Abstract
The study of sex differences in the brain is a topic of neuroscientific study that has broad reaching implications for culture, society and biomedical science. Recent research in rodent models has led to dramatic shifts in our views of the mechanisms underlying the sexual differentiation of the brain. These include the surprising discoveries of a role for immune cells and inflammatory mediators in brain masculinization and a role for epigenetic suppression in brain feminization. How and to what degree these findings will translate to human brain development will be questions of central importance in future research in this field.
Historically, the study of sex differences in brain and behaviour was not considered to be broadly important to neuroscience but was instead walled off under the rubric ‘reproductive endocrinology’. This niche area focused on the neurophysiology of ovulation and lactation and the essential behaviours of mating and parenting. Nevertheless, there were persistent reports of males and females differing in non-reproductive parameters, such as performance on learning tasks1–4, and associated neurophysiological correlates like hippocampal long-term potentiation (LTP)2 and cortical synapse number5.
Parallel to animal studies was the advent of functional MRI, which allowed for assessment of human brain activity in real time and provided the opportunity to revisit the ageless question ‘do men and women think differently?’ Functional MRI studies revealing such differences have involved tasks assessing verbal recall, spatial learning, non-verbal reasoning, emotional face reading, fear and anxiety, and responses to sexually explicit stimuli, as well as the impact of stress on most of these tasks (for a review, see REF. 6). Similarly, the emergence of diffusion tensor imaging revealed sex differences in the connectome: girls and young women were found to have stronger interhemispheric connections, whereas boys and young men had greater intrahemispheric connections7. In a follow-up study in a subset of individuals, a male bias in performance in sensorimotor tasks was found to correlate with a higher degree of connectivity between motor and sensory cortex nodes, whereas a female bias in performance in tasks involving social cognition and non-verbal reasoning corresponded to greater connectivity across the hemispheres of subcortical regions8. However, the authors of this study themselves noted the apparent ‘playing to stereotype’ of these findings, as have others9, who have cautioned that a correlation in measures is not indicative of causation.
Arguments about the validity of using imaging to determine sex differences in humans continue to this day and are likely to persist for some time. This is particularly apparent when the confound of gender is considered8,10. Gender is a uniquely human construct that combines self and societal awareness of one’s sex, thereby including the influence of cultural norms, implicit bias and parental expectations (according to the World Health Organization definition of gender; see Further Information). This makes it difficult, if not impossible, to isolate a purely biological contribution to sex differences in human brain and behaviour. Indeed, some would argue that we should not even try as there is potential to do real harm by lending scientific credence to well-entrenched stereotypes10.
Despite these legitimate concerns, there are two reasons that an understanding of such a biological contribution continues to be sought. The first is that animal research unequivocally demonstrates that sex is a crucial variable that modulates fundamental neurobiological processes that range from rates of neurogenesis to synaptic physiology (TABLE 1). The second is that clinical research unequivocally demonstrates a gender bias in the relative frequency and severity of neuropsychiatric, neurological and neurodegenerative disorders (TABLE 2). This gender bias shifts markedly across the lifespan, with boys being much more likely to suffer adverse outcomes as a result of these disorders in early life than girls, whereas women disproportionately suffer in adulthood. This coherent pattern obliges us to explore how the biology of males and females contributes to disease risk based on gender, as well as the developmental events that lead to these biological differences.
Table 1.
Examples of neuroanatomical, neurochemical and physiological sex differences in the brains of rodents
| End point measured | Measurements displaying male bias | Measurements displaying female bias | Measurements displaying context-, age- or region-specific biases |
|---|---|---|---|
| Volume | Differences in medial amygdala volume are dependent on circulating testosterone in adult males124 | ||
| Fibre density | AVPV (kisspeptin fibres)128 | None known | |
| Synapses | Axodendritic synapses in the arcuate nucleus132 | ||
| Branching | Dendritic branching in the agranular insular cortex137 | ||
| Neurochemical phenotype | Differences in aromatase activity and progesterone receptor expression are region specific146–148 | ||
| Cell number | |||
| Activational state | Duration of developmental excitatory actions of GABA154 | Hormonal control of nigrostriatal dopamine system156 | |
| Cell genesis | Neurogenesis in CA1 and dentate gyrus157,158 | Addition of new cells to sexually dimorphic AVPV and medial amygdala at puberty depends on gonadal steroids161 |
The categorization of sex differences in the rodent brain for over 40 years makes a comprehensive list unwieldy. Here, we note some examples of the range of types of sex differences that are found throughout the brain. AVPV, anteroventral periventricular nucleus; BNST, bed nucleus of the stria terminalis; LC, locus coeruleus; mPOA, medial preoptic area; VMN, ventromedial nucleus of the hypothalamus.
Table 2.
Sex differences in neuropsychiatric, neurological and neurodegenerative conditions
| Condition | Sex differences in prevalence | Sex differences in onset | Sex differences in phenotype | Refs |
|---|---|---|---|---|
| Neuropsychiatric conditions with origins in development | ||||
| Autism spectrum disorder | Four to five times higher in males than in females | None | More social impairment in males; more affective symptoms in females | 87,88, 162,163 |
| Conduct disorder oppositional defiance disorder | Three times higher in males than in females | Earlier onset in males | More externalizing symptoms in males; more affective symptoms in females | 164,165 |
| ADHD | Two to three times higher in males than in females | None | More hyperactivity, externalizing and impulsivity in males; more internalizing, inattention and intellectual impairment in females | 166,167 |
| Schizophrenia | 1.42 times higher in males than in females | Earlier onset in males than in females (early onset is more likely in males; late onset is more likely in females) | More language disruption, positive symptoms and severe course of illness in males; more affective symptoms in females | 98,99, 168–170 |
| Neurological developmental conditions | ||||
| Dyslexia and/or reading impairment | Two to three times higher in males than in females | None | None known | 104,105 |
| Stuttering | 2.3 times higher in males than in females | Adolescent onset four times higher in males than females | None known | 103 |
| Tourette syndrome | Three to four times higher in males than in females | Earlier onset in males | Greater tic severity in adulthood in females | 106 |
| Adult onset neuropsychiatric conditions | ||||
| Major depression | None before puberty; two times higher in females than in males post-puberty | None | None known | 171–175 |
| Bipolar disorder | None for bipolar I; bipolar II higher in females than in males | Earlier onset in males | Sex by genotype interaction | 176–179 |
| Generalized anxiety | Two times higher in females than in males | None | Higher chronicity and comorbidity with major depression in females | 174, 180–182 |
| Panic disorder | 2.5 times higher in females than in males | None | None known | 180 |
| OCD | 1.5 times higher in females than in males | None | None known | 183 |
| PTSD | Two times higher in females than in males | None | More likely in females than in males following childhood trauma | 184,185 |
| Anorexia nervosa | Three times higher in females than in males | Unknown | None known | 186–189 |
| Bulimia | Three to four times higher in females than in males | Unknown | None known | 186–189 |
| Alcoholism or substance abuse | Higher in males than in females | Earlier in females than males | Females progress to addiction more quickly than males | 190 |
| Adult neurological conditions | ||||
| Migraine | None pre-puberty, but three times higher in females than males post-puberty | None | None known | 191,192 |
| Stroke | Higher in males than females before age 85, but higher in females than males after age 85 | Males 4 years before females | None known | 193,194 |
| Neurodegenerative disease | ||||
| MS (with exception of primary progressive MS) | Two times higher in females than males | Earlier onset in females | More severe in males | 195–197 |
| Alzheimer disease | 1.5–2 times higher in females than males, especially in those over 80 years | Earlier onset in females | More tangles and global pathology in females; pathology more highly correlated with clinical score in females | 198–201 |
| Parkinson disease | 1.5 times higher in males than females | Males 2 years before females | None known | 202 |
| ALS | Three times higher in males than females | Earlier onset in males | None known | 197,203 |
| Myasthenia gravis | Four times higher in females than males | Earlier onset in females | None known | 196 |
ADHD, attention deficit hyperactivity disorder; ALS, amyotrophic lateral sclerosis; Bipolar I, bipolar spectrum disorder characterized by at least one manic or mixed episode; Bipolar II, bipolar spectrum disorder characterized by at least one episode of major depression lasting two or more weeks and at least one hypomanic episode; MS, multiple sclerosis; OCD, obsessive compulsive disorder; PTSD, post-traumatic stress disorder.
The goal of this Review is to highlight recent findings that provide new investigative avenues for identifying the sources of gender-biased vulnerability and resilience. Sex differences in the brain are found at the macro and micro level: entire brain regions can vary in size according to sex, or a small subnucleus might exhibit a robust sex difference in the number and type of synapses found therein. The numbers of specific cell types can vary, as can the magnitude of projections between and within brain regions. Even the signal transduction pathways invoked following neuronal activation can be markedly different in males versus females (for a review, see REF. 11). Connecting these sex differences to specific behaviours and physiological responses is most easily achieved for those directly tied to reproduction, with the neural circuits controlling luteinizing hormone release from the anterior pituitary and sexual behaviour being the best characterized. Although these reproductive end points might seem far removed from neuropsychiatric, neurological and neurodegenerative disorders, we argue that discovery of the fundamental mechanisms establishing those sex differences are providing important insights into the sources of gender biases in vulnerability and resilience.
