Abstract
BACKGROUND:
Recently epidemiological studies showed that low vitamin D is linked to airway hyperresponsiveness, decreased lung function, poor asthma control, and steroid–resistant asthma.
AIM:
We investigated the relationship between Vitamin D, inflammation with circulating IL-33 and lung function in 30 patients with severe uncontrolled asthma.
MATERIALS AND METHODS:
The study included 30 patients with severe uncontrolled asthma. In each of them were measured serum levels of IL-33 and Vitamin D by the ELISA method. The pulmonary function is measured by basic spirometry parameters, FEV1. The results were statistically elaborated according to the Pearson’s Correlation Tests.
RESULTS:
The results showed statistically insignificant correlation between Vitamin D and IL-33, and Vitamin D with FEV1 (Vit.D/IL-33; r = 0.11323, p = 0.551); (Vit.D/FEV1; r = -0.1005; p = 0.597) Correlation between IL-33 and FEV1 is negative but statistically significant (IL-33/FEV1; r = -0.5248; p = 0.003).
CONCLUSION:
Because there are little studies about the link between vitamin D and asthma, further research to clarify the mechanism how vitamin D control the activity of CD4+ T cells and the related Th2-type cytokines in the parthenogenesis of asthma.
Keywords: asthma, Vitamin D, IL-33, FEV1, Th2-type cytokines
Introduction
Asthma is the leading chronic respiratory diseases which affect more than 334 million people worldwide [1-3]. It is estimated that the number of people with asthma will grow by more than 100 million by 2025. Women were more likely than men and boys more likely than girls to have asthma [4]. Approximately 500,000 annual hospitalisations are due to asthma, and 250 000 deaths annually [3]. Five percentages or 100,000 of the Macedonian population have asthma [5, 6].
Characteristic mechanisms of asthma include inflammation, hyperresponsiveness and structural changes in the airways and the lung. Many cellular elements and different cells play a role (T lymphocytes, neutrophils, eosinophils, mast cells, macrophages, and dendritic cells). Recurrent episodes of wheezing, chest tightness, coughing and breathlessness, particularly at night or in the early morning are a clinical expression of this disease. These episodes are usually variable, and the airflow obstruction within the lung is often reversible with treatment or spontaneously [7].
Chronic inflammation of the asthmatic airway leads to epithelial desquamation, infiltration of the airway wall with T cells especially dominated Th2 helper CD4+ lymphocytes, smooth muscle, hypertrophy and hyperplasia, vascular congestion, oedema due to plasma leakage and mucus plugging [8-11]. All these changes could lead to thickening of the airway walls due to subepithelial fibrosis and reduction of their lumen [12, 13].
More than 100 mediators and markers of inflammation are involved in this inflammation. Cytokines take place in complex pathophysiological process, and they are produced by cells like T cells, Tc, Th, Th1, Th2, NK, dendritic cells, B cells, endothelial cells, plasma cells, mast cells, bone marrow, tumor cells and thymus, together with, leukocytes, macrophages, fibroblasts, and monocytes [14, 15]. The interleukins are cytokines which can stimulate the proliferation and differentiation of immune cells. In asthma, T cells take a central place in coordinating the immune response. Cytokines present during activation of naïve CD4 + T cells from pathogens and many types of antigens differentiate them into subpopulations of T cells in T-follicular effector cells, Th1, Th2, Th9, Th17, and Th22. Th2 cells synthesise IL-4, IL-5, IL-13, IL-25, IL-31, IL-33 which are associated with asthma and allergic diseases [16-19].
Interleukin 33 (IL-33) is a new cytokine which was found in 2005. It belongs to the IL-1 family consisting of 11 members, high proinflammatory cytokines which activate the function of inflammation cell in the early asthmatic responses. IL-33 is a potent type 2 inducing cytokine. It is bind to ST2 receptors, which are expressed on mast cells and Th2 cells, and some various cells including epithelial, bronchial and endothelial cells, fibroblasts and some immune cells, as well as dendritic cells and macrophages [20-23]. It is assumed that IL-33 is one of the earliest released mediators and can orchestrate the immune cascade of asthma and stands out as an attractive candidate for discovering various therapeutic modalities, especially a new targeted therapy.
