Abstract
BACKGROUND:
Lung disease is the most common cause of death in the world. The last stage of pulmonary diseases is lung transplantation. Limitation and shortage of donor organs cause to appear tissue engineering field. Decellularization is a hope for producing intact ECM in the development of engineered organs.
AIM:
The goal of the decellularization process is to remove cellular and nuclear material while retaining lung three-dimensional and molecular proteins. Different concentration of detergents was used for finding the best approach in lung decellularization.
MATERIAL AND METHODS:
In this study, three-time approaches (24, 48 and 96 h) with four detergents (CHAPS, SDS, SDC and Triton X-100) were used for decellularizing rat lungs for maintaining of three-dimensional lung architecture and ECM protein composition which have significant roles in differentiation and migration of stem cells. This comparative study determined that variable decellularization approaches can cause significantly different effects on decellularized lungs.
RESULTS:
Results showed that destruction was increased with increasing the detergent concentration. Single detergent showed a significant reduction in maintaining of three-dimensional of lung and ECM proteins (Collagen and Elastin). But, the best methods were mixed detergents of SDC and CHAPS in low concentration in 48 and 96 h decellularization.
CONCLUSION:
Decellularized lung tissue can be used in the laboratory to study various aspects of pulmonary biology and physiology and also, these results can be used in the continued improvement of engineered lung tissue.
Keywords: Decellularization, CHAPS, SDS, SDC and Triton X-100
Introduction
The mortality of pulmonary diseases is increasing but mortality of other diseases such as heart disease, cancer and so on is decreasing for example 400 thousand of American people die every year [1, 2]. Lung transplantation is the only definitive treatment for some lung diseases such as CF, COPD and IPF. But, patient survival is only 50% during 5 years and 24% during 10 years after lung transplantation [3]. On the other hand, unfortunately, many patients on the waiting list for lung transplant pass out before suitable donor organ get found because of the critical shortage of transplantable donor lungs. Moreover, immunosuppression is another lifelong drawback for transplant recipients [4]. Due to this problem in transplantation, lung tissue engineering research is increasing and demand for engineered lung tissue has been increased for transplantation. There are advantages of engineered lung tissue such as using a patient’s own cells, so avoiding the need for immunosuppression which causes many problems including neurologic disorders and infection and so on [5, 6]. Tissue engineering is a multidisciplinary field which tries to combine cellular, molecular, technological and medical advances to make replacement tissues for implantation [7-10]. However, the lung is a complex tissue and ECM scaffolds are complex bio-structures constructed of a variety of structural and functional compositions that are specially organized and suitable for the intended tissue [11]. One of lung tissue engineering strategies is that to make appropriate scaffolds for lung tissue engineering [12]. Decellularization is a new technique for removing all cellular materials and produces an intact whole lung, which is an important characteristic of a three-dimensional matrix for cellular differentiation and migration [13, 14]. Decellularization methods have been used for producing scaffolds for a variety of tissue engineering applications such as heart valves, trachea, liver and heart and so on [7, 15-19]. Decellularized organs have several advantages as s tissue engineering scaffold. The decellularized scaffold maintains the suitable 3D organization essential for tissue function, such as a vascular system and airway network in the complex structure of lung [20]. Native ECM has the optimal substrate for cell attachment, spreading, growth and differentiation. The aim of the decellularization method is to remove cellular and nuclear material while maintaining 3D and ECM protein compositions and also, diminishing any damage to lung ECM. There are various types of decellularization approaches such as physical, chemical and enzymatic methods [16, 17]. Physical approaches can cause the destruction of the delicate lung ECM which this method includes freezing, pressure, sonication and agitation, while enzymatic approaches cannot be cost-effective due to large volumes of liquid would be needed for decellularization of a whole organ such as proteases, nucleases, and calcium chelators [21]. Trypsin has been used in the enzymatic method which it can remove fibronectin, laminin, and elastin. Because of the importance of these compositions, enzymatic approaches have not been mostly used in decellularization. But, chemical approaches are cost-effective and have several of techniques such as alkaline or acidic solutions, detergents (SDS, SDC, CHAPS and …) hypotonic or hypertonic solutions, and chelators [16, 17, 21]. A hypertonic NaCl solution can efficiently lyse cells, while it cannot help in removing cellular components from the tissue. CHAPS are a zwitterionic detergent which can be an effective solubilization for removing of cellular material [21]. The objectives of this research are to demonstrate that decellularized lung is promising substrates for producing an engineered lung scaffold and as the noted 3D and ECM protein compositions are important characteristics for cellular differentiation and migration.