Mechanisms of sexual differentiation
Gonadal hormones and early life programming
The reproductive behaviour of adult males and females has long been known to be dependent on the gonadal steroid hormone milieu, which is unique to each sex. Males have higher circulating levels of testosterone derived from the testis, whereas females have a cyclic pattern of oestrogens and progestins arising from the ovary. In the ideal conditions of the laboratory (that is, in the presence of continuous food, water, light and shelter), males are always sexually active, but females will only readily engage in mating at or around the time of ovulation. What was not initially obvious is that the concordance of adult hormonal milieu and mating behaviour actually depends on early life programming, a concept that has been historically referred to as the organizational hypothesis12.
In rodents, this programming is initiated prenatally in males when the fetal testes produce androgens that gain access to the brain, are converted to oestrogens in large amounts and initiate brain masculinization. Both male and female fetuses express high levels of the steroid-binding globulin α-fetoprotein, which sequesters the maternal oestrogens that are present in fetal circulation and thus protects the brain from the influence of maternal steroids. As the female fetal ovary remains quiescent, this sequestration of maternal oestrogens means that only males experience high intracerebral oestrogen levels (as a result of the aromatization of testicular androgens). If α-fetoprotein is ablated, maternal oestradiol penetrates the fetal brain during the critical period, and females are subsequently masculinized13.
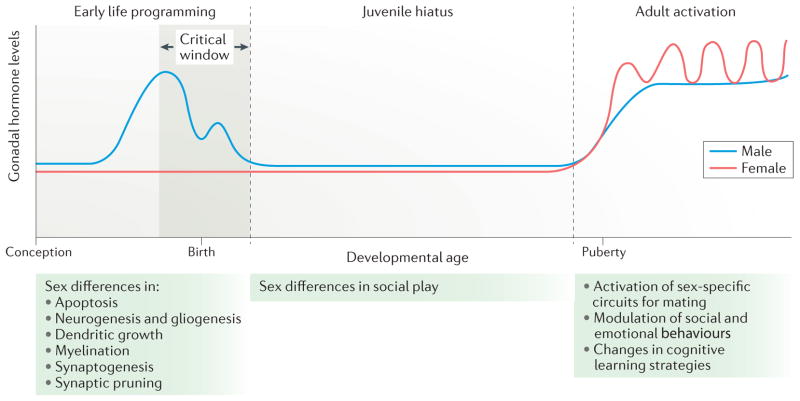
The processes of brain masculinization and feminization both occur during a critical period (FIG. 1). In rodents, the critical period begins prenatally at the time of the surge in male fetal androgen production. The end of the critical period is defined as the time at which the female phenotype can no longer be diverted to male by treatment with exogenous steroid hormones, which, in rats and mice, is one week to 10 days after birth. The existence of this critical window provides a tool for studying the process of masculinization. Injecting female rat or mouse pups with testosterone or its aromatized metabolite, oestradiol, for 1 or 2 days immediately after birth initiates many (although not necessarily all) of the same gene expression profiles and cell signalling cascades that occur normally in males in utero (for a review, see REF. 11). Although this is not a perfect model of masculinization, it provides an excellent means to interrogate both the short-term processes and long-term consequences of brain sexual differentiation by comparing males, females and masculinized females. There is an apparently separate sensitive period for rodent brain feminization that occurs weeks after birth; however, this remains less well characterized than the critical window for masculinization14.
Figure 1. Early life programming of adult sex differences.
The role of hormones in the establishment of sex differences in brain and behaviour can be broadly divided into three stages. In the rodent, early life programming occurs during a perinatal sensitive period (the critical window) that is marked by the onset of gonadal steroid production in males. This occurs prenatally and around birth, a period during which there is no corresponding steroidogenesis in females. During this sensitive period, numerous neuroanatomical end points are differentiated in males versus females in a region-specific manner. In some areas, there are sex differences in apoptosis, in others, there are differences in synaptogenesis or neurogenesis. The juvenile hiatus begins shortly after birth and extends until puberty. It is characterized by low to non-existent steroid levels; however, during this time, social play behaviour is more frequent among males than among females, a behavioural sex difference that is organized during early life programming. The adult period is characterized by sex-specific hormonal milieus with males having high and steady levels of testosterone (with some daily and annual variations), whereas females undergo cyclic changes in hormone levels that are associated with ovulation. The steroid milieu of each sex activates the neural circuits of mating that were organized during the perinatal period. In this way, the gonadal phenotype and neural phenotype controlling mating behaviour are synchronized. Non-reproductive behaviours such as stress and anxiety, spatial learning, locomotion and social affiliation are also modulated by steroids in adulthood, and this modulation may or may not depend on earlier programming effects during the sensitive period.
In addition to the adult consequences of the effects of gonadal hormones on brain development (known as ‘organizational’ effects), many sex differences in adult animals (and probably in humans too) are the result of the markedly different gonadal hormonal milieus that each experiences in adulthood. These are often referred to as ‘activational’ hormonal effects and may or may not depend on prior organizational hormonal effects.
Chromosome complement: XX versus XY matters too
Not all hormonal effects in adults are preordained developmentally, and not all sex differences are due to hormones. Besides its influence on gonadal differentiation and steroid hormone production, chromosome complement is an additional contributing variable to sex differences in brain and behaviour15,16 (FIG. 2). The potential for direct sex chromosome effects was evident in a comparison of embryonic neural stem cells derived from male and female mouse embryos. When compared before the onset of gonadal steroid production, more than 100 transcripts were differentially expressed between female (XX) and male (XY) stem cells. Intriguingly, stem cells of both sexes were responsive to testosterone treatment but not in the same way: thousands more transcripts varied in response to testosterone in XX stem cells than in XY stem cells17.
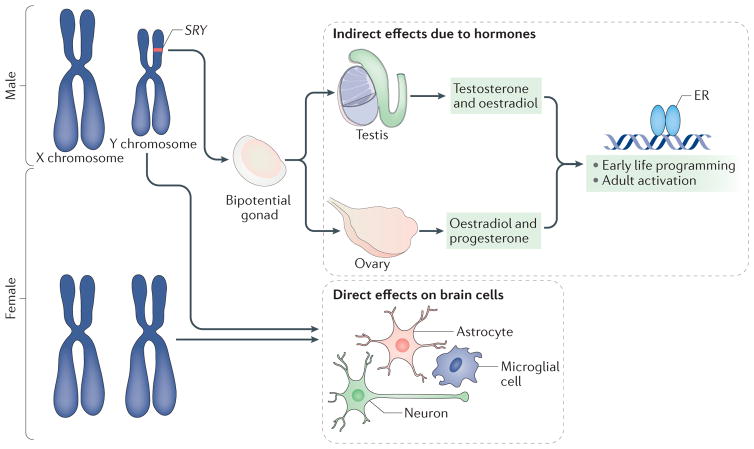
Figure 2. Sex chromosomes affect brain development directly and indirectly.
The differentiation of the bipotential gonad into a testis versus an ovary depends on the SRY gene, which is located on the Y chromosome. If present, the gonad will become a testis, and, if not, it will become an ovary18. Testosterone and its aromatized end product oestradiol produced by the embryonic testis bind to their cognate receptors, which regulate gene transcription and exert an organizing influence on cells in the developing brain. Ovarian and testicular steroids also activate sex-specific physiology and behaviour in adulthood. Moreover, every cell in the brain is XY in male mammals and XX in female mammals. Genes on the X chromosome may be expressed at different levels in males and females (in cases in which they escape from X inactivation in females)113, whereas genes on the Y chromosome (such asSRY) are expressed only in the brain of males20. In females, the presence of a second X chromosome could create a heterochromatic sink by monopolizing the cellular machinery used for epigenetic regulation and thus alter the expression of other genes in a manner that is distinct from males. Thus, the sex chromosomes can exert both specific and broad influences on the developing brain. ER, oestrogen receptor.
The SRY gene on the Y chromosome is best known for its role as the essential driver of testis development18 but also promotes catecholamine production by dopaminergic neurons of the substantia nigra of the mouse19 and by human NT2 cells differentiated to be dopaminergic. As only males carry the SRY gene, this is a true sex dimorphism and is speculated to contribute to the higher susceptibility of males to dopamine disorders such as Parkinson disease and schizophrenia20.
A genetically modified mouse line in which Sry has been translocated from the Y chromosome to an autosome allows for generation of XX individuals with testis and XY individuals with ovaries, thereby separating sex genotype and gonadal phenotype21. This powerful tool is called the four-core genotype and has shown that multiple sex differences in brain and behaviour can be attributed to chromosome complement rather than gonadal phenotype. These include differences in the incidence of neural tube defects22, pain perception23 and many additional phenotypes (for a review, see REF. 24).
The four-core genotype was also exploited to dissect the sources of increased vulnerability of females to auto-immune disorders, focusing on multiple sclerosis (which is easily modelled in rodents in a paradigm referred to as experimental autoimmune encephalomyelitis). The severity of symptoms and degree of myelination were greater in XX mice compared with XY, regardless of gonadal phenotype25, suggesting a genetic origin of disease vulnerability. However, it is important to note that the four-core genotype involves every cell in the body, preventing the dissociation of effects in the periphery from those in the CNS. This conundrum was addressed by the use of bone marrow transplantation to generate subjects with an XX nervous system and XY peripheral immune system and vice versa, thereby revealing that an XY nervous system was more sensitive to injury than an XX one26. The opposing effects of sex chromosome complement on the peripheral immune system and CNS, when combined with the sex-specific modulatory effects of gonadal steroids (for a review, see REF. 27), reveal the enormous complexity of gender bias in disease and the importance of determining the contribution of sex as a biological variable.
