Abstract
BACKGROUND:
Dental erosion is a common problem in modern societies, owing to the increased consumption of acid drinks such as soft drinks, sports drinks, fruit juice. Examining the enamel surface with the Atomic Force Microscopy (AFM) enables more precise registering and defining the changes of enamel surface structure and microhardness. This method can be used to compare the efficiency of application of different preventive and therapy materials and medicaments in dentistry. The chronic regular consumption of low pH cola drinks encouraged the erosion of the teeth. The loss of anatomy and sensitivity are direct results of acid cola dissolving coronal tooth material. Under the influence of coca cola, a change of crystal structure and nanomorphology on enamel surface occurs.
AIM:
This paper reflects dental damage from abusive cola drinking, and the clinical presentation can be explained from data presented in this thesis.
MATERIAL AND METHODS:
The trial was conducted on a total of 40 extracted teeth which were divided into two groups treated with the solution of coca cola during 5 minutes, and then prepared and tested with a standard AFM procedure, type SPM-5200. Quantitative analysis was performed by comparing the roughness parameters (Ra) of the treated and non-treated sample.
RESULTS:
Based on the test of a hypothesis of the existence of differences between the treated and untreated sample, with an application of a t-test, it is shown that there are statistically highly significant differences between Ra of the treated sample with a 5-minute treatment of coca cola and Ra of the same sample without the treatment.
CONCLUSION:
Use of AFM enables successful monitoring of changes on enamel surface as well as the interpretation of the ultrastructural configuration of the crystal stage and the damage created under the influence of different external factors.
Keywords: Nanomorphology, enamel surface, AFM procedure, roughness parameters
Introduction
In the last three decades, the consumption of “Sugary” or “Sugar-sweetened Beverages” (SSBs) including carbonated “soda” soft drink, sports and energy beverages, fruit drinks, and sweetened bottled waters have increased dramatically. SSB is consumed by 66% of children and 77% of adolescent’s daily. Including adults and adolescents, males have been found to consume more SSBs than females. Older females consume the lowest (42 calories/day) amount, with the highest consumption rates (70%) by male adolescents, 12-19 years old (273 calories/day). Consumption of SSBs has also been associated with poor dietary habits, weight gain, obesity, and type 2 diabetes, predominately in adults, but also recognised in children and adolescents. Continuously sipping soda creates an acid bath for teeth. This softened area is ideal for bacteria to enter. As well, the sugar content of the soda is converted to acid by the bacteria on the teeth. There is no question that erosion causes significant damage to dental enamel, particularly among young people.
Dental erosion is defined as the irreversible loss of dental structure due to a chemical process and without the involvement of microorganisms. This process is the result of the action of acids whose pH is lower than 4.5.
Enamel is the hardest substance in the body, and it protects the crowns of the teeth. However, it is susceptible to demineralisation from acids. Acids are produced when certain bacteria colonise the tooth surface and metabolise carbohydrates. If this process continues, it may eventually lead to the development of carious lesions in the enamel and dentin. Another source of acid is dietary. Many foods and beverages contain acids that also can lead to demineralisation of the enamel.
Tooth enamel is made of a billion crystals of carbonised hydroxyapatite that are packed in individual prisms winding from enamel-dentin border toward the tooth surface.
Enamel prisms are formed by the complex interaction of ectodermal and ectomesenchymal tissues that coordinate the action of ameloblasts (cells responsible for their synthesis).
Each enamel prism is a product of a single ameloblast and stretches uninterruptedly from the enamel-dentin border to tooth surface.
Enamel surface is not flat. It has a wavy structure because at places where Retzius’ striae end such striae overlap in the form of steps, with the appearance of shallow grooves referred to as perikymata.
Although enamel has pronounced hardness, it is also fragile at the same time and similar to glass, so that for these reasons it could appear to be susceptible to breaking. Despite that, enamel can take loads higher than 1000 N several times during the day. The overall enamel microstructure is formed in such a way to adjust to such loads. This is also contributed by the support of elastic dentin and the structures such as enamel tufts at the dentin-enamel junction.
Besides the fact that it represents the hardest biological tissue, tooth enamel may be quickly damaged under the influence of various factors. Enamel damage can be divided into two large groups, i.e. infective and non-infective [1, 2].