Material and Methods
Animals and lung extraction
Fourteen male healthy rats (250–300 gr) were purchased from animal researcher centre of Baqiyatallah University of Medical Sciences and housed in this centre. Rats were controlled in a standard environment (humidity and temperature) on a 12/12 hours light/dark cycle with standard food and water, following experimental procedures approved by the Ethical Committee for Animal Research of Baqiyatallah University of Medical Sciences. The rats were anaesthetized with intraperitoneal with Ketamine and Xylazine (100 mg/kg and 10 mg/kg). A midline incision was made in the throat, and the trachea was exposed. Right ventricle perfusion was perfused with 250cc phosphate buffered saline (PBS) containing Heparin 5000 u/ml and 1% penicillin and streptomycin (P/S) (Figure 1). Lung was sliced to use in decellularization processes.
Figure 1.
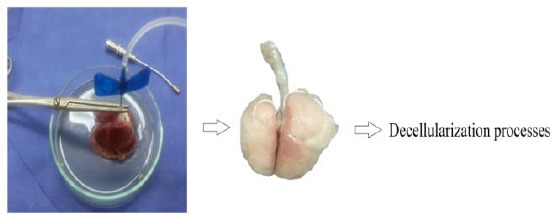
Perfusion of PBS, Heparin and P/S
Twenty four hour decellularization approaches
Sliced lungs were similarly rinsed and treated with SDS with different concentrations at 24 which is shown in Table 1. Lastly, sliced lungs were rinsed and treated with 1M sodium chloride for 1 h at room temperature for single detergent. Sliced lungs were flushed with PBS before they were used for experiments.
Table 1.
24h decellularization approaches for rat lung decellularization
| Approach | Methods | Decellularization approaches |
|---|---|---|
| 24h | 1 | SDC 0.5% 23h, NaCl 1 M 1h |
| 2 | SDC 1% 23h, NaCl 1 M 1h | |
| 3 | SDC 1.5% 23h, NaCl 1 M 1h | |
| 4 | SDC 2% 23h, NaCl 1 M 1h |
Forty-eight-hour decellularization approaches
In this time, sliced lungs were rinsed and treated by mixed detergents and single detergent of SDC. These detergents were used at 48 h which is shown in Table 2. Sliced lungs were flushed with PBS before they were used for experiments.
Table 2.
48h decellularization approaches for rat lung decellularization
| Approach | Methods | Decellularization approaches | ||
|---|---|---|---|---|
| 24 h | 24 h | |||
| 48h | 5 | SDC 0. 5% 47h, NaCl 1 M 1h | ||
| 6 | SDC 1% 47h, NaCl 1 M 1h | |||
| 7 | SDC 1.5% 47h, NaCl 1 M 1h | |||
| 8 | SDC 2% 47h, NaCl 1 M 1h | |||
| 9 | SDC 0. 5% | + | SDS 0.05% | |
| 10 | SDC 1% | + | SDS 0.1% | |
| 11 | SDC 1.5% | + | SDS 0.15% | |
| 12 | SDC 2% | + | SDS 0.2% | |
| 13 | SDC 1% | + | SDS 0.05% | |
| 14 | SDC 1.5% | + | SDS 0.1% | |
| 15 | SDC 0. 5% | + | Triton X-100 0.05% | |
| 16 | SDC 1% | + | Triton X-100 0.1% | |
| 17 | SDC 1.5% | + | Triton X-100 0.15% | |
| 18 | SDC 2% | + | Triton X-100 0.2% | |
| 19 | SDC 1% | + | Triton X-100 0.05% | |
| 20 | SDC 1.5% | + | Triton X-100 0.1% | |
| 21 | SDC 0. 5% | + | CHAPS 2mM | |
| 22 | SDC 1% | + | CHAPS 4mM | |
| 23 | SDC 1.5% | + | CHAPS 6mM | |
| 24 | SDC 2% | + | CHAPS 8mM | |
| 25 | SDC 1% | + | CHAPS 2mM | |
| 26 | SDC 1.5% | + | CHAPS 4mM | |
Ninety-six-hour decellularization approaches
In this time, sliced lungs were rinsed and treated by mixed detergents and single detergent of SDC. These detergents were used at 96 h which is shown in Table 3. Sliced lungs were flushed with PBS before they were used for experiments.