Neuroimmunity and brain sex differences
Steroid hormones largely exert their effects by binding to intracellular receptors, which dimerize and dock at hormone response elements on gene promoters to regulate gene transcription28–30. Surprisingly, however, relatively few genes have been identified that are directly induced by steroid hormones and mediate masculinization. Instead, over the past decade, immune mediators have been strongly implicated as drivers of the masculinization process.
The brain is considered to be protected from systemic immune responses and pathogens by the blood–brain barrier. However, this does not mean that the brain is immunodeficient. Instead, the brain receives information from the periphery about immune status from various sources, including the vagal nerve, humoral signalling and cytokine transport into the brain, as well as meningeal and epithelial signalling across the blood–brain barrier31–33. The brain is also populated by resident immunocompetent cells, known as microglia, that engage in immune surveillance. These cells produce a host of neuroinflammatory mediators that are crucial regulators of brain development and homeostasis, including cytokines, prostanoids, purines and reactive oxygen species. Many of these neuroimmune mediators act in a manner that is indistinguishable from the functions of neuromodulators or neurohormones34–36.
Microglia seed the brain early in fetal development, beginning at around 4.5 gestational weeks in humans and embryonic day 8–9.5 in mice and rats37–40. Microglia derive from yolk-sac progenitor cells, colonize the brain before the blood–brain barrier is formed and then locally proliferate across the remainder of fetal development and into the early postnatal period41. This process differs in males and females. On embryonic day 17, just before the fetal androgen surge in males, male and female rats have the same number of microglia in the brain. One week later, males have significantly more microglia than females in several brain regions, including the parietal cortex, hippocampus, amygdala and paraventricular nucleus of the hypothalamus42. Similarly, in the developing medial preoptic area (mPOA), male rats have significantly more microglia than females in the early postnatal period and twice as many microglia that are in an activated state (based on their ameboid-like morphology)43. Treating newborn females with a masculinizing dose of oestradiol increases both microglia number and activational state to male levels.
In recent years, the functional significance of these sex differences in neuroimmune signalling and microglial number has become apparent (FIG. 3). The mPOA is a crucial brain region for expression of male sexual behaviour. Olfactory cues from sexually receptive females are detected by the male olfactory system and projected to the amygdala, which in turn projects to the mPOA. From there, signals are integrated with the neural circuits of motivation and reward, as well as projected to the hypothalamus and midbrain for execution of mating behaviour44. Dendrites of mPOA neurons are studded with dendritic spines, and, in males, the density of these spines is twofold higher than in females, resulting in greater excitation in response to olfactory input45. A crucial molecular driver of the sex difference in the density of dendritic spine synapses in the rodent mPOA is the prostanoid prostaglandin E2 (PGE2). The surge in oestradiol levels in the male brain46 during the critical period triggers an upregulation in the expression of cyclooxygenase 2 (COX2), the enzyme that leads to prostaglandin synthesis47,48. Elevated COX2 leads to increased PGE2 in the mPOA, which is necessary for the induction of both dendritic spine patterning and male typical copulatory behaviour in adulthood48. The spinogenic effect of PGE2 involves activation of the prostanoid receptors EP2 and EP4 (REF. 49), which are linked to protein kinase A activation50, resulting in phosphorylation and mobilization of AMPA-type glutamate receptors to the membrane of both neurons and astrocytes51, and glutamate-dependent formation of dendritic spines50,51.
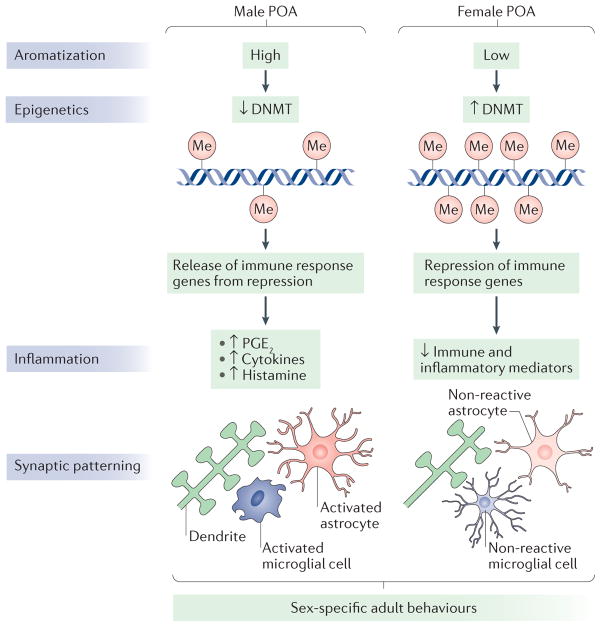
Figure 3. Neuroepigenetic and neuroinflammatory contributions to sex differences in the preoptic area.
The rodent preoptic area (POA) is robustly sexually dimorphic and is therefore an ideal model system to understand the role of neuroepigenetics and neuroinflammation in sexual differentiation. The activity of DNA methyltransferase (DNMT) enzymes is higher in the developing POA of female rats than in that of males. This is because it is reduced in males, through unknown mechanisms, by the elevated oestradiol levels that are present owing to the aromatization of testosterone made in the testis. As a result, males have less DNA methylation (in the figure, indicated by ‘Me’) in POA cells79. Indirect evidence suggests that the lower DNA methylation in males releases immune response genes from epigenetic repression, resulting in elevation of inflammatory signalling molecules such as prostaglandin E2 (PGE2), histamine and cytokines79. PGE2 in particular is known to activate a signal transduction cascade that results in the formation of dendritic spine synapses on the dendrites of POA neurons. This process requires the participation of both astrocytes, as a putative source of glutamate, and neighbouring microglia, which provide much of the prostaglandin. In females, immune response genes are epigenetically silenced by higher levels of DNA methylation. PGE2 levels are therefore low, and both microglia and astrocytes are in a non-reactive state. As a result, females have half the density of dendritic spine synapses in the POA compared with males. These neuroanatomical sex differences undergird sex-specific reproductive behaviours, including male copulatory behaviour (both motivation to copulate and actual sexual performance), social scent marking, female oestrous cycling and maternal behaviour (for reviews, see REFS 44,114).
We now know that microglia are crucial for the PGE2-mediated induction of male-typical synaptic patterning in the mPOA of rats and the resultant male- typical copulatory behaviour in adulthood. Treating females with oestradiol increases ameboid microglia numbers to male-typical levels, as well as the number of dendritic spine-like protrusions on mPOA neurons. Oestradiol or PGE2 treatment will also masculinize POA neurons in a dish, as indicated by an increased density of spine-like processes. However, this response does not occur if the culture is deprived of microglia43. In vivo, oestradiol-induced release of PGE2 in the mPOA is blunted by minocycline, an antibiotic known to inhibit microglia activity, confirming the requirement for microglia in the inflammatory signalling that is responsible for male-typical synaptic patterning. Furthermore, masculinization of copulatory behaviour in females by neonatal oestradiol treatment can be prevented by co-treatment with minocycline43. Even brief microglial ablation from the neonatal brain of males leads to complete loss of male typical copulatory behaviour in adulthood52, along with other sex-specific effects53.
In the nearby anteroventral periventricular nucleus (AVPV) of the POA, a parallel process of sexual differentiation is underway during the sensitive period. In this region, oestradiol increases cell death in males, significantly reducing the size of the AVPV in comparison to that of females54. Once again, sex differences in inflammatory signalling are responsible for masculinization. An unbiased screen of AVPV gene expression revealed that several members of the tumour necrosis family of cytokines — tumour necrosis factor (TNF), TNF receptor 2 (TNFR2; also known as TNFRSF1B) and nuclear factor-κB (NF-κB) — are constitutively active in neurons of the female AVPV, but this activity is blocked in males by testosterone-induced expression of E3 ubiquitin-protein ligase TRAIP, which allows apoptosis to proceed55,56.
Resident immune cells may not be the only immune players in the sexual differentiation process. Indirect evidence supports a role for immune cells derived from the bone marrow and thymus. T cells from the thymus reside in the meninges, and recent studies have found that T cell-deficient mice have impairments in adult behaviours, including spatial learning and social behaviour57,58. A separate study examined the role of T cells on brain development59. In adulthood, the bed nucleus of the stria terminalis (BNST), a brain region that is contiguous with the POA and is both highly sexually dimorphic and sexually differentiated by steroid hormones during development60, was masculinized, that is, increased in size, in female mice congenitally lacking T cells. The BNST is necessary for sociosexual behaviours, including territoriality and olfactory investigation, as well as mood- related behaviours. The T cell-deficient females showed a decrease in anxiety behaviour that may or may not be a result of the increased volume of the BNST. Social and sexual behaviours have not been investigated in these animals to date but certainly warrant future inquiry. Overall, this study suggests T cells are active participants in brain feminization, but the mechanism by which they influence physiology in the CNS remains unclear.
