Abstract
One of the most insidious features of cocaine addiction is a high rate of relapse even after extended periods of abstinence. A wide variety of drug-associated stimuli, including the context in which a drug is taken, can gain incentive motivational properties that trigger drug desire and relapse to drug-seeking. Both animal and clinical studies suggest that extensive cocaine exposure may induce a transition from cortical to striatal control over decision-making as compulsive drug-seeking emerges. Using an animal model of relapse to cocaine-seeking, the present study investigated the expression patterns of three different activity-related genes (c-fos, zif/268, and arc) in cortical and striatal brain regions implicated in compulsive drug-seeking in order to determine the neuroadaptations that occur during context-induced relapse following brief or prolonged abstinence from cocaine self-administration. Re-exposure to the environment previously associated with cocaine self-administration following 22 h or 15 days of abstinence produced a significant increase in zif/268 and arc, but not c-fos mRNA, in the caudate-putamen and nucleus accumbens. With the exception of arc mRNA levels following 15 days of abstinence, all three genes were increased in the anterior cingulate cortex of animals with a cocaine history when they were re-exposed to the operant chamber. Additionally, c-fos, zif/268, and arc expression was differentially affected in the motor and sensory cortices at both timepoints. Together, these results support convergent evidence that drug-seeking induced by a cocaine-paired context changes the activity of corticostriatal circuits.
Keywords: Arc, C-fos, Cingulate cortex, Cocaine, Relapse, Self-administration, Striatum, Zif/268
Introduction
Cocaine addiction is a progressive disease in which individuals become increasingly focused on obtaining the drug, even in the face of reduced positive reinforcement and the presence of detrimental consequences. One of the most refractive components of cocaine addiction is the high degree to which addicts return to drug-seeking (Gawin 1991; O'Brien et al. 1998). This relapse behavior is often associated with feelings of drug craving triggered by environmental cues that have become associated with the drug over time (Ehrman et al. 1992). Likewise, in animal models of relapse, re-exposure to a conditioned context previously associated with drug self-administration is sufficient to trigger drug-seeking behavior, even after prolonged periods of abstinence (Crombag and Shaham 2002; Fuchs et al. 2005, 2006). In fact, the ability of a cocaine-paired context to elicit drug-seeking may actually increase during the first several months of abstinence in animals whose behavior has not been specifically extinguished (Neisewander et al. 2000; Lu et al. 2004). The critical brain areas and neurobiological substrates underlying this phenomenon are unknown but it is likely that a combination of many associative and sensory-motor circuits mediate this conditioned behavior based on past experience. Thus, like other forms of learning, it is apparent that relapse to drug-seeking involves the formation and retrieval of context-associated memories (Hyman 2005) that are processed by interconnected cortical and subcortical structures over time (Macey et al. 2004).
Frontal corticostriatal projections mediate many aspects of reward-related learning that eventually become automated. As associative learning becomes more habitual in nature, activity in the corticostriatal network shifts from a prefrontal cortical-dorsomedial striatal circuit to a sensorimotor cortical-dorsolateral striatal circuit (Jog et al. 1999; Packard and Knowlton 2002; White and McDonald 2002; Balleine et al. 2007). Since the process of addiction is thought to reflect the progressive development of compulsive behavior and/or loss of inhibitory control over impulsive behavior (Tiffany 1990; Jentsch and Taylor 1999; Everitt et al. 2001; Vanderschuren and Everitt 2004), the role of the striatum, particularly the dorsolateral caudate-putamen (dlCPu), in the compulsive aspects of addiction has become a major focus of study (Everitt and Wolf 2002; Ito et al. 2002; Everitt and Robbins 2005). Thus, extensive cocaine exposure may induce a transition from corticolimbic to corticostriatal control over decision-making and choice behavior as compulsive drug-seeking emerges (Porrino et al. 2004; Volkow et al. 2006).
As alluded to above, the duration of abstinence may be a critical determinant of the motivation to seek cocaine. Cortical and striatal activation in response to cocaine-paired cues has been observed after varying periods of abstinence (Breiter and Rosen 1999; Childress et al. 1999; Volkow et al. 2006). Because the variable durations of drug-taking and abstinence in clinical studies may underlie differences in the brain areas activated by cue- or context-induced drug-seeking, in this study, we investigated whether distinct cortical and striatal regions would be activated by re-exposure to a cocaine-paired environment after short (22 h) or long (15 days) periods of abstinence from cocaine exposure.
Induction of immediate early genes (IEGs) is commonly used to identify brain regions activated by psychostimulants. IEGs, like the transcription factors, c-fos and zif/268, and the effector, activity-regulated cytoskeleton-associated gene (arc), contribute to the promotion and maintenance of synaptic plasticity (Rial Verde et al. 2006), associative learning (Davis et al. 2003; Malkani et al. 2004), and long-term storage and retrieval of memories (Guzowski et al. 2000; Hall et al. 2001; Thomas et al. 2002; Bozon et al. 2003; Plath et al. 2006). The expression of all of these IEGs is transiently induced by cocaine (Graybiel et al. 1990; Young et al. 1991; Moratalla et al. 1992; Bhat and Baraban 1993; Daunais and McGinty 1994, 1995; Fosnaugh et al. 1995; Tan et al. 2000; Fumagalli et al. 2006) and cocaine-associated cues (Brown et al. 1992; Crawford et al. 1995; Everitt and Robbins 2000; Neisewander et al. 2000; Ciccocioppo et al. 2001; Thomas et al. 2003: Zavala et al. 2007). Moreover, cocaine-induced behavioral sensitization and conditioned place preference are absent in zif/268 knockout mice (Valjent et al. 2006). However, because IEGs are regulated by complex neurotransmitter interactions, they are distinguished by differences in their induction thresholds and responses to stimuli (Worley et al. 1993; West et al. 2002). Thus, in this study, alterations in c-fos, zif/268, and arc mRNA expression were used to identify activation of associative and sensory-motor cortices as well as their striatal targets in response to a cocaine-associated context after a short or long period of abstinence. Further, to determine whether instrumental responding affected IEG responses in different brain regions, rats were either allowed to press a lever previously associated with cocaine or saline delivery or they were prevented from making this instrumental response in a chamber where the levers were retracted.
