Abstract
AIM:
The current study was designed to investigate the effectiveness of locally applied Strontium ranelate to induce bone formation.
MATERIALS AND METHODS:
Forty-eight female rats were divided into six groups (eight rats in each group): The three test groups included Strontium (SR) 2.5 mg, 5 mg and 10 mg that was dissolved in methylcellulose gel. The control groups included methylcellulose, simvastatin 5 mg and a negative control where the defect was left to heal without any intervention. At 44 days the groups were sacrificed, and the bone defects were assessed histomorphometically to assess bone formation. The data was statistically analysed.
RESULTS:
There was a statistically significant difference in the amount of new bone formation between all groups, where the 2.5 mg SR group showed the highest median bone percentage, is 41.95 %, followed by the 5, and 10 mg SR demonstrating a median bone are a percentage of 39.89%, and 30.19% respectively. Simvastatin showed a median bone percentage of 36.07 %, while the methylcellulose and the negative control groups demonstrated the lowest median area percentage of 23.12 and 20.70 % respectively.
CONCLUSIONS:
The study showed that the local application of an SR could up-regulate the bone formation and may prove to be a cost-effective method of bone regeneration.
Keywords: Strontium ranelate, Bone formation, Critical defect, Animal experiment
Introduction
Epidemiological studies have shown that 50% of the adult population between the ages of 45 and 65 years exhibited bone loss around teeth that are caused by periodontal disease. Various materials have been proposed to treat the bone defects which are a hallmark of the periodontal disease. The stimulation of local bone formation could positively affect the healing of isolated bony defects [1, 2]. Growth factors have exhibited the ability to induce bone formation [3]. On the other hand host tissues as well as a possible antibody response decreased the appeal for growth factor utilization.
The local application of systemic bone modulating drug that is commonly used to treat bone disease could offer a plausible alternative to growth factors. The advantage of local drug delivery is releasing the drug directly into the site of infection for a sufficient period without systemic exposure preventing both bacterial resistance and drug-related systemic side effects [4].
Various drugs have been studied using local delivery to improve the periodontal health and to achieve periodontal regeneration. Pharmacological agents offer great promise in this direction. Simvastatin, a widely used cholesterol-lowering drug, was shown to stimulate bone both in vitro and in vivo when applied locally, and its effects on bone metabolism favour its use in the treatment of periodontal defects. Additional advantages of simvastatin are its antioxidant and anti-inflammatory properties which could further facilitate healing of periodontal intrabony defects [5, 6].
Strontium ranelate (SR), a drug licensed for the treatment of postmenopausal osteoporosis is composed of an organic moiety (ranelic acid) and two atoms of stable strontium. In contrast to most currently used drugs currently that inhibit bone resorption rather than stimulating bone formation, SR has a novel mode of action, both increasing bone formation and reducing bone resorption, rebalancing bone turnover in favor of bone formation and increases bone strength It has a dual mode of action, both increasing bone formation and decreasing bone resorption [7-9]. The mechanism behind its dual mode of action was explained in several studies as by enhancing osteoblastic cell replication and activity and decreasing preosteoblast differentiation and osteoclastic activity [9, 10].
In an experiment that was conducted to examine the cytotoxicity of SR on human periodontal ligament (PDL) fibroblasts, SR was found to be non-toxic at appropriate concentrations. In the experiment, PDL cells were treated with SR at 20, 10, 5 and 2.5 mg/ml. The highest SR concentrations (20 and 10 mg) had significantly lower cell viability and cell numbers than those in 5 and 2.5 mg/ml. The author recommended the need for preclinical tests to further assess its safety and effectiveness before clinical use [11].
Methylcellulose has been used as a drug carrier for releasing in several pharmaceutical preparations because it is a harmless, nontoxic material that does not sensitise the tissues [12].
The critical-size rat calvarial defect is a convenient model for evaluating bone regenