Abstract
Hypothesis: Palmitate causes insulin resistance (IR) in insulin target tissue. Pioglitazone (an anti-hyperglycemic agent) and epigallocatechin gallate (EGCG, a dietary supplement) can be used for the treatment of type 2 diabetes. However, their molecular effects on gluconeogenesis remain unclear.
Objective: Hence, we aimed to investigate the simultaneous effect of these anti-hyperglycemic agents on gluconeogenesis through in vitro experiments.
Methods: HepG2 cells were treated with 0.5 mM palmitate, 10 μM pioglitazone, and 40 μM epigallocatechin gallate (EGCG). Gene expression assay was used to investigate the underlying mechanism. Glucose production assay was applied in culture medium to evaluate the activity of gluconeogenesis pathway.
Results: Palmitate induced IR could significantly increase G6Pase and PEPCK gene expressions by 58 and 30%, respectively, compared to the control. EGCG reduced the expression of PEPCK and G6Pase by 53 and 67%, respectively. Pioglitazone reduced the mRNA level of PEPCK and G6Pase by 58 and 62% respectively. Combined treatment of insulin-resistant cells with EGCG and pioglitazone significantly decreased the mRNA level of PEPCK and G6Pase by 73 and 80%, respectively. Treatment with palmitate increased glucose production by 50% in HepG2 cells. When the insulin resistant HepG2 cells were treated alone with EGCG and pioglitazone, the glucose production reduced by 50 and 55%, respectively. The combined treatment with EGCG and pioglitazone resulted in 69% reduction in glucose production compared to the palmitate treated HepG2 cells.
Conclusions: These data suggest the additive inhibitory effect of co-treatment with pioglitazone and EGCG on the gluconeogenesis pathway in palmitate-induced insulin resistance HepG2 cells.
Keywords: gluconeogenesis, epigallocatechin gallate (EGCG), pioglitazone, phosphoenolpyruvate carboxykinase, glucose 6-phosphatase
Introduction
Nowadays, calorie rich dietary habits and sedentary lifestyle are possible common predisposing factors resulting in diabetes [1]. Insulin resistance is a central feature of type 2 diabetes, which results from reduced responsiveness of insulin target tissues such as liver, adipose and muscle to normal insulin levels [2][3]. Increased level of free fatty acids (FFAs) has been proposed as an important etiology of insulin resistance [2][4]-[7]. It has been shown that lipid oversupply can induce insulin resistance in muscle and liver, which is mediated by increased level of FFAs [2][8][9]. The activity of gluconeogenesis pathway is increased in insulin resistance and type 2 diabetes resulting in increased fasting hepatic glucose production [10]. Given the destructive role of increased gluconeogenesis in diabetic patients, phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase), two key enzymes in gluconeogenesis, would most likely be affected [4][6]. Unfortunately, until now, the synthetic drugs could not completely overcome diabetes complications [11].
In the last decades, a large number of studies focused on introducing herbal drugs for the treatment of diabetes, but the simultaneous effect of herbal and synthetic drugs on the hepatic cells could be given outstanding results [12]. It has been reported that the green tea is one of the important herbal agents that can lower the blood sugar [5]. Epigallocatechin gallate (EGCG) is the most important fractions of green tea, which has been shown to decrease the blood sugar. Thiazolidinediones (TZDs), the peroxisome proliferator-activated receptor γ (PPARγ) agonists, are one of the insulin-sensitive drugs that have been used for the treatment of type 2 diabetes [4][13]. At the molecular level, TZDs activate PPARγ, a ligand-activated nuclear transcription factor that modulates the expression of a number of genes encoding proteins involved in glucose and lipid metabolism. PPARγ receptors are most strongly expressed in adipose tissue and the vascular wall, with secondary benefits on insulin sensitivity in skeletal muscle and liver. It has been reported that pioglitazone enhances glucose uptake by muscle and adipose tissue, and to a lesser degree, reduces hepatic gluconeogenesis. It also promotes adipogenesis, resulting in an increased uptake of FFAs and glucose, and a decreased release of FFAs into the circulation [14], leading to reduced hepatic and muscular FFA flux and further reduction of gluconeogenesis, and increased glucose uptake. More recent evidence also suggests that pioglitazone improves short-and long-term pancreatic beta cell function [8], thus reducing the functional stress associated with chronic hyperinsulinemia in type 2 diabetes. However, the simultaneous effects of EGCG and pioglitazone, in hepatic cell gluconeogenesis, have not been studied yet, and this study aimed to investigate the simultaneous effects of EGCG and pioglitazone on gluconeogenesis pathway in palmitate-induced insulin resistant HepG2 cells.
Methods
Cell culture
HepG2 cells were purchased from The Pasteur Institute of Iran and were cultured in low-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Berlin, Germany) supplemented with 10% FBS,2 mM glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin and maintained under 5% CO2 and 37°C.
Palmitate treatment
Insulin resistance was induced by palmitate treatment as described in previous studies [2][15]-[20]. In brief, sodium palmitate was dissolved in prewarmed 50% (v/v) ethanol, then diluted in prewarmed DMEM containing 1% (w/v) fatty acid-free BSA to final concentration and placed in an incubator for 2 hr with shaking. The same concentration of ethanol mixed BSA (1%) was administrated to control cells. EGCG and pioglitazone were added 2–3 hr before the incubation of the cells with palmitate.
Gene expression analysis
Total RNA was extracted using RNeasy mini kit. Total RNA (1 μg) was reverse transcribed using Fermentase reverse transcriptase. Real-time PCR was conducted using a RotorGene Q instrument (Corbett Research, Australia). The complementary DNA was amplified in duplicate using QuantiTect primers and QuantiFast SYBR Green PCR Master Mix. The data were normalized against β-actin transcript level and analyzed by delta-delta Ct method. The primer sequences of target genes used in this study are listed in Table 1.
Table 1.
Primer sequence of target genes
| Forward 5 to 3 | Reverse 5 to 3 | |
|---|---|---|
| PEPCK | TGACAACTGCTGGTTGGCT | TGGTGCGACCTTTCATGC |
| G6Pase | GGGAAAGATAAAGCCGACCTAC | CAGCAAGGTAGATTCGTGACAG |
| β-actin | TTCTACAATGAGCTGCGTGTG | GGGGTGTTGAAGGTCTCAAA |
Glucose production assay
Glucose production assay was used to evaluate the activity of gluconeogenesis pathway [21]. Briefly, cells were seeded in 24-well plates and washed three times with PBS to remove glucose, incubated with 1 nM insulin for 16 hr in 300 μl of glucose production medium (glucose- and phenol red-free DMEM containing gluconeogenic substrates, 20 mM sodium lactate, and 2 mM sodium pyruvate). A quantity of 250μl of the medium was sampled for the measurement of glucose concentration using a glucose assay kit (Sigma–Aldrich). Glucose concentration was normalized with the total protein content determined from the whole cell lysates.
Statistical Analysis
The data are presented as mean ± SD of at least three independent experiments. The statistical analyses were applied using SPSS 19.0 (SPSS, Chicago, IL). Comparisons between all groups were performed with one-way analysis of variance (ANOVA) test. If significant differences were found, Tukey’s post hoc test was applied. Values of p<0.05 were considered statistically significant.
Results
EGCG and pioglitazone treatment reduced gluconeogenesis activity in insulin-resistant HepG2 cells
The mRNA level of G6Pase and PEPCK genes were measured to evaluate the effect of EGCG and pioglitazone on gluconeogenesis. First, the cells were incubated in the presence of 0.5 mM palmitate to induce insulin resistance. As shown in Fig. 1, palmitate increased mRNA level of G6Pase and PEPKC by 58 and 30% respectively compared to control. EGCG 40 μM reduced the expression of PEPCK and G6Pase by 53 and 67%, respectively (Fig. 1 and Fig. 2). As shown in Fig. 1 and 2, 10 μM pioglitazone reduced the mRNA level of PEPCK and G6Pase by 58 and 62%, respectively. Combined treatment of insulin-resistant cells with EGCG and pioglitazone significantly reduced the mRNA level of PEPCK and G6Pase by 73 and 80%, respectively (Fig. 1 and Fig. 2).
Fig. 1.
The effect of EGCG and pioglitazone on mRNA level of PEPCK. HepG2 cells were incubated for 24 h with palmitate (0.5 mM), 10 μM pioglitazone and 40 μM EGCG. Total RNA extracted, and then, the level of PEPCK expression was determined by real-time PCR and normalized to endogenous β-actin. *p < 0.05 versus untreated cells, **p < 0.05 versus just palmitate-treated cells, ≠<0.05 Combined treated cells versus the cells individually treated with palmitate and EGCG or Pioglitazone. The figure shows representative data gained from mean ± SD of three independent experiments.
Fig. 2.
The effect of EGCG and pioglitazone on mRNA level of G6Pase. HepG2 cells were incubated for 24 h with palmitate (0.5 mM), 10 μM pioglitazone and 40 μM EGCG. Total RNA extracted, and then, the level of G6Pase expression was determined by real-time PCR and normalized to endogenous β-actin. *p < 0.05 versus untreated cells, **p < 0.05 versus just palmitate-treated cells, ≠<0.05 Combined treated cells versus the cells individually treated with palmitate and EGCG or Pioglitazone. The figure shows representative data gained from mean ± SD of three independent experiments.
EGCG and Pioglitazone significantly reduced glucose production in HepG2 cells under insulin resistance condition
As shown in Fig. 3, the treatment of the cells with 0.5 mM palmitate increased glucose production by 50% of the control. When the cells were pretreated alone with EGCG and pioglitazone, the glucose production reduced by 50 and 55%, respectively. The combined treatment with EGCG and pioglitazone resulted in 69% reduction in glucose production compared to control HepG2 cells (Fig. 3).
Fig. 3.
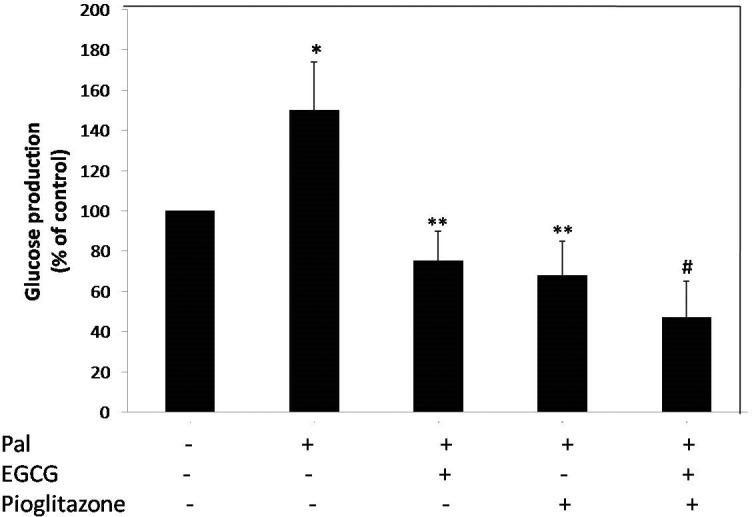
The effect of EGCG and pioglitazone on glucose production in HepG2 cells. The cells were incubated for 24 h with palmitate (0.5 mM), 10 μM pioglitazone and 40 μM EGCG in serum-free DMEM. After incubation for 24 h, the medium was replaced with glucose-free DMEM, and then the cells were incubated with 1 nM insulin for 16 h. The glucose level was determined using a glucose assay kit as described in the Materials and methods section. *p < 0.05 versus untreated cells, **p < 0.05 versus just palmitate-treated cells, ≠<0.05 Combined treated cells versus the cells individually treated with palmitate and EGCG or Pioglitazone. The figure shows representative data gained from mean ± SD of three independent experiments.
