Abstract
Objective
Controversy exists regarding the optimal energy prescription to promote successful long-term behavioral management of obesity. Prescribing intake of 1,000 (vs. 1,500) kcal/day may produce larger initial weight reduction, but long-term advantage remains unclear. The effects of prescribing 1,000 versus 1,500 kcal/day on 6- and 12-month weight changes within behavioral treatment of obesity were examined.
Design and Methods
Participants were 125 obese women (mean ± SD; BMI ¼ 37.84 ± 3.94 kg/m2) randomly assigned goals of 1,000 or 1,500 kcal/day.
Results
From months 0 to 6, participants prescribed 1,000 kcal/day lost more weight than those prescribed 1,500 kcal/day (mean ± SE = −10.03 ± 0.92g vs. −6.23 ± 0.94 kg, P = 0.045); however, from months 7 through 12, only the 1,000 kcal/day condition experienced a significant weight regain (1.51 ± 0.77 kg, P ¼ 0.025). Baseline caloric consumption moderated the effect of treatment on regain; participants with baseline intakes ≥2,000 kcal/day who were assigned 1,000 kcal/day were significantly more susceptible to weight regain than those assigned 1,500 kcal/day (P = 0.049). At month 12, a significantly greater percentage of 1,000 kcal/day participants achieved weight reductions of 5% or more than those prescribed 1,500 kcal/day.
Conclusion
Encouraging obese individuals in behavioral treatment to adhere to a 1,000 kcal/day intake may increase their likelihood of achieving clinically meaningful weight losses.
Introduction
Lifestyle interventions for obesity that employ behavioral principles to accomplish changes in diet and physical activity commonly pro- duce body weight reductions of 7–10% that are accompanied by decreases in risk factors for heart disease and diabetes (1–3). Nonetheless, behavioral changes initiated during lifestyle treatment often are poorly maintained and regaining of lost weight is common (1,4,5), thereby diminishing health benefits of weight loss (6). As a variety of biological and environmental influences make it difficult to maintain large dietary changes, a number of researchers and professional organizations (7–9) have proposed a “small change” approach to weight management, arguing that small sustainable changes will produce better long-term weight control than larger changes that are unlikely to be sustained. Alternatively, other researchers (10–14) have observed that larger initial dietary changes, and the greater, more rapid weight losses they produce, are more likely to reinforce the weight-change process and lead to better long-term weight-loss outcomes. Thus, controversy exists regarding the optimal energy intake prescription needed to promote long-term success in behavioral management of obesity.
To address this issue, the current randomized trial examined the effects of prescribing 1,000 versus 1,500 kcal/day on 6- and 12- month weight losses in obese adults participating in lifestyle treatment for obesity. The primary aim was to determine whether assigning participants daily intake goals of 1,000 versus 1,500 kcal would result in differential weight reductions after 6 and 12 months of treatment. We hypothesized that participants prescribed 1,000 kcal/day would demonstrate greater short- and long-term weight losses.
As body weight reductions of 5% or more decrease risk for diabetes and heart disease (2,3,15), our second aim was to assess whether assigning participants to 1,000 versus 1,500 kcal/day would result in differential percentages of participants achieving body weight reductions of 5% or more after 6 and 12 months of treatment. We hypothesized that, compared with the 1,500 kcal/day condition, the 1,000 kcal/day intervention would produce a significantly greater percentage of participants achieving clinically significant reductions in body weight (5% or more) at 6 and 12 months.
A final aim was to explore whether pretreatment caloric intake moderated the effects of prescribing 1,000 versus 1,500 kcal/day goals. We were particularly interested in determining whether participants prescribed 1,000 kcal/day who were asked to make “very large” changes in caloric intake (i.e., reduce daily consumption by more than 50%) would experience difficulty sustaining such changes long-term and thereby experience significant weight regain during months 7 through 12.
Methods
Participants
Participants were 125 obese women between the ages of 25 and 75 years who weighed between 91 and 136 kg and had BMIs between 30 and 45 kg/m2. Recruitment occurred via direct mailings and announcements in local newspapers. Interested women underwent a brief telephone screening and were invited to an in-person assessment visit to complete informed consent, height and weight measurements, demographic information, and medical history. Potential participants were required to obtain written consent from their primary care providers stating that there were no medical contraindications to their participation in weight-loss intervention.