Materials and methods
Animals
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 300–325 g at the start of the experiment, were individually housed in a temperature- and humidity-controlled environment on a reversed light/dark cycle. Rats received *25 g of rat chow per day, maintaining them at approximately 85–90% of free feeding body weight, and were allowed water ad libitum. The housing and treatment of the rats were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996). Formal approval to conduct the experiments was obtained from the MUSC IACUC.
Experimental design
Details of the self-administration and relapse to cocaine-seeking methodology have been previously described (Hearing et al. 2008) for the cohort of rats in experiments 1 and 2 from which the in situ hybridization data in this publication was derived. Only essential behavioral information is provided below to facilitate understanding of the experiments. The experimental design is illustrated in Fig. 1a. Following 10 days of cocaine (0.6 mg/kg, i.v.) self-administration or yoked-saline administration during daily 2 h sessions, the rats remained drug-free for 22 h (experiment 1) or 15 days (experiment 2) before being returned to the operant chamber for 1 h. In order to assess whether changes in gene expression were independent of instrumental behavior and to better isolate the effects of context-recognition on gene expression, levers previously associated with cocaine (active) or saline delivery (inactive), or those previously associated with no consequences were either presented or were retracted for the duration of the rat's re-exposure to the self-administration chamber in both experiments. In experiment 2, after 7 days of abstinence in the home cage, all rats were transported to a distinctly different procedure room (alternate environment) and were placed in clear Plexiglas cages for 2 h/day for 7 days. The “alternate environment” group was included in this experiment to control for any IEG induction due to transporting rats to a non-homeroom environment and to equalize the handling of animals among all groups. On day 15 of abstinence, one-third of the saline and cocaine-treated rats were placed in the alternate environment while the remainder of the subjects were re-exposed to the self-administration chamber with or without the levers available for 1 h. Lever presses were recorded, but had no programmed consequences. At the end of the 1 h test session, rats were anesthetized with Equithesin and the brains removed and frozen for in situ hybridization. Using this experimental design, we were able to assess the effects on gene expression of a reward-paired context, a context where no learning of reward contingencies had occurred (yoked-saline), and a context that has minimal conditioned associations or novelty (alternate environment) on activity-regulated gene expression.
Fig. 1.
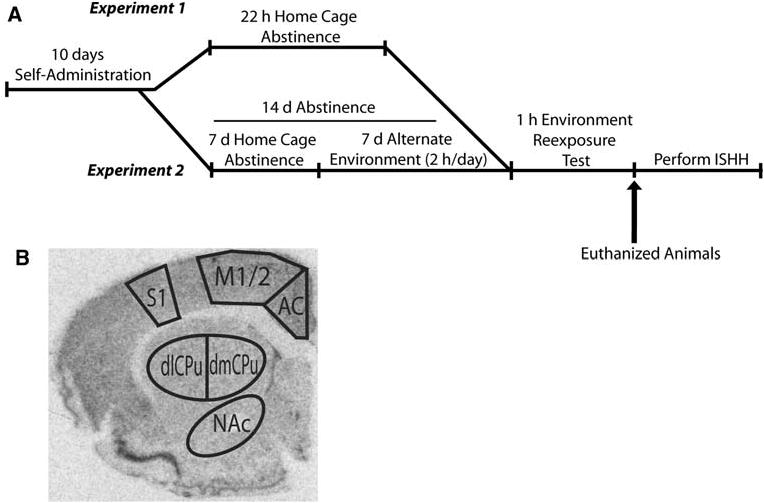
a Schematic representing the experimental design for experiment 1 and 2 (adapted from Hearing et al. 2008). SA and CA rats were not included in the design of experiment 1 because exposure to an alternate environment on day 1 of abstinence would have constituted a novel experience. In experiment 2, all rats were habituated to the alternate environment before the test day. b Audioradiographic image from tissue labeled with 35S-dATP-labeled zif/268 oligonucleotide illustrating brain regions analyzed. Regions of interest selected for measurement according to Paxinos and Watson 2007. AC anterior cingulate, dlCPu dorsolateral striatum, dmCPu dorsomedial striatum, M1/2 motor1/2 cortex, NAc nucleus accumbens core, S1FL sensory 1 forelimb cortex, SN saline alternate environment, CA cocaine alternate environment
In situ hybridization histochemistry
In situ hybridization histochemistry was performed as described previously (Hearing et al. 2008) using 35S-dATP-labeled 48 mer antisense oligodeoxynucleotide probes [Integrated DNA Technologies (IDT), Coralville, IN] complementary to rat c-fos, zif/268, and arc mRNA (see Hearing et al. 2008 for sequence details). Twelve μm tissue sections cut through the anterior-posterior extent of the striatum were mounted onto slides and pretreated, hybridized, and washed. Hybridized slides were dried and placed in an X-ray film cassette along with 14C standards (ARC, St Louis, MO) and Biomax MR film (Eastman Kodak, Rochester, NY). Films were developed at several time intervals in order to establish linearity and an optimal signal:noise ratio.
Image analysis
Quantitation of film autoradiograms was performed using the Macintosh-based NIH Image program as previously described (Wang and McGinty 1995; Hearing et al. 2008). The mean density and number of pixels per area were measured in the selected cortical and subcortical areas (Fig. 1b, nomenclature of Paxinos and Watson 2007) independently from three adjacent sections per rat. The measurements were expressed as integrated density (number of pixels per area × mean density).
Statistical analysis
The number of lever responses during the 1 h test session was analyzed for total active and inactive lever responding using a one-way ANOVA. Statistically significant interaction effects were further investigated using Tukey's honestly significant difference (HSD) tests. Measurements of the hybridization signals (integrated density) were strongly correlated and could not be treated independently. To account for this correlation, the data were fit with a hierarchical linear model using integrated density (ID) as the response variable with treatment and environment (as well as a treatment by environment interaction) as fixed effects explanatory variables and rat as a random effect (mixed model SAS 9.1) followed by planned multiple comparison tests [Tukey−Kramer HSD (experiment 1 in which less than 4 pairwise comparisons were made) or a Bonferonni correction (experiment 2 in which more than 4 pairwise comparisons were made) ] when an interaction was found or to further analyze the source of main effects. A Pearson's correlation coefficient between total lever pressing during the 1 h test and gene expression in the cocaine-levers available groups in experiments 1 and 2 was determined by a simple regression analysis using GraphPad Prism 4.