Mast cells are granulocytes of myeloid lineage derived from the bone marrow and are distributed throughout the body, including the brain61,62. Some mast cells synthesize gonadotropin-releasing hormone63, a peptide crucial for the control of the anterior pituitary and gonadal function. Mast cell numbers increase in specific brain regions in both male and female rodents when exposed to sexually receptive stimuli of the other sex63,64. Furthermore, the transcriptome of peripheral mast cells is markedly different in males and females65. Mast cells have not yet been implicated directly in brain sexual differentiation, but, given their involvement in reproductive responses, the potential for such a role is high.
Neuroepigenetics and brain sex differences
The term ‘epigenetics’ describes modifications (epigenetic marks) to the DNA or associated histones that do not involve alterations of nucleotide sequences but have an impact on levels of gene expression in the long term. Canonical epigenetic modifications are methylation of the 5′ carbon of cytosines that are proximal to guanines (CpG) and post-translational modifications of histone tails, the most prevalent of which are acetylation and methylation. Epigenetic mechanisms also include additional sites of DNA methylation and other modifications of histones along with the actions of non-coding RNAs and the generation of a constellation of epigenetic marks that inactivate the X chromosome. The observation that epigenetic modifications in the nervous system happen rapidly and are reversible within a lifespan has led to a re-synthesis of the traditional view of cell fate determination and is aptly named neuroepigenetics66.
A neuroepigenetic contribution to sex differences is at once obvious and mysterious. For example, every cell in the female nervous system has one epigenetically inactivated X chromosome, but how that contributes to functional sex differences remains obscure. As mentioned above, it is obvious that sex chromosome composition is a contributing variable to sex differences in the brain16,67, but it is not clear whether X-chromosome genes that escape X inactivation in females drive these differences or whether the effect is due to the heterochromatic ‘sink’ that is created when large amounts of epigenetic regulatory proteins are required to maintain silencing of the second X chromosome: that is, the reduction in available epigenetic modifiers in cells with two X chromosomes may dilute the capacity for other forms of epigenetic regulation. It is also important not to forget the potential direct effects of Y chromosome genes (see above).
When contemplating an early life programming event that only manifests in adulthood, such as hormone- mediated sexual differentiation, it seems to be obvious that some form of cellular ‘memory’ must be established. Steroids bind to nuclear transcription factors (which interact directly with DNA), as well as to larger transcriptional complexes that include histone-modifying enzymes68,69, thereby increasing the likelihood that epigenetics is involved. Indeed the early life programming scenario outlined above predicts that masculinization is driven by direct transcriptional regulation of a set of masculinizing genes that are turned on early (and possibly continuously), thereby differentiating the male brain. There are hints that this is true, to at least some degree, in the developing rat amygdala. Male adult rats have more vasopressin mRNA in their amygdala than females, a sex difference that is determined developmentally by higher androgen levels in males70,71. It has been shown that an experimentally induced transient reduction in the levels of the DNA methyl-binding protein methyl-CpG-binding protein 2 (MeCP2) during the sensitive period for sexual differentiation in males can permanently reduce amygdala vasopressin expression to the level of females, suggesting that an epigenetic modification (DNA methylation and the subsequent binding of MeCP2) establishes and maintains the sex difference in amygdala vasopressin levels72 (FIG. 4). The amygdala is a site of multiple neuroanatomical sex differences and is central to the control of multiple behaviours that differ in males and females, including juvenile social play (which is displayed at higher levels by males across a wide range of species)73.
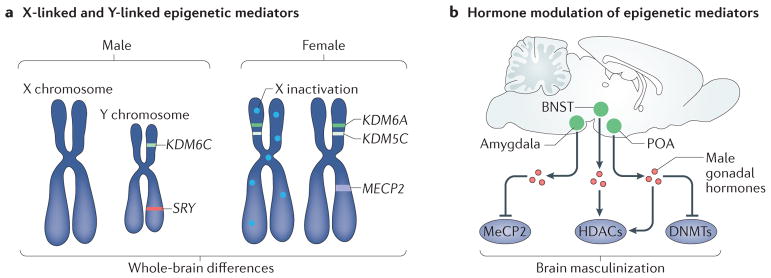
Figure 4. Epigenetic sources of brain sex differences.
a | Several crucial epigenetic mediators are X chromosome and Y chromosome linked. These include the histone lysine demethylases KDM6A (also known as UTX) and KDM5C, which escape X inactivation and are thus expressed at higher levels in the female brain115,116. The Y-linked homologue of KDM6A, KDM6C (also known as UTY), is expressed at higher levels in the male brain116. Sex-specific expression of epigenetic modifiers such as these has the potential to establish widespread sex differences in the chromatin landscape and gene expression and thus to drive structural and functional sex differences in the brain. X-linked chromatin-binding proteins, such as methyl CpG binding protein 2 (MeCP2), have also been shown to be important for establishment of brain sex differences. b | Several epigenetic mediators are regulated by sex specific gonadal hormones. For example, male gonadal hormones reduce the expression of MeCP2 in the amygdala72, reduce DNA methyltransferase (DNMT) activity and methylation genome-wide in the preoptic area (POA)79, and alter methylation on specific promoters related to brain masculinization such as the oestrogen and progesterone receptors74,75. Hormonal modulation at the level of histone methylation and acetylation has also been demonstrated in the POA and bed nucleus of the stria terminalis (BNST), potentially mediating both active and repressive chromatin states80,83. HDACs, histone deacetylases.
Epigenetic regulation of vasopressin and MeCP2 expression is presumed to be downstream of steroid hormone action, but what about regulation of the steroid receptors themselves? Direct modulation of these transcription factor genes should provide a wide ranging and enduring regulation of hormonal responsiveness in adulthood. There have been studies of promoter methylation of both isoforms of the oestrogen receptor and the progesterone receptor in rats74,75. However, there is little agreement about the existence, direction and magnitude of any sex differences in promoter methylation76,77. In the few instances in which more than one time point was measured, sex-specific epigenetic patterns established early in life were replaced by different but still sex-specific patterns later in life75. This was most dramatically seen in a genome-wide analysis of genomic areas that are highly methylated. When comparing male mice, female mice and female mice treated with testosterone at birth to induce masculinization, the investigators found relatively few genes for which methylation levels were different in newborn males and females or that were modified by testosterone treatment of females. But, when animals treated the same way were assessed in adulthood, there were hundreds of genes that were differentially methylated by the testosterone treatment78. Among the challenges to epigenetic research is the inability to comprehensively assess DNA methylation and histone modifications at the same time on the same gene, and — even more importantly — to do so retrospectively. Thus, when an epigenetic modification is observed, it is impossible to know if it happened long ago and endured until now or was placed there just yesterday. Instead, we are limited to a single snapshot in time of a single end point. Nevertheless, these studies suggest that the epigenetic impacts of early life hormone exposure are enduring, but dynamic, generating an epigenetic ‘echo’ that grows and distorts across the lifespan but retains aspects of its original form.
As noted above, steroid-mediated transcription is an obvious means by which males could be differentiated from females. In addition, a recent study79 showed that DNA methyltransferase (DNMT) activity in the POA of female rats was higher than that in males and that treating females with a masculinizing dose of steroid reduced enzymatic activity to that of males. This sex difference lasted for only the first few days after birth. DNA isolated from the female mPOA had more global methylation and a higher number of 100% methylated CpG sites than that isolated from both males and masculinized females, consistent with higher DNMT activity. But is this differential methylation important? RNA-sequencing analyses of the transcriptome from neonatal males and females with and without treatment that reduces DNA methylation was employed to explore this question. As expected, reducing DNA methylation increased the number of genes upregulated in females more than it did in males. More interestingly, when the transcriptome of males and females was directly compared, a surprisingly small number of genes (70) were differentially expressed. Furthermore, about half were expressed at higher levels in males, whereas half were higher in females. This is surprising because a scenario in which steroid receptor-induced transcription differentiates males from females would predict increased gene expression in males. Moreover, most sex differences in differential gene expression were reversed in animals in which DNA methylation was reduced, confirming that epigenetics was the basis for the sex-specific transcriptomes. Further proof was found in the reversal of sex differences in mPOA neuron synaptic density and adult mating behaviour in females subject to reduced DNA methylation as neonates. The mechanism by which steroids reduce DNMT activity is not known, but the capacity is lost by the end of the first week of life, coincident with the closing of the critical period. However, if females are treated with a demethylating agent at the normal end of the critical period, the critical period remains open and females are masculinized. Thus, DNA methylation seems to be essential to both the initiation and maintenance of sexual differentiation of the mPOA.
In contrast to the effects of DNA methylation (which prevent masculinization), histone deacetylation- mediated repression of gene expression is required for masculinization80. This is again surprising, as DNA methylation and histone deacetylation usually work in concert to suppress gene expression. Even more interestingly, there is evidence that these two opposing forces are at work in the same general brain region. As described above, the BNST is larger in males than in females because more cells die in females. This sex difference can be reversed by giving females a masculinizing dose of steroid during the critical period, indicating that steroids support survival60. It takes several days for the pro-survival effects of steroids to manifest, suggesting that an epigenetic change is induced by the steroids that then protects the cells from a subsequent apoptotic programme. Treatment of neonatal mouse pups with a histone deacetylase (HDAC) inhibitor increased histone acetylation and prevented the masculinization of the BNST, suggesting that pro-death genes that are normally suppressed in males were activated81. A functional impact of neonatal HDAC inhibition is evident in the impaired sexual ability of adult males80, an effect that is attributed at least in part to dysregulation of the Cyp19a1 gene, which encodes the enzyme aromatase, responsible for converting testosterone to oestradiol.