Table 3.
96h decellularization approaches for rat lung decellularization
| Approach | Methods | Decellularization approaches | ||
|---|---|---|---|---|
| 48 h | 48 h | |||
| 96h | 27 | SDC 0. 5% | + | SDS 0.05% |
| 28 | SDC 1% | + | SDS 0.1% | |
| 29 | SDC 1.5% | + | SDS 0.15% | |
| 30 | SDC 2% | + | SDS 0.2% | |
| 31 | SDC 1% | + | SDS 0.05% | |
| 32 | SDC 1.5% | + | SDS 0.1% | |
| 33 | SDC 0. 5% | + | Triton X-100 0.05% | |
| 34 | SDC 1% | + | Triton X-100 0.1% | |
| 35 | SDC 1.5% | + | Triton X-100 0.15% | |
| 36 | SDC 2% | + | Triton X-100 0.2% | |
| 37 | SDC 1% | + | Triton X-100 0.05% | |
| 38 | SDC 1.5% | + | Triton X-100 0.1% | |
| 39 | SDC 0. 5% | + | CHAPS 2mM | |
| 40 | SDC 1% | + | CHAPS 4mM | |
| 41 | SDC 1.5% | + | CHAPS 6mM | |
| 42 | SDC 2% | + | CHAPS 8mM | |
| 43 | SDC 1% | + | CHAPS 2mM | |
| 44 | SDC 1.5% | + | CHAPS 4mM | |
Lung histology
H&E staining protocol was utilized to tissue assay. Sliced lungs tissue (before and after decellularization) were fixed in paraformaldehyde 10% after fixation transferred to tissue processor device to dehydration and clearing. Finally, they were embedded in paraffin and sectioned in 5-micron thickness. Sections were stained with H&E, Trichrome- Masson staining and Elastin staining and then, they were observed under a light microscope [22-26]. H&E staining was utilized to visually quantify the nuclei loss and three-dimensional lung architecture by comparing nuclei counts in paraffin sections of normal and decellularized sliced lungs. Nuclei were counted from three random images at 100 × magnification using a counting tool. Trichrome- Masson staining and Elastin staining were done for evaluating the level of collagen and elastin, respectively.
Statistical analysis
All studies were conducted at least in biological triplicate. Data were statistically analyzed by t-test. P values less than 0.05 were considered significant.
Results
Analysis of a 24-h decellularized rat lung by Hematoxylin and Eosin, Trichrome- Masson and Elastin staining
This 24-h decellularization process could not completely preserve the architecture of the lung and also, could not completely eliminate the nuclei utilizing SDC detergent. But, method 1 was better than other methods in 24 h decellularization which this method maintained the three-dimensional structure at very low levels and also. This method could not preserve the proteins ECM composition such as Collagen and Elastin (Figure 2).
Figure 2.
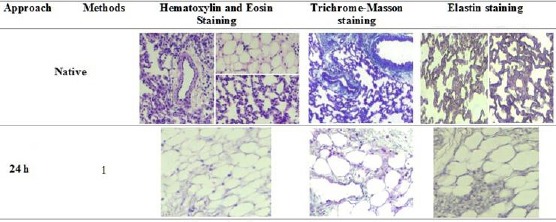
Hematoxylin and Eosin Staining showed structures and nuclei present in native and decellularized lungs. Trichrome-Masson staining showed collagen levels in native and decellularized lungs (Blue colour). Elastin staining showed elastin levels in native and decellularized lungs (Blue colour to black colour). Representative images for all conditions are shown (100 × magnification)
Analysis of a 48-h decellularized rat lung by Hematoxylin and Eosin, Trichrome- Masson and Elastin staining
SDC detergent
In this approach, SDC detergent was not able to maintain the three-dimensional, Collagen and Elastin. The architecture of the lung was destroyed by increasing the concentration of SDC from 0.5% to 2%. In addition, a significant reduction was seen in Collagen and Elastin. Method 5 was better than other methods in single SDC detergent in 48 h decellularization (Figure 3) and it could relatively preserve the three-dimensional but could not maintain Collagen and Elastin.
