximately 10-fold) increases in 2-AG brain levels (Ignatowska-Jankowska et al., 2015). Such prolonged and complete MAGL inhibition leads to functional CB1 receptor tolerance and dependence (Burston et al., 2016; Kinsey et al., 2013). Conversely, partial inhibition of MAGL produces maximal anti-allodynic effects, retains its anti-allodynic effects, and does not lead to CB1 receptor downregulation/desensitization or physical dependence (Burston et al., 2016; Kinsey et al., 2013). Thus, the results of the present study suggest that low to moderate doses of MAGL inhibitors may effectively treat chronic pain conditions, while minimizing effects associated with cannabinoid abuse.
Supplementary Material
Acknowledgments
The authors thank the members of the Lichtman laboratory and Beardsley laboratory (Lesley O'Brien, Giulia Donvito, Zachary Curry, Jenny Wilkerson, Travis Grimm, Jason Wiebelhaus, Matthew Walentiny) for helpful discussions. The authors greatly appreciate the National Institute on Drug Abuse (NIDA) for supplying CP55,940, rimonabant, diazepam, JZL184, and PF3845, and thank Organix Inc. (Woburn, MA) for supplying AEA. This paper is dedicated to the memory of Dr. Toby Jarbe, a pioneer in the field.
Funding: This research was supported by the National Institutes of Health [Grants: R01DA026449, R01DA032933, R01DA030404, R01DA03672, P30DA033934, T32DA007027].
Footnotes
Chemical compounds: CP55,940 (PubChem CID: 104895)
Rimonabant (PubChem CID: 104850)
PF-3845 (PubChem CID: 25154867)
JZL184 (PubChem CID: 25021165)
MJN110 (PubChem CID: 71722059)
SA-57 (PubChem CID: 44589122)
KT182 (PubChem CID: 53364491)
Nicotine (PubChem CID: 24278591)
Diazepam (PubChem CID: 3016)
Valdecoxib (PubChem CID: 119607)
Authorship contributions: Participated in research design: Owens, Mustafa, Ignatowska-Jankowska, Beardsley, Damaj, Wiley, Cravatt, Lichtman.
Conducted experiments: Owens, Mustafa.
Contributed new reagents or analytic tools: Niphakis, Cravatt.
Performed data analysis: Owens, Mustafa, Lichtman.
Wrote or contributed to the writing of the manuscript: Owens, Mustafa, Ignatowska-Jankowska, Wiley, Beardsley, Damaj, Lichtman.
Appendix A. Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2017.06.032.
References
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WB, Gould MJ, Torres RD, Mitchell VA, Vaughan CW. Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine inflammatory pain model. Neuropharmacology. 2014;81:224–230. doi: 10.1016/j.neuropharm.2013.12.018. http://dx.doi.org/10.1016/j.neuropharm.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Differential generalization to pentobarbital in rats trained to discriminate lorazepam, chlordiazepoxide, diazepam, or triazolam. Psychopharmacol Berl. 1989;98:20–30. doi: 10.1007/BF00442001. [DOI] [PubMed] [Google Scholar]
- Blankman J, Simon G, Cravatt B. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. http://dx.doi.org/10.1016/j.chembiol.2007.11.006.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston JJ, Mapp PI, Sarmad S, Barrett DA, Niphakis MJ, Cravatt BF, Walsh DA, Chapman V. Robust anti-nociceptive effects of mono-acylglycerol lipase inhibition in a model of osteoarthritis pain. Br J Pharmacol. 2016;173:3134–3144. doi: 10.1111/bph.13574. http://dx.doi.org/10.1111/bph.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Lectures on Biostatistics: An Introduction to Statistics with Applications in Biology and Medicine. Clarendon Press; Oxford, UK: 1971. [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. http://dx.doi.org/10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- de Moura FB, McMahon LR. The contribution of α4β2 and non-α4β2 nicotinic acetylcholine receptors to the discriminative stimulus effects of nicotine and varenicline in mice. Psychopharmacol Berl. 2017;234:781–792. doi: 10.1007/s00213-016-4514-4. http://dx.doi.org/10.1007/s00213-016-4514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, Kunos G. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem. 1999;264:258–267. doi: 10.1046/j.1432-1327.1999.00631.x. http://dx.doi.org/10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. http://dx.doi.org/10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Ghosh S, Kinsey SG, Liu QS, Hruba L, McMahon LR, Grim TW, Merritt CR, Wise LE, Abdulla Ra, Selley DE, Sim-Selley L, Cravatt BF, Lichtman AH. Full FAAH inhibition combined with partial monoacylglycerol lipase inhibition: augmented and sustained antinociceptive effects with negligible cannabimimetic side effects in mice. J Pharmacol Exp Ther. 2015;354:111–120. doi: 10.1124/jpet.115.222851. http://dx.doi.org/10.1124/jpet.115.222851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. http://dx.doi.org/10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Morales AJ, Thomas BF, Wiley JL, Endres GW, Negus SS, Lichtman AH. Apparent CB1 receptor rimonabant affinity estimates: combination with THC and synthetic cannabinoids in the mouse in vivo triad model. J Pharmacol Exp Ther jpet. 2017;117:240192. doi: 10.1124/jpet.117.240192. http://dx.doi.org/10.1124/jpet.117.240192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre-and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. http://dx.doi.org/10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Harris RT, Balster RL. Stimulus Properties of Drugs. Springer; New York, Boston, MA: 1971. An Analysis of the Function of Drugs in the Stimulus Control of Operant Behavior; pp. 111–132. http://dx.doi.org/10.1007/978-1-4757-0788-5_7. [Google Scholar]