Results
Cocaine self-administration and cocaine-seeking 22 h and 15 days after the end of self-administration
Experiment 1
The mean (±SEM) cocaine intake for the last 3 days of self-administration was 21.4 ± 2.3 mg/(kg day). The mean (±SEM) number of active lever responses over the final 3 days of self-administration for cocaine-treated animals was 40.87 ± 4.0, which was significantly different from yoked-saline active lever responding [7.53 ± 1.5; F(1,28) = 66.79, P < 0.0001] during self-administration (Fig. 2a). The mean (±SEM) number of active lever responses during the 1 h test period was significantly greater in cocaine-treated animals than yoked-saline rats [49 ± 6.4 vs. 6.8 ± 2.3; F(1,13) = 43.04, P < 0.0001; Fig. 2b]. Inactive lever pressing was not significantly different during self-administration or testing between cocaine and yoked-saline animals (for details, see Hearing et al. 2008).
Fig. 2.
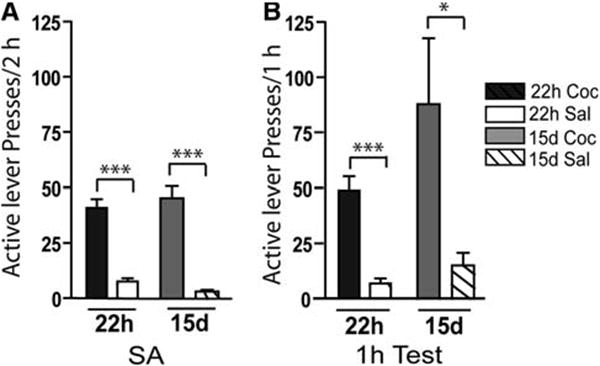
Active lever pressing during self-administration and relapse testing. a Mean number of active lever presses from the last 3 days of 2 h self-administration (SA) sessions for the 22 h abstinence group (experiment 1 left) and 15 day abstinence group (experiment 2 right) of cocaine and yoked-saline treated animals. b Mean number of active lever presses during the 1 h test session (test) of cocaine and yoked-saline treated animals after 22 h (left) and 15 days of abstinence (right). *P < 0.05, ***P < 0.001; cocaine versus saline
Experiment 2
The mean (±SEM) cocaine intake for the last 3 days of self-administration was 17.4 ± 1.4 mg/(kg day). The mean (±SEM) number of active lever responses over the last 3 days of self-administration was 46.13 ± 5.4 for cocaine-treated rats, which was significantly greater than yoked-saline active lever responding [5.10 ± 0.84; F(1,28) = 41.03, P < 0.0001; Fig. 2a]. After a 15 day abstinence period, the mean (±SEM) number of active lever responses during the 1 h test period was significantly greater in cocaine-treated animals than yoked-saline rats [87 ± 29.6 vs. 15 ± 5.7; F(1,8) = 5.83, P = 0.04; Fig. 2b]. Inactive lever pressing during the 1 h test was significantly greater in animals with a cocaine history compared to yoked-saline treated animals (for details, see Hearing et al. 2008). This difference in inactive lever responding likely represents an alternative means of obtaining drug when the lever previously associated with cocaine self-administration no longer provides the drug (Fuchs et al. 2006; Berglind et al. 2007).
Experiment 1: re-exposure to a cocaine-paired environment 22 h after the end of cocaine or yoked-saline administration increased cortical and striatal IEG expression
Anterior cingulate, motor, and sensory cortex
Arc, zif/268, and c-fos expression was induced in the anterior cingulate (AC) cortex of rats that were re-exposed to the operant chamber previously associated with cocaine, independent of lever availability, 22 h after the end of cocaine or yoked-saline administration (Fig. 3a–c). Oneway ANOVA revealed a significant main effect of treatment (cocaine, saline) for all IEGs [arc (F(1,23) = 125.98, P < 0.0001); zif/268 (F(1,24) = 21.73, P < 0.0001); c-fos (F(2,27) = 16.72, P < 0.0004) ]. Tukey–Kramer multiple comparison tests revealed significantly higher mRNA levels of all three genes within the AC of cocaine no (CN) lever available and cocaine lever (CL) available groups as compared to saline no (SN) lever available and saline lever (SL) available groups, respectively (Fig. 3d, f, h, left). In the M1/2 motor cortex, re-exposure to the operant chamber significantly increased IEG expression of rats with a cocaine history independent of lever availability. One-way ANOVA revealed a significant main effect of drug treatment for arc [F(1,25) = 72.60, P < 0.0001], zif/268 [F(1,24) = 24.41, P < 0.0001], and c-fos [F(1,23) = 22.83, P<0.0001] mRNA levels. Tukey–Kramer multiple comparison tests revealed significantly greater levels of all three genes in M1/2 cortex of CN and CL groups compared to the SN and SL groups, respectively (Fig. 3d, f, h, middle). In the S1FL sensory cortex, re-exposure to the operant chamber increased arc, but not c-fos or zif/268 mRNA of rats with a cocaine history. One-way ANOVA revealed a significant main effect of drug treatment for arc [F(1,24) = 34.87, P = 0.0001], zif/268 [F(1,24) = 9.02, P = 0.006], and c-fos [F(1,25) = 9.59, P = 0.005]. Additionally, a significant main effect of lever (NL or LA) was seen with zif/268 [F(1,24) = 8.75, P = 0.007]. Tukey–Kramer multiple comparison tests revealed a significant increase in arc mRNA expression within the S1FL cortex of the CN and CL groups compared to SN and SL groups, respectively (Fig. 3d, f, h, right).
Fig. 3.