Figure 3.
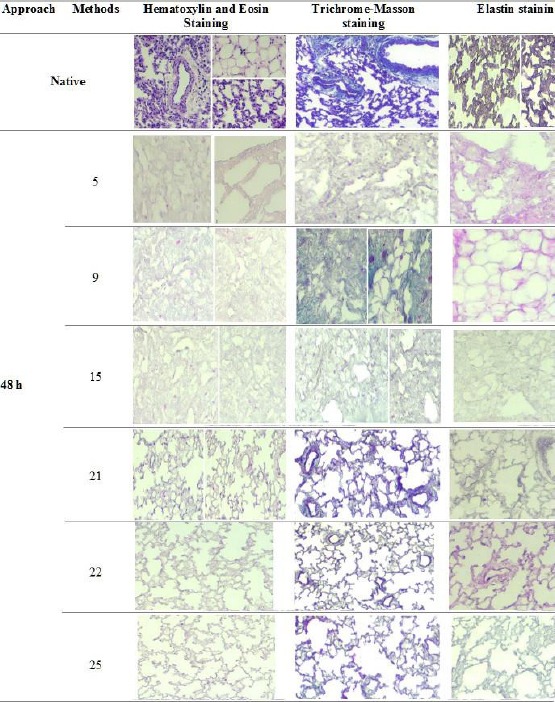
Hematoxylin and Eosin Staining showed structures and nuclei present in native and decellularized lungs. Trichrome-Masson staining showed collagen levels in native and decellularized lungs (Blue colour). Elastin staining showed elastin levels in native and decellularized lungs (Blue colour to black colour). Representative images for all conditions are shown (100 × magnification)
Figure 3.
Hematoxylin and Eosin Staining showed structures and nuclei present in native and decellularized lungs. Trichrome-Masson staining showed collagen levels in native and decellularized lungs (Blue colour). Elastin staining showed elastin levels in native and decellularized lungs (Blue colour to black colour). Representative images for all conditions are shown (100 × magnification)
Mixed detergents of SDC with SDS, Triton X-100 or CHAPS
Results showed that methods 9, 15, 21, 22 and 25 were considered as the best methods in maintaining of 3D and protein ECM composition (Collagen and Elastin) (Figure 3). Methods 9 and 15 could relatively preserve the three-dimensional and remove nuclei from lung tissue but Collagen and elastin were not seen in SDC+SDS and SDC+Triton X-100 detergent, respectively.
Methods 21, 22 and 25 relatively maintained proteins ECM (Collagen and Elastin) and also, a significant reduction in the nuclei was seen in 48 h decellularization of SDC+ CHAPS. In addition, Method 22 maintained the 3D, Collagen and elastin better than methods 21 and 25 (Figure 3).
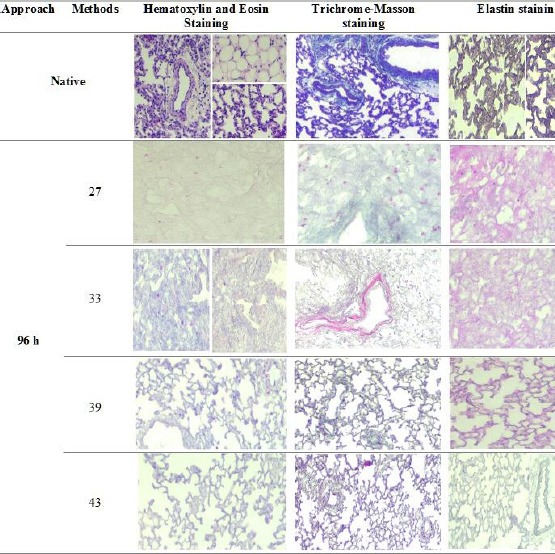
Analysis of a 96-h decellularized rat lung by Hematoxylin and Eosin, Trichrome - Masson and Elastin staining
In Ninety-six hour decellularization, methods 27, 33, 39 and 43 were the best decellularization approaches which methods 27 and 33 maintained the three-dimensional structure at very low levels and a significant reduction were not seen in the nuclei in SDC+SDS and SDC+Triton X-100 approaches, respectively. Methods 39 and 43 preserved the architecture of the lung while eliminating the nuclei in SDC+CHAPS approaches and also, the portions ECM composition (Collagen and Elastin) were decreased with increasing the time from 48 h to 96 h decellularization.
