Abstract
AIM:
This study aims to evaluate the algorithm of procalcitonin (PCT) and its role on the duration of antibiotics prescription for intra-abdominal infections.
MATERIALS AND METHODS:
This study is a prospective controlled study that is conducted in groups of 50 hospitalised patients and 50 controlled group patients.
RESULTS:
The results indicated that the average duration of antibiotic delivery to the PCT group was -10.6 days (SD ± 6.6 days), while in the control group -13.2 days (SD ± 4.2 days). These data showed a significant difference in the duration of antibiotic therapy and the monitoring role of PCTs in the prediction success of antibiotic treatment. The antibiotic delivery was longer in the septic shock 17 (SD ± 11.7) that corresponds to high PCT values of 67.8 (SD ± 50.9). Recurrence of the infection after the cessation of antibiotics occurred in 2 cases (4%) in the standard group, while it occurred in 3 cases (6%) in the control group.
CONCLUSION:
The treatment of the intra-abdominal infections based on the PCT algorithm shortens the duration of antibiotic treatment and does not pose a risk for the recurrence of the infection.
Keywords: intraabdominal infections, procalcitonin, antibiotic, microbiological examination, CRP
Introduction
Intra-abdominal infections are a group of diseases with a relatively high degree of mortality and morbidity. Two determinants for the successful treatment of intra-abdominal infections are the identification of the source of the infection and the speed at which the empirical delivery of antibiotics begins, circumstances that reduce the risk of complications and mortality [1]. Reduction of mortality is not possible without effective control of the source of infection [2]. The mortality rate of intra-abdominal infections was about 90% at the end of the 19th century, where management was mostly non-operative. In recent years, surgical intervention in intra-abdominal infections has influenced the control of the source of infection by reducing the mortality rate about 30% in cases of severe sepsis and septic shock [3-5]. Reduction of mortality rate is achieved with the earliest diagnostics of these infections and timely and adequate treatment [6-8].
Antibiotic therapy should start immediately after the intraabdominal infection diagnosis, while the duration of administration of antibiotics is a matter of ongoing discussion and there is still no consensus [8]. Treatment protocols recommend empirical antimicrobial therapy that is effective against the enterococcus, staphylococcus and the candida [6, 7]. If the empirical antibiotic is inadequate, it is followed by failure of treatment, an extension of hospitalisation and death. Non-rational antibiotic delivery leads to increased resistance to antibiotics and the appearance of intrahospital infections [8]. Therefore it is recommended to restrict the use of antibiotics and apply the biomarker algorithm in treatment to monitor the success of antibiotic therapy and discontinuation of their ordination as earliest [9, 10]. In Intensive Care Department, the procalcitonin (PCT) serial measurement can be used as a guide to facilitate the most rapid interruption of antibiotics [11]. PCT is the calcitonin prohormone produced by the C-cell of the thyroid gland. The PCT inflammatory processes do not happen in thyroid C cells and do not depend on the calcium concentration, but its production depends on the microbial endotoxins and indirect from specific cytokines such as IL-1, IL-6 and TNF [12]. Indeed, it has been reported that the PCT threshold of 0.25 ng/mL to 0.50 ng/mL or a reduction of at least 80% of the initial value [13, 14] allows the antibiotic to stop earlier without changing the clinical outcome [15-18]. The highest PCT values reach 24-48 h after infection, and proper management causes the PCT values to drop by about 50% within about one to one-half days after the infection decoction is interrupted. In some clinical cases, interferences with PCT values have been introduced, as in patients with renal failure, where half-life elimination is prolonged, but PCT accumulation does not occur [19]. Data from the literature suggests that if stimulation is discontinued its concentration decreases progressively and returns to baseline values on Day 5 or 7 [20]. An exceptional PCT situation is presented if clinical symptoms indicate possible sepsis but PCT values are low, then sepsis therapy should begin anyway, PCT measurement should be repeated (after 12, 24, 36 h) until the final diagnosis is clarified [19]. Despite the important information, the determination of PCT values that have a diagnostic and predictive role in the mentioned diagnoses is not yet definitive and is considered to be investigated further in other issues of surgical infections.