Potential participants were excluded for the following reasons: the presence of a major psychiatric disorder, excessive alcohol intake, unable to read English at a sixth grade level, or unavailable or unwilling to attend weekly group meetings, self-monitor daily intake, adhere to the prescribed caloric goal, or provide informed consent. Potential participants were also excluded if they lost 4.5 kg or more during the preceding 6 months, were participating in another randomized trial, or previously participated in a behavioral weight-loss program. Approval for this study was obtained from the University of Florida Institutional Review Board.
Procedure
Eligible participants were randomly assigned to intake goals of 1,000 or 1,500 kcal/day, respectively. Both dietary prescriptions were implemented within a standard behavioral lifestyle intervention for weight management that included two phases: Months 0-6 involved an initial treatment period of 24 weekly group sessions; Months 7-12 entailed an extended-care phase with six monthly group sessions. Intervention groups were led by master’s level graduate students with experience in conducting behavioral weight-management groups. The interventionists (assigned to treatment conditions in a counter-balanced fashion) were supervised by a licensed psychologist with extensive experience in obesity management. During months 0-6, participants were instructed to follow their prescribed condition-specific energy intake goal and adhere to a balanced diet according to recommendations from the U.S. Department of Agriculture and the National Institutes of Health’s Dietary Approaches to Stop Hypertension (16). Participants in both conditions were provided pedometers to monitor daily step counts. Based on the American College of Sports Medicine recommendations (17), participants were encouraged to increase walking to 10,000 steps per day (or by 3,000 steps above baseline levels). To assist in accomplishing these behavioral goals, participants were instructed to maintain detailed daily written records of dietary intake and physical activity.
Treatment included training in cognitive and behavioral skills for weight management including stimulus control, self-reinforcement, cognitive restructuring, and problem solving. Each group session involved a private weigh-in, review of participants’ progress toward goals, feedback, and encouragement from group leaders and other group members, and a brief presentation related to nutrition, physical activity, stress management, or behavioral management of eating and physical activity.
During months 7-12, participants were asked to attend monthly in- person group sessions and maintain caloric intake goals and exercise behaviors prescribed during the initial treatment phase. If a participant obtained a BMI of <25 kg/m2, she was instructed to gradually increase caloric intake to achieve weight maintenance. Participants were also instructed to continue monitoring dietary intake and physical activity through written logs.
Measures
Height
During the baseline assessment visit, participants’ heights, without shoes, were measured with a Seca (model 213) portable stadiometer.
Body weight
Weights were measured to the nearest 0.1 kg using a Tanita (model BWB 800S) digital scale. Participants were weighed wearing light indoor clothing, without shoes, and with empty pockets. An independent staff member weighed participants at months 0, 6, and 12; interventionists weighed participants at group sessions.
Energy intake
Mean caloric intake was calculated by averaging daily values provided by participants on self-monitoring logs from months 0 to 6 of weekly intervention and also from months 7 to 12 of monthly extended-care. One week prior to treatment initiation, staff members instructed participants regarding appropriate methods of self-monitoring food and caloric intake. Participants received self-monitoring logs, measuring cups, a food scale, and a reference book containing caloric values of foods. Participants were asked to record their intake for 7 days without changing usual eating patterns. Data from this initial 7-day period constituted the baseline assessment of caloric consumption. Intake estimates derived from self- monitoring logs have modest positive correlations with energy values derived from doubly labeled water assessments and tend to underestimate energy consumption (18–20).
Attendance
A treatment session was recorded as completed if the participant attended the group session and was weighed by a staff member, or if the participant attended an individual make-up session with the group leader within 1 week of the original group session.
Self-monitoring adherence
Participants were asked to complete daily food and physical activity records during the baseline assessment week and throughout months 0-6. During months 7-12, they were asked to complete food and physical activity records 3 days per week.
Statistical analyses
Primary aim
The sample size was selected to provide a statistical power of 0.80 to detect a 3.0% difference in weight reduction between groups (two-tailed test with Bonferroni adjustments; 4,21,22). Intent-to-treat analyses were conducted using multiple imputations to account for missing data. A missing-not-at-random procedure was used to complete data for participants who did not attend assessments at month 6 or month 12. Based on the documented pattern of weight regain after lifestyle treatment (4,21–23), these participants were assumed to have regained on average 0.3 kg/month from their last recorded weight. The variance for imputing the missing values was taken to be the appropriate conditional variance estimated under an assumption of missing at random. Differences in weight reduction by treatment condition at months 6 and 12 were analyzed using a multivariate normal model with appropriate variance adjustment for the multiple imputations (24). Statistical analyses were conducted using SAS version 9.2 (25).