- Henriksson BG, Järbe T. D9-Tetrahydrocannabinol used as discriminative stimulus for rats in position learning in a T-shaped water maze. Psychon Sci. 1972;27:25–26. http://dx.doi.org/10.3758/BF03328876. [Google Scholar]
- Hruba L, Seillier A, Zaki A, Cravatt BF, Lichtman AH, Giuffrida A, McMahon LR. Simultaneous inhibition of Fatty Acid amide hydrolase and monoacylglycerol lipase shares discriminative stimulus effects with delta9-tetrahydrocannabinol in mice. J Pharmacol Exp Ther. 2015;353:261–268. doi: 10.1124/jpet.115.222836. http://dx.doi.org/10.1124/jpet.115.222836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KL, Tsuboi K, Chang JW, Whitby LR, Speers AE, Pugh H, Cravatt BF. Discovery and optimization of Piperidyl-1, 2, 3-Triazole ureas as potent, selective, and in vivo-active inhibitors of α/β-Hydrolase domain containing 6 (ABHD6) J Med Chem. 2013;56:8270–8279. doi: 10.1021/jm400899c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska B, Wilkerson JL, Mustafa M, Abdullah R, Niphakis M, Wiley JL, Cravatt BF, Lichtman AH. Selective monoacylglycerol lipase inhibitors: antinociceptive versus cannabimimetic effects in mice. J Pharmacol Exp Ther. 2015;353:424–432. doi: 10.1124/jpet.114.222315. http://dx.doi.org/10.1124/jpet.114.222315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, O'Neal ST, Walentiny DM, Wiley JL, Cravatt BF, Lichtman AH. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol. 2014;171:1392–1407. doi: 10.1111/bph.12298. http://dx.doi.org/10.1111/bph.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe T, Lamb R, Lin S, Makriyannis A. (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacol Berl. 2001;156:369–380. doi: 10.1007/s002130100730. http://dx.doi.org/10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Henriksson BG. Discriminative response control produced with hashish, tetrahydrocannabinols (delta 8-THC and delta 9-THC), and other drugs. Psychopharmacologia. 1974;40:1–16. doi: 10.1007/BF00429443. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Henriksson BG. Open-field behavior and acquisition of discriminative response control in delta 9-THC. Experientia. 1973;29:1251–1253. doi: 10.1007/BF01935102. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Henriksson BG, Ohlin GC. Delta9-THC as a discriminative cue in pigeons: effects of delta8-THC, CBD, and CBN. Arch Int Pharmacodyn Thérapie. 1977;228:68–72. [PubMed] [Google Scholar]
- Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, Lichtman AH. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1—mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther. 2013;345 doi: 10.1124/jpet.112.201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the GABAA positive allosteric modulator diazepam and Δ -THC in humans discriminating Δ -THC. Drug Alcohol Depend. 2014;143:141–148. doi: 10.1016/j.drugalcdep.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009a;5:37–44. doi: 10.1038/nchembio.129. http://dx.doi.org/10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009b;106:20270–20275. doi: 10.1073/pnas.0909411106. http://dx.doi.org/10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition J Biol Chem. 1999;274:22903–22906. doi: 10.1074/jbc.274.33.22903. http://dx.doi.org/10.1074/JBC.274.33.22903. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating D9-tetrahydrocannabinol. Psychopharmacol Berl. 2009;203:219–228. doi: 10.1007/s00213-008-1230-8. http://dx.doi.org/10.1007/s00213-008-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Niphakis MJ, Cognetta AB, Chang JW, Buczynski MW, Parsons LH, Byrne F, Burston JJ, Chapman V, Cravatt BF. Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci. 2013;4:1322–1332. doi: 10.1021/cn400116z. http://dx.doi.org/10.1021/cn400116z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niphakis MJ, Johnson DS, Ballard TE, Stiff C, Cravatt BF. O-hydroxyacetamide carbamates as a highly potent and selective class of endocannabi-noid hydrolase inhibitors. ACS Chem Neurosci. 2012;3:418–426. doi: 10.1021/cn200089j. http://dx.doi.org/10.1021/cn200089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endo-cannabinoid hydrolysis generates brain prostaglandins that promote neuro-inflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton DA. State-dependent learning produced by depressant and atropine-like drugs. Psychopharmacologia. 1966;10:6–31. doi: 10.1007/BF00401896. http://dx.doi.org/10.1007/BF00401896. [DOI] [PubMed] [Google Scholar]