Indeed, most of the research has evaluated PCT’s interest in determining the duration of antibiotic delivery to conservatively treated patients [9, 11], while there is no or insufficient research on the role of PCT in intra-abdominal infections diagnosed as peritonitis, therefore, the aim of this study is to evaluate the predictive role of PCT in the duration of antibiotic delivery and treatment priorities based on the PCT algorithm in relation to standard treatment.
Materials and Methods
This is a clinically prospective, controlled trial involving hospitalised patients in the Clinical Surgery department at University Clinical Hospital of Kosovo in Prishtina, registered in the period from March 2016 to January 2017. The study was performed with simple randomisation, and 100 patients were included out of which, 50 patients were used for testing the PCT algorithm efficacy in discontinuing antibiotics. The study included a control group of 50 patients as a comparator in the duration of antibiotic delivery while comparing the complications following the discontinuation of antibiotics in both groups. The Ethics Committee of the University of Prishtina – Faculty of Medicine, has approved the research protocol and all participants or their family members completed the consent form.
Inclusion and exclusion criteria: Patients over the age of 18 diagnosed with acute abdomen with Systemic Inflammatory Response Syndrome (SIRS), who underwent urgent surgical intervention and Index Manheim Peritonitis > 10 points, were eligible for enrollment in the study. Patients with a) autoimmune disease b) acute hepatic insufficiency c) diabetes d) immunosuppression e) pregnant women f) patients treated with corticoids g) when the patient or family members have refused to be included in the study were excluded.
Protocol and Treatment: PCT levels were measured before surgery on the day 1, 4, and 7 and the following days as needed. The measurement was made with the Elecsys BRAHMS PCT method. The restraints of the values measured by this method are from 0.02 to 100 ng/mL. Values below the detectable limit are reported as < 0.02 ng/mL, whereas those over the upper limit are reported >100 ng/mL [21]. PCT values in healthy people are low <0.1ng/mL [22]. To exclude sepsis and systemic inflammation PCT < 0.2 ng/mL concentration is a useful reference. As the cut off for the diagnosis of sepsis the PCT values > 0.5 ng/mL are interpreted as abnormal and suggest for sepsis [20].
Recommendations based on the PCT algorithm are discontinuation of the antibiotics when PCT decreases > 80% of the initial value or PCT < 0.5 ng/mL, reduction by the clinical condition and other laboratory parameters.
Antibiotic delivery has begun in all patients with acute abdominal symptoms and SIRS presence. Interventions have been performed by experienced surgeons. After the intervention, all participants continued empirically given antibiotics, to the group with SIRS/sepsis and severe sepsis ceftriaxone 1 gram was ordinated intravenously every 12 hours and metronidazole 500 mg intravenously every 8 hours, while in patients with septic shock imipenem 500 mg three times daily and metronidazole 500 mg intravenous every 8 hours.
After receiving the microbiological examination results, antibiogram treatment continued. Termination of the antibiotics in the study group was conducted according to the protocol of the study. The basic criteria for the antibiotic discontinuation in control group were the normalization of leukocyte values followed by improvement of the clinical condition. After the antibiotic discontinuation, the condition of all patients was monitored for the identification of possible complications.
Statistical Analysis
Stratification of the results was conducted based on the criteria of the severity of intraabdominal infections (SIRS/sepsis, Severe sepsis, Septic shock). We included age, gender, APACHE II, MODS, SOFA and INDEX MANNHEIM PERITONITIS.
The statistical processing of the data was conducted with the statistical package SPSS 22.0. The arithmetic average, the standard deviation, the minimum and the maximum value were calculated from statistical parameters. Qualitative data testing was done with the χ2-test and the exact Fisher test of quantitative data that had a normal distribution with T-test and One Way ANOVA, and those with a nonnormal distribution with the Mann-Whitney test or Kruskal Wallis test.
Results
In both study groups the male patients were slightly more than females but without a significant statistical difference (X2 = 0.176, P = 0.675). Patients of both groups were in different age groups - the youngest was 17 years old, and the oldest was 86 years old. The average age of the patients was 43.2 years (DS ± 19.5 years), ranging from 17 to 86 years.
According to the demographic characteristics of patients, there is no significant difference between the study and control groups. In both groups, according to the diagnosis structure, the acute perforative appendicitis and perforated duodenal ulcer were a more frequent diagnosis of patients.