Secondary aim
A chi-square analysis was conducted to assess whether a difference existed in the percent of participants who achieved a 5% or greater weight loss between conditions at months 6 and 12.
Additional analyses
Independent sample t-tests were utilized to assess between-group differences in caloric intake, attendance, and adherence. We investigated whether baseline caloric intake moderated treatment effects by adding baseline caloric intake as an inter- action with condition and time into the main model.
Results
Participant flow
Among the 182 interested women assessed on site, 44 did not meet eligibility requirements and were excluded. Of the 138 women eligible for randomization, 13 decided against participation prior to initiation of lifestyle intervention. Therefore, the study sample consisted of 125 obese women (mean 6SD; age 51.98 610.85 years; weight 104.83 ± 10.58 kg; BMI = 37.84 ± 3.94 kg/m2). Of these, 65 were randomly assigned the 1,000 kcal/day goal and 60 were assigned the 1,500 kcal/day goal. Overall, 90% of those who initiated treatment (n = 112) completed the month 6 assessment visit and 89% (n =111) finished the month 12 assessment visit (for a Consolidated Standards of Reporting Trials [CONSORT] diagram documenting participant flow, see Figure 1). No statistically significant baseline differences existed between 1,000 and 1,500 kcal/day conditions in terms of age, weight, BMI, race/ethnicity, education, or household income (Table 1).
FIGURE 1.
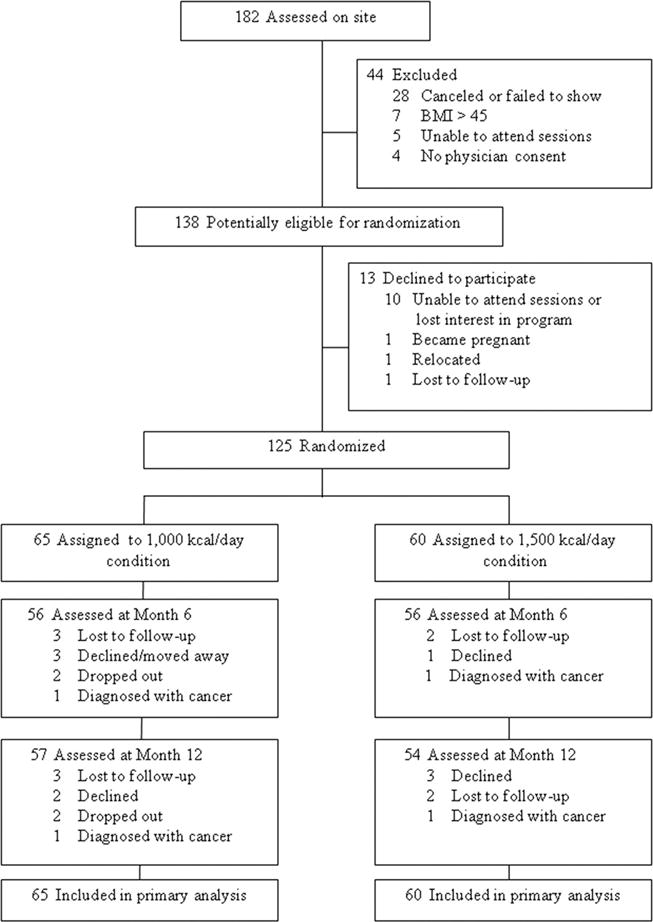
Participant flow over the course of the trial.
TABLE 1.
