Abstract
In select areas of the brain, neural stem cells produce new neurons throughout life. An elegant new study in this issue of Cell reveals the origins of a stem cell population that persists into adulthood and uncovers a surprising relationship between neurons born in the mature brain and those generated early in development.
The rediscovery and confirmation of adult neurogenesis in the mid-1990s has led to an explosion of research focused on establishing the functions of newly generated neurons in the adult brain. However, the rules that govern the generation of adult neural stem cells (NSCs) and their relationship to stem cells and neurons born early in development have remained elusive. A new study by Alvarez-Buylla and colleagues now reveals the exquisite specificity with which the neurogenic niche is established and the remarkable association between the precursors of adult born neurons and neurons born during embryogenesis (Fuentealba et al., 2015).
Adult neurogenesis occurs primarily in two areas: the subgranular zone (SGZ) of the hippocampal dentate gyrus and the ventricular-subventricular zone (V-SVZ), a band of tissue that lines the lateral ventricles (Ming and Song, 2011). In the hippocampus, radial glial-like stem cells give rise to dentate granule neurons, which functionally integrate into local circuits. The V-SVZ hosts NSCs with astrocytic features, named B1 cells, which generate different classes of adult-born interneurons that migrate to the olfactory bulb (OB) (Doetsch et al., 1999). It is known that B1 cells are generated from radial glial (RG) cells during embryonic development (Merkle et al., 2004) and that different types of OB interneurons arise from B1 cells according to their position in the V-SVZ (Merkle et al., 2007). However, it is uncertain when this spatial determination of cell fate occurs and whether B1 cells derive from the same neural precursors that are responsible for embryonic neurogenesis. Alvarez-Buylla and colleagues set out to answer these questions by defining the origins of B1 cells, revealing along the way the common origins of adult-born OB interneurons and embryonically generated forebrain neurons (Figure 1) and finding that adult NSCs are generated very early in embryonic brain development but remain quiescent until they are reactivated in adulthood.
Figure 1. Using Genetic Barcodes to Tracethe Family Tree of Adult-Born Olfactory Neurons.
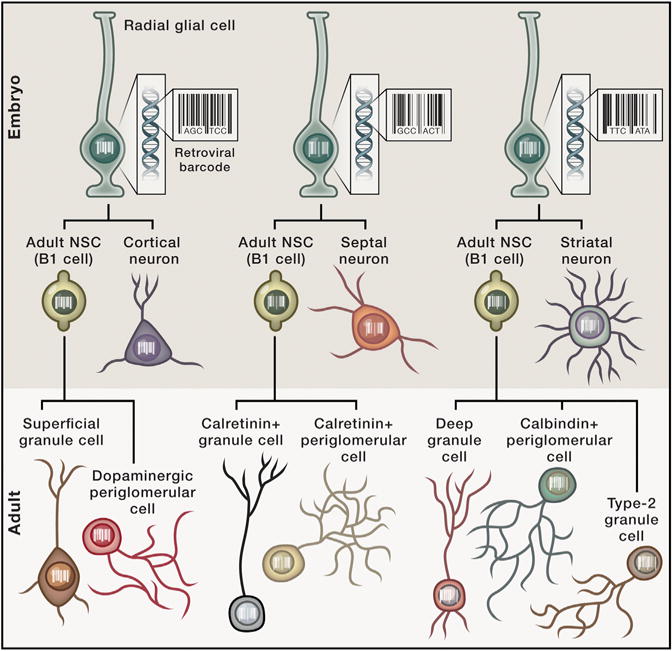
Adult NSCs (B1-cells) arise from progenitors that give rise to specific subsets of forebrain neurons whose identity is defined by embryonic position of the RG cell early in development. This level of specificity generates a direct relationship between adult-born OB neurons and embryonically generated forebrain neurons. This “family tree” allows tracing the relationships between adult-born neurons and those born in utero.
To determine the exact period during embryonic development when adult NSCs are generated, the authors combined transgenic reporter lines of mice with BrdU and retroviral labeling of dividing cells. They found that B1 cells arise from embryonic cells dividing on embryonic days 13.5 to 15.5., which is the time when other classes of forebrain neurons appear, raising the exciting possibility that they all hail from the same RGs. To test this idea, the authors adopted an ingenious retroviral approach, labeling dividing cells with a membrane-bound GFP (mGFP) and a “genetic barcode,” a 24-base-pair oligonucleotide unique to each retrovirus (Golden et al., 1995). By labeling progenitors during the embryonic period, all cells originating from a single progenitor would inherit the same barcode, enabling cell lineage determination. In adulthood, newborn neurons were also labeled with tdTomato, allowing the identification of cells carrying both labels. Importantly, cells’ barcodes allowed the authors to determine whether adult and embryonically generated neurons originated from the same progenitor. In other words, if adult-born OB neurons had the same ancestor as neurons born early in development, the neurons would share the same barcode. Indeed, the results of the study demonstrate that the progenitors of forebrain neurons generated early in embryonic development also give rise to B1 cells that, in turn, generate adult-born neurons in the OB (Figure 1).
Adult NSCs in distinct regions of the V-SVZ produce different classes of OB neurons (Merkle et al., 2007). The authors therefore investigated whether this regional specification was established early in development, reasoning that classes of OB neurons born in adulthood would be related to distinct subtypes of forebrain neurons according to where they were generated. By reading the barcodes of labeled cells, they indeed found that the position of the embryonic progenitor indicated the class of adult-born OB neuron it produced and to which type of forebrain neuron it was clonally related. For example, adult-born superficial GCs and dopaminergic periglomerular cells in the OB were clonally related to embryonically generated cortical cells (Figure 1).
This elegant study provides a characterization of the origins and lineage of a key type of neural precursor in the adult brain, as well as the methodology for future studies aimed at understanding the origin of NSCs in another adult neurogenic niche, the SGZ (Li et al., 2013), and the lineages they share with embryonically generated neurons. In addition, by revealing the exquisite selectivity in embryonic determination of cell fate, these findings set the stage for efforts aimed at understanding the malleability of the system and how extrinsic and intrinsic factors acting during development may affect cell types generated in adulthood.
In addition to identifying the relationship between adult-born and embryonically generated neurons, these data have important implications for the ongoing efforts to identify the self-renewal properties of adult NSCs. In the SGZ, selfrenewal of adult NSCs was shown (Bonaguidi etal., 2011), but heterogeneity among adult NSCs means that some might have limited self-renewal capacity (Calzolari et al., 2015; Encinas et al., 2011). In this study, the authors rarely found clones of both B1 cells and OB neurons, indicating that individual cohorts of B1 cells that are activated in adulthood eventually become exhausted and that new neurons arise only from distinct B1 cohorts. Therefore, future studies employing the powerful lineage-tracing strategies used here might be used to elucidate mechanisms of self-renewal in the hippocampal SGZ. In addition, employing in vivo imaging technologies to track individual adult NSCs across time may cast further light on their capacity for renewal.
Clarifying the identity of adult NSC precursors and the factors that generate the myriad of neuronal subtypes in the adult brain is essential if we wish to harness their power for treatments of brain injury and neurological and neuropsychiatric disorders. Indeed, while we know that neurogenesis occurs in adult brains, this study reveals that it is also critically influenced by events that occur before birth and that the exquisite spatial and temporal dynamics of progenitor cell generation in the embryo is the key determinant of the adult born neurons’ fate.
References
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari F, Michel J, Baumgart EV, Theis F, Götz M, Ninkovic J. Nat Neurosci. 2015;18:490–92. doi: 10.1038/nn.3963. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Ricardo R, Cepko CL, Alvarez-Buylla A. Cell. 2015;161:1644–1655. doi: 10.1016/j.cell.2015.05.041. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JA, Fields-Berry SC, Cepko CL. Proc Natl Acad Sci USA. 1995;92:5704–5708. doi: 10.1073/pnas.92.12.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Fang L, Fernández G, Pleasure SJ. Neuron. 2013;78:658–672. doi: 10.1016/j.neuron.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Proc Natl Acad Sci USA. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