There are two approaches to discover sex differences in the epigenome. One is the use of broad based surveys that assay most or all potential targets. The second approach is to focus on specific candidate genes or epigenetic marks. An advantage of broad survey approaches is that intragenic regions, promoters, regulatory elements and reading frames of genes are all included in the analyses. Whole-genome bisulfite sequencing analysis of the neonatal POA demonstrated that most sex differences in DNA methylation levels are in the intergenic region, the functional significance of which remains a mystery79. A more focused view was gained by analysing an epigenetic mark known to cluster at transcription start sites, histone H3 lysine 4 trimethylation (H3K4me3), which is generally permissive of gene expression82. Chromatin immunoprecipiation followed by sequencing (CHIP–seq) established that most genes of males and females exhibited the same number of H3K4me3 marks but that ~200 genes differed in the number of H3K4me3 marks carried (most of them being higher in females)83. Thirteen genes were confirmed by quantitative polymerase chain reaction to be differentially expressed at the moment of assay, and two of these were located on the X chromosome, where they escape X inactivation. Together, these findings highlight the importance of considering the ‘sexome’ — the constellation of gene expression changes related to sex — rather than sex differences in individual genes84.
Intersection of neuroimmunity and neuroepigenetics
Given the findings outlined above, it is natural to question whether there is crosstalk between neuroimmune and neuroepigenetic mechanisms of sex differentiation. The X chromosome has the highest concentration of immune-related genes of any chromosome85. Thus, X-chromosome inactivation in females is crucial to keeping overexpression of these immune-related genes repressed, and dysregulated X-chromosome silencing is proposed as a possible reason that females suffer more auto-inflammatory conditions85.
Gene ontology analyses of the genes regulated by DNA methylation in the POA showed an almost fourfold enrichment of immune-related genes in female rat pups treated with a DNMT inhibitor (FIG. 3). These included cytokines, chemokines and their receptors, microglia- specific and/or macrophage-specific genes, genes related to phagocytosis, complement-related proteins and antigen presentation and receptor genes79. There were also many gene isoforms differentially expressed in males and females. Among these were many immune-related genes, including intriguingly a mast cell protease79, implicating this non-neuronal cell type in the process of sexual differentiation.
A complementary possibility is that immunocompetent cells possess sexually differentiated epigenetic tags. Exposure to early life stress via neonatal handling of rat pups induces microglial-specific decreases in methylation of the anti-inflammatory immune gene interleukin-10, resulting in long-term increases in cytokine levels and subsequent resilience to morphine-induced conditioned place preference86. Although no sex differences were reported in this study, future exploration of immune cell-specific sex differences in the methylome or histone modifications would answer the question of whether the epigenetic regulation of these cells contributes to or maintains sexual differentiation of the brain.
Gender biased human neuropathology
A diagnosis of autism spectrum disorder (ASD) is 4–5 times more common in males than in females87–89 and is greater in individuals exposed to higher levels of fetal testosterone as a result of maternal conditions such as polycystic ovarian syndrome90,91. Higher levels of several steroid hormones present in amniotic fluid during pregnancy, including cortisol, progesterone and testosterone, predict higher rates of autism diagnosis92. Low maternal levels of the oestrogen oestriol and both low and high levels of the placental hormone human chorionic gonadotropin during pregnancy have been associated with a higher risk of autism in male, but not female, offspring93. Thus, too much exposure to hormones involved in sexual differentiation may lead to ASD and possibly contribute to the male bias in autism risk. However, the role of these alterations in sex hormone signalling in brain processes associated with autism is not known.
The centrality of gonadal steroids to the developmental process of brain masculinization was the basis for the ‘extreme male brain’ theory of autism94; however, in the absence of a thorough understanding of the cellular mechanisms by which steroids masculinize the brain, it is difficult to evaluate what is ‘extreme’. As reviewed above, neuroinflammatory mediators direct many sex differences in the brains of rodents. New analysis of existing gene expression profiles from post-mortem human cerebral cortex95,96 reveals that many markers associated with activated astrocytes and microglia are higher in developing males than in females, suggesting that these cells have a role in normal human brain masculinization97. More interestingly, when the gene expression profile of post-mortem tissue of adult males with ASD is compared with that of neurotypical males, neuroimmune genes are expressed at even higher levels in those with ASD97. The paucity of females with a diagnosis of ASD precludes analyses of sex differences among affected individuals at this time but is an important future goal. Nonetheless, these data make a persuasive case that the normal biology of male-typical brain development seen in rodents also applies to humans and interacts with autism risk genes or environmental factors to make males more vulnerable.
Schizophrenia too is diagnosed more often in males than in females (in the order of 1.5 male diagnoses:1 female diagnosis)98, with an earlier onset and greater severity in males that include more positive and negative symptoms99. By contrast, females tend towards more affective symptoms and a spike in psychosis around menopause, which has been hypothesized to depend on loss of the antipsychotic and antidepressant properties of oestrogens100. Neuroimaging indicates that regions of the cerebral cortex that exhibit sex differences in neurotypical individuals also show the largest abnormalities in individuals with schizophrenia101, suggesting that sexual differentiation of the brain is disrupted in individuals that go on to develop schizophrenia.
Behavioural disorders that begin in childhood, including attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder, conduct disorder102, stuttering103, dyslexia104,105 and Tourette syndrome106, are also more common in males. Of these disorders, ADHD has been the most extensively studied in terms of sex differences. As with schizophrenia, males and females show phenotypic differences in presentation, with girls showing less impulsivity, hyperactivity and externalizing relative to boys, as well as more mood disturbances and inattention107,108. Neuroimaging reveals sex differences, with ADHD diagnosed females having a smaller prefrontal cortex and ADHD males having a smaller premotor cortex109, as well as significant sex differences in white matter tracts110. Certain ADHD risk alleles, such as catechol O-methyltransferase (COMT), an enzyme that helps to degrade neurotransmitters such as dopamine and noradrenaline, seem to play a stronger part in ADHD incidence in males than in females (others alleles, including monoamine oxidase type A (MAOA) and sodium-dependent serotonin transporter (SERT; also known as SLC6A4) seem to have a greater role in female ADHD)111. As of yet, the role of these genes in normal sex-specific brain development or risk of ADHD has not been well studied in animal models.
The only neurodevelopmental disorder diagnosed more often in females than in males is Rett syndrome, an X-linked genetic disorder that is embryonically lethal in males112 (thus explaining its sex bias). This highlights a very important caveat to any discussion of sex differences in neurodevelopmental disorders: it is important to note that the measured incidence of a particular disorder does not account for cases in which the disrupted development underlying the disorder is embryonically fatal. Thus, it must be considered that any disorder that seems to be more biased towards males may be the result of more males exposed to a particular genetic or environmental risk surviving through fetal development than females.
Conclusions
The impact of sex and gender on health and disease is complex, multifactorial and pervasive. Discriminating biological origins of sex differences in disease susceptibility is one tool among many for improving the diagnosis and treatment of conditions that affect both men and women. Dissociating the many variables contributing to sex differences in brain and behaviour is particularly challenging, but animal models are uniquely powerful in this regard. Recent discoveries hint at novel biological sources of sex differences in the brain that involve use of inflammatory mediators as natural agents for differentiation between males and females. Divergent neuroepigenetic profiles in males and females seem to partner with differences in neuroinflammation, and, although the rules of engagement remain elusive, they surely warrant further study.
Acknowledgments
M.M.M. is supported by US National Institutes of Health (NIH) grant R01MH052716-19, R01DA039062 and R01MH091424. K.M.L. is supported by NIH grants F32NS076327 and R21MH105826, and NARSAD Young Investigator Award 2382.
Glossary
- Functional MRI
The detection of changes in regional brain activity through their effects on blood flow and blood oxygenation that in turn affect the brightness of magnetic resonance images.
- Diffusion tensor imaging
An MRI technique that provides a three-dimensional image of water diffusion in the brain. As water diffuses more readily along the axis of myelinated nerve fibre tracts, this method can be used to obtain a non-invasive estimate of anatomical connectivity between brain areas.
- Connectome
A comprehensive map of neural connections within the nervous system of an organism.
- Steroid hormone
A signalling molecule that is synthesized from cholesterol and is released into the circulation from endocrine glands including the gonads and adrenals. It binds to nuclear transcription factor receptors and can directly and indirectly modify gene expression.
- Early life programming
The phenomenon whereby events during development, such as stress, altered nutrition or endogenous hormone exposure, exert enduring influences on the nervous system in anticipation of the adult environment and experiences.
- Critical period
A developmental window during which specific cellular events must occur or will be forever precluded. Sexual differentiation of the brain mediated by steroid hormones occurs during a perinatal critical period in the rodent and prenatally in humans.
- Hormone response elements
Sequences of DNA in promoter regions that are recognized and bound to by steroid hormone receptors after binding of hormone, thereby promoting transcription.
- Humoral signalling
Signalling molecules released into the blood stream that then act at a distance, such as steroid hormones.
- Cytokine
Originally defined as an immune system protein that modifies biological responses; cytokines are now known to be released by most cells and are important in regulating intercellular communication, cell function and cell survival.