Baseline demographics according to treatment condition
| 1,000 kcal (n = 65)
|
1,500 kcal (n = 60)
|
Total sample (N = 125)
|
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age (years) | 51.49 | 11.73 | 52.53 | 9.79 | 51.98 | 10.85 |
| Weight (kg) | 104.99 | 10.63 | 104.70 | 10.72 | 104.83 | 10.58 |
| BMI (kg/m2) | 38.09 | 4.02 | 37.59 | 3.84 | 37.84 | 3.94 |
| n | % | n | % | n | % | |
|
|
||||||
| Race/ethnicity | ||||||
| Caucasian | 48 | 73.85 | 44 | 73.33 | 92 | 73.60 |
| African American | 9 | 13.85 | 12 | 20.00 | 21 | 16.80 |
| Hispanic American | 4 | 6.15 | 0 | 0.00 | 4 | 3.20 |
| Other | 3 | 4.62 | 1 | 1.67 | 4 | 3.20 |
| Declined to respond Education | 1 | 1.54 | 3 | 5.00 | 4 | 3.20 |
| 12 Years or less | 3 | 4.62 | 3 | 5.00 | 6 | 4.80 |
| 13–15 Years | 33 | 50.77 | 28 | 46.67 | 61 | 48.80 |
| 16 Years or more | 29 | 44.62 | 29 | 48.33 | 58 | 46.40 |
| Household yearly income <$35,000 | 12 | 18.46 | 15 | 25.00 | 27 | 21.60 |
| $35,000–$49.999 | 10 | 15.38 | 12 | 20.00 | 22 | 17.60 |
| $50,000–$74,999 | 21 | 32.31 | 12 | 20.00 | 33 | 26.40 |
| ≥$75,000 | 18 | 27.69 | 18 | 30.00 | 36 | 28.80 |
| Not reported | 4 | 6.15 | 3 | 5.00 | 7 | 5.60 |
Weight change outcomes
Body weights at months 0, 6, and 12, according to the treatment condition, are shown in Figure 2. An examination of the effect of treatment condition on weight changes over the study period showed a significant time-by-condition interaction effect (X2(2)= 8.91, P = 0.012, Cramer’s V= 0.27, 95% confidence interval [CI] for V = 0.14, 0.45, Table 2). At month 6, conditions differed significantly in weight change with participants prescribed 1,000 kcal/day, demonstrating significantly greater weight losses than those prescribed 1,500 kcal/day (mean ± SE difference = −3.52 ± 2.07 kg, z =1.70, P = 0.045). However, from months 7 to 12, only participants in the 1,000 kcal/day condition experienced a significant weight regain, and at month 12, whereas those prescribed 1,000 kcal/day showed a greater net weight change than the 1,500 kcal/day condition, the between-group difference was not statistically significant (mean ± SEdifference= −2.40 ± 2.42kg, P =0.322;Table 2).
FIGURE 2.
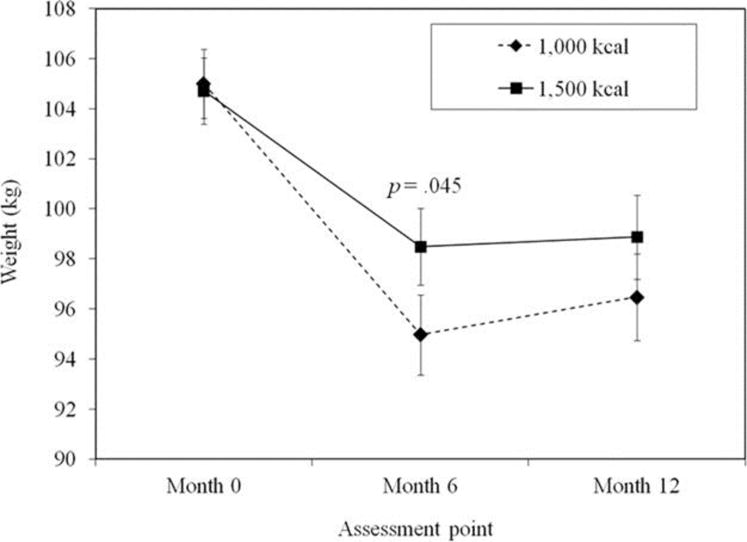
Body weights (mean ± SE) for the 1,000 and 1,500 kcal/day conditions over the course of the trial.
TABLE 2.
Weight changes in kilograms according to the treatment condition
| 1,000 kcal
|
1,500 kcal
|
|||
|---|---|---|---|---|
| M | SE | M | SE | |
| Months 0–6 | −10.03* | 0.92 | −6.23* | 0.94 |
| Months 7–12 | 1.51** | 0.77 | 0.38 | 0.72 |
| Months 0–12 | −8.52* | 1.17 | −5.84* | 1.11 |
P < 0.001
P = 0.025 for within-condition weight change across the specified time periods.
Achievement of clinically significant weight reductions
At month 6, 71% of the participants prescribed 1,000 kcal/day achieved weight losses of 5% or greater, compared to 58% pre- scribed 1,500 kcal/day (P = 0.146). However, at month 12, significantly more participants in the 1,000 kcal/day intervention achieved weight reductions of 5% or more compared to the 1,500 kcal/day condition (62 vs. 43%, respectively; X2(1) = 4.15, P = 0.042, Cramer’s V = 0.18, 95% CI of V = 0.09, 0.37).
Caloric prescription, self-monitoring, and attendance
The 1,000 and 1,500 kcal/day conditions did not differ significantly in baseline self-reported energy intake (mean ± SD kcal/day = 1,915 ± 523 vs. 1,930 ± 475, respectively, P=0.869). During months 0-6, participants prescribed 1,000 kcal/day reportedly consumed significantly fewer calories (1,164 ± 170 kcal/day) compared with 1,500 kcal/day participants (1,518 ± 222 kcal/day; t (122)=10.01, P < 0.001, Cohen’s d =1.81, 95% CI of d= 2.21, −1.37). During months 0-6, the 1,000 and 1,500 kcal/day conditions did not differ in rates of attendance (sessions attended 19.1 ± 4.5 vs. 17.1 ± 6.3, respectively, P= 0.377) or number of weeks with completed self-monitoring logs (14.6 ± 7.2 vs. 14.7 ± 8.3, respectively, P = 0.973).
During months 7-12, the 1,000 kcal/day condition reported an intake of 1,247 ± 246 kcal/day compared to 1,488 ± 208 kcal/day for participants prescribed 1,500 kcal/day (t(56)= −4.00, P < 0.001, Cohen’s d = −1.07, 95% CI of d = −1.60, −0.50). One participant prescribed 1,000 kcal/day achieved a BMI of <25 kg/m2 at month 6 and was instructed to increase caloric intake until an energy balance was achieved for weight maintenance. During months 7-12, the 1,000 and 1,500 kcal/day conditions did not differ in number of monthly sessions attended (3.7 ± 1.8 vs. 3.3 ± 2.3, respectively, P = 0.670) or number of weeks with completed self-monitoring logs (4.8 ± 7.2 vs. 6.8 ± 9.7, respectively, P=0.182). In addition, conditions did not significantly differ in level of physical activity (i.e., average step counts) achieved during months 0-6 (P=0.798) and months 7-12 (P = 0.163).
Baseline caloric intake and weight loss
An examination of whether baseline caloric intake moderated the effect of treatment condition on weight outcome showed a significant interaction effect during months 7-12 (X2(2) = 14.73, P < 0.001, Cramer’s V = 0.34, 95% CI for V = 0.19, 0.53). To decompose this interaction, baseline caloric intake was dichotomized; participants who consumed 2,000 or more calories at baseline were considered to have “high” baseline caloric intake, whereas those who consumed fewer than 2,000 calories were considered to have “low” baseline intake. Participants categorized with “high” and “low” intakes did not differ on any key demographic variables (all Ps > 0.05), and the percentages of participants with “high” baseline intakes were similar in each condition (36.9 and 43.3% for the 1,000 and 1,500 kcal/day conditions, respectively). Figure 3 shows weight changes from months 7-12 by treatment condition for participants with “high” versus “low” baseline caloric intake. Post hoc testing indicated baseline caloric intake significantly impacted weight regain only for participants assigned the 1,000 kcal/day condition, such that significant regaining of weight during months 7-12 was observed only among those participants with “high” baseline intakes who were prescribed 1,000 kcal/day (P = 0.049; Figure 3).
FIGURE 3.
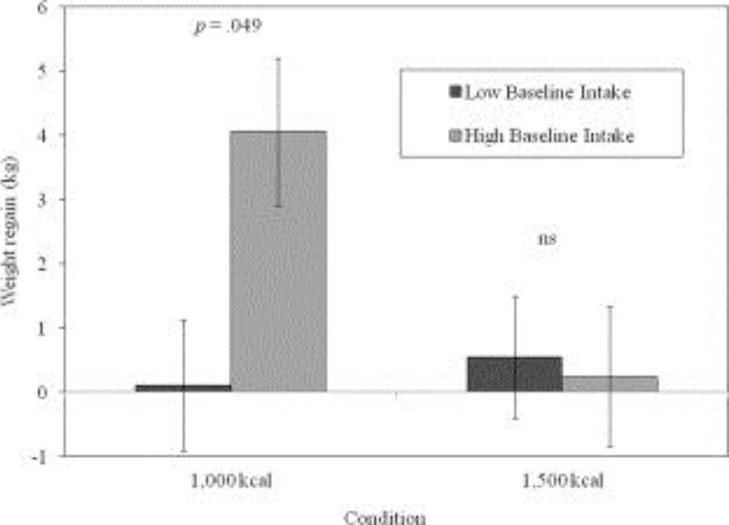
Weight regain (mean ± SE) from months 7 to 12 according to the treatment condition and baseline caloric intake level.
Discussion
This randomized trial demonstrated four key findings. First, obese participants prescribed 1,000 kcal/day achieved significantly greater weight losses after 6 months of lifestyle treatment than those prescribed 1,500 kcal/day. Second, during extended-care treatment (months 7-12), the 1,000 kcal/day condition experienced significant weight regain, whereas the 1,500 kcal/day condition demonstrated no significant weight change. Third, baseline caloric consumption moderated the effect of treatment condition on weight regain. The subset of participants who reported consuming >2,000 kcal/day at baseline and were assigned 1,000 kcal/day were significantly more susceptible to weight regain during the extended-care period as compared with their counterparts prescribed 1,500 kcal/day. Fourth, at trial conclusion, a significantly greater percentage of participants prescribed 1,000 kcal/day evidenced clinically significant body weight reductions (5% or greater) than those assigned 1,500 kcal/day.
The significantly greater initial weight losses achieved by participants prescribed 1,000 versus 1,500 kcal/day (means 10.03 vs. 6.23 kg, respectively) may be attributed to the significantly lower energy intake levels maintained by participants in the 1,000 kcal/day intervention during the first 6 months of treatment (means 1,164 vs. 1,518 kcal/day, respectively). Similar results have been found with the usage of very low calorie diets (26), in which meal replacements providing 400-800 kcal/day are often utilized to promote larger amounts of initial weight loss than with low calorie diets. For most obese individuals, achievement of large weight reductions represents the major motivation for their involvement in weight-loss programs (27). Thus, consistent with operant models of obesity management (28, 29), larger initial weight losses may reinforce changes in weight management behaviors and thereby contribute further to superior weight-loss outcomes.
Nonetheless, weight loss usually slows following 6 months of behavioral treatment, and weight regain ensues (1, 4, 5). Indeed, for many participants prescribed 1,000 kcal/day, the degree of dietary restraint required to sustain this level of dietary intake may have been too difficult to maintain, thereby resulting in consumption exceeding the prescribed caloric goal and subsequent weight regain (30). In contrast, participants prescribed 1,500 kcal/day did not regain significant weight during extended-care. Daily calorie goals of 1,500 may have been easier to achieve, resulting in less susceptibility to lapses in dietary restraint. Wadden et al. (26) documented similar weight change patterns in which participants prescribed severe caloric restriction regained significantly more weight than those prescribed a moderate, balanced-deficit diet of 1,200 kcal/day. In addition, participants consuming the moderate, balanced-deficit diet generally maintained lost weight, but did not demonstrate continued weight loss during extended-care. Our 1,500 kcal/day intervention also did not produce the continued weight loss observed in prior trials, examining the “small change” approach (8,9). After generating a weight loss of 6.23 kg, a goal of 1,500 kcal/day may not entail a large enough caloric deficit to produce additional weight loss. Moreover, a slow rate of weight loss may have caused participants prescribed 1,500 kcal/day to view the “costs” of weight management behaviors as exceeding the “benefits” associated with modest weight changes (5).
Although net weight changes were not statistically different between conditions at month 12 (−8.52 vs. −5.84 kg for the 1,000 vs. 1,500 kcal/day conditions, respectively), a significantly greater percentage of participants prescribed 1,000 versus 1,500 kcal/day successfully achieved weight losses of 5% or greater at 12 months (62 vs. 43%, respectively). Weight reductions of 5% or more have been associated with decreased risk of developing diabetes (2) and with reductions in cardiovascular risk factors such as hypertension and hyperlipidemia (3, 15). Conversely, when weight regain occurs and the net reduction in body weight is <5%, participants often lose beneficial health effects associated with weight loss (6). The finding that a greater percentage of 1,000 kcal/day participants accomplished long-term body weight reductions of 5% or more highlights the potential clinical benefit of prescribing a lower rather than higher daily calorie goal in weight management programs.