The average duration of antibiotic delivery to all patients was 11.1 days (SD ± 5.5 days), ranging from 3.0 to 31.0 days. In the test group, the average duration was 10.6 days (SD ± 6.6 days), ranging from 3.0 to 31.0 days. In the control group, the average duration was 13.2 days (SD ± 4.2 days), ranging from 3.0 to 29.0 days. With t-test, we revealed significant statistical significance (t-test), (P = 0.028), (Table 1).
Table 1.
Characteristics of Patients included in the study
| Study Group | Control Group | p | |
|---|---|---|---|
| Age | 39.4 ± 19.6 | 47.0 ± 18.8 | 0.064a |
| Female | 16 | 19 | 0.675b |
| Male | 34 | 31 | 0.675b |
| Etiology of the disease | |||
| Appendicitis acuta perforativa | 23 | 34 | |
| Ulcus bulbi duodeni perforans | 9 | 10 | |
| Perforatio intestini (ilei, jejuni, sygmae) | 11 | 5 | |
| Cholecystitis acuta perforativa | 4 | 1 | |
| St. post op.Wiple (Dehiscencio anastomosis pancreatico-jejuni) | 1 | ||
| Perforatio diverticulum Meckeli | 2 | ||
| Duration of Antibiotic Delivery | 10.6 ± 6.6 | 13.2 ± 4.2 | 0.028c |
| Reconnaissance of infection after antibiotic termination | 2 (4%) | 3 (6%) | |
A = Man Whitney test; b = χ2-test; c-T-test.
According to duration of antibiotic administration in the study group, we have noted this structure of administration: 8% - 4 days, 4% -5 days, 16% - 6 days, 10% - 7 days, 62% > 7 days. The criteria of PCT decreased > 80% of the initial value was met in 8 patients; 8 (16%) patients did not receive the antibiotics as per PCT < 0.5 ng/mL algorithm. This group of patients had an average of 4 days more (SD ± 3) administered antibiotic due to unsatisfactory clinical condition and laboratory parameters, while in other 4 patients we had an initial decrease of PCT in < 0.5 ng/mL, and after that an increase of PCT values. Two of these patients had pulmonary problems, and the other two were indispensable for reintervention. Contrary, in the control group, only 4 (8%) patients had antibiotics < 7 days (Table 1).
MODS-Multiple Organ Dysfunction Score, IMP-Index Mannheim peritonitis, APACHE-Acute Physiology and Chronic Health Evaluation, SOFA-Sequential Organ Failure Assessment, PCT-procalcitonin, CRP-C reactive protein, TG-Triglycerides, WBC-White Blood Cell.
Based on the results we have found a significant correlation of the values of SOFA, APACHE II, MODS, PCT, and TG with the duration of antibiotic delivery, while the values of CRP and WBC do not correspond to the duration of antibiotic delivery (Table 2).
Table 2.
Duration of Antibiotics Delivery in report to SOFA, APACHE II, IMP, MODS, PCT, CRP, WBC in admission of patients to the study group
| Abdominal Urgencies (n=50) | ||||
|---|---|---|---|---|
| Parameters | Septic Shock | Severe Sepsis | SIRS/Sepsis | p |
| Duration of antibiotic administration | 17.0 (SD ± 11.7) | 15.9 (SD ± 8.4) | 8.6 (SD ± 5.7) | <0.001a |
| SOFA | 17.8 (SD ± 7.6) | 10.5 (SD ± 3.8) | 5.2 (SD ± 2.4) | <0.0001a |
| APACHE II | 34 (SD ± 9.5) | 19,5 (SD ± 6.3) | 9.9 (SD ± 5.1) | <0.0001a |
| MODS | 12.8 (SD ± 3.8) | 7.3 (SD ± 3.6) | 2.3 (SD ± 1.3) | <0.184a |
| PCT | 67.8 (SD ± 50.9) | 22.5 (SD ± 36.7) | 3.7 (SD ± 5.6) | 0.00015a |
| CRP | 117.9 (SD ± 123.7) | 154.2 (SD ± 72.3) | 133.1 (SD ± 90.5) | 0.431a |
| WBC | 11.5 (SD ± 8.2) | 18.4 (SD ± 11.7) | 14.2 (SD ± 4.1) | 0.132b |
= Kruskal Wallis test;
= one way ANOVA test.
PCT algorithm shows that after surgical treatment and initiation of antibiotic therapy in a group with SIRS/sepsis and severe sepsis the values are reduced until in the septic shock PCT values decreases about the initial values but remain high enough to prevent the discontinuation of antibiotics (Fig. 1).
Figure 1.
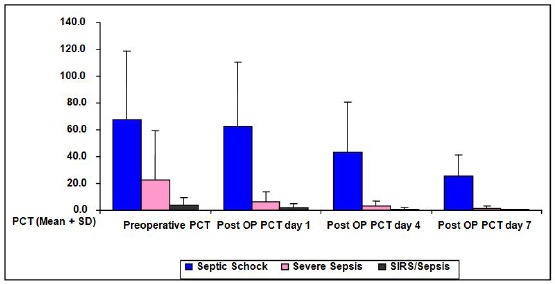
PCT algorithm according to the severity of the infection
In most of the patients of the study group and control group, E. coli in the form of mono-culture or co-infection with Streptococcus pyogenes and Pseudomonas aeruginosa were isolated. In the structure of isolated microorganisms, monocultures of Pseudomonas aeruginosa, Klebsiella pneumoniae and Candida were also identified microbiological cultures. However, the culture of bacteria was not isolated in 34.0% of cases in the study group and 38% in the control group (Table 3).
Table 3.
Results of microbiological examination in the study group and control group
| Microbiological results | Study Group | Control Group | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Escherichia coli | 13 | 26.0 | 15 | 30.0- | 28 | 28.0 |
| Escherichia coli streptococcus pyogenes | 2 | 4.0 | 3 | 6.0 | 5 | 5.0 |
| Escherichia coli Pseudomonas aeruginosa | 1 | 2.0 | 2 | 4.0 | 3 | 3.0 |
| Candida SPP | 4 | 8.0 | 2 | 4.0 | 6 | 6.0 |
| Klebsiella pneumoniae | 4 | 8.0 | 2 | 4.0 | 6 | 6.0 |
| Pseudomonas aeruginosa | 5 | 10.0 | 2 | 4.0 | 7 | 7.0 |
| Staphylococcus aureus | 2 | 4.0 | 3 | 6.0 | 5 | 5.0 |
| Peptostreptococcus | 1 | 2.0 | 2 | 4.0 | 3 | 3.0 |
| Acinetobacter SP | 1 | 2.0 | - | - | 1 | 1.0 |
| No culture of bacteria is isolated | 17 | 34.0 | 19 | 38.0 | 36 | 36.0 |
| Total | 50 | 100.0 | 50 | 100.0 | 100 | 100.0 |
The PCT values in the diagnosis structure are quite variable with the signaling scale for P < 0.001 the highest values were recorded in mycotic infections, then the gram negative ones, mixed gram negative and gram positive infections and even lower in gram positive which show a significant statistical difference (Table 4). For the treatment of gram negative infections, seven different forms of antibiotics were administered where Metronidazole and Ceftriaxone were dominated, whereas other forms of infections were administered with fewer antibiotics.
Table 4.