- Owens RA, Ignatowska-jankowska B, Mustafa M, Beardsley PM, Wiley JL, Jali A, Selley DE, Niphakis MJ, Cravatt BF, Lichtman AH. Discriminative Stimulus Properties of the Endocannabinoid Catabolic Enzyme Inhibitor SA-57 in Mice. 2016:306–314. doi: 10.1124/jpet.115.229492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Cassidy MP, Martin BR, Sim-Selley LJ. Long-term administration of Delta9-tetrahydrocannabinol desensitizes CB1-, adenosine A1-, and GABAB-mediated inhibition of adenylyl cyclase in mouse cerebellum. Mol Pharmacol. 2004;66:1275–1284. doi: 10.1124/mol.104.000604. http://dx.doi.org/10.1124/mol.104.000604. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Herling S. Discriminative stimulus effects of diazepam in rats: evidence for a maximal effect. J Pharmacol Exp Ther. 1983;227:160–166. [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1:1194–1206. doi: 10.1038/nprot.2006.167. http://dx.doi.org/10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabi-noid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. http://dx.doi.org/10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. The fatty acid amide hydrolase inhibitor URB 597: interactions with anandamide in rhesus monkeys. Br J Pharmacol. 2011;164:655–666. doi: 10.1111/j.1476-5381.2011.01388.x. http://dx.doi.org/10.1111/j.1476-5381.2011.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostagl Leukot Essent Fat Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. http://dx.doi.org/10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tanda G. Preclinical studies on the reinforcing effects of cannabinoids. A tribute to the scientific research of Dr. Steve Goldberg. Psychopharmacol Berl. 2016 doi: 10.1007/s00213-016-4244-7. http://dx.doi.org/10.1007/s00213-016-4244-7. [DOI] [PMC free article] [PubMed]
- Vann RE, Walentiny DM, Burston JJ, Tobey KM, Gamage TF, Wiley JL. Enhancement of the behavioral effects of endogenous and exogenous cannabinoid agonists by phenylmethyl sulfonyl fluoride. Neuropharmacology. 2012;62:1019–1027. doi: 10.1016/j.neuropharm.2011.10.011. http://dx.doi.org/10.1016/j.neuropharm.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentiny DM, Gamage TF, Warner Ja, Nguyen TK, Grainger DB, Wiley JL, Vann RE. The endogenous cannabinoid anandamide shares discriminative stimulus effects with Δ9-tetrahydrocannabinol in fatty acid amide hydrolase knockout mice. Eur J Pharmacol. 2011;656:63–67. doi: 10.1016/j.ejphar.2011.01.056. http://dx.doi.org/10.1016/j.ejphar.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. Cannabis: discrimination of “internal bliss”? Pharmacol Biochem Behav. 1999;64:257–260. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Wiley J, Balster R, Martin B. Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol. 1995;276:49–54. doi: 10.1016/0014-2999(95)00010-i. http://dx.doi.org/10.1016/0014-2999(95)00010-I. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR. Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology. 1995a;34:669–676. doi: 10.1016/0028-3908(95)00027-4. http://dx.doi.org/10.1016/0028-3908(95)00027-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995b;275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Matthew Walentiny D, Vann RE, Baskfield CY. Dissimilar cannabinoid substitution patterns in mice trained to discriminate D9-tetrahydrocannabinol or methanandamide from vehicle. Behav Pharmacol. 2011;22:480–488. doi: 10.1097/FBP.0b013e328348eced. http://dx.doi.org/10.1097/FBP.0b013e328348eced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Owens RA, Lichtman AH. Discriminative stimulus properties of phytocannabinoids, endocannabinoids, and synthetic cannabinoids. Curr Top Behav Neurosci. 2016 doi: 10.1007/7854_2016_24. http://dx.doi.org/10.1007/7854_2016_24. [DOI] [PubMed]
- Wiley JL, Walentiny DM, Wright MJ, Beardsley PM, Burston JJ, Poklis JL, Lichtman AH, Vann RE. Endocannabinoid contribution to Δ9-tetrahy-drocannabinol discrimination in rodents. Eur J Pharmacol. 2014;737:97–105. doi: 10.1016/j.ejphar.2014.05.013. http://dx.doi.org/10.1016/j.ejphar.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JL, Ghosh S, Mustafa M, Abdullah RA, Niphakis MJ, Cabrera R, Maldonado RL, Cravatt BF, Lichtman AH. The endocannabinoid hydrolysis inhibitor SA-57: intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice. Neuropharmacology. 2017;114:156–167. doi: 10.1016/j.neuropharm.2016.11.015. http://dx.doi.org/10.1016/j.neuropharm.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