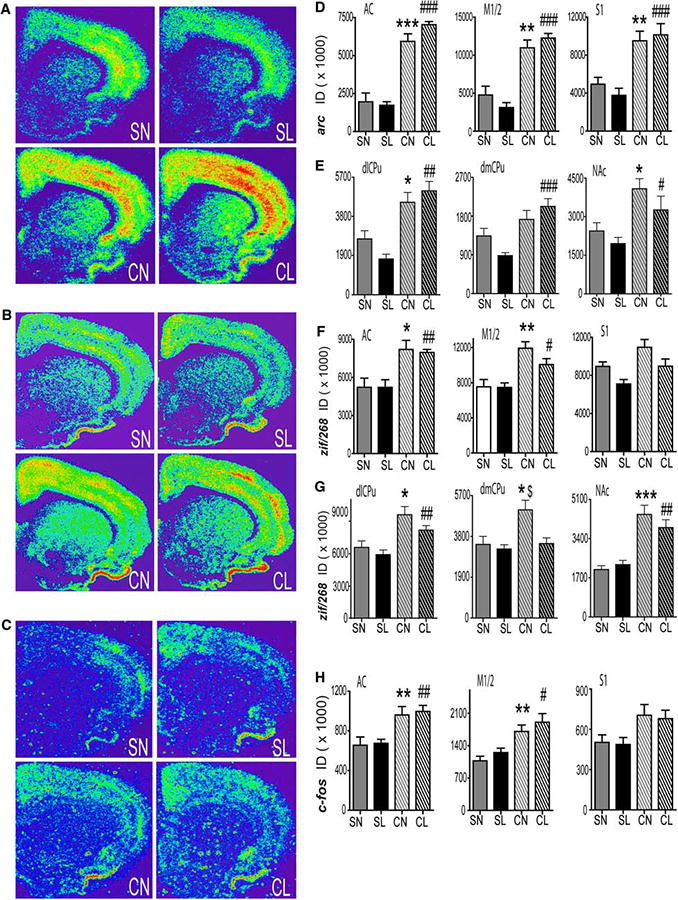
IEG expression at the end of a 1 h extinction test, 23 h after the last cocaine or yoked-saline administration. Representative coronal hemi-sections illustrating the expression pattern of (a) arc, (b) zif/268, and (c) c-fos hybridization signals. d–h Quantitative analysis of the integrated density of the hybridization signal for (d, e) arc, (f, g) zif/268 and (h) c-fos mRNA in the AC (d, f, h, left), M1/2 (d, f, h, middle), and S1 (d, f, h, right) cortices or the dlCPu (e, g, left), dmCPu (e, g, middle), and NAc core (e, g, right). N = 4–5 per group. *P < 0.05, **P < 0.01, *** P < 0.001 versus SL, ##P < 0.05, ##P < 0.01, ###P < 0.001 versus SN. ID integrated density, SL saline lever available, SN saline no lever, CN cocaine no lever, CL cocaine lever available
Striatum
Zif/268 and arc, but surprisingly, not c-fos, mRNA was induced in the dorsal and ventral striatum of rats with a cocaine history re-exposed to the operant chamber, independent of lever availability, 22 h after the end of cocaine or yoked-saline administration (Fig. 3a–c). One-way ANOVA revealed a significant main effect of treatment for both arc [F(1,24) = 39.56, P<0.0001] and zif/268 [F(1,23) = 18.26, P = 0.0003] in the dlCPu. Tukey–Kramer multiple comparison tests revealed significantly higher mRNA levels of zif/268 and arc in the dlCPu of CL and CN versus SL and SN rats, respectively (Fig. 3e, g, left). In the dmCPu, zif/268 and arc mRNA expression was differentially increased in cocaine-treated rats compared to yoked-saline rats returning to the operant chamber, depending on the availability of the lever during testing. One-way ANOVA revealed a significant interaction between drug treatment and lever availability for both arc [F(1,25) = 5.13, P = 0.03] and zif/268 [F(1,24) = 4.48, P = 0.05] mRNA levels. Tukey–Kramer multiple comparison tests revealed that arc was significantly greater in the CL group compared to the SL group whereas zif/268 mRNA was significantly higher in the CN versus SN and CL groups (Fig. 3e, g, middle). In the NAc core, zif/268 and arc mRNA was significantly greater in rats re-exposed to the operant chamber previously associated with cocaine, independent of lever availability, when compared to rats re-exposed to the operant chamber previously associated with yoked-saline infusions. One-way ANOVA revealed a significant main effect of treatment (cocaine, saline) for arc [F(1,25) = 14.29, P = 0.0009] and zif/268 [F(1,24) = 46.25, P<0.0001] mRNA. Tukey–Kramer multiple comparison tests revealed significantly higher mRNA levels of zif/268 and arc in the NAc core of CL and CN vs. SL and. SN groups, respectively (Fig. 3e, g, right).
Experiment 2: re-exposure to a cocaine-paired environment 15 days after the end of cocaine or yoked-saline administration increased cortical and striatal IEG expression
Anterior cingulate, motor, and sensory cortex
Following 15 days of abstinence, there was a more robust response of zif/268 and c-fos mRNA than arc mRNA in the AC of rats re-exposed to the cocaine-paired chamber than those subjects re-exposed to saline-paired chambers or to the alternate environment on the test day (Fig. 4a–c). Two-way ANOVA revealed a significant interaction of drug treatment (cocaine, saline) by test environment (operant chamber with levers available, operant chamber without levers available, alternate environment) for c-fos [F(2,22) = 4.28, P = 0.03] and zif/268 [F(2,22) = 4.77, P = 0.02] in the AC overlying the striatum. A significant main effect of drug treatment [F(1,20) = 17.76, P = 0.0004] and test environment [F(2,20) = 4.76, P = 0.02] was found for arc mRNA in the AC. Multiple comparison tests for arc mRNA in the AC revealed a significant difference between the CA and SA group only (Fig. 4d, f, h, left) although there was a trend toward a greater arc expression in the CL versus SL (P< 0.06). Multiple comparison tests revealed significantly greater mRNA levels of c-fos and zif/268 in the AC of CL and CN groups than in rats with a cocaine history placed in the alternate environment for testing (CA). Additionally, c-fos and zif/268 mRNA levels were higher in the CL and CN groups than in the SL and SN groups, respectively. Zif/268 mRNA was also greater in the SL group than in the saline alternate environment (SA) group. In contrast, arc mRNA levels were significantly greater in the CA than in SA animals.Inthe M1/2motorcortex,two-wayANOVArevealed a significant drug treatment by test environment interaction for c-fos [F(2,22) = 15.82, P < 0.0001]. A significant main effect of environment was found for zif/268 [F(2,21) = 5.50, P = 0.01] and a significant drug treatment [F(1,22) = 6.01, P = 0.02] and environment [F(2,22) = 10.00, P = 0.0008] main effect for arc mRNA. Multiple comparison tests revealed significantly higher levels of arc and zif/268 mRNA in the motor cortex of the CL group than in the CA group. In contrast, c-fos mRNA was significantly greater in both the CL and CN groups than in the CA group. Additionally, c-fos mRNA levels in the M1/2 cortex were significantly greater in the CL and CN groups compared to the SL and SN groups, respectively, and in CL as compared to CN animals (Fig. 4d, f, h, middle). In the S1FL sensory cortex, two-way ANOVA revealed a significant interaction of drug treatment by test environment for c-fos [F(2,20) = 4.88, P = 0.02], zif/268 [F(2,21) = 3.40, P = 0.05], and arc [F(2,20) = 4.29, P = 0.03] mRNA levels. Multiple comparison tests revealed that arc mRNA expression was significantly greater in the CL and CN groups than in the CA group and in the CL group than in the SL group with a trend toward significantly more expression in the CL versus CN group (P = 0.06). Arc expression was also greater in the sensory cortex of the SL group compared to both SN and SA groups (Fig. 4d, f, h, right). Zif/268 mRNA levels were significantly greater in the CL group than in the CA and SL groups, and in the SN than in the SA group. Multiple comparison tests revealed significantly higher levels of c-fos mRNA in both the CL and CN animals than in the CA group.
Fig. 4.
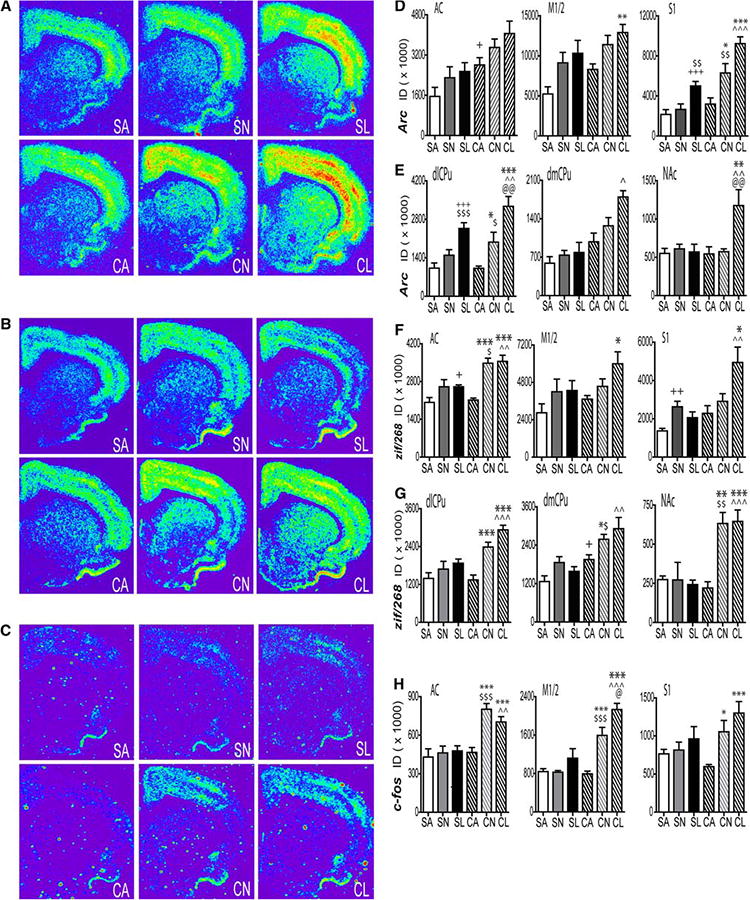
IEG expression at the end of a 1 h extinction test, 15 days after the last cocaine or yoked-saline administration. Representative coronal hemi-sections illustrating the expression pattern of (a) arc, (b) zif/268, and (c) c-fos hybridization signals. Quantitative analysis of the integrated density of the hybridization signal for (d) arc (f) zif/268 and (h) c-fos, mRNA in the rat AC (d, f, h, left), M1/2 (d, f, h, middle), and S1 (d, f, h, right) cortices or in the dlCPu (e, g, left), dmCPu (e, g, middle), and NAc core (e, g, right). N = 4–5 per group. *P<0.05,**P < 0.01, ***P<0.001 versus CA, @@P<0.01, @@@P<0.0001 versus CN, +P<0.05, +++P<0.001 versus SA, ^P<0.05,^̂P<0.01, ^̂̂P < 0.001 versus SL, $P < 0.05, $$$P < 0.001 versus SN. ID integrated density, SA saline alternate environment, SL saline lever available, SN saline no lever, CA cocaine alternate environment, CN cocaine no lever, CL cocaine lever available
Striatum
Following 15 days of abstinence from cocaine self-administration or yoked-saline infusions, there was a differential increase in zif/268 and arc, but not c-fos, mRNA in striatal subregions of rats re-exposed to the cocaine-associated chamber as compared to an alternative environment on the test day (Fig. 4a–c). Two-way ANOVA revealed a significant drug treatment by test environment interaction for both zif/268 [F(2,22) = 5.37, P = 0.01] and arc [F(2,21) = 4.23, P = 0.03] within the dlCPu. Multiple comparison tests revealed significantly higher levels of arc and zif/268 mRNA in CL and CN groups than in the CA group. Additionally, arc mRNA levels were significantly greater in CL and CN groups than in SL and SN groups, respectively. However, zif/268 mRNA was greater in the CL group than in the SL group but the comparison between the CN and SN groups did not reach significance (P < 0.07). In addition, arc mRNA levels were higher in the SL group than in the SN and SA groups (Fig. 4e, g, left). In the dmCPu, alterations in IEG expression were less prominent than those in the dlCPu. Two-way ANOVA revealed significant main effects of drug treatment [zif/268 (F(1,20) = 28.16, P<0.0001); arc (F(1,21) = 11.81, P = 0.003) ] and test environment [zif/268 (F(2,20) = 8.10, P = 0.003); arc (F(2,21) = 3.97, P = 0.03) ] on mRNA levels with no interactions. Multiple comparison tests revealed a significant increase in arc mRNA levels in the CL group than in the SL group only, (Fig. 4e, g, middle) whereas zif/268 mRNA was significantly greater in the CN group than in the CA group, and and in the CN group versus the SN group. In the NAc core, zif/268 and arc mRNA expression was significantly increased in rats re-exposed to the cocaine-paired operant chamber. The increase in zif/268 mRNA was independent of lever availability whereas changes in arc mRNA were specific to rats with access to levers during testing. Two-way ANOVA revealed a significant drug treatment by test environment interaction for zif/268 [F(2,20) = 12.79, P = 0.0001] and arc [F(2,21) = 5.08, P = 0.02] in the NAc core. Multiple comparison tests revealed that arc mRNA was significantly greater in the CL group than in the CN, CA, and SL groups (Fig. 4e, right) whereas zif/268 mRNA levels were significantly greater in CL and CN groups than in the CA group and in the CL and CN groups versus the SL and SN groups, respectively.