Vitamin D plays a role in the pathogenesis of asthma. Vitamin D is a potent immune system modulator and in the form of 1,25-dihydroxy vitamin D has been shown that it can be involved in the suppression of dendritic cell maturation and the development of consecutive Th1 cell [24-27]. Vitamin D may suppress the production of IL-12, which reduces the production of Th1 cells and potentially leading to increased proliferation of Th2 cells [25]. Additionally, studies with treatment of 1,25-dihydroxy vitamin D in mice showed reduced secretion of Th1-type cytokines IL-2 and IFN-γ and an increase in Th2-type IL-4. In asthmatic children, low vitamin D levels are associated with airway hyperresponsiveness, decreased lung function, worse asthma control, and steroid-resistant asthma and exacerbations. It remains unknown whether vitamin D is an association with increased airway hyperresponsiveness (AWH), inflammatory markers of asthma, decreased lung function, poor asthma control and steroid resistance in adult asthma patients [28-30].
We aimed to investigate the relationship between Vitamin D, inflammation with circulating IL-33 and lung function in 30 patients with severe uncontrolled asthma.
Material and Methods
The study included 30 patients with asthma. They are diagnosis and treated at the Clinic of Pulmonology and Allergy in Skopje, Macedonia. All of them are a classification of uncontrolled moderate persistent asthma. In all patients, serum IL-33 level was measured by the ELISA, enzyme-linked immunosorbent assay method according to the manufacture’s protocol at the Institute of Immunobiology and Human Genetics, Faculty of Medicine, in Skopje, Macedonia. Values within the reference range for IL-33 are 0pg/ml. Serum 25-hydroxyvitamin D was measured by ELISA method according to the manufacture’s protocol at University Clinic of Clinical biochemist, Skopje, Macedonia.
At the Clinic of pulmonology and allergy in Skopje, was done the spirometry with turbine spirometer - Spirobank G, according to the standard methodology. In all patients was done and analysed FEV1 - Forced expiratory volume in the first second. Every patient is done three manoeuvres with three acceptable and three reproducible curves, according to guidelines for the measurement of Spirometry, with Flow-Volume and Time-Volume curve. The best curve has been automatically selected from the spirometer [31-33].
Inclusion Criteria: the patients are diagnosed and classified in uncontrolled moderate persistent asthma at PHI University Clinic of Pulmonology and Allergy according to the actual version of the Global Initiative for Asthma guidelines – GINA [7] and Guidelines for the Diagnosis and Management of Asthma (EPR-3) of NAEPP, National Asthma Education Prevention Program [33]. Uncontrolled asthma defined as at least one of the following [34].
Poor asthma symptom control: Asthma Control Questionnaire (ACQ) consistently >1.5, or Asthma Control Test <20 (or “not well controlled” by GINA - NAEPP guidelines over three months of evaluation:
two or more bursts of systemic CS (3 days each) in the previous year - frequent severe exacerbations;
at least one hospitalisation, required to stay on ICU or mechanical ventilation in the previous year - serious exacerbations; and
after an appropriate bronchodilator withhold FEV1<80% predicted - airflow limitation.
All patients have stable asthma because there has been no increase asthma symptoms or need for add another asthma medication for at least the past four weeks. Some of the patients have arterial hypertension, and they did not have other comorbidities, that could increase the IL-33 level. The age range of patients was 20-71 years old.
Exclusion criteria were severe diseases of renal, neurological, haematological, cardiac, gastrointestinal, endocrine or immune system, psychiatric disorders, and neoplastic diseases. Pregnancy is also exclusion criteria.
Statistical analysis
The results were statistically analysed by the statistical program SPSS for Windows 17.0. The results were statistically analyzed according to the Pearson’s Correlation Tests. The significances values were taken p < 0.05.
Results
The study included 30 patients with severe uncontrolled asthma. The majority were females, 19 women (63.33%), and 11 were men (36.67%). The age of the patients was 20-71 years. The obtained values showed (Table 1):
Table 1.
Mean values for Vitamin D, IL-33 and FEV1
| N-30 | mean | Std.Dv |
|---|---|---|
| Vitamin D | 15.260 | 5.808 |
| IL-33 | 33.461 | 8.851 |
| FEV1 | 44.367 | 14.207 |
All patients had low serum vitamin D and their correlation with IL-33 showed statistically insignificant correlation (Vit.D/IL-33; r = 0.11323; p = 0.551) (Figure 1).
Figure 1.
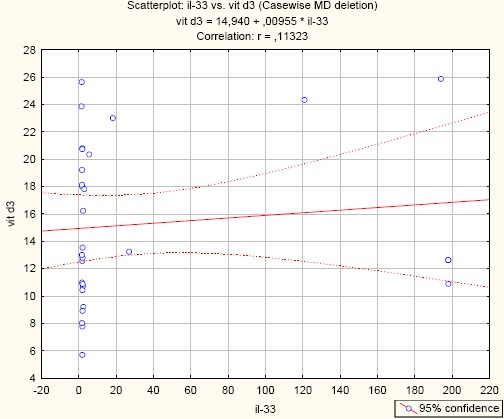
Correlation between Vitamin D and IL-33 in asthma patients. (r = -0.11323; p = 0.551)
Negative significant correlation between IL-33 and FEV1 (IL-33/FEV1; r = -0.5248; p = 0.003) (Figure 2).
Figure 2.
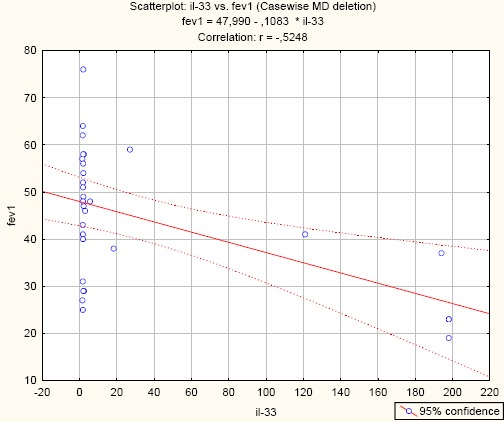
Correlation between IL-33 and FEV1 in asthma patients. (r = -0.5248; p = 0.003)
Negative insignificant correlation between serum levels of vitamin D and FEV1 (Vit.D/ FEV1; r = -0.1005; p = 0.597) (Figure 3)
Figure 3.
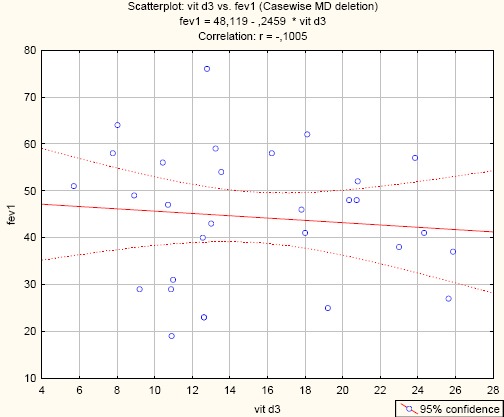
Correlation between Vitamin D and FEV1 in asthma patients. (r= -0.1005; p = 0.597)
Discussion
The studies which investigate vitamin D status, inflammatory markers of asthma and lung function are scarce. The studies which investigated the direct mechanistic links between vitamin D and lung diseases are limited. Cross section of data indicate that low level of vitamin D in patients with mild and moderate asthma is associated with more frequent exacerbations, poor asthma control, decreased lung function, steroid–resistant asthma, and consequent increased steroid use [24-26, 35-37].
A couple study presented that the low level of vitamin D has an association with airway obstruction and corticosteroid requirement, influencing the severity in asthmatic patients. Our study showed that serum vitamin D level was lower (level of vitamin D < 30 ng/ml) in these 30 patients with asthma but their correlation with IL-33 was statistically insignificantly. Anna Bonanno et al. in their study showed that Vitamin D it is not involved in IL-33/IL-31, Th2-type cytokines activity implicated in bronchial and nasal allergic disease [24]. Felicia M.A. et al. in their study they did not find a link between allergy markers (allergic rhinitis, eosinophil count, total IgE) and vitamin D levels [38].
Vitamin D inhibits the synthesis and releases cytokines which are Th1-associated and some other molecules, like IL-17, which lead to decreased inflammation and proliferation of smooth muscle cell [35, 38, 39]. Recent studies have shown that low level of vitamin D is linked with increased expression of the pro-inflammatory cytokine TNF-α, enhancing a pro-inflammatory effect in patients with asthma [35, 40]. This vitamin promotes regulatory T cells and also increases synthesis of IL-10, which lead to an inhibition of Th2 responses as well as airway inflammation and airway hyperresponsiveness [35, 41].
Larose et al. in HUNT, prospective cohort study, found that low serum 25(OH) D level was not correlated with airway obstruction in most asthmatics adults except men with asthma but without allergic rhinitis [42]. In our study, there was an insignificant correlation between serum levels of vitamin D and FEV1. Laura et al. also showed in their study that the vitamin D level did not correlate with lung function and markers of allergy in asthmatic patients [43]. Some studies show a significant direct relation between vitamin D level and both FEV1 and FEV1/FVC [43, 44].
In this study, we found that serum IL-33 was increased in patients with asthma and they positively correlated with asthma severity with the significant negative correlation between IL-33 and FEV1. In human studies of allergic asthma, serum IL-33, bronchoalveolar lavage fluid and lung tissue have found to be higher in patients with asthma compared to healthy controls and correlate with asthma severity [45-50], which was confirmed in our study
In conclusion, despite the limitation of this study with a small number of patients, we found an increased serum IL-33 in patients with asthma and it is positively related to the severity of asthma (FEV1), but we did not find a correlation between levels of vitamin D and FEV1 and IL-33. Because there are little studies about the link between asthma and vitamin D, further studies are necessary to explain the mechanism how vitamin D can control the activity of CD4+ T cells and the related Th2 type cytokines in the pathogenesis of the allergic disease, including asthma.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma:executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. https://doi.org/10.1111/j.1398-9995.2004.00526.x PMid:15080825. [DOI] [PubMed] [Google Scholar]
- 2.Beasley R. The Global Burden of Asthma Report, Global Initiative for Asthma (GINA) Available from http://www.ginasthma.org.2004 .
- 3.World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases:a comprehensive approach. 2007 [Google Scholar]
- 4.Centers for Disease Control and Prevention, Vital Signs, May 2011 [Google Scholar]
- 5.Gjorcev A. Makedonski nacionalen plan i program za dijag-nostikai lekuvanje na bronhilajnata asthma-realnost i vizija. 1996 [Google Scholar]
- 6.Vladimir Cvetanov. Alergiskite bolesti vo R. Makedonija. 2006:151–174. [Google Scholar]
- 7.Global Initiative for Asthma (GINA), GINA Report 2017, Global Strategy for Asthma Management and Prevention updated 2017. http://www.ginasthma.org .
- 8.Holgate ST. Genetic and environmental interaction in Allergy and asthma. J Allergy Clin Immunol. 1999;104(6):1139–1146. doi: 10.1016/S0091-6749(99)70005-9. https://doi.org/10.1016/S0091-6749(99)70005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas MJ, Kemeny DM. Novel CD4 and CDS T-cell subsets. Allergy. 1998;53:1122–1132. doi: 10.1111/j.1398-9995.1998.tb03831.x. https://doi.org/10.1111/j.1398-9995.1998.tb03831.x. [DOI] [PubMed] [Google Scholar]
- 10.Heard BE, Hossain S. Hyperplasia of bronchial muscle in asthma. J Pathol. 1973;110:319–331. doi: 10.1002/path.1711010212. https://doi.org/10.1002/path.1711100406. [DOI] [PubMed] [Google Scholar]
- 11.Shiels IA, Bowler SD, Taylor SM. Airway smooth muscle proliferation in asthma:the potential of vascular leakage to contribute to pathogenesis. Med Hypotheses. 1995;45:37–40. doi: 10.1016/0306-9877(95)90198-1. https://doi.org/10.1016/0306-9877(95)90198-1. [DOI] [PubMed] [Google Scholar]
- 12.Dirkje S Postma, Wim Timens. Remodeling in Asthma and Chronic Obstructive Pulmonary Disease. American Thoracic Society. 2006;3(5):434–439. doi: 10.1513/pats.200601-006AW. https://doi.org/10.1513/pats.200601-006AW PMid:16799088. [DOI] [PubMed] [Google Scholar]
- 13.Christopher L. Grainge, Laurie C.K. Lau, Jonathon A. Ward, et al. Effect of bronchoconstriction on Airway Remodeling in Asthma. N Engl J Med. 2011;364:2006–2015. doi: 10.1056/NEJMoa1014350. https://doi.org/10.1056/NEJMoa1014350 PMid:21612469. [DOI] [PubMed] [Google Scholar]
- 14.Barnes PJ. Pathophysiology of asthma. Eur Respir Mon. 2003;23:84–113. [Google Scholar]