Discussion
In the percent century, pulmonary diseases are becoming world healthcare problems [27]. Chronic obstructive pulmonary diseases (COPD) such as emphysema and chronic obstructive bronchitis have caused more than 120,000 deaths in adults in the US in one year [28]. The last stage of pulmonary diseases is lung transplantation which has many challenges such as transplant rejection, immunosuppression, and a shortage of donor organ. Waitlist of lung transplantation is increasing and many patients on the waiting list for lung transplant pass out before suitable donor organ get found because of the critical shortage of transplantable donor lungs [2, 4]. Lung tissue has a complex bio-structure which has various structural, functional compositions [11] and proteins ECM composition including collagen, laminin, elastin, fibronectin and so on [4]. Many types of research have been done for producing a lung scaffold from biomaterial but it has a lot of limitations. Recently, decellularization is appearing as decellularized biomimetic scaffold to support differentiated stem cells which it can be a significant treatment for lung diseases [13, 22]. In this regard, lung organ is decellularized and lung ECM’s own cells are removed from the extracellular matrix and also, proteins ECM composition and growth factor are maintained which they are necessary for proliferation, differentiation and migration of stem cells but decreasing them during decellularization can be a major problem [4]. Native extracellular matrix has the optimum substrate for cell attachment, spreading, growth and differentiation.
The goal of the decellularization approach is to remove cellular and nuclear material while maintaining three dimensional and ECM protein compositions and also, reducing any damage to lung ECM. There are several types of decellularization methods such as physical, chemical and enzymatic methods [16, 17]. Physical approaches can destruct the complex lung ECM which this method includes freezing, pressure, sonication and agitation, while enzymatic approaches cannot be cost-effective due to large volumes of liquid would be needed for decellularization of a whole organ such as proteases, nucleases, and calcium chelators [21].
Trypsin has been used in the enzymatic method which it can remove fibronectin, laminin, and elastin. Because of the importance of these compositions, enzymatic approaches have not been mostly used in decellularization. But, chemical approaches are cost-effective and have several of techniques such as alkaline or acidic solutions, detergents (SDS, SDC, CHAPS and …) hypotonic or hypertonic solutions, and chelators [16, 17, 21]. Jensen et al (2012) studied on the evaluation of 24h decellularization process (Triton-X100 0.1% and sodium deoxycholate 2%) which they showed lung scaffolds with preservation of key ECM proteins collagen I, collagen IV, fibronectin, and laminin, but with the loss of nuclear proteins [13]. Price et al (2015) used pig lung for decellularization by an automated method and compared to manual method and they found that an automated method offered more consistent matrix and reduced the decellularization procedure required time [29]. Neill et al (2013) used CHAPS 8 mM and SDS 1.8 mM for human and porcine decellularization and they found that Porcine lungs could be decellularized using CHAPS to produce lung ECM scaffolds with properties resembling those of human lungs, for pulmonary tissue engineering and they proposed that porcine lung ECM can be an excellent screening platform for the envisioned human tissue engineering applications of decellularized lungs [30].
In this study, three-time approaches were used for decellularization (24, 48 and 96 h). In 24 h decellularization, method 1 could relatively maintain 3D and remove nuclei but all methods could not preserve Collagen and Elastin and also, there was significant of destruction with increasing the concentration of SDC from 0.5% to 2%. In 48 h decellularization, methods 5, 9, 15 could not preserve the integrity of the 3D and ECM proteins but they were better than other approaches in SDC, SDC+SDS and SDC+Triton X-100, respectively. On the other hand, methods 21, 22 and 25 were the best decellularization approaches which preserved the architecture of the lung while eliminating the nuclei and maintained a considerable of Collagen and Elastin.