Behavioral correlations with gene expression
In order to determine if changes in gene expression were related to the number of lever presses as a measure of drugseeking, a correlational analysis was used to obtain a Pearson's correlation coefficient for gene expression and total lever presses during the 1 h test day session. No significant correlations were found between lever pressing and gene expression in any brain regions following 22 h of abstinence (data not shown). However, following a 15 day period of abstinence, the number of total lever presses was positively correlated with zif/268 mRNA expression within the dlCPu (Pearson's r = 0.901; P = 0.05;), dmCPu (Pearson's r = 0.954; P = 0.02), and NAc core (Pearson's r = 0.986; P = 0.007). The total number of lever presses was also significantly correlated with arc mRNA expression, in the dlCPu (Pearson's r = 0.904; P = 0.02), NAc core (Pearson's r = 0.915; P = 0.04), and the M1/2 motor cortex (Pearson's r = 0.945; P = 0.01) (Fig. 5).
Fig. 5.
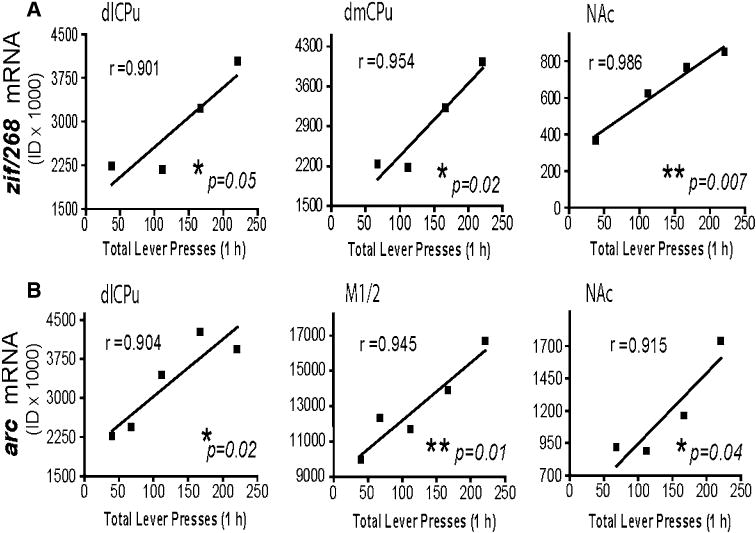
Correlations between lever pressing during relapse testing and gene expression in CL rats after 15 days of abstinence. a Zif/268 mRNA levels within the dlCPu (left), dmCPu (middle) and NAc core (right) were significantly correlated with total lever pressing. b Arc mRNA levels (ID × 1,000) in the dlCPu (left), M1/2 motor cortex (middle) and NAc (right) were significantly correlated with test day total lever pressing. *P < 0.05, **P < 0.01. ID integrated density
Discussion
The present study demonstrated that the mRNA of three activity-related genes (arc, zif/268, and c-fos) was elevated in cortical and/or striatal regions implicated in drug-seeking when cocaine abstinent rats were re-exposed to an environment associated with cocaine self-administration. The data indicate that re-exposure to a previously cocaine-paired environment produced robust increases in (1) arc and zif/268, but not c-fos, mRNA in the CPu and NAc core at 22 h or 15 days, (2) arc, zif/268, and c-fos in the AC and M1 motor cortex but only arc mRNA in the S1 sensory cortex at 22 h, and (3) zif/268, and c-fos mRNA in the AC cortex, c-fos mRNA in the M1/2 motor cortex, and all three genes in S1 sensory cortex at 15 days. Furthermore, lever pressing was a particularly strong inducer of arc mRNA in the NAc core and zif/268 mRNA in the S1 cortex. Finally, increased gene expression was significantly correlated with lever pressing during relapse testing only after 15 days of abstinence. Together, these data suggest that following chronic cocaine self-administration, associative and sensorimotor cortical and striatal regions become involved in relapse to drug-seeking behavior, that conditioned responding becomes compulsive after chronic cocaine administration, and that the motivational salience of a drug-paired context is maintained and exacerbated following prolonged abstinence.