Infective damage or tooth caries occurs as a consequence of demineralisation caused by the bacteria organised in a special ecological formation: oral biofilm – dental plaque. In certain conditions, the so-called cariogenic bacteria (specific species of streptococci) dominate on the tooth surface. They can create organic acids but can survive in acidic conditions. They suppress neutral or useful bacteria. For acidogenic biofilms to form and exert a cariogenic effect, the presence of sugar is necessary. Sugars originate from food and can be obvious such as those from candies, refined buns, snacks, beverages, or hidden, like for example in juices.
Beverages that decrease pH in the oral cavity and on the tooth surface, thus potentially leading to dental erosions, include fruit juices, soft drinks, sports beverages, other fizzy drinks, as well as various pickled vegetables (due to the content of acetic acid). Ideally, the pH of saliva lies within the range of 5.5–6.5; a pH of 5.5 is accepted as the threshold level for the development of dental caries.
When wear between enamel surfaces occurs at low pH, stress cracks are generated and propagate within the enamel, releasing particles. This particulate debris becomes trapped between the contacting surfaces, causing the two-bodyabrading system to transform into ahighwear, three-body abrasion system. This transformation does not appear to happen in low-pH media because the opposing surfaces have a smoother appearance; in fact, it appears that erosion modulates attrition to the extent that wear is reduced by an apparent polishing effect on the contacting surfaces. Degradation of enamel is a complex phenomenon, but erosion appears to be the predominating factor at low pH levels.
Table 1.
Beverage characteristics
| Group | Composition | pH (s.d.) | TA (s.d.) |
|---|---|---|---|
| Coca-Cola Classic (Coke) | Carbonated water, High fructose corn syrup, Caramel colour, Phosphoric acid, Natural flavours, Caffeine | 2.49 (.006) | 9.57 (1.87) |
| Diet Coke | Carbonated water, Caramel colour, Aspartame, Phosphoric acid, Potassium benzoate, Natural flavours, Citric acid, Caffeine | 3.16 (.015) | 9.11 (1.63) |
| Red Bull | Water, Sucrose, Glucose, Sodium citrate, Taurine Glucuronolactone, Caffeine, Inositol, Niacinamide, Calcium-Pantothenate, Pyridoxine HCL, Vitamin B12 | 3.32 (.006) | 28.99 (4.17) |
| Tap Water (Water) | Water, various minerals | 7.55 (.010) | ---- |
pH: potential (power) of hydrogen, TA: titratable acidity, s.d.: standard deviation
Excessive consumption of drinks with an acid pH tends to cause the demineralisation of the dental enamel, though this effect may be reversible given the saliva’s ability to remineralise 12-13 the teeth. Individuals consuming citric fruits more than twice a day have a 37 times greater risk of developing lesions through erosion than those who do not. Similar risks appear to occur with the consumption of apple vinegar (10 times higher), sports drinks (4 times higher) or sodas (4 times higher), when consumed every day. The advancing loss of dental structure through erosion could be as much as around 1 µm per day.
Signs of soda pop erosion:
Broad shiny concavities on smooth surface enamel
Glazed appearance
Wide buccal concavities in mandibular premolars and molars
Concavities with an enamel cuff at the free gingival margin
Deep shiny concavities occlusally in premolars and molars
Restorations that ‘rise’ above the occlusal surface
Sealants that ‘rise’ above the occlusal surface
Thin maxillary central incisors
Increased incisal translucency in maxillary central incisors
Surface characteristics missing
Loss of surface detail in the primary dentition
In dentistry, the development of which has been greatly influenced both by the knowledge of and observing the biological and mechanical properties of hard (mineralised) tissues, the research with using this precise technique has started only in recent years. The results of the analyses of oral cavity tissue introduced the dental science into nano-era [3-6]. Only with the development of AFM technologies, it was possible to observe more subtle surface changes of enamel. Also, AFM studies start to be more and more used in researches in dentistry too, that observe the surface changes such as the dental plaque and mineralised and coloured deposits, surface properties of different materials and morphological and mechanical changes on mineralised tissues [7-9]. In recent years AFM has been more and more used to investigate erosions and early stadiums of demineralisation on enamel surface – the first results show that this is a very convenient tool. For the investigations to be comparable as much data as possible should be collected about the enamel nanomorphology in different biological and pathological conditions [10-13].
We chose to use the average roughness (Ra) of our samples as a measure because it has been most commonly used in previous studies, so it was easier to make a proper comparison of our results [14].