- Immune surveillance
The hypothesized process by which the immune system constantly monitors the body for both invading pathogens and aberrant cell pathology, such as that seen in cancer.
- Neuromodulators
Endogenous chemical substances that change the intrinsic properties of a neuron and the dynamics and strength of neurotransmission. Neuromodulators can modify neuronal responses to synaptic inputs on potentially long timescales.
- Neurohormones
Steroid hormones that are further modified in the brain or synthesized de novo from cholesterol in the brain and are thus distinguished from those synthesized in the endocrine glands. Other neurohormones are peptides synthesized in the brain and released into the periphery such as oxytocin and vasopressin.
- T cells
Lymphocytes produced by the thymus gland that actively participate in the immune response.
- Mast cells
Multigranular cells that function as stores for several key inflammatory and/or pain mediators (including nerve growth factor, tumour necrosis factor, chemokines and histamine) and that originate in bone marrow but have a resident population in the brain.
- Epigenetic marks
Modifications to the genome that do not change the nucleotide sequence but have an impact on gene regulation. Methylation groups added to cytosine nucleotides or histones on the chromatin, along with other chemical groups, are examples of epigenetic marks.
- Chemokines
A subfamily of inflammatory molecules that were initially described as regulators of the chemotaxis of inflammatory cells but that also have important roles in other processes, such as cell growth and differentiation.
Footnotes
Competing interests statement
The authors declare no competing interests.
FURTHER INFORMATION
World Health Organization definition of gender: http://www.who.int/gender-equity-rights/understanding/gender-definition/en/
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Griew S. Age and sex differences in probability learning of rats in a swimming T-maze. Gerontologia. 1968;14:197–203. doi: 10.1159/000211658. [DOI] [PubMed] [Google Scholar]
- 2.Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 3.Perrot-Sinal TS. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- 4.Roof RL. Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze perfromance in prebubescent and adult rats. Behav Brain Res. 1993;53:1–10. doi: 10.1016/s0166-4328(05)80261-x. [DOI] [PubMed] [Google Scholar]
- 5.Munoz-Cueto JA, Garcia-Segura LM, Ruiz-Marcos A. Developmental sex differences and effect of ovariectomy on the number of cortical pyramidal cell dendritic spines. Brain Res. 1990;515:64–68. doi: 10.1016/0006-8993(90)90577-x. [DOI] [PubMed] [Google Scholar]
- 6.Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging. 2013;31:366–375. doi: 10.1016/j.mri.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Ingalhalikar M, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA. 2014;111:823–828. doi: 10.1073/pnas.1316909110. In this study, diffusion tensory imaging of nearly 1,000 human brains reveals sex differences in intra-hemispheric versus inter-hemispheric connectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tunc B, et al. Establishing a link between sex-related differences in the structural connectome and behaviour. Phil Trans R Soc B. 2016;371:20150111. doi: 10.1098/rstb.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joel D, Tarrasch R. On the mis-presentation and misinterpretation of gender-related data: the case of Ingalhalikar’s human connectome study. Proc Natl Acad Sci USA. 2014;111:E637. doi: 10.1073/pnas.1323319111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joel D, Fausto-Sterling A. Beyond sex differences: new approaches for thinking about variation in brain structure and function. Phil Trans R Soc B. 2016;371:20150451. doi: 10.1098/rstb.2015.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy M, De Vries G, Forger N. In: Hormones, Brain and Behavior. Pfaff DW, Joëls M, editors. Elsevier; 2017. pp. 3–32. [Google Scholar]
- 12.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 13.Bakker J, et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 14.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold AP, et al. The importance of having two X chromosomes. Phil Trans R Soc B. 2016;371:20150113. doi: 10.1098/rstb.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bramble MS, et al. Sex-specific effects of testosterone on the sexually dimorphic transcriptome and epigenome of embryonic neural stem/progenitor cells. Sci Rep. 2016;6:36916. doi: 10.1038/srep36916. This study shows that, in mouse embryonic stem cells, the transcriptional response to testosterone exposure is different in males versus females in both expression levels and numbers of genes responding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodfellow PN, Lovell-Badge R. SRY and sex determination in mammals. Annu Rev Genet. 1993;27:71–92. doi: 10.1146/annurev.ge.27.120193.000443. [DOI] [PubMed] [Google Scholar]
- 19.Dewing P, et al. Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Czech DP, et al. The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J Neurochem. 2012;122:260–271. doi: 10.1111/j.1471-4159.2012.07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vries GJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, et al. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev Neurobiol. 2008;68:265–273. doi: 10.1002/dneu.20581. [DOI] [PubMed] [Google Scholar]
- 23.Gioiosa L, Chen X, Watkins R, Umeda EA, Arnold AP. Sex chromosome complement affects nociception and analgesia in newborn mice. J Pain. 2008;9:962–969. doi: 10.1016/j.jpain.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold AP, et al. Minireview: sex chromosomes and brain sexual differentiation. Endocrinology. 2004;145:1057–1062. doi: 10.1210/en.2003-1491. [DOI] [PubMed] [Google Scholar]
- 25.Smith-Bouvier DL, et al. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du S, et al. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2014;111:2806–2811. doi: 10.1073/pnas.1307091111. This study shows that the impact of chromosome complement on disease progression in an animal model of multiple sclerosis is opposite in peripheral versus central nervous system tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voskuhl R. Preclinical studies of sex differences: a clinical perspective. Biol Sex Differ. 2016;7:7. doi: 10.1186/s13293-016-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimm SL, Hartig SM, Edwards DP. Progesterone receptor signaling mechanisms. J Mol Biol. 2016;428:3831–3849. doi: 10.1016/j.jmb.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Stanisic V, Lonard DM, O’Malley BW. Modulation of steroid hormone receptor activity. Prog Brain Res. 2010;181:153–176. doi: 10.1016/S0079-6123(08)81009-6. [DOI] [PubMed] [Google Scholar]
- 30.Katzenellenbogen BS, et al. Structure-function relationships in estrogen receptors and the characterization of novel selective estrogen receptor modulators with unique pharmacological profiles. Ann NY Acad Sci. 2001;949:6–15. doi: 10.1111/j.1749-6632.2001.tb03998.x. [DOI] [PubMed] [Google Scholar]
- 31.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 32.McAllister AK, van de Water J. Breaking boundaries in neural–immune interactions. Neuron. 2009;64:9–12. doi: 10.1016/j.neuron.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 35.Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- 36.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 37.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 38.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuadros MA, Navascues J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56:173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 40.Verney C, Monier A, Fallet-Bianco C, Gressens P. Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J Anat. 2010;217:436–448. doi: 10.1111/j.1469-7580.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz J, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. This is the first report of a sex difference in microglia, showing that males have higher numbers of microglia that are more activated at early stages and that there is a switch to female preponderance around puberty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J Neurosci. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. This study establishes a functional role for more activated microglia in the developing POA mediating masculinization of synaptic profile and adult copulatory behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 46.Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 47.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. This study identifies a prostaglandin as both necessary and sufficient for masculinizing the synaptic profile of POA neurons and adult copulatory behaviour. [DOI] [PubMed] [Google Scholar]
- 49.Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol. 2008;68:1406–1419. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright CL, McCarthy MM. Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling. J Neurosci. 2009;29:13274–13282. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenz KM, Wright CL, Martin RC, McCarthy MM. Prostaglandin E regulates AMPA receptor phosphorylation and promotes membrane insertion in preoptic area neurons and glia during sexual differentiation. PLoS ONE. 2011;6:e18500. doi: 10.1371/journal.pone.0018500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanRyzin JW, Yu SJ, Perez-Pouchoulen M, McCarthy MM. Temporary depletion of microglia during the early postnatal period induces lasting sex-dependent and sex-independent effects on behavior in rats. eNeuro. 2016 doi: 10.1523/ENEURO.0297-16.2016. http://dx.doi.org/10.1523/ENEURO.0297-16.2016. [DOI] [PMC free article] [PubMed]
- 53.Nelson LH, Lenz KM. Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav Brain Res. 2017;316:279–293. doi: 10.1016/j.bbr.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci. 2009;29:9714–9718. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci USA. 2009;106:16692–16697. doi: 10.1073/pnas.0906293106. This study shows that the higher cell death in the developing male AVPV is mediated by the suppression of the TNF receptor-associated cell survival pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petersen SL, Krishnan S, Aggison LK, Intlekofer KA, Moura PJ. Sexual differentiation of the gonadotropin surge release mechanism: a new role for the canonical NfκB signaling pathway. Front Neuroendocrinol. 2012;33:36–44. doi: 10.1016/j.yfrne.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Radjavi A, Smirnov I, Kipnis J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav Immun. 2014;35:58–63. doi: 10.1016/j.bbi.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Filiano AJ, et al. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–429. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rilett KC, et al. Loss of T cells influences sex differences in behavior and brain structure. Brain Behav Immun. 2015;46:249–260. doi: 10.1016/j.bbi.2015.02.016. In this study, null mutations in T-cell receptors both reveal and eliminate sex differences in brain and behaviour in mice. [DOI] [PubMed] [Google Scholar]