- 15.Kuipers H, Lambrecht BN. The interplay of dendritic cells, Th2cells and regulatory T cells in asthma. Curr Opin Immunol. 2004;16(6):702–708. doi: 10.1016/j.coi.2004.09.010. https://doi.org/10.1016/j.coi.2004.09.010 PMid:15511661. [DOI] [PubMed] [Google Scholar]
- 16.Abbas AK, Lichtman Ah, Pober JS. Cellular and Molecular Immunology. (4th ed) 2000:235–69. [Google Scholar]
- 17.Roitt IM, Delves PJ. Essential Immunology. (10th ed) 2001:177–199. [Google Scholar]
- 18.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–463. doi: 10.1067/mai.2003.169. https://doi.org/10.1067/mai.2003.169 PMid:12642820. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Hidehiro Y, William EP. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. https://doi.org/10.1146/annurev-immunol-030409-101212 PMid:20192806 PMCid:PMC3502616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borish L, Steinke JW. Interleukin-33 in asthma:how big of a role does it play? Curr Allergy Asthma Rep. 2011;11:7–11. doi: 10.1007/s11882-010-0153-8. https://doi.org/10.1007/s11882-010-0153-8 PMid:20931364 PMCid:PMC3068600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T-helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. https://doi.org/10.1016/j.immuni.2005.09.015 PMid:16286016. [DOI] [PubMed] [Google Scholar]
- 22.Kakkar R, Lee RT. The IL-33/ST2 pathway:therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. https://doi.org/10.1038/nrd2660 PMid:18827826 PMCid:PMC4277436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm. 2011;8:22. doi: 10.1186/1476-9255-8-22. https://doi.org/10.1186/1476-9255-8-22 PMid:21871091 PMCid:PMC3175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anna Bonanno, Sebastiano G, Stefania La G, et al. 25-Hydroxyvitamin D, IL-31, and IL-33 in Children with Allergic Disease of the Airways Mediators Inflamm. 2014;2014 doi: 10.1155/2014/520241. ID 520241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benson AA, Toh JA, Vernon N, Jariwala SP. The role of vitamin D in the immunopathogenesis of allergic skin diseases. Allergy. 2012;67(3):296–301. doi: 10.1111/j.1398-9995.2011.02755.x. https://doi.org/10.1111/j.1398-9995.2011.02755.x PMid:22171613. [DOI] [PubMed] [Google Scholar]
- 26.Annesi-Maesano I. Perinatal events, vitamin D, and the development of allergy. Pediatric Research. 2002;52(1):3–5. doi: 10.1203/00006450-200207000-00003. https://doi.org/10.1203/01.PDR.0000017228.29635.EF. [DOI] [PubMed] [Google Scholar]
- 27.Meyts I, Hellings PW, Hens G, et al. IL-12 contributes to allergen-induced airway inflammation in experimental asthma. Journal of Immunology. 2006;177(9):6460–6470. doi: 10.4049/jimmunol.177.9.6460. https://doi.org/10.4049/jimmunol.177.9.6460. [DOI] [PubMed] [Google Scholar]
- 28.Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression:enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. The Journal of Allergy and Clinical Immunology. 2003;112(3):585–592. doi: 10.1016/s0091-6749(03)01855-4. https://doi.org/10.1016/S0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 29.Wu AC, Kelan T, Lingling L, et al. The effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit. Care Med. 2012;186(6):508–513. doi: 10.1164/rccm.201202-0351OC. https://doi.org/10.1164/rccm.201202-0351OC PMid:22798322 PMCid:PMC3480528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hala Mohamed ShS, Amany RE, Eman N. Vitamin D and markers of airway inflammation in asthma. Egyptian Journal of Chest Diseases and Tuberculosis. 2015;64:779–783. https://doi.org/10.1016/j.ejcdt.2015.03.027. [Google Scholar]
- 31.George RB, Light RW, Matthay RA, Matthay MA Clinical Pulmonary Function Testing, Exercise Testing, and Disability Evaluation. In Chest Medicine:Essentials Of Pulmonary And Critical Care Medicine. (5th edition) 2005 May; [Google Scholar]
- 32.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. https://doi.org/10.1183/09031936.05.00034805 PMid:16055882. [DOI] [PubMed] [Google Scholar]