In addition, among these approaches, method 22 was remarkable in maintaining of 3D and ECM proteins (Collagen and Elastin). In 96 h decellularization, methods 27 and 33 could not maintain the 3D, Collagen and elastin but they were better than other approaches in SDC+SDS and SDC+Triton X-100, respectively. Methods 39 and 43 preserved the architecture of the lung while eliminating the nuclei and relatively maintained Collagen and Elastin. Maghsoudlou et al (2013) used rat lung for decellularization using the intermittent intra-tracheal flow of detergent-enzymatic treatment and they presented a methodology which was applicable to rat and sheep lungs, preserved lung architecture and ECM and reduced the generating time [31]. Petersen et al (2012), two different techniques were examined using either the detergent 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) or sodium dodecyl sulphate (SDS). CHAPS-based decellularization resulted in the elimination of the majority of cellular components (including DNA) and maintenance of collagen and elastin, whereas SDS-based decellularization caused collagen depletion and less mechanical stability [16].
Cortiella et al (2010) demonstrated that the first attempt to produce and use whole decellularized lung and they compared the influence of decellularized lung by SDS 0.1%, Gelfoam, Matrigel, and a collagen I hydrogel matrix on the mESC attachment, differentiation and subsequent formation of complex tissue and they found that decellularized lung allowed for better retention of cells with more differentiation of mESCs into epithelial and endothelial lineages [32]. In this research, we used single and mixed detergents for decellularization and results showed that destruction was increased with increasing the concentration of detergent. Single detergent showed a significant reduction in maintaining of three-dimensional of lung and ECM proteins (Collagen and Elastin). But, the best methods were mixed detergents of SDC and CHAPS in low concentration in 48 and 96 h decellularization. On the other hand, there was a considerable loss of Collagen and elastin and also, damage in 3D with increasing time. We conclude that the best method for decellularization was approach 22 which related to SDC 1%+CHAPS 4mM in 48 h decellularization. Finally, engineered scaffold of lung tissue can be used in the laboratory to study a wide variety of important aspects of pulmonary biology and physiology.
Acknowledgements
This study is extracted from PhD thesis which has been approved by Baqiyatallah University of Medical Sciences, Tehran, Iran. This work was supported by the Iran National Science Foundation (INSF).
Footnotes
Funding: This work was supported by the Iran National Science Foundation (INSF).
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.ALA. Data American Lung Association: Lung Disease Data. 2008:1–188. [Google Scholar]
- 2.Tebyanian H, Karami A, Motavallian E, Aslani J, Samadikuchaksaraei A, Arjmand B, and Nourani MR. Histologic analyses of different concentrations of TritonX-100 and Sodium dodecyl sulfate detergent in lung decellularization. Cellular and Molecular Biology. 2017;63(7):46–51. doi: 10.14715/cmb/2017.63.7.8. https://doi.org/10.14715/cmb/2017.63.7.8 PMid:28838339. [DOI] [PubMed] [Google Scholar]
- 3.Mulligan MS, Shearon TH, Weill D, Pagani FD, Moore J, Murray S. Heart and lung transplantation in the United States. American Journal of Transplantation. 2008;8(4p2):977–987. doi: 10.1111/j.1600-6143.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 4.Prakash YS, Tschumperlin DJ, Stenmark KR. Coming to terms with tissue engineering and regenerative medicine in the lung. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2015;309(7):L625–L638. doi: 10.1152/ajplung.00204.2015. https://doi.org/10.1152/ajplung.00204.2015 PMid:26254424 PMCid:PMC4593835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols JE, Niles JA, Cortiella J. Design and development of tissue engineered lung:Progress and challenges. Organogenesis. 2009;5(2):57–61. doi: 10.4161/org.5.2.8564. https://doi.org/10.4161/org.5.2.8564 PMid:19794900 PMCid:PMC2710526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichenspurner H. Overview of tacrolimus-based immunosuppression after heart or lung transplantation. The Journal of heart and lung transplantation. 2005;24(2):119–130. doi: 10.1016/j.healun.2004.02.022. https://doi.org/10.1016/j.healun.2004.02.022 PMid:15701425. [DOI] [PubMed] [Google Scholar]
- 7.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix:using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–221. doi: 10.1038/nm1684. https://doi.org/10.1038/nm1684 PMid:18193059. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt D, Mol A, Odermatt B, Neuenschwander S, Breymann C, Gössi M, Genoni M, Zund G, and Hoerstrup SP. Engineering of Biologically Active Living Heart Valve Leaflets Using Human Umbilical Cord–Derived Progenitor Cells. Tissue engineering. 2006;12(11):3223–3232. doi: 10.1089/ten.2006.12.3223. https://doi.org/10.1089/ten.2006.12.3223 PMid:17518636. [DOI] [PubMed] [Google Scholar]
- 9.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284(5413):489–493. doi: 10.1126/science.284.5413.489. https://doi.org/10.1126/science.284.5413.489 PMid:10205057. [DOI] [PubMed] [Google Scholar]
- 10.Atala A, Bauer SB, Soker S, Yoo JJ, and Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. The Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. https://doi.org/10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 11.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering:decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. https://doi.org/10.1146/annurev-bioeng-071910-124743 PMid:21417722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vertrees RA, Zwischenberger JB, Boor PJ, Popov V, McCarthy M, Goodwin T, Solley T. Cellular differentiation in three-dimensional lung cell cultures. Cancer biology & therapy. 2008;7(3):404–411. doi: 10.4161/cbt.7.3.5368. https://doi.org/10.4161/cbt.7.3.5368. [DOI] [PubMed] [Google Scholar]
- 13.Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, Jaworski DM, Hatton C, Weiss DJ, and Finck C. A Rapid Lung De-cellularization Protocol Supports Embryonic Stem Cell Differentiation In Vitro and Following Implantation. Tissue Engineering. Part C, Methods. 2012;18(8):632–646. doi: 10.1089/ten.tec.2011.0584. https://doi.org/10.1089/ten.tec.2011.0584 PMid:22404373 PMCid:PMC3401389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-Engineered Lungs for in Vivo Implantation. Science (New York, N.Y.) 2010;329(5991):538–541. doi: 10.1126/science.1189345. https://doi.org/10.1126/science.1189345 PMid:20576850 PMCid:PMC3640463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirsadraee S, Wilcox HE, Watterson KG, Kearney JN, Hunt J, Fisher J, and Ingham E. Biocompatibility of acellular human pericardium. Journal of Surgical Research. 2007;143(2):407–414. doi: 10.1016/j.jss.2007.01.026. https://doi.org/10.1016/j.jss.2007.01.026 PMid:17574597. [DOI] [PubMed] [Google Scholar]
- 16.Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix Composition and Mechanics of Decellularized Lung Scaffolds. Cells, Tissues, Organs. 2012;195(3):222–231. doi: 10.1159/000324896. https://doi.org/10.1159/000324896 PMid:21502745 PMCid:PMC3696368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen TH. In vitro development of engineered lung tissue. 2009 [Google Scholar]
- 18.Lin YM, Boccaccini AR, Polak JM, Bishop AE, Maquet V. Biocompatibility of Poly-DL-lactic acid (PDLLA) for Lung Tissue Engineering. Journal of Biomaterials Applications. 2006;21(2):109–118. doi: 10.1177/0885328206057952. https://doi.org/10.1177/0885328206057952 PMid:16443629. [DOI] [PubMed] [Google Scholar]
- 19.Conconi MT, Coppi PD, Liddo RD, Vigolo S, Zanon GF, Parnigotto PP, Nussdorfer GG. Tracheal matrices, obtained by a detergent-enzymatic method, support in vitro the adhesion of chondrocytes and tracheal epithelial cells. Transplant international. 2005;18(6):727–734. doi: 10.1111/j.1432-2277.2005.00082.x. https://doi.org/10.1111/j.1432-2277.2005.00082.x PMid:15910302. [DOI] [PubMed] [Google Scholar]
- 20.Bernard MP, Chu ML, Myers JC, Ramirez F, Eikenberry EF, Prockop DJ. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983;22(22):5213–5223. doi: 10.1021/bi00291a023. https://doi.org/10.1021/bi00291a023 PMid:6689127. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. https://doi.org/10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, Jaworski DM, Allen GB, Weiss DJ. Initial binding and re-cellularization of de-cellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2011;18(1-2):1–16. doi: 10.1089/ten.tea.2011.0301. https://doi.org/10.1089/ten.tea.2011.0301 PMid:21756220 PMCid:PMC4066256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babavalian H, Latifi AM, Shokrgozar MA, Bonakdar S, Shakeri F, and Tebyanian H. Healing Effects of Synthetic and Commercial Alginate Hydrogel Dressings on Wounds: A Comparative Study. Trauma Mon. 2016 Aug; (In press). https://doi.org/10.5812/traumamon.38941. [Google Scholar]
- 24.Babavalian H, latifi AM, Shokrgozar MA, Bonakdar S, Tebyanian H, and Shakeri F. Cloning and expression of recombinant human platelet-derived growth factor-BB in Pichia Pink. Cellular and Molecular Biology. 2016;62(8):45–51. PMid:27545214. [PubMed] [Google Scholar]
- 25.Karami A, Tebyanian H, Goodarzi V, and Shiri S. Planarians:an in vivo model for regenerative medicine. International journal of stem cells. 2015;8(2):128. doi: 10.15283/ijsc.2015.8.2.128. https://doi.org/10.15283/ijsc.2015.8.2.128 PMid:26634061 PMCid:PMC4651277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakeri F, Tebyanian H, Karami A, Babavalian H, and Tahmasbi MH. Effect of Topical Phenytoin on Wound Healing. Trauma Mon. 2017;22(5) [Google Scholar]
- 27.Lal MK, Manktelow BN, Draper ES, Field DJ. Chronic lung disease of prematurity and intrauterine growth retardation:a population-based study. Pediatrics. 2003;111(3):483–487. doi: 10.1542/peds.111.3.483. https://doi.org/10.1542/peds.111.3.483 PMid:12612225. [DOI] [PubMed] [Google Scholar]
- 28.Mini-o AM, Murphy SL, Xu J, Kochanek KD. Deaths:final data for 2008. National vital statistics reports:from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2011;59(10):1–126. [PubMed] [Google Scholar]
- 29.Price AP, Godin LM, Domek A, Cotter T, D'Cunha J, Taylor DA, Panoskaltsis-Mortari A. Automated Decellularization of Intact, Human-Sized Lungs for Tissue Engineering. Tissue Engineering. Part C, Methods. 2015;21(1):94–103. doi: 10.1089/ten.tec.2013.0756. https://doi.org/10.1089/ten.tec.2013.0756 PMid:24826875 PMCid:PMC4290793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neill JD, Anfang R, Anandappa A, Costa J, Javidfar JJ, Wobma HM, Singh G, Freytes DO, Bacchetta MD, Sonett JR, Vunjak-Novakovic G. Decellularization of Human and Porcine Lung Tissues for Pulmonary Tissue Engineering. The Annals of thoracic surgery. 2013;96(3):1046–1056. doi: 10.1016/j.athoracsur.2013.04.022. https://doi.org/10.1016/j.athoracsur.2013.04.022 PMid:23870827 PMCid:PMC4033908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maghsoudlou P, Georgiades F, Tyraskis A, Totonelli G, Loukogeorgakis SP, Orlando G, Shangaris P, Lange P, Delalande J-M, Burns AJ, Cenedese A, Sebire NJ, Turmaine M, Guest BN, Alcorn JF, Atala A, Birchall MA, Elliott MJ, Eaton S, Pierro A, Gilbert TW, De Coppi P. Preservation of micro-architecture and angiogenic potential in a pulmonary acellular matrix obtained using intermittent intra-tracheal flow of detergent enzymatic treatment. Biomaterials. 2013;34(28):6638–6648. doi: 10.1016/j.biomaterials.2013.05.015. https://doi.org/10.1016/j.biomaterials.2013.05.015 PMid:23727263 PMCid:PMC3988964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, and Nichols JE. Influence of Acellular Natural Lung Matrix on Murine Embryonic Stem Cell Differentiation and Tissue Formation. Tissue Engineering Part A. 2010;16(8):2565–2580. doi: 10.1089/ten.tea.2009.0730. https://doi.org/10.1089/ten.tea.2009.0730 PMid:20408765. [DOI] [PubMed] [Google Scholar]