- 60.del Abril A, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Dev Brain Res. 1987;32:295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- 61.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silver R, Curley JP. Mast cells on the mind: new insights and opportunities. Trends Neurosci. 2013;36:513–521. doi: 10.1016/j.tins.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Khalil MH, Silverman AJ, Silver R. Mast cells in the rat brain synthesize gonadotropin-releasing hormone. J Neurobiol. 2003;56:113–124. doi: 10.1002/neu.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kriegsfeld LJ, et al. Brain mast cells are influenced by chemosensory cues associated with estrus induction in female prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:377–384. doi: 10.1016/j.yhbeh.2003.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mackey E, et al. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol Sex Differ. 2016;7:60. doi: 10.1186/s13293-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sweatt JD. The emerging field of neuroepigenetics. Neuron. 2013;80:624–632. doi: 10.1016/j.neuron.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reardon PK, et al. An allometric analysis of sex and sex chromosome dosage effects on subcortical anatomy in humans. J Neurosci. 2016;36:2438–2448. doi: 10.1523/JNEUROSCI.3195-15.2016. This study reveals that sex differences in the size of two subcortical structures are further modified by sex chromosome aneuploidies in both men and women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torchia J, Glass C, Rosenfeld MG. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 69.Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids. 2000;65:227–251. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 70.De Vries GJ, et al. Sexual differentiation of vasopressin innervation of the brain: cell death versus phenotypic differentiation. Endocrinology. 2008;149:4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z, De Vries GJ. Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats. J Neuroendocrinol. 1995;7:827–831. doi: 10.1111/j.1365-2826.1995.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 72.Forbes-Lorman RM, Rautio JJ, Kurian JR, Auger AP, Auger CJ. Neonatal MeCP2 is important for the organization of sex differences in vasopressin expression. Epigenetics. 2012;7:230–238. doi: 10.4161/epi.7.3.19265. In this study, a transient reduction in MeCP2 early in life permanently removed the sex difference in medial amygdala vasopressin expression by reducing it in males to female levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Argue KJ, McCarthy MM. Characterization of juvenile play in rats: importance of sex of self and sex of partner. Biol Sex Differ. 2015;6:16. doi: 10.1186/s13293-015-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nugent BM, McCarthy MM. Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology. 2011;93:150–158. doi: 10.1159/000325264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nugent BM, Schwarz JM, McCarthy MM. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: implications for sexual differentiation. Horm Behav. 2010;59:338–344. doi: 10.1016/j.yhbeh.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghahramani NM, et al. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ. 2014;5:8. doi: 10.1186/2042-6410-5-8. In this study, the short-term effects of neonatal hormone treatment were modest, but the number of genes with altered methylation increased by 20-fold in adulthood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nugent BM, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. This study demonstrates that higher levels of DNA methylation in the POA of females repress the gene expression programme that is required for normal masculinization, an effect that can be reversed by hormone treatment and is dependent on DNMT activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuda KI, et al. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology. 2011;152:2760–2767. doi: 10.1210/en.2011-0193. This study shows that inhibition of HDAC2 and HDAC4 early in development prevents normal male sex behaviour in adulthood, perhaps in part by modifying sex-specific patterns of acetylation of oestrogen receptor-α and aromatase promoter regions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–4247. doi: 10.1210/en.2009-0458. In this study, treatment with the HDAC inhibitor valproic acid prevented the increased cell survival that normally occurs in males resulting in a BNST volume similar to that of females. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 83.Shen EY, et al. Epigenetics and sex differences in the brain: a genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Exp Neurol. 2015;268:21–29. doi: 10.1016/j.expneurol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arnold AP, Lusis AJ. Understanding the sexome: measuring and reporting sex differences in gene systems. Endocrinology. 2012;153:2551–2555. doi: 10.1210/en.2011-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. 2012;38:J187–J192. doi: 10.1016/j.jaut.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 86.Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 88.Halladay AK, et al. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism. 2015;6:36. doi: 10.1186/s13229-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26:146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kosidou K, et al. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry. 2016;21:1441–1448. doi: 10.1038/mp.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ingudomnukul E, Baron-Cohen S, Wheelwright S, Knickmeyer R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm Behav. 2007;51:597–604. doi: 10.1016/j.yhbeh.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 92.Baron-Cohen S, et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2015;20:369–376. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Windham GC, Lyall K, Anderson M, Kharrazi M. Autism spectrum disorder risk in relation to maternal mid-pregnancy serum hormone and protein markers from prenatal screening in California. J Autism Dev Disord. 2016;46:478–488. doi: 10.1007/s10803-015-2587-2. [DOI] [PubMed] [Google Scholar]
- 94.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6:248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 95.Voineagu I, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gupta S, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun. 2014;5:5748. doi: 10.1038/ncomms6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Werling DM, Parikshak NN, Geschwind DH. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat Commun. 2016;7:10717. doi: 10.1038/ncomms10717. Re-analyses of existing transcriptomic data sets reveal sex differences in the fetal brain, including an increase in microglia-associated and activated astrocyte-associated genes in males. The same pattern was observed in adult males with ASD compared to neurotypic males. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- 99.Castle DJ, Murray RM. The neurodevelopmental basis of sex differences in schizophrenia. Psychol Med. 1991;21:565–575. doi: 10.1017/s0033291700022194. [DOI] [PubMed] [Google Scholar]
- 100.Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Horm Behav. 2006;50:612–622. doi: 10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 101.Goldstein JM, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- 102.Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Dev Psychopathol. 2001;13:355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- 103.Craig A, Hancock K, Tran Y, Craig M, Peters K. Epidemiology of stuttering in the community across the entire life span. J Speech Lang Hear Res. 2002;45:1097–1105. doi: 10.1044/1092-4388(2002/088). [DOI] [PubMed] [Google Scholar]
- 104.Rutter M, et al. Sex differences in developmental reading disability: new findings from 4 epidemiological studies. JAMA. 2004;291:2007–2012. doi: 10.1001/jama.291.16.2007. [DOI] [PubMed] [Google Scholar]
- 105.Quinn JM, Wagner RK. Gender differences in reading impairment and in the identification of impaired readers: results from a large-scale study of at-risk readers. J Learn Disabil. 2015;48:433–445. doi: 10.1177/0022219413508323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang HS, Kuo MF. Tourette’s syndrome in Taiwan: an epidemiological study of tic disorders in an elementary school at Taipei County. Brain Dev. 2003;25(Suppl 1):S29–S31. doi: 10.1016/s0387-7604(03)90005-2. [DOI] [PubMed] [Google Scholar]
- 107.Biederman J, et al. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry. 2002;159:36–42. doi: 10.1176/appi.ajp.159.1.36. [DOI] [PubMed] [Google Scholar]
- 108.Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5:143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- 109.Dirlikov B, et al. Distinct frontal lobe morphology in girls and boys with ADHD. Neuroimage Clin. 2015;7:222–229. doi: 10.1016/j.nicl.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jacobson LA, et al. Sex-based dissociation of white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:938–946. doi: 10.1016/j.jaac.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Biederman J, et al. Sexually dimorphic effects of four genes (COMT, SLC6A2, MAOA, SLC6A4) in genetic associations of ADHD: a preliminary study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1511–1518. doi: 10.1002/ajmg.b.30874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 113.Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X inactivation. Hum Genet. 2011;130:237–245. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 115.Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS ONE. 2008;3:e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J Neurosci. 2008;28:4521–4527. doi: 10.1523/JNEUROSCI.5382-07.2008. This study shows that sex chromosome paralogue genes that escape X inactivation are differentially expressed in males and females in a brain region-specific manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 118.Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol Jpn. 1983;30:227–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- 119.Reid SNM, Juraska JM. Sex differences in the neuron number in binocular area of rat visual cortex. J Comp Neurol. 1992;321:448–455. doi: 10.1002/cne.903210311. [DOI] [PubMed] [Google Scholar]
- 120.Mizukami S, Nishizuka M, Arai Y. Sexual difference in nuclear volume and its ontogeny in the rat amygdala. Exp Neurol. 1983;79:569–575. doi: 10.1016/0014-4886(83)90235-2. [DOI] [PubMed] [Google Scholar]
- 121.Van Eden CG, Uylings HB, Van Pelt J. Sex-difference and left-right asymmetries in the prefrontal cortex during postnatal development in the rat. Brain Res. 1984;314:146–153. doi: 10.1016/0165-3806(84)90186-x. [DOI] [PubMed] [Google Scholar]
- 122.Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- 123.Guillamon A, de Blas MR, Segovia S. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 1988;468:306–310. doi: 10.1016/0165-3806(88)90143-5. [DOI] [PubMed] [Google Scholar]
- 124.Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci USA. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Vries GJ, Buds MR, Swaab DF. Ontogeny of vassopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain — presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- 126.Simerly RB, Swanson LW, Gorski RA. Reversal of the sexually dimorphic distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus by treatment with perinatal androgen. Brain Res. 1985;340:91–98. doi: 10.1016/0006-8993(85)90777-2. [DOI] [PubMed] [Google Scholar]