- 33.National Asthma Education, Prevention Program (National Heart, Lung, Blood Institute) Second Expert Panel on the Management of Asthma. Expert panel report 2:Guidelines for the diagnosis and management of asthma. DIANE Publishing; 1998. [Google Scholar]
- 34.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. European Respiratory Journal. 2013:erj02020–2013. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 35.Sandhu MS, Casale TB. The role of vitamin D in asthma. Ann Allergy Asthma Immunol. 2010;105:191–199. doi: 10.1016/j.anai.2010.01.013. https://doi.org/10.1016/j.anai.2010.01.013 PMid:20800785. [DOI] [PubMed] [Google Scholar]
- 36.Stephanie K, Hübner M, Matthias J, Maria B, Roland B. Severe and uncontrolled adult asthma is associated with vitamin D insufficiency and deficiency. Respir Res. 2013;14:25. doi: 10.1186/1465-9921-14-25. https://doi.org/10.1186/1465-9921-14-25 PMid:23432854 PMCid:PMC3648461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. PMid:16825677. [DOI] [PubMed] [Google Scholar]
- 38.Felicia Montero-Arias, Giovanni Sedó-Mejía, Allan Ramos-Esquivel. Vitamin D Insufficiency and Asthma Severity in Adults From Costa Rica. Allergy Asthma Immunol Res. 2013;5(5):283–288. doi: 10.4168/aair.2013.5.5.283. https://doi.org/10.4168/aair.2013.5.5.283 PMid:24003384 PMCid:PMC3756174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression:enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112:585–92. doi: 10.1016/s0091-6749(03)01855-4. https://doi.org/10.1016/S0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 40.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. https://doi.org/10.1164/rccm.200911-1710OC PMid:20075384 PMCid:PMC2868500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lange NE, Litonjua A, Hawrylowicz CM, Weiss S. Vitamin D, the immune system and asthma. Expert Rev Clin Immunol. 2009;5:693–702. doi: 10.1586/eci.09.53. https://doi.org/10.1586/eci.09.53 PMid:20161622 PMCid:PMC2812815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larose TL, Arnulf L, Yue Chen, et al. Serum 25-hydroxyvitamin D levels and lung function in adults with asthma:the HUNT Study. Eur Respir J. 2015;45(4):1019–26. doi: 10.1183/09031936.00069714. https://doi.org/10.1183/09031936.00069714 PMid:25395037. [DOI] [PubMed] [Google Scholar]
- 43.Laura T, Edita G, Simona L, et al. Evaluation of vitamin D levels in allergic and non-allergic asthma. Medicine. 2015;51(6):321–327. doi: 10.1016/j.medici.2015.11.003. https://doi.org/10.1016/j.medici.2015.11.003 PMid:26739673. [DOI] [PubMed] [Google Scholar]
- 44.Lin Z, Li W. The roles of vitamin D and its analogues in inflammatory diseases. Current topics in medicinal chemistry. 2016;16(11):1242–61. doi: 10.2174/1568026615666150915111557. https://doi.org/10.2174/1568026615666150915111557 PMid:26369816. [DOI] [PubMed] [Google Scholar]
- 45.Li YM, Cheng XM. Clinical significance of IL-18 and IL-33 levels changes in the treatment of bronchial asthma with acute attack stage by glucocorticoids. Practical Pharmacy and Clinical Remedies. 2013;16:897–9. [Google Scholar]
- 46.Azazi EA, Elshora AE, Tantawy EA, Elsayd MA. Serum levels of Interleukin-33 and its soluble receptor ST2 in asthmatic patients. Egyptian Journal of Chest Diseases and Tuberculosis. 2014;63(2):279–84. https://doi.org/10.1016/j.ejcdt.2013.11.005. [Google Scholar]
- 47.Liu SP, Ma R, Li XA. The relationship between interleukin 33 promotes and lung inflammation in asthmatic children. Chin J Diffic and Compl Cas. 2013;12:422–4. [Google Scholar]
- 48.Préfontaine D, Nadigel J, Chouiali F, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–4. doi: 10.1016/j.jaci.2009.12.935. https://doi.org/10.1016/j.jaci.2009.12.935 PMid:20153038. [DOI] [PubMed] [Google Scholar]
- 49.Préfontaine D. Lajoie-Kadoch S, Foley Set al. Increased expression of IL-33 in severe asthma:evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–103. doi: 10.4049/jimmunol.0802387. https://doi.org/10.4049/jimmunol.0802387 PMid:19801525. [DOI] [PubMed] [Google Scholar]
- 50.Liao XM. Changes of plasma interleukin 33 in asthmatic patients and its clinical significance. Journal of Clinical Pulmonary Medicine. 2014;19:33–5. [Google Scholar]