Discussions
Insulin resistance is the most important feature of T2DM, which is characterized by reducing sensitivity or responsiveness of muscle, liver, and adipose tissue to the metabolic actions of insulin [22][23]. Increased plasma levels of FFA in obesity and T2DM has been shown to be correlated with insulin resistance [15]-[17][24][25]. Elevated hepatic glucose production is one of the pathophysiological consequences of insulin resistance in the liver, which is the inability of insulin to inhibit hepatic glucose production or gluconeogenesis [4][7][22][26][28]. Different approaches have been suggested to attenuate the insulin resistance-induced hepatic glucose production, including synthetic and herbal drugs. In the present study, we aimed to examine the simultaneous effect of EGCG and pioglitazone on gluconeogenesis activity in HepG2 cells under insulin resistance condition. We first showed that palmitate causes insulin resistance in HepG2 cells. This finding is in agreement with the previous study [2]. Then we investigated the role of insulin resistance on key regulators of gluconeogenesis pathway, G6Pase and PEPCK [29]. IR significantly increased the expression of both enzymes compared to control. This finding was in line with the previous study [30][31].
In the next step, we determined the effect of EGCG on glucose production and expression level of G6Pase and PEPCK and in HepG2 cells under IR condition. EGCG is the main catechin of green tea that has an inhibitory effect on gluconeogenesis, and previous studies have shown a negative effect of EGCG on gluconeogenesis pathway [32]. Waltner-Law and colleagues reported that EGCG could repress hepatic glucose production in H4IIE rat hepatoma cells [33]. Collins et al. also reported that lower concentrations of EGCG could suppress hepatic gluconeogenesis through an independent insulin-signaling pathway by stimulation of 5' AMP-activated protein kinase (AMPK) [32]. It has also been reported that EGCG can increase insulin secretion and sensitivity in β and liver cells respectively [34][35] and inhibit hepatic gluconeogenesis in an insulin-independent manner [36]. Li et al. showed that EGCG ameliorated FFAs-induced peripheral insulin resistance in vivo, and this might be through decreasing oxidative stress and protein kinase Cθ (PKCθ) membrane translocation, activating the AMPK pathway and improving insulin-signaling pathway in vivo [22]. Cordero-Herrera et al. showed that Cocoa and epicatechin decreased PEPCK gene expression in HepG2 cells. They found that Cocoa and epicatechin had insulin-like effects and decreased glucose production and suppressed PEPKC through protein kinase B (PKB, also known as AKT) and AMPK pathways [37]. Similar findings were also reported for EGCG both in vitro and in vivo [38][39]. In agreement with these studies, we also found that EGCG down-regulates the expression of PEPCK and G6Pase and reduces glucose production under IR condition in HepG2 cells. Previous studies have shown that thiazolidinediones activate PPARs that are extensively expressed in adipose tissue, vascular endothelium, muscles, and liver and sensitize liver and muscle to insulin action [40]. Thus, we evaluated the effect of pioglitazone on gluconeogenesis pathway and glucose production in HepG2 cells under IR condition. In 2004, Scheen et al. reported that pioglitazone is an oral antihyperglycemic drug for the treatment of type 2 diabetes mellitus [41]. Pioglitazone was also shown to improve the long and short-term beta cell function and increase the sensitivity of liver, adipose tissue, and muscular system to insulin [8][14][42]. It has been reported that PPARγ stimulation results in increased glucose consumption and insulin sensitivity and decreased glucose production [10]. It has also been reported that pioglitazone improved glucose absorption by muscular and adipose tissues and decreased hepatic gluconeogenesis through downregulation of PEPKC and G6Pase [43]. An animal study reported that troglitazone resulted in a dose-dependent reduction in glucagon-stimulated gluconeogenesis in the absence of insulin. In addition, combined treatment of troglitazone with insulin produced an additive inhibition of gluconeogenesis during glucagon-stimulated conditions [38]. These findings are in line with ours, in which thiazolidinedione decreased gene expressions. As reported by these studies, we also found that pioglitazone down-regulated the expression of PEPCK and G6Pase and glucose production in HepG2 cells under insulin-resistance condition. In addition to these findings, we also evaluated the combined effect of pioglitazone and EGCG on gluconeogenesis pathway in HepG2 cells under insulin resistant condition. It has been reported that combined treatment of pioglitazone with metformin or sulfonylurea resulted in significant reduction of HbA1c and blood glucose concentration [44][45]. For the first time, we found in this study that the combined treatment of HepG2 cells with pioglitazone and EGCG results in significant reduction of PEPCK and G6Pase and glucose production compared to individual treatment in HepG2 cells under insulin resistance condition. We also found that the effect of EGCG is somehow identical to pioglitazone.