Procalcitonin, duration, and types of antibiotics according to microbial infection
| Parameters | Microbiological Infections | |||||
|---|---|---|---|---|---|---|
| Gram positive and Gram negative | Gram negative | Gram positive | Mycotic | Not culture isolated | p | |
| PCT before OP (ng/mL) | 4.2 ± 0 | 18.8 ± 34.6 | 0.9 ± 0.3 | 29.5 ± 47.2 | 3.5 ± 5.2 | 0.001 |
| Duration of antibiotic delivery (d) | 12 ± 1.4 | 11.6 ± 5.4 | 19 ± 16.9 | 2.5 ± 0.7 | 0.41 | |
| Metronidazole | 2 (5%) | 25 (62.5%) | 1 (2.5%) | 4 (10%) | 8 (20%) | |
| Ceftriaxone | - | 25 (62.5% | 1 (2.5%) | 2 (5%) | 12 (30%) | |
| Imipenem and enzyme inhibitor | 3 (20%) | 6 (40%) | 3 (20%) | - | 3 (20%) | |
| Gentamicin | 1 (100%) | |||||
| Tazobactam | 1 (100%) | |||||
| Lincomycin | 2 (50%) | 2 (50%) | ||||
| Amoxicillin/clavulanic acid | 4 (100%) | |||||
Discussion
This randomized study evaluated the role of PCT algorithm in the duration of antibiotic delivery and monitored the success of antibiotics after surgeries of intra-abdominal infections with peritonitis. Our findings indicate a significant difference in the duration of antibiotic delivery between the study group and the control group, with a reduction in the duration of antibiotic delivery to patients of the study group by 2.6 days without risking a recurrence of infection. Our findings do not correspond to the study conducted by Juliette [21], which states that in general there is no significant difference in the duration of antibiotic delivery between the study group and the control group. They revealed the difference by structure of pathogenesis of infections, concluding that there is a difference in the duration of antibiotic delivery between groups of patients with peritonitis due to gastrointestinal perforation (7 days in the PCT and 10-days with control group for p = 0.065), while in patients with localized peritonitis and postoperative peritonitis caused by intestinal dehiscences, there was no reduction of antibiotics with PCT algorithm.
Other authors have stated that the PCT algorithm has a role in reducing the duration of antibiotic delivery of peritonitis. Ting Shuo Huang has concluded that this algorithm reduces the use of antibiotics for three days and provides a 50% relative reduction in the duration of antibiotic delivery [22].
Also, Schroeder et al. in a comparative study with 19 patients with peritonitis after abdominal surgery, stated that antibiotics were reduced to the research group in relation to the control group in significant values (6.6 ± 1.1 days versus 8.3 ± 0.7 days) [23]. Hochreiter et al. recorded patients with confirmed infection or high suspicions for a bacterial infection that were treated with antibiotics and were hospitalized in Intensive Care [24]. Both authors had established antibiotic discontinuity criteria when there was clinical improvement and PCT levels < 1.0 ng/mL or decreased by 25% to 35% of the initial value within three days by making measurements every day. In the results of Hochreiter with authors, the duration of antibiotic delivery was significantly shorter in 57 patients compared to the control group 53 patients (5.9 ± 1.7 days versus 7.9 ± 0.5 days: p < 0.001) without negative effects on results.
In our study, the discontinuation of the antibiotics in the control group was done based on the value of the leukocytes and the clinical condition of the patients. In our study, measurement was done every successive day, and this approach has had an impact on the duration of antibiotic delivery. The PCT algorithm does not affect the reduction of antibiotics in septic shock and severe sepsis. Neither the threshold of PCT of 0.5 ng/mL nor the reduction of at least 80% of the initial value can accurately predict the response to treatment for cases with intra-abdominal infections, where there are a septic shock and severe sepsis corresponding to the results of other authors [25].
Findings of our study show that relation between high PCT, TG and high scores of APACHE II, SOFA, IMP, and MODS and antibiotic treatment duration, whereas preoperative leukocyte and C – reactive protein values do not correspond to the duration of antibiotic administration. Other studies report that age, hypoalbuminemia, malnutrition, comorbidities, APACHE II score ≥ 15 affect the failure of management of infection source and the appearance of infectious complications [26-28].
In the microbiological examination of intraperitoneal fluid, our findings indicate that PCT values have varied between infection groups and have been higher in mycotic infections than in gram-negative ones, mixed gram negative and positive infections, and lower in gram positive infections. However, our data regarding mycotic infections do not correspond to the findings of other authors Martini et al., [29] in his study of 48 surgical patients with mycotic infections risk found that the cut off of PCT for these infections was 2.0 ng/mL. According to Leli, a cut-off of PCT > 1.3 ng/mL can help us in diagnosing mycotic infection [30]. Considering that PCT in mycotic infections are not usually increased since the PCT values are too high in our results, we can conclude that these infections are accompanied by bacterial infections that have not been detected microbiologically in our facilities. In our study PCT cut-off of 18.8 ng/mL is important for predicting sepsis with gram negative bacteria, whereas some studies refer to data linking high PCT values with gram negative sepsis but with cut off values that differ from our values. In the study of Brodská et al. [31], it is described that the PCT cut-off of 15 ng/mL can affect the sepsis caused by gram negative bacteria from sepsis caused by gram positive bacteria and mycotic sepsis with 87.8% specificity. While in the study conducted by Leli, the findings indicate that the cut-off of PCT 10.8 ng/mL may be significant in predicting the infection caused by gram-negative bacteria with a specificity of 82.5%. Other authors have also found that PCT levels were higher in gram negative than in gram positive sepsis [32, 33]. In our study, the PCT average of sepsis with the gram positive bacteria was 0.64 ng/mL (SD = 0.3), corresponding to the data of the author Shuhua [33], where the PCT value was 0.48 ng/mL.
The results from our study and other authors suggest that PCT can help in the ordination of proper antibacterial initiation therapy, considering that 24-48 hours should be expected for microbiological results.
The structure of microorganisms isolated on microbiological samples is similar to the results of other authors, who reported similar infections of the gastrointestinal tract [34, 28].
Overall, we conclude that monitoring of PCT value may impact in shortening of treatment antibiotic duration in patients with intra-abdominal infections. Although PCT is considered to be a very important predictive factor in intra-abdominal infections, in the presence of clinical signs of sepsis, the role PCT is diminished, and antibiotic therapy should be continued according to clinical signs and other predictive parameters. Also, we conclude that further studies are needed to validate the complex role of PCTs in antibiotic monitoring through pharmacoepidemio-logical and pharmacoeconomic studies of antibiotic consumption in both groups that would enable valuable data to be obtained for the role of this algorithm in reducing resistance to antibiotics.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Marshall JC. Principles of source control in the early management of sepsis. Current infectious disease reports. 2010;12(5):345–53. doi: 10.1007/s11908-010-0126-z. https://doi.org/10.1007/s11908-010-0126-z PMid:21308516. [DOI] [PubMed] [Google Scholar]
- 2.Grundmann RT, Petersen M, Lippert H, Meyer F. [The acute (surgical) abdomen - epidemiology, diagnosis and general principles of management] Zeitschrift fur Gastroenterologie. 2010;48(6):696–706. doi: 10.1055/s-0029-1245303. https://doi.org/10.1055/s-0029-1245303 PMid:20517808. [DOI] [PubMed] [Google Scholar]
- 3.Schoeffel U, Jacobs E, Ruf G, Mierswa FB, von Specht U, Farthmann EH. Intraperitoneal micro-organisms and the severity of peritonitis. The European journal of surgery = Acta chirurgica. 1995;161(7):501–8. [PubMed] [Google Scholar]
- 4.Wacha H, Hau T, Dittmer R, Ohmann CP Peritonitis Study Group. Risk factors associated with intraabdominal infections:a prospective multicenter study. Langenbeck's archives of surgery. 1999;384(1):24–32. doi: 10.1007/s004230050169. https://doi.org/10.1007/s004230050169 PMid:10367626. [DOI] [PubMed] [Google Scholar]
- 5.Mulier S, Penninckx F, Verwaest C, Filez L, Aerts R, Fieuws S, Lauwers P. Factors affecting mortality in generalized postoperative peritonitis:multivariate analysis in 96 patients. World journal of surgery. 2003;27(4):379–84. doi: 10.1007/s00268-002-6705-x. https://doi.org/10.1007/s00268-002-6705-x PMid:12658477. [DOI] [PubMed] [Google Scholar]
- 6.Mazuski JE. Antimicrobial treatment for intra-abdominal infections. Expert opinion on pharmacotherapy. 2007;8(17):2933–45. doi: 10.1517/14656566.8.17.2933. https://doi.org/10.1517/14656566.8.17.2933 PMid:18001254. [DOI] [PubMed] [Google Scholar]
- 7.Adkins AL, Robbins J, Villalba M, Bendick P, Shanley CJ. Open abdomen management of intra-abdominal sepsis. Am Surg. 2004;70(2):137–40. PMid:15011916. [PubMed] [Google Scholar]
- 8.Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, Marsch S, Azzola A, Rakic J, Mueller B, Tamm M. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia:a randomised study. European Respiratory Journal. 2009;34(6):1364–75. doi: 10.1183/09031936.00053209. https://doi.org/10.1183/09031936.00053209 PMid:19797133. [DOI] [PubMed] [Google Scholar]
- 9.Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Critical care medicine. 2006;34(10):2596–602. doi: 10.1097/01.CCM.0000239116.01855.61. https://doi.org/10.1097/01.CCM.0000239116.01855.61 PMid:16915118. [DOI] [PubMed] [Google Scholar]
- 10.Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, von Spiegel T, Schroeder S. Procalcitonin to guide duration of antibiotic therapy in intensive care patients:a randomized prospective controlled trial. Critical Care. 2009;13(3):R83. doi: 10.1186/cc7903. https://doi.org/10.1186/cc7903 PMid:19493352 PMCid:PMC2717450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, Bohuon CL. Procalcitonin increase after endotoxin injection in normal subjects. The Journal of Clinical Endocrinology & Metabolism. 1994;79(6):1605–8. doi: 10.1210/jcem.79.6.7989463. PMid:7989463. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, Von Spiegel T. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis:results of a prospective randomized study. Langenbeck's archives of surgery. 2009;394(2):221–6. doi: 10.1007/s00423-008-0432-1. https://doi.org/10.1007/s00423-008-0432-1 PMid:19034493. [DOI] [PubMed] [Google Scholar]
- 13.Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions:a systematic review of randomized controlled trials and recommendations for clinical algorithms. Archives of internal medicine. 2011;171(15):1322–31. doi: 10.1001/archinternmed.2011.318. https://doi.org/10.1001/archinternmed.2011.318 PMid:21824946. [DOI] [PubMed] [Google Scholar]
- 14.Schuetz P, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database of Systematic Reviews. 2012;(9):CD007498. doi: 10.1002/14651858.CD007498.pub2. https://doi.org/10.1002/14651858.CD007498.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units:a systematic review. Clinical Infectious Diseases. 2011;53(4):379–87. doi: 10.1093/cid/cir408. https://doi.org/10.1093/cid/cir408 PMid:21810753. [DOI] [PubMed] [Google Scholar]
- 16.Kopterides P, Siempos II, Tsangaris I, Tsantes A, Armaganidis A. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit:a systematic review and meta-analysis of randomized controlled trials. Critical care medicine. 2010;38(11):2229–41. doi: 10.1097/CCM.0b013e3181f17bf9. https://doi.org/10.1097/CCM.0b013e3181f17bf9 PMid:20729729. [DOI] [PubMed] [Google Scholar]
- 17.Rau BM, Frigerio I, Büchler MW, Wegscheider K, Bassi C, Puolakkainen PA, Beger HG, Schilling MK. Evaluation of procalcitonin for predicting septic multiorgan failure and overall prognosis in secondary peritonitis:a prospective, international multicenter study. Archives of Surgery. 2007;142(2):134–42. doi: 10.1001/archsurg.142.2.134. https://doi.org/10.1001/archsurg.142.2.134 PMid:17309964. [DOI] [PubMed] [Google Scholar]
- 18.Meisner M. Update on procalcitonin measurements. Annals of laboratory medicine. 2014;34(4):263–73. doi: 10.3343/alm.2014.34.4.263. https://doi.org/10.3343/alm.2014.34.4.263 PMid:24982830 PMCid:PMC4071182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meisner M, Tschaikowsky K, Schnabel S, Schmidt J, Katalinic A, Schüttler J. Procalcitonin-influence of temperature, storage, anticoagulation and arterial or venous asservation of blood samples on procalcitonin concentrations. Clinical Chemistry and Laboratory Medicine. 1997;35(8):597–602. doi: 10.1515/cclm.1997.35.8.597. https://doi.org/10.1515/cclm.1997.35.8.597. [DOI] [PubMed] [Google Scholar]
- 20.Omar AS, ElShawarby A, Singh R. Early monitoring of ventriculostomy-related infections with procalcitonin in patients with ventricular drains. Journal of clinical monitoring and computing. 2015;29(6):759–65. doi: 10.1007/s10877-015-9663-1. https://doi.org/10.1007/s10877-015-9663-1 PMid:25638513. [DOI] [PubMed] [Google Scholar]