Although the majority of participants prescribed 1,000 kcal/day achieved clinically meaningful weight losses at 12 months, results showed that a subset of participants may not benefit from this level of caloric prescription. Indeed, baseline caloric intake moderated the effect of treatment condition on long-term weight change. Participants with “high” baselinecaloricintake(≥ 2,000kcal/day)regainedmore weight during months 7-12 if assigned 1,000 kcal/day than those with “low”baseline caloric intake (≤ 2,000 kcal/day). For individuals who consumed “high” levelsofbaselinecalories,theprescribedintakeof 1,000 kcal/day required a reduction in energy consumption of 50% or more—a level that may be unsustainable long term. This finding holds important treatment-matching implications. At the start of life- styleinterventions,participantsreporting “high” baselinecalorielevels may benefit from energy prescriptions based on either a percent- age of their baseline intake (e.g., 25-50% reduction) or a projected amount of weight change per week (e.g., 0.50-0.75 kg) rather than a fixed energy intake, such as 1,000 kcal/day. Furthermore, after initial weight loss, less restrictive calorie goals might be set for extended- care treatment. Gradually moving participants from 1,000 to 1,250 to 1,500 kcal/day goals or allowing a range of acceptable intake goals may increase self-efficacy and reduce all-or-nothing attitudes that often lead to weight regain (31).
This study has several notable strengths. The trial employed a randomized design and prospectively prescribed caloric intake levels to promote weight loss at different rates in accordance with the recommendations of advocates of the small- (8,9) and large-change approaches (10,11,13,14). All participants received a structured life- style intervention that incorporated state-of-the-art behavioral strategies to enhance treatment adherence, and the study employed a conservative intent-to-treat analyses, which assumed that missing data were not at random (i.e., that participants who dropped out had poorer outcomes than those who remained in the study).
Moreover, this study demonstrated strong treatment fidelity. In two prior trials that assessed impact of caloric prescriptions (32,33), poor adherence to prescribed energy intake goals precluded valid testing of intervention effects. In contrast, participants of this study reported significantly different energy intakes that were consistent with their randomized prescriptions. Furthermore, although conditions differed in energy consumption, no significant between-group differences were observed in baseline characteristics or in rates of attendance or adherence to self-monitoring records. Collectively, these findings suggest that the observed between-group differences in weight reduction were attributable to the experimental prescriptions of 1,000 versus 1,500 kcal/day.
This study also has several limitations. First, the assessment of energy intake was based on the data collected via self-monitoring logs completed by participants. Self-monitoring data are susceptible to social desirability (18) and commonly underestimate actual caloric intake as compared with objective measurement via doubly labeled water (19,20). In addition, self-monitoring adherence decreased throughout the trial. Second, participants were generally well-educated, middle- class, white women. It is unclear whether findings are generalizable to men or to women with different demographic characteristics. Finally, the trial was conducted over the course of 12 months. The effects of the caloric prescriptions beyond 1 year are unknown.
Conclusion
In conclusion, findings from this randomized trial suggest that pre- scribing 1,000 versus 1,500 kcal/day in the context of behavioral treatment produces greater initial weight losses, but a 1,000 kcal/day prescription may be more difficult to sustain, particularly in individuals for whom energy reductions entail a decrease of 50% or more from their pretreatment eating patterns. Nonetheless, results of this trial indicate that prescribing an energy intake of 1,000 kcal/day to obese individuals in behavioral treatment may increase the likelihood of their achieving clinically significant levels of weight loss.
Acknowledgments
Funding agencies: SA is supported by a K23 AT004251-01A2, an Early Career Investigator Award from the American Heart Association (09CRP2390173) and Thomas H. Maren Foundation.
Footnotes
Author contributions: LN, MP, SA, and PD designed the study. LN and PD served as group interventionists and collected data. MP and SA supervised interventionists. KM and MD conducted statistical analyses and interpretation. LN, MP, KM, MD, and SA contributed substantially to the writing of the manuscript.
References
- 1.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34:841–859. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffery RW, Drewnowski A, Epstein LH, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 5.Perri MG, Foreyt JP, Anton SD. Prevention of weight regain after weight loss. In: Bray GA, Bouchard C, editors. Handbook of Obesity Treatment: Clinical Applications. Informa Healthcare; New York: 2008. pp. 249–268. [Google Scholar]
- 6.Krebs JD, Evans S, Cooney L, et al. Changes in risk factors for cardiovascular dis- ease with body fat loss in obese women. Diab Obes Metab. 2002;4:379–387. doi: 10.1046/j.1463-1326.2002.00231.x. [DOI] [PubMed] [Google Scholar]
- 7.Hill JO. Can small-changes approach help address the obesity epidemic? A report of the Joint Task Force of the American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. Am J Clin Nutr. 2009;89:477–484. doi: 10.3945/ajcn.2008.26566. [DOI] [PubMed] [Google Scholar]
- 8.Lutes LD, Winett RA, Barger SD, et al. Small changes in nutrition and physical activity promote weight loss and maintenance: three-month evidence from the ASPIRE randomized trial. Ann Behav Med. 2008;35:351–357. doi: 10.1007/s12160-008-9033-z. [DOI] [PubMed] [Google Scholar]
- 9.Sbrocco T, Nedegaard RC, Stone JM, Lewis EL. Behavioral choice treatment pro- motes continuing weight loss: preliminary results of a cognitive-behavioral decision-based treatment for obesity. J Consult Clin Psychol. 1999;67:260–266. doi: 10.1037//0022-006x.67.2.260. [DOI] [PubMed] [Google Scholar]
- 10.Astrup A, Rössner S. Lessons from obesity management programmes: greater initial weight loss improves long-term maintenance. Obes Rev. 2000;1:17–19. doi: 10.1046/j.1467-789x.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 11.Carels RA, Cacciapaglia HM, Douglass OM, Rydin S, O’Brien WH. The early identification of poor treatment outcome in a women’s weight loss program. Eat Behav. 2003;4:265–282. doi: 10.1016/S1471-0153(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 12.Elfhag K, R€ossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 13.Jeffery RW, Wing RR, Mayer RR. Are smaller weight losses or more achievable weight loss goals better in the long term for obese patients? J Consult Clin Psychol. 1998;66:641–645. doi: 10.1037//0022-006x.66.4.641. [DOI] [PubMed] [Google Scholar]
- 14.Nackers LM, Ross KM, Perri MG. The impact of rate of initial weight loss on long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas PR. Weighing the Options: Criteria for Evaluating Weight Management Programs. National Academy Press; Washington, DC: 1995. [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services and US Department of Agriculture. Dietary Guidelines for Americans, 2005. U.S. Government Printing Office; Washington, DC: 2005. [Google Scholar]
- 17.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 18.Hebert JR, Hurley TG, Peterson KE, et al. Social desirability trait influences on self-reported dietary measures among diverse participants in a multicenter multiple risk factor trial. J Nutr. 2008;138:226S–234S. doi: 10.1093/jn/138.1.226S. [DOI] [PubMed] [Google Scholar]
- 19.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133:895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 20.Livingstone MB, Prentice AM, Strain J, et al. Accuracy of weighed dietary records in studies of diet and health. Br Med J. 1990;300:708–712. doi: 10.1136/bmj.300.6726.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadden TA, Berkowitz RJ, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Arch Intern Med. 2001;161:218–227. doi: 10.1001/archinte.161.2.218. [DOI] [PubMed] [Google Scholar]
- 22.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 23.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural set- tings (TOURS) randomized trial. Arch Intern Med. 2008;168:2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; New York: 1987. [Google Scholar]
- 25.SAS Institute, Inc. SAS System, Version 9.2. SAS Institute, Inc; Cary, NC: 2008. [Google Scholar]
- 26.Wadden TA, Foster GD, Letizia KA. One-year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol. 1994;62:165–171. doi: 10.1037//0022-006x.62.1.165. [DOI] [PubMed] [Google Scholar]
- 27.Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol. 1997;65:79–85. doi: 10.1037//0022-006x.65.1.79. [DOI] [PubMed] [Google Scholar]
- 28.Ferster CB, Nurnberger JI, Levitt EB. The control of eating. J Math. 1962;1:87–109. [PubMed] [Google Scholar]
- 29.Stuart RB. Behavioral control of overeating. Behav Res Ther. 1967;5:357–365. [Google Scholar]
- 30.Herman CP, Polivy J. Restrained eating. In: Stunkard AJ, editor. Obesity. WB Saunders; Philadelphia, PA: 1980. pp. 208–225. [Google Scholar]
- 31.Ruderman AJ. Dietary restraint: a theoretical and empirical review. Psychol Bull. 1986;99:247–262. [PubMed] [Google Scholar]
- 32.Das SK, Saltzman E, Gilhooly CH, et al. Low or moderate dietary energy restriction for long-term weight loss: what works best? Obesity. 2009;17:2019–2024. doi: 10.1038/oby.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toplak H, Ziegler O, Keller U, et al. X-PERT: Weight reduction with orlistat in obese subjects receiving a mildly or moderately reduced-energy diet. Early response to treatment predicts weight maintenance. Diab Obes Metab. 2005;7:699–708. doi: 10.1111/j.1463-1326.2005.00483.x. [DOI] [PubMed] [Google Scholar]