Overall, these findings extend the potential utility of combined treatment of HepG2 cells with pioglitazone and EGCG for the treatment of diabetes complications. However, further in vivo and clinical trial studies are required to demonstrate whether this combination therapy can efficiently prevent gluconeogenic pathway and hepatic glucose production under insulin resistance condition.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hu FB. Globalization of Diabetes. The role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishii M, Maeda A, Tani S, Akagawa M. Palmitate induces insulin resistance in human HepG2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. Arch Biochem Biophys. 2015;566:26–35. doi: 10.1016/j.abb.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Robertson RP, Harmon AJ, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 4.Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- 5.Kim MJ, Yoo KH, Park HS, Chung SM, Jin CJ, Lee Y, Shin YG, Chung CH. Plasma adiponectin and insulin resistance in Korean type 2 diabetes mellitus. Yonsei Med J. 2005;45:42–50. doi: 10.3349/ymj.2005.46.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasaki K, Cripe TP, Koch SR, Andreone TL, Petersen DD, Beale EG, Granner DK. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984;259:15242–15251. [PubMed] [Google Scholar]
- 7.Wilding J. Thiazolidinediones, insulin resistance and obesity: Finding a balance. Int J Clin Pract. 2006;60:1272–1280. doi: 10.1111/j.1742-1241.2006.01128.x. [DOI] [PubMed] [Google Scholar]
- 8.Campbell IW. Pioglitazone — an oral antidiabetic agent and metabolic syndrome modulator. Can theory translate into practice? Br J Diabetes Vasc Dis. 2005;5:209–216. [Google Scholar]
- 9.Hu X, Wang S, Xu J, Wang DB, Chen Y, Yang GZ. Triterpenoid saponins from Stauntonia chinensis ameliorate insulin resistance via the AMP-activated protein kinase and IR/IRS-1/PI3K/Akt pathways in insulin-resistant HepG2 cells. Int J Mol Sci. 2014;15:10446–10458. doi: 10.3390/ijms150610446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 11.Larsen TM, Toubro S, Astrup A. PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord. 2003;27:147–161. doi: 10.1038/sj.ijo.802223. [DOI] [PubMed] [Google Scholar]
- 12.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology. 2013;4:177–177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblatt S, Miskin B, Glazer NB, Prince MJ, Robertson KE. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron Artery Dis. 2001;12:413–423. doi: 10.1097/00019501-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Tan MH, Baksi A, Krahulec B, Kubalski P, Stankiewicz A, Urquhart R, Edwards G, Johns D. Comparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with type 2 diabetes. Diabetes Care. 2005;28:544–550. doi: 10.2337/diacare.28.3.544. [DOI] [PubMed] [Google Scholar]
- 15.Bakhtiyari S, Haghani K, Basati G, Karimfar MH. siRNA therapeutics in the treatment of diseases. Ther Deliv. 2013;4:45–57. doi: 10.4155/tde.12.136. [DOI] [PubMed] [Google Scholar]
- 16.Bakhtiyari S, Meshkani R, Taghikhani M, Larijani B, Adeli K. Protein tyrosine phosphatase-1B (PTP-1B) knockdown improves palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Lipids. 2010;45:237–244. doi: 10.1007/s11745-010-3394-3. [DOI] [PubMed] [Google Scholar]