Regional changes in gene expression
Cerebral cortex
The AC cortex plays an integral role in cognitive and affective processes frequently associated with motivation, stimulus-reinforcement associations, and reward-based decision-making (Bush et al. 2001), all of which are thought to be impaired during drug addiction. Moreover, evidence suggests that alterations in dopaminergic innervation of the AC is involved in compulsive drug-taking and deficient self-control (Volkow et al. 2004). Further, the AC becomes activated in cocaine abusers exposed to visual cues depicting drug-related stimuli and this activity correlates with subjective craving for cocaine (Childress et al. 1999). Thus, it is likely that increases in AC activity-regulated gene expression in the present study reflect recognition of a previously drug-paired context that contributes or responds to drug-seeking. The AC is also highly implicated in error detection, becoming more activated in response to conflict between behavior and goals (Carter et al. 1998; Kerns 2006). Therefore, it is possible that the observed increases in AC gene expression specific to cocaine-treated animals returning to the previously drug-paired chamber may represent a discrepancy between drug availability and the context, as it is no longer predictive of cocaine availability. Additionally, AC activation may reflect increased communication of the AC with other regions of the PFC in order to encode the altered conditioned reinforcing properties of the self-administration chamber in the absence of cocaine availability.
As the continued pairing of environmental cues and subsequent behaviors associated with obtaining a reward (lever pressing) throughout self-administration becomes habitual, activity in sensory- and motor-related cortices may exert more control of striatal function through convergent corticostriatal projections. In the present study, 22 h after the end of self-administration, re-exposure to the test chamber that was previously associated with cocaine self-administration produced parallel increases in all three genes, regardless of lever availability, in the M1/2 cortex whereas only arc mRNA was significantly increased in the S1 sensory cortex. Therefore, cortical sensory-motor activation occurred whether or not rats made an instrumental response when re-exposed to the cocaine-paired chamber 1 day after the end of self-administration. In addition to displaying a consistent response across all three cortical regions, arc also displayed the most robust increases in sensory-motor cortex at this timepoint, suggesting that synaptic activity was more involved than nuclear transcription events under these conditions.
Following a 15 day period of abstinence, c-fos expression was increased in the S1 and M1/2 cortical regions of rats with a cocaine history that were re-exposed to the self-administration environment, regardless of lever availability. However, an increase in arc in M1/2 cortex and zif/268 mRNA in S1 and M1/2 cortex was only detected in rats that pressed the lever. This pattern of cortical expression suggests that c-fos responded more to general behavioral activity elicited by the context associated with cocaine whereas zif/268 and arc expression encoded more selective events associated with instrumental responding.
Striatum
In the present study, re-exposure to a drug-paired context following either 22 h or 15 days of abstinence resulted in a robust upregulation of zif/268 and arc, but not c-fos, mRNA within the CPu and NAc core. Thus, in sharp contrast to the induction of all three genes in cortical regions, zif/268 and arc mRNAs appeared to be driven by a pattern of activity downstream from cell surface receptors in the CPu that did not drive c-fos. Indeed, there is evidence that kainate/AMPA or NMDA receptor antagonists block zif268 expression but only NMDA receptor antagonists block c-fos expression in the striatum after cocaine or amphetamine administration (Torres and Rivier 1993; Wang et al. 1994a, b). Therefore, it is possible that stimulation of both kainate/AMPA and NMDA receptors facilitates the expression of all three IEGs in the cortex whereas a differential stimulation of kainate/AMPA receptors selectively drives the expression of arc and zif/268 mRNA in the CPu. The lack of c-fos induction in the striatum is consistent with a study by Neisewander et al. (2000) that reported no significant increase in Fos protein immunoreactivity in the CPu of rats re-exposed to a drug-paired environment following prolonged forced abstinence, and a slight, but significant, increase in the NAc shell and core. In their more recent study, however, there was a small but significant increase in Fos immunoreactivity in the CPu, NAc core, and NAc shell in rats 90 min after re-exposure to the cocaine self-administration environment (Zavala et al. 2007). Because of the small magnitude of this signal, it is likely that this level of response, if reflected at the mRNA level using in situ hybridization, would not be enough to be detected above background on X-ray films.
The transition to addiction is associated with a shift from goal-directed learning to habitual/compulsive use patterns in addicts (Tiffany 1990; Berke and Hyman 2000; Everitt et al. 2001). This behavioral shift is accompanied and/or driven by a shift from prefrontal cortical to striatal control of behavior (Everitt and Wolf 2002; Ito et al. 2002; Porrino et al. 2004). The CPu becomes activated during cue-elicited cocaine craving in humans (Garavan et al. 2000; Volkow et al. 2006). Similarly, the duration and severity of addiction has been correlated with self-reported craving measures and dopamine activation in the CPu (Garavan et al. 2000; Volkow et al. 2006). Additionally, inhibition of activity or blockade of dopamine or AMPA receptors in the dlCPu attenuated cocaine-seeking under a second-order schedule of cocaine reinforcement (Vanderschuren et al. 2005) or cue- or context-induced reinstatement after daily extinction or abstinence from chronic cocaine self-administration (Fuchs et al. 2005, 2006). Therefore, it is likely that the CPu is involved in mediating the presumed habitual nature of drug-seeking behavior following chronic cocaine self-administration.
Following 22 h of abstinence, re-exposure to the self-administration chamber produced significant increases in zif/268 and arc expression within the dlCPu and NAc core of cocaine-treated vs. saline-treated rats. The increased gene expression was not due to activity associated with lever pressing because zif/268 and arc mRNA was increased independent of lever availability in both the dlCPu and NAc core. Surprisingly, zif/268 mRNA was increased in the dmCPu of cocaine-treated rats denied access to levers during testing whereas arc mRNA was increased exclusively in the dmCPu of rats that lever-pressed. Additionally, no correlation was found between total lever pressing during the 1 h test and increased zif/268 or arc mRNA expression for any of these brain regions at this timepoint.
Following 15 days of abstinence, both zif/268 and arc expression was increased in the CPu and NAc core by re-exposure to a previously drug-paired context. Interestingly, zif/268 displayed more of a general response to the context that was independent of lever availability whereas changes in arc appeared to be more specific, showing more robust lever-dependent increases in the dlCPu and NAc core. The finding that gene expression patterns were similar after both short and prolonged abstinence in the dlCPu whereas activation in the dmCPu was less consistent and robust extends the idea that stimulus-response actions are preferentially mediated by the dlCPu (Packard and Knowlton 2002; White and McDonald 2002).