- 127.Miller MA, Vician L, Clifton DK, Dorsa DM. Sex differences in vassopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides. 1989;10:615–619. doi: 10.1016/0196-9781(89)90152-6. [DOI] [PubMed] [Google Scholar]
- 128.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raisman G, Field PM. Sexual dimorphism in the preoptic area of the rat. Science. 1971;173:731–733. doi: 10.1126/science.173.3998.731. [DOI] [PubMed] [Google Scholar]
- 130.Raisman G, Field PM. Sexual dimorphism in the neuropil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Res. 1973;54:1–29. doi: 10.1016/0006-8993(73)90030-9. [DOI] [PubMed] [Google Scholar]
- 131.Gould E, Westlind-Danielsson A, Frankfurt M, McEwen BS. Sex differences and thyroid hormone sensitivity of hippocampal pyramidal neurons. J Neurosci. 1990;10:996–1003. doi: 10.1523/JNEUROSCI.10-03-00996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsumoto A, Arai Y. Sexual dimorphism in ‘wiring pattern’ in the hypothalamic arcuate nucleus and its modification by neonatal hormonal environment. Brain Res. 1980;19:238–242. doi: 10.1016/0006-8993(80)91173-7. [DOI] [PubMed] [Google Scholar]
- 133.Matsumoto A, Arai Y. Male-female differences in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinolgy. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- 134.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galea LAM, et al. Sex differences in dendrtic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 137.Kolb B, Stewart J. Sex-related differences in dendritic branching of cells in prefrontal cortex of rats. J Neuroendocrinol. 1991;3:95–99. doi: 10.1111/j.1365-2826.1991.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 138.Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Juraska JM, Fitch JM, Henderson C, Rivers N. Sex differences in the dendritic branching of dentate granule cells following differential experience. Brain Res. 1985;29:73–80. doi: 10.1016/0006-8993(85)90125-8. [DOI] [PubMed] [Google Scholar]
- 140.Verhovshek T, Buckley KE, Sergent MA, Sengelaub DR. Testosterone metabolites differentially maintain adult morphology in a sexually dimorphic neuromuscular system. Dev Neurobiol. 2010;70:206–221. doi: 10.1002/dneu.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol. 2003;54:502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- 142.Shah NM, et al. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 143.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 144.Kauffman AS, et al. Sexual differentiation of Kiss1 expression in the brain of the rat. Endocrinology. 2006;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 145.Carlsson M, Svensson K, Eriksson E, Carlsson A. Rat brain serotonin: biochemical and functional evidence for a sex difference. J Neural Transm. 1985;63:297–313. doi: 10.1007/BF01252033. [DOI] [PubMed] [Google Scholar]
- 146.Roselli CE, Resko JA. Aromatase activity in the rat brain: hormonal regulation and sex differences. J Steroid Biochem Mol Biol. 1993;44:499–508. doi: 10.1016/0960-0760(93)90254-t. [DOI] [PubMed] [Google Scholar]
- 147.Yang CF, et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. This study demonstrates that progesterone receptor-expressing neurons subserve distinct but related roles in males and females. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002;143:3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- 149.Garcia-Segura LM, et al. The distribution of glial fibrillary acidic protein in the adult rat brain is influenced by the neonatal levels of sex steroids. Brain Res. 1988;456:357–363. doi: 10.1016/0006-8993(88)90239-9. [DOI] [PubMed] [Google Scholar]
- 150.Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol. 2008;511:599–609. doi: 10.1002/cne.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tobet SA, Fox TO. Sex- and hormone-dependent antigen immunoreactivity in developing rat hypothalamus. Proc Natl Acad Sci USA. 1989;86:382–386. doi: 10.1073/pnas.86.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marin-Husstega M, Muggironi M, Raban D, Skoff RP, Casacia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. 2004;26:245–254. doi: 10.1159/000082141. [DOI] [PubMed] [Google Scholar]
- 153.Pfau DR, Hobbs NJ, Breedlove SM, Jordan CL. Sex and laterality differences in medial amygdala neurons and astrocytes of adult mice. J Comp Neurol. 2016;524:2492–2502. doi: 10.1002/cne.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nunez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Diaz S, et al. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–440. doi: 10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- 157.Zhang JM, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bowers JM, Waddell J, McCarthy MM. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ. 2010;1:8. doi: 10.1186/2042-6410-1-8. This is the first report of a sex difference in cell genesis and its modulation by endogenous possibly locally synthesized oestradiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci USA. 2010;107:20535–20540. doi: 10.1073/pnas.1005003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pinos H, et al. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull. 2001;56:73–78. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- 161.Ahmed EI, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. This study shows that volumetric sex differences in three brain regions seem to be actively maintained by the addition of new cells during the peripubertal period. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gillberg C, Cederlund M, Lamberg K, Zeijlon L. Brief report: “the autism epidemic”. The registered prevalence of autism in a Swedish urban area. J Autism Dev Disord. 2006;36:429–435. doi: 10.1007/s10803-006-0081-6. [DOI] [PubMed] [Google Scholar]
- 163.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 164.Loeber R, Burke JD, Lahey BB, Winters A, Zera M. Oppositional defiant and conduct disorder: a review of the past 10 years, part I. J Am Acad Child Adolesc Psychiatry. 2000;39:1468–1484. doi: 10.1097/00004583-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 165.Trepat E, Ezpeleta L. Sex differences in oppositional defiant disorder. Psicothema. 2011;23:666–671. [PubMed] [Google Scholar]
- 166.Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36:1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 167.Arcia E, Conners CK. Gender differences in ADHD? J Dev Behav Pediatr. 1998;19:77–83. doi: 10.1097/00004703-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 168.Bergen SE, et al. Genetic modifiers and subtypes in schizophrenia: investigations of age at onset, severity, sex and family history. Schizophr Res. 2014;154:48–53. doi: 10.1016/j.schres.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Walder DJ, et al. Sex differences in language dysfunction in schizophrenia. Am J Psychiatry. 2006;163:470–477. doi: 10.1176/appi.ajp.163.3.470. [DOI] [PubMed] [Google Scholar]
- 170.Castle DJ, Abel K, Takei N, Murray RM. Gender differences in schizophrenia: hormonal effect or subtypes. Schizophr Bull. 1995;21:1–12. doi: 10.1093/schbul/21.1.1. [DOI] [PubMed] [Google Scholar]
- 171.Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- 172.Weissman MM, et al. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 173.Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch Gen Psychiatry. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- 174.Alonso J, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;109:21–27. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 175.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 176.Robb JC, Young LT, Cooke RG, Joffe RT. Gender differences in patients with bipolar disorder influence outcome in the medical outcomes survey (SF-20) subscale scores. J Affect Disord. 1998;49:189–193. doi: 10.1016/s0165-0327(98)00003-2. [DOI] [PubMed] [Google Scholar]
- 177.Leibenluft E. Women with bipolar illness: clinical and research issues. Am J Psychiatry. 1996;153:163–173. doi: 10.1176/ajp.153.2.163. [DOI] [PubMed] [Google Scholar]
- 178.Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26:595–620. doi: 10.1016/s0193-953x(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 179.Dao DT, et al. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 2010;68:801–810. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kessler RC, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 182.Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatry Res. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- 183.Bogetto F, Venturello S, Albert U, Maina G, Ravizza L. Gender-related clinical differences in obsessive-compulsive disorder. Eur Psychiatry. 1999;14:434–441. doi: 10.1016/s0924-9338(99)00224-2. [DOI] [PubMed] [Google Scholar]
- 184.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 185.Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- 186.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Raevuori A, Keski-Rahkonen A, Hoek HW. A review of eating disorders in males. Curr Opin Psychiatry. 2014;27:426–430. doi: 10.1097/YCO.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 188.Bulik CM, et al. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry. 2006;63:305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 189.Woodside DB, et al. Comparisons of men with full or partial eating disorders, men without eating disorders, and women with eating disorders in the community. Am J Psychiatry. 2001;158:570–574. doi: 10.1176/appi.ajp.158.4.570. [DOI] [PubMed] [Google Scholar]
- 190.Ceylan-Isik AF, McBride SM, Ren J. Sex difference in alcoholism: who is at a greater risk for development of alcoholic complication? Life Sci. 2010;87:133–138. doi: 10.1016/j.lfs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 192.Buse DC, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–1299. doi: 10.1111/head.12150. [DOI] [PubMed] [Google Scholar]
- 193.Petrea RE, et al. Gender differences in stroke incidence and poststroke disability in the Framingham Heart Study. Stroke. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep. 2010;12:6–13. doi: 10.1007/s11886-009-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Orton SM, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5:932–936. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 196.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 197.Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96:457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 198.Fratiglioni L, et al. Very old women at highest risk of dementia and Alzheimer’s disease: incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997;48:132–138. doi: 10.1212/wnl.48.1.132. [DOI] [PubMed] [Google Scholar]
- 199.Ott A, Breteler MM, van Harskamp F, Stijnen T, Hofman A. Incidence and risk of dementia. The Rotterdam Study. Am J Epidemiol. 1998;147:574–580. doi: 10.1093/oxfordjournals.aje.a009489. [DOI] [PubMed] [Google Scholar]
- 200.Di Carlo A, et al. Incidence of dementia, Alzheimer’s disease, and vascular dementia in Italy. The ILSA Study. J Am Geriatr Soc. 2002;50:41–48. doi: 10.1046/j.1532-5415.2002.50006.x. [DOI] [PubMed] [Google Scholar]
- 201.Barnes LL, et al. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62:685–692. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 202.Haaxma CA, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend Med. 2010;7:557–570. doi: 10.1016/j.genm.2010.11.010. [DOI] [PubMed] [Google Scholar]