- 17.Gorgani-Firuzjaee S, Bakhtiyari S, Golestani A, Meshkani R. Leukocyte antigen-related inhibition attenuates palmitate-induced insulin resistance in muscle cells. J Endocrinol. 2012;215:71–77. doi: 10.1530/JOE-12-0160. [DOI] [PubMed] [Google Scholar]
- 18.Haghani K, Pashaei S, Vakili S, Taheripak G, Bakhtiyari S. TNF-alpha knockdown alleviates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Biochem Biophys Res Commun. 2015;460:977–982. doi: 10.1016/j.bbrc.2015.03.137. [DOI] [PubMed] [Google Scholar]
- 19.Parvaneh L, Meshkani R, Bakhtiyari S, Mohammadtaghvaie N, Gorganifiruzjaee S, Taheripak G, Golestani A, Foruzandeh M, Larijani B, Taghikhani M. Palmitate and inflammatory state additively induce the expression of PTP1B in muscle cells. Biochem Biophys Res Commun. 2010;396:467–471. doi: 10.1016/j.bbrc.2010.04.118. [DOI] [PubMed] [Google Scholar]
- 20.Taheripak G, Bakhtiyari S, Rajabibazl M, Pasalar P, Meshkani R. Protein tyrosine phosphatase 1B inhibition ameliorates palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells. Free Radic Biol Med. 2013;65:1435–1446. doi: 10.1016/j.freeradbiomed.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Gao D, Nong S, Huang X, Lu Y, Zhao H, Lin Y, Man Y, Wang S, Yang J, Li J. The Effects of Palmitate on Hepatic Insulin Resistance Are Mediated by NADPH Oxidase 3-derived Reactive Oxygen Species through JNK and p38MAPK Pathways. J Biol Chem. 2010;285:29965–29973. doi: 10.1074/jbc.M110.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Zhao S, Zhang W, Zhao P, He B, Wu N, Han P. Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes Research and Clinical Practice. 2011;93:205–214. doi: 10.1016/j.diabres.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tripathy D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care. 2009;32:S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karimfar MF, Haghani K, Babakhani A, Bakhtiyari S. Rosiglitazone, but not epigallocatechin-3-gallate, attenuates the decrease in PGC-1alpha protein levels in palmitate-induced insulin-resistant C2C12 cells. Lipids. 2015;50:521–528. doi: 10.1007/s11745-015-4016-x. [DOI] [PubMed] [Google Scholar]
- 25.Meshkani R, Sadeghi A, Taheripak G, Zarghooni M, Gerayesh-Nejad S, Bakhtiyari S. Rosiglitazone, a PPARgamma agonist, ameliorates palmitate-induced insulin resistance and apoptosis in skeletal muscle cells. Cell Biochem Funct. 2014;32:683–691. doi: 10.1002/cbf.3072. [DOI] [PubMed] [Google Scholar]
- 26.Boden G, Cheung P, Stein TP, Kresge K, Mozzoli M. FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am J Physiol Endocrinol Metab. 2002;283:E12–E19. doi: 10.1152/ajpendo.00429.2001. [DOI] [PubMed] [Google Scholar]
- 27.Clore JN, Allred J, White D, Li J, Stillman J. The role of plasma fatty acid composition in endogenous glucose production in patients with type 2 diabetes mellitus. Metabolism. 2002;51:1471–1477. doi: 10.1053/meta.2002.35202. [DOI] [PubMed] [Google Scholar]
- 28.Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab. 2003;284:E863–E873. doi: 10.1152/ajpendo.00033.2003. [DOI] [PubMed] [Google Scholar]