By using special research nanoprobes, it is possible nowadays to measure resolutions and movements at the level of nanometers and picomolar [15]. This also enables precise registering of physical properties of healthy – unchanged enamel, dentin and cement of the tooth root, as well as carrying out of the analyses of chemical processes and biological transformations that until now were impossible to perform [3, 4, 15, 16].
This study aimed to investigate dental damage from abusive cola drinking, and the clinical presentation can be explained from data presented in this thesis.
Material and Methods
The study was carried out on a total of 40 extracted teeth divided into two groups from which samples were taken (3 mm x 2 mm x 2 mm in size) and which were treated with the solution of coca cola and then prepared and tested with the standard procedure using AFM of JSPM-5200 type. One group of samples was not treated, while the other was treated for 5 minutes, with the analysis of enamel nanostructure performed after the treatment. Every sample was fixed to the microscope holder by using cyanoacrylate adhesive.
Images were made with the very slowed down scanning of the surface of every 25.0 μm2, and with 0.1 Hz scanning frequency with 256 lines per sample, to avoid damaging of the probe.
Surface roughness was measured by average roughness (Ra) automatically on WinSPM software.
The average roughness is defined as an average distance of the centerline when they are observed as if they are local minimums and local maximums. This roughness is defined by the following formula :
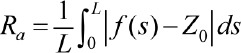
where Z0 is the centerline of the profile of the length L:
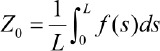
Figure 1 presents an image of the profile with absolute values about the centreline. The distance of their average height from the height of the centreline profile is defined as average roughness.
Figure 1.
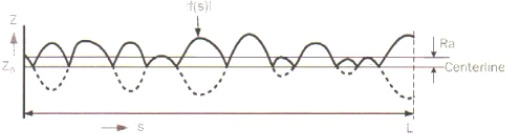
Average roughness
Competence at the level of AFM nanometry can be observed in the same way as in conventional measurements because the average roughness (Ra) at one measurement point is the arithmetic mean of local maximums and minimums of enamel surface calculated for that measurement point. All our measurement points have a surface of 5 x 5 μm, i.e. 256 x 256 or 512 x 512 pixels.
Results
Enamel surface morphology has been described both regarding quality and quantity. Quantitative analysis was based on a comparison of Ra roughness parameter between different samples and on statistical analysis of obtained values.
In the initial assumption of the study, we intended to start analysing the effect of coca cola on the enamel surface during a 60-second interval. Preliminary tests have not identified any significant difference between the treated and non-treated samples. Therefore, a study with a 5-minute treatment was undertaken.
Figure 2 and three present untreated samples and samples that were treated with coca cola for 5 minutes.
Figure 2.

Untreated control surface
On smaller magnification, the untreated surface (Figure 2) is covered with an amorphous layer which may point to aprismatic enamel and parts of the pellicle. No clear borders between prisms nor existence of larger depressions can be seen. Enamel shows signs of compactness. The surface is flatter and protrusions that can be seen have a uniform structure. The granules are arranged in parallel rows, and no pores on enamel surface can be seen.
In contrast to that, slight porosity on the surface treated for 5 minutes with coca cola can be seen. Depressions and the grid-like structure of granules can be seen (Figure 3).
Figure 3.

Surface treated with coca cola for 5 min
Higher magnifications confirm the compactness of untreated surface (Figure 4). The structure of densely compacted crystals on the untreated surface is more prominent, where no border can be seen between the prisms. On the treated surface depressions between the prisms are more prominent, with protruding prism heads and depressions around them.
Figure 4.

Untreated control surface Figure 5. Surface treated with coca-cola for 5 minutes
On higher magnitudes, depressions can be seen (the arrow) between the „prismatic structures” in treated samples (Figure 6B), while on the untreated surface (Figure 6A) there is a filled-in area, which would correspond to compacted crystals. The findings point to the primary action in the area of less mineralised prism rims, where dilatation of the area can be seen.
Figure 6.
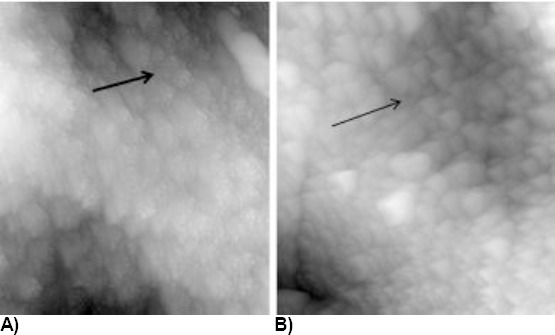
A) Untreated control surface; B) Surface treated with coca cola for 5 minutes
By the analysis of all measurement points, the following mean values of average roughness, expressed in Ra, were obtained:
Table 2.
Values of average roughness
| Untreated sample-N | Sample treated with coca cola | |||
| Arithmetic mean of Ra values of all measurement points Xsr | 34.66 | 76.28 | ||
| Variables | temp | tgr | Statistical significance | P |
| N-Ra /CC5-Ra | -237.9093 | 3.291 | VSZ | < 0.001 |
Based on the test of a hypothesis of existence of differences between the treated and untreated sample, with an application of a t-test, it is shown that there are statistically highly significant differences between Ra of the treated sample with a 5-minute treatment of coca cola and Ra of the same sample without the treatment, because the absolute value t is empirically higher than t – border value temp =|-237.9093|> tgr = 3.291 for p = 0.001.
Discussion
The selected studies are extremely important because non-carious damages of teeth are coming more and more in focus with the trend to place caries under control in developed countries. While in the past a great deal of such damage would even pass unnoticed, today, in developed countries, it is among the most frequent chronic diseases with the children of 5-17 years of age [2, 11, 17].
Carbonated beverages are dominant among soft drinks, with coca cola taking a leading place. An increased consumption is accounted for by the recession and a lower price of coca cola [18, 19].
The increased consumption of carbonated beverages is correlated with an increase in erosive damages of enamel [20].
Numerous studies have shown the advantage of using AFM analyses to monitor both qualitative and quantitative changes in enamel surface [21-26].
Speaking of qualitative and quantitative monitoring of enamel dissolution, the paper [27] demonstrates correspondence of AFM examination technique with other techniques (SEM, profilometry, nano carving), while the paper [28] confirms AFM as the most precise technique for such analyses.
The analysis of changes on our sample after five minutes of action of coca cola shows that there are no densely compacted crystals which are, on the contrary, clearly seen on untreated samples; this is an indication of initial demineralisation and creation of depressions, most probably on places of the lower density of crystals. Other authors have also shown similar results [29].
Many other researchers, who used different techniques, also showed negative effects of carbonated beverages on both tooth enamel and composite fillings [30-33]. In the paper [30] it was demonstrated that carbonated beverages reduce the physical properties of enamel (hardness and elasticity module) as a consequence of enamel erosion. The paper also shows that such drinks have a more prominent effect compared to, for example, orange juice. Some authors have found that unpolished enamel is less susceptible to erosions compared to the polished one and that the enamel structure itself in vitro conditions can significantly influence the progression of erosions. This particularly relates to the presence of aprismatic enamel, cracks and perikymata [34].
The chemical composition of acidic beverages is certainly significant in the modification of the mineral structure and thereby of the mechanical properties of enamel [34]. That acidity of carbonated cola drinks primarily originates from the phosphoric acid has been shown in the paper [32]. It has been known from earlier researches that the drinks with citric acid cause higher enamel erosion compared to the drinks that contain only phosphoric acid, such as in coca cola [34]. In addition to pH, the liquid environment around the enamel and temperature are also relevant, and they all affect the physical properties such as the elasticity module, hardness and surface roughness of human enamel.
The results obtained in in vitro studies, such as ours, can only be partly transferred on what is going in clinical conditions, which largely depends on the dynamics of distortion of demineralisation-remineralisation kinetics. Remineralization periods that lead to enamel recovery, reduction of roughness and microhardness increase have not been included in our studies [21, 26, 35]. Likewise, certain studies have shown increased resistance with the application of remineralisation pastes or alternating presence of enamel in saliva and the drink [21, 27].
In conclusion, the analysis of the samples after five minutes of coca cola action shows both the qualitative and quantitative difference. On treated enamel samples, a lower density of crystals with larger areas between prisms is seen. The surface is more irregular with higher depressions. The analysis of Ra of treated and untreated surfaces confirms such findings as well as the higher roughness of enamel surface treated with coca cola. The initial irregularity (after five minutes of treatment) is the consequence of the partial destruction of the aprismatic layer and the attack with the crystals of less compacted parts, such as perikymata or lamellae. The findings correlate with the other studies [29, 36].
The results obtained indicate that the treated surfaces are statistically significantly rougher (higher Ra), which is also confirmed with the morphological signs of depressions and decrease of enamel crystals.
Under the influence of coca cola, there is statistically significant disruption of the integrity of the crystal grid which is observed after five minutes of action, which corresponds to the most common way of consumption of this carbonated drink. Enamel damages are primarily related to the decrease of the crystal thickness and creation of fissures which increase the overall roughness of the surface. Higher roughness leads to greater contact with acids thus increasing the possibility of further damage.
Our AFM researches indicate the irregular surface structure of enamel in physiological conditions, which has a certain degree of roughness, depending on the histological properties, the presence of pellicle and compactness of crystal units in prisms.
Despite its limitations, our studies have proved that the use of AFM enables successful monitoring of changes on enamel surface as well as the interpretation of the ultrastructural configuration of the crystal stage and the damage created under the influence of different external factors.
Likewise, we believe that coca cola, as the most common carbonated beverage, exerts aggressive influence on enamel surface thus endangering the mineral structure.
Further research should be directed toward the changes of ultrastructural enamel during the period two h after the action of the examined agent, which should be correlated with the physiological conditions in the oral cavity; subsequently, causal relationship between them should be established.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Al-Majed I, Maguire A, Murray JJ. Risk factors for dental erosion in 5–6 year old and 12–14 year old boys in Saudi Arabia. Community Dent Oral Epidemiol. 2002;30:38–46. doi: 10.1034/j.1600-0528.2002.300106.x. https://doi.org/10.1034/j.1600-0528.2002.300106.x PMid:11918574. [DOI] [PubMed] [Google Scholar]
- 2.Attin T, Meyer K, Hellwig E, et al. Effect of mineral supplements to citric acid on enamel erosion. Arch Oral Biol. 2003;48:753–759. doi: 10.1016/s0003-9969(03)00156-0. https://doi.org/10.1016/S0003-9969(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 3.Attin T, et al. Impact of modified acidic soft drinks on enamel erosion. Oral Diseases. 2005;11:7–12. doi: 10.1111/j.1601-0825.2004.01056.x. https://doi.org/10.1111/j.1601-0825.2004.01056.x PMid:15641960. [DOI] [PubMed] [Google Scholar]
- 4.Bozec L, de Groot J, Odlyha M, Nicholls B, Horton MA. Mineralised tissues as nanomaterials:Analysis by atomic force microscopy. IEEE Proc. Nanobiotechnol. 2005;152:183–186. doi: 10.1049/ip-nbt:20050004. [DOI] [PubMed] [Google Scholar]
- 5.Barbour ME, Finke M, Parker D.M, Hughes JA, Allen GC, Addy M. The relationship between enamel softening and erosion caused by soft drinks at a range of temperatures. J Dent. 2006;34:207–213. doi: 10.1016/j.jdent.2005.06.002. https://doi.org/10.1016/j.jdent.2005.06.002 PMid:16112333. [DOI] [PubMed] [Google Scholar]
- 6.Barbour ME, Finke M, Parker DM, Hughes JA, Allen GC, Addy M. The relationship. between softening and erosion caused by soft drinks at a range of temperatures. J Dent. 2006;34:207–213. doi: 10.1016/j.jdent.2005.06.002. https://doi.org/10.1016/j.jdent.2005.06.002 PMid:16112333. [DOI] [PubMed] [Google Scholar]
- 7.Barbour ME, Parker DM, Allen GC, Jandt KD. Human enamel erosion in constant composition citric acid solutions as a function of degree of saturation with respect to hydroxyapatite. Journal of Oral Rehabilitation. 2005;32:16–21. doi: 10.1111/j.1365-2842.2004.01365.x. https://doi.org/10.1111/j.1365-2842.2004.01365.x PMid:15634296. [DOI] [PubMed] [Google Scholar]
- 8.Barbour M, Shellis RP. An investigation using atomic force microscopy nanoindentation of dental enamel demineralization as a function of undissociated acid concentration and differential buffer capacity. Phys Med Biol. 2007;52:899–910. doi: 10.1088/0031-9155/52/4/003. https://doi.org/10.1088/0031-9155/52/4/003 PMid:17264360. [DOI] [PubMed] [Google Scholar]
- 9.Badra VV, Faraoni JJ, Ramos RP, Palma-Dibb RG. Influence of different beverages on the microhardness and surface roughness of resin composites. Oper Dent. 2005;30:213–219. PMid:15853107. [PubMed] [Google Scholar]
- 10.Chandra S, et al. Textbook of Dental and Oral Histology with Embryology and MCQS 2/E. 2nd edition. Jaypee Brothers Medical Publishers (P) Ltd; 2010. [Google Scholar]
- 11.Devlin H, Bassiouny MA, Boston D. Hardness of enamel exposed to Coca-Cola and artificial saliva. J Oral Rehabil. 2006;33(1):26–30. doi: 10.1111/j.1365-2842.2006.01533.x. https://doi.org/10.1111/j.1365-2842.2006.01533.x PMid:16409513. [DOI] [PubMed] [Google Scholar]
- 12.Fzhen-Jiang Cheng, et al. The enamel softening and loss during early erosion studied by AFM, SEM and nanoindentation 2009 Biomed. Mater. 2009;4:015020. doi: 10.1088/1748-6041/4/1/015020. https://doi.org/10.1088/1748-6041/4/1/015020 PMid:19193971. [DOI] [PubMed] [Google Scholar]
- 13.Field J, Waterhouse P, Germa M. Quantifying and qualifying surface changes on dental hard tissues in vitro. Journal of dentistry. 2010;38:182–19. doi: 10.1016/j.jdent.2010.01.002. https://doi.org/10.1016/j.jdent.2010.01.002 PMid:20079800. [DOI] [PubMed] [Google Scholar]
- 14.Freitas RA., Jr Nanodentistry. J Am Dent Assoc. 2000;131:1559–1565. doi: 10.14219/jada.archive.2000.0084. https://doi.org/10.14219/jada.archive.2000.0084 PMid:11103574. [DOI] [PubMed] [Google Scholar]
- 15.Gimzewski J, Miles MJ. High-speed atomic force microscopy of dental enamel dissolution in citric acid. Arch Histol Cytol. 2009;72:328–335. doi: 10.1679/aohc.72.209. [DOI] [PubMed] [Google Scholar]
- 16.Holme B, Hove LH, Tveit AB. Using white light interferometry to measure etching of dental enamel. Measurement. 2005;38:137–147. https://doi.org/10.1016/j.measurement.2005.04.003. [Google Scholar]
- 17.Humphris ADL, Miles MJ, Hobbs JK. A mechanical microscope:High-speed atomic force microscopy. Appl Phys Lett. 2005;86:281–288. https://doi.org/10.1063/1.1855407. [Google Scholar]
- 18.Kimyai S, et al. (2011), Effect of three prophylaxis methods on surface roughness of giomer. Medicina Oral, Patología Oral y Cirugía Bucal. 2011;16(1):e110–e114. doi: 10.4317/medoral.16.e110. https://doi.org/10.4317/medoral.16.ne110. [DOI] [PubMed] [Google Scholar]
- 19.Lee GJ, et al. A quantitative AFM analysis of nano-scale surface roughness in various orthodontic brackets. Micron. 2010;41(7):775–782. doi: 10.1016/j.micron.2010.05.013. https://doi.org/10.1016/j.micron.2010.05.013 PMid:20646928. [DOI] [PubMed] [Google Scholar]
- 20.Lussy A, Jaeggi T, Jaeggi-Scharer S. Prediction of the erosive potential of some beverages. Caries Res. 1995;29:349–354. doi: 10.1159/000262091. https://doi.org/10.1159/000262091. [DOI] [PubMed] [Google Scholar]
- 21.Machado C, Lacefield W, Catledge A. Human Enamel Nanohardness, Elastic Modulus and Surface Integrity after Beverage Contact. Braz Dent J. 2008;19(1):68–72. doi: 10.1590/s0103-64402008000100012. https://doi.org/10.1590/S0103-64402008000100012 PMid:18438563. [DOI] [PubMed] [Google Scholar]
- 22.Maupome G, Diez de Bonilla J, Torres-Villasenor G, Andrade-Delgado LC, Casta-o VM. In vitro quantitative assessment of enamel microhardness after exposure to eroding immersion in cola drink. Caries Res. 1998;32:148–153. doi: 10.1159/000016445. https://doi.org/10.1159/000016445 PMid:9580392. [DOI] [PubMed] [Google Scholar]
- 23.Meurman JH, Frank RM. Progression and surface ultrastructure of in vitro caused erosive lesions in human and bovineenamel. Caries Res. 1991;25:81–8. doi: 10.1159/000261348. https://doi.org/10.1159/000261348. [DOI] [PubMed] [Google Scholar]
- 24.Mirjanic Dj MirjanicV, Vojinovic J. AFM analysis of nanostructure enamel after the aggressive beverage. Contemporary materials. 2015;24:603–617. [Google Scholar]
- 25.Neville B, Damm DD, Allen CM, Bouquot J. Oral and Maxillofacial Pathology. 3rd Edition. Saunders; 2008. [Google Scholar]
- 26.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–1583. https://doi.org/10.1557/JMR.1992.1564. [Google Scholar]
- 27.Poggio C, et al. Impact of two toothpastes on repairing enamel erosion produced by a soft drink:an AFM in vitro study. Journal of Dentistry. 2010;38(11):868–874. doi: 10.1016/j.jdent.2010.07.010. https://doi.org/10.1016/j.jdent.2010.07.010 PMid:20673785. [DOI] [PubMed] [Google Scholar]
- 28.Mythri H, Chandu GN, Prashant GM, Reddy VS. Effect of four fruit juices on pH of dental plaque-A four period cross-over study. Journal of Indian Association of Public Health Dentistry. 2008;6(11):53. [Google Scholar]
- 29.Roveri N, Battistella E, Bianchi CL, Foltran I, Foresti E, Iafisco M, Lelli M, Naldoni A, Palazzo B, Rimondini L. Surface enamel remineralization:biomimetic apatite nanocrystals and fluoride ions different effects. Journal of Nanomaterials. 2009;2009:8. https://doi.org/10.1155/2009/746383. [Google Scholar]
- 30.Schmid T, Burkhard J, Yeo BS, Zhang W, Zenobi R. Towards chemical analysis of nanostructures in biofilms I:Imaging of biological nanostructures. Anal. Bioanal. Chem. 2008;391:1899–1905. doi: 10.1007/s00216-008-2100-2. https://doi.org/10.1007/s00216-008-2100-2 PMid:18427786. [DOI] [PubMed] [Google Scholar]
- 31.Schlueter N, Hara A, Shellis RP, Ganss C. Methods for the measurement and characterization of erosion in enamel and dentine. Caries Res. 2011;45(Suppl 1):13–23. doi: 10.1159/000326819. https://doi.org/10.1159/000326819 PMid:21625129. [DOI] [PubMed] [Google Scholar]
- 32.Smith A. The Oxford companion to American food and drink. Oxford University Press US. 2007:451. [Google Scholar]
- 33.Sigusch BW, Beyer M, Heurich E, Jandt KD. Erosion des Zahnhartgewebes - Neue pathogenetische und diagnostische Aspekte durch Atom-Kraft-Mikroskopie und Nanoindentation. ZWR. 2008;117:152–158. https://doi.org/10.1055/s-2008-1076779. [Google Scholar]
- 34.Sigusch BW, Beyer M, Heurich E, Jandt KD. Erosion des Zahnhartgewebes - Neue pathogenetische und diagnostische Aspekte durch Atom-Kraft-Mikroskopie und Nanoindentation. ZWR. 2008;117:152–158. https://doi.org/10.1055/s-2008-1076779. [Google Scholar]
- 35.Šaćirović S, Imamović M, Ketin S, Milešević T, Biočanin R. Elements of Water Bacteriology with Special Reference to Public Drinking Water. Acta Medica Mediane. 2015 [Google Scholar]
- 36.Quartarona E, et al. Surface kinetic roughening caused by dental erosion:An atomic force microscopy study. J Appl Phys. 2008;103:104702. https://doi.org/10.1063/1.2927386. [Google Scholar]
- 37.Zeng Q, Zhang YH, Xing ZH, Zhao XJ. Comparisons of the Apatite Presentation and Demineralization in Enamel and Cementum by Atomic Force Microscopy. InKey Engineering Materials. 2007;330:733–736. https://doi.org/10.4028/0-87849-422-7.733. [Google Scholar]
- 38.U.S. Department of Health and Human Services. Preventing Chronic Diseases:Investing Wisely in Health. National Center for Chronic Disease Prevention and Health. 2007 [Google Scholar]