Functional significance of gene expression induction
Several studies have demonstrated a time-dependent, conditioned-cue-induced increase in drug-seeking (Neisewander et al. 2000; Lu et al. 2004). In the present study, a significant correlation was seen between the total number of lever presses during testing and increased zif/268 and arc mRNA expression in the dlCPu and NAc core exclusively following 15 days of abstinence. Further, there was a significant correlation between lever pressing and arc induction in the M1/M2 cortex and zif/268 induction in the dmCPu. Thus, the salience of the previously cocaine-active lever was greater after 15 days than after 1 day. This finding suggests that the number of lever presses more selectively reflects drug salience than does the general context of the operant chamber after prolonged abstinence and that the intensity of arc and zif/268 expression encodes this salience.
Different patterns of IEG expression were also revealed under different conditions in certain brain regions. As pointed out above, arc expression was greater than that of zif/ 268 and c-fos in the S1 cortex and arc mRNA was induced in CL rats whereas zif/268 mRNA was induced in CN rats in the dmCPu at 22 hr. Further, zif/268 and c-fos mRNA was greater than arc mRNA in the AC at 15 days and arc was induced only in CL rats vs. zif/268 in CL and CN rats in the NAc at 15 days. These different patterns of IEG expression may be driven by different stimuli and they may mediate distinct components of learning and memory processes associated with drug-seeking. For example, arc mRNA is rapidly expressed and delivered to activated synapses where it alters AMPA receptor endocytosis and trafficking (Chowdhury et al. 2006; Rial Verde et al. 2006) whereas c-fos and zif/268 induction in the nucleus leads to the transcription of late-effector genes and longterm changes in gene and protein expression (Curran and Morgan 1995; James et al. 2005). These transcriptional (c-fos, zif/268) and effector (arc) genes share a critical dependence on intracellular signaling pathways that connect nuclear and synaptic events underlying neuronal plasticity and the formation of long-term memories associated with cocaine seeking. In general, by gaining a clearer understanding of the selective regional patterns of activity-regulated genes under specific environmental conditions, new directions for molecular targets may be developed for the pharmacological treatment of cocaine addiction.
Acknowledgments
The authors thank Shannon Ghee and Anthony Carnell for excellent technical support and Scott W. Miller for statistical consultation. This research was supported by P50 DA15369 and CO6RR015455.
References
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine-seeking in rats. Eur J NeuroSci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exper Ther. 1993;267:496–505. [PubMed] [Google Scholar]
- Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Phil Trans R Soc Lond. 2003;358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, GreveD D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 2001;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytotic machiinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50, 488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology. 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Curran T, Morgan JL. Fos: an immediate-early transcription factor in neurons. J Neurobiol. 1995;26:403–412. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF. Acute and chronic cocaine administration differentially alters striatal opioid and nuclear transcription factor mRNAs. Synapse. 1994;18:35–45. doi: 10.1002/syn.890180106. [DOI] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Brain Res. 1995;29:201–210. doi: 10.1016/0169-328x(94)00246-b. [DOI] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM. Activation of arc, a putative “effector” immediate early gene, by cocaine in rat brain. J Neurochem. 1995;64:2377–2380. doi: 10.1046/j.1471-4159.1995.64052377.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Differential neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Frasca A, Di Pasquale L, Racagni G, Riva MA. Corticostriatal up-regulation of activity-regulated cytoskeletal-associated protein expression after repeated exposure to cocaine. Mol Pharmacol. 2006;70:1726–1734. doi: 10.1124/mol.106.026302. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippo-campal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1090-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AB, Conway AM, Morris BJ. Genomic profiling of the neuronal target genes of the plasticity-related transcription factor-Zif268. J Neurochem. 2005;95:796–810. doi: 10.1111/j.1471-4159.2005.03400.x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications ht e control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuro-pharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Rice WN, Freedland CS, Whitlow CT, Porrino LJ. Patterns of functional activity associated with cocaine self-administration in the rat change over time. Psychopharmacology. 2004;172:384–392. doi: 10.1007/s00213-003-1676-7. [DOI] [PubMed] [Google Scholar]
- Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr–1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear-conditioning. Learn Mem. 2004;11:617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Robertson HA, Graybiel AM. Dynamic regulation of NGFI-A (zif268, egr1) gene expression in the striatum. J Neurosci. 1992;12:2609–2622. doi: 10.1523/JNEUROSCI.12-07-02609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Lau LF, Huganir RL. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th. Academic Press; San Diego: 2007. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/ arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, Moratalla R, Lyford GL, Worley P, Graybiel AM. The activity-regulated cytoskeletal-associated protein arc is expressed in different striosome-matrix patterns following exposure to amphetamine and cocaine. J Neurochem. 2000;74:2074–2078. doi: 10.1046/j.1471-4159.2000.0742074.x. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Hall J, Everitt BJ. Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur J Neurosci. 2002;16:1789–1796. doi: 10.1046/j.1460-9568.2002.02247.x. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci. 2003;17:1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Torres G, Rivier C. Cocaine-induced expression of c-fos in the rat is inhibited by NMDA receptro antagonisyts. Brain Res Bull. 1993;30:173–176. doi: 10.1016/0361-9230(93)90055-g. [DOI] [PubMed] [Google Scholar]
- Valjent E, Aubier B, Corbille AG, Brami-Cherrier K, Caboche J, Topilko P, Girault JA, Herve D. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci. 2006;26:4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alteration in zif/268 and preprodynorphin mRNA expression induced by amphetamine or methamphetamine in rat forebrain. J Pharmacol Exper Ther. 1995;273:909–917. [PubMed] [Google Scholar]
- Wang JQ, Daunais JB, McGinty JF. NMDA receptors mediate amphetamine-induced upregulation of zif268 and preprodynorphin mRNA expression in rat striatum. Synapse. 1994a;18:343–353. doi: 10.1002/syn.890180410. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Daunais JB, McGinty JF. Role of kainate/AMPA receptors in induction of zif268 and preprodynorphin mRNA by a single injection of amphetamine. Mol Brain Res. 1994b;27:118–126. doi: 10.1016/0169-328x(94)90192-9. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. J Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]