- 29.Kuo T, McQueen A, Chen TC, Wang JC. Regulation of Glucose Homeostasis by Glucocorticoids. Adv Exp Med Biol. 2015;872:99–126. doi: 10.1007/978-1-4939-2895-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cang X, Wang X, Liu P, Wu X, Yan J, Chen J, Wu G, Jin Y, Xu F, Su J, Wan C, Wang X. PINK1 alleviates palmitate induced insulin resistance in HepG2 cells by suppressing ROS mediated MAPK pathways. Biochemical and Biophysical Research Communications. 2016;478:431–438. doi: 10.1016/j.bbrc.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Tang Z, Zhang W, Wan C, Xu G, Nie X, Zhu X, Xia N, Zhao Y, Wang S, Cui S, Wang C. TRAM1 protect HepG2 cells from palmitate induced insulin resistance through ER stress-JNK pathway. Biochemical and Biophysical Research Communications. 2015;457:578–584. doi: 10.1016/j.bbrc.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5'-AMP-activated protein kinase. J Biol Chem. 2007;282:30143–30149. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem. 2002;277:34933–34940. doi: 10.1074/jbc.M204672200. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, Collins HW, Matschinsky FM, Stanley CA, Smith TJ. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J Biol Chem. 2006;281:10214–10221. doi: 10.1074/jbc.M512792200. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RA, Polansky MM. Tea enhances insulin activity. J Agric Food Chem. 2002;50:7182–7186. doi: 10.1021/jf020514c. [DOI] [PubMed] [Google Scholar]
- 36.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab. 2007;292:E1378–E1387. doi: 10.1152/ajpendo.00698.2006. [DOI] [PubMed] [Google Scholar]
- 37.Cordero-Herrera I, Chen X, Ramos M, Devaraj M. (-)-Epicatechin attenuates high-glucose-induced inflammation by epigenetic modulation in human monocytes. Eur J Nutr. 2015 doi: 10.1007/s00394-015-1136-2. [DOI] [PubMed] [Google Scholar]
- 38.Raman P, Judd RL. Role of glucose and insulin in thiazolidinedione-induced alterations in hepatic gluconeogenesis. Eur J Pharmacol. 2000;409:19–29. doi: 10.1016/s0014-2999(00)00806-2. [DOI] [PubMed] [Google Scholar]
- 39.Yasui K, Tanabe H, Miyoshi N, Suzuki T, Goto S, Taguchi K, Ishigami Y, Paeng N, Fukutomi R, Imai S, Isemura M. Effects of (-)-epigallocatechin-3-O-gallate on expression of gluconeogenesisrelated genes in the mouse duodenum. Biomed Res. 2011;32:313–320. doi: 10.2220/biomedres.32.313. [DOI] [PubMed] [Google Scholar]
- 40.Quinn CE, Hamilton PK, Lockhart CJ, McVeigh GE. Thiazolidinediones: effects on insulin resistance and the cardiovascular system. Br J Pharmacol. 2008;153:636–645. doi: 10.1038/sj.bjp.0707452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheen AJ. Combined thiazolidinedione-insulin therapy: should we be concerned about safety? Drug Saf. 2004;27:841–856. doi: 10.2165/00002018-200427120-00002. [DOI] [PubMed] [Google Scholar]
- 42.Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y, DeFronzo RA. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52:1364–1370. doi: 10.2337/diabetes.52.6.1364. [DOI] [PubMed] [Google Scholar]
- 43.Campbell IW. The Clinical Significance of PPAR Gamma Agonism. Curr Mol Med. 2005;5:349–363. doi: 10.2174/1566524053766068. [DOI] [PubMed] [Google Scholar]
- 44.Einhorn D, Rendell M, Rosenzweig J, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled study. The Pioglitazone 027 Study Group. Clin Ther. 2000;22:1395–1409. doi: 10.1016/s0149-2918(00)83039-8. [DOI] [PubMed] [Google Scholar]
- 45.Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111:10–17. doi: 10.1016/s0002-9343(01)00713-6. [DOI] [PubMed] [Google Scholar]