- 21.Slieker JC, Aellen S, Eggimann P, Guarnero V, Schäfer M, Demartines N. Procalcitonin-Guided Antibiotics after Surgery for Peritonitis:A Randomized Controlled Study. Gastroenterology Research and Practice. 2017;2017 doi: 10.1155/2017/3457614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang TS, Huang SS, Shyu YC, Lee CH, Jwo SC, Chen PJ, Chen HY. A procalcitonin-based algorithm to guide antibiotic therapy in secondary peritonitis following emergency surgery:a prospective study with propensity score matching analysis. PloS one. 2014;9(3):e90539. doi: 10.1371/journal.pone.0090539. https://doi.org/10.1371/journal.pone.0090539 PMid:24594916 PMCid:PMC3942439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, Von Spiegel T. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis:results of a prospective randomized study. Langenbeck's archives of surgery. 2009;394(2):221–6. doi: 10.1007/s00423-008-0432-1. https://doi.org/10.1007/s00423-008-0432-1 PMid:19034493. [DOI] [PubMed] [Google Scholar]
- 24.Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, von Spiegel T, Schroeder S. Procalcitonin to guide duration of antibiotic therapy in intensive care patients:a randomized prospective controlled trial. Critical Care. 2009;13(3):R83. doi: 10.1186/cc7903. https://doi.org/10.1186/cc7903 PMid:19493352 PMCid:PMC2717450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung B, Molinari N, Nasri M, Hajjej Z, Chanques G, Jean-Pierre H, Panaro F, Jaber S. Procalcitonin biomarker kinetics fails to predict treatment response in perioperative abdominal infection with septic shock. Critical Care. 2013;17(5):R255. doi: 10.1186/cc13082. https://doi.org/10.1186/cc13082 PMid:24156734 PMCid:PMC4056026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S. Diagnosis and management of complicated intra-abdominal infection in adults and children:guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clinical infectious diseases. 2010;50(2):133–64. doi: 10.1086/649554. https://doi.org/10.1086/649554 PMid:20034345. [DOI] [PubMed] [Google Scholar]
- 27.Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery:a multi-institutional study. Annals of surgery. 2010;252(2):325–9. doi: 10.1097/SLA.0b013e3181e9819a. https://doi.org/10.1097/SLA.0b013e3181e9819a PMid:20647925. [DOI] [PubMed] [Google Scholar]
- 28.Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011;3(6):637–82. doi: 10.3390/nu3060637. https://doi.org/10.3390/nu3060637 PMid:22254115 PMCid:PMC3257638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martini A, Gottin L, Menestrina N, Schweiger V, Simion D, Vincent JL. Procalcitonin levels in surgical patients at risk of candidemia. Journal of Infection. 2010;60(6):425–30. doi: 10.1016/j.jinf.2010.03.003. https://doi.org/10.1016/j.jinf.2010.03.003 PMid:20226210. [DOI] [PubMed] [Google Scholar]
- 30.Leli C, Ferranti M, Moretti A, Al Dhahab ZS, Cenci E, Mencacci A. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Disease markers. 2015;2015 doi: 10.1155/2015/701480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodská H, Malíčková K, Adámková V, Benáková H, Šťastná MM, Zima T. Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clinical and experimental medicine. 2013;13(3):165–70. doi: 10.1007/s10238-012-0191-8. https://doi.org/10.1007/s10238-012-0191-8 PMid:22644264. [DOI] [PubMed] [Google Scholar]
- 32.Charles PE, Ladoire S, Aho S, Quenot JP, Doise JM, Prin S, Olsson NO, Blettery B. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC infectious diseases. 2008;8(1):38. doi: 10.1186/1471-2334-8-38. https://doi.org/10.1186/1471-2334-8-38 PMid:18366777 PMCid:PMC2289831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, Rong H, Guo Q, Chen Y, Zhang G, Yang J. Serum procalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. Journal of research in medical sciences:the official journal of Isfahan University of Medical Sciences. 2016;21 doi: 10.4103/1735-1995.183996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical care medicine. 2006;34(6):1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. https://doi.org/10.1097/01.CCM.0000217961.75225.E9 PMid:16625125. [DOI] [PubMed] [Google Scholar]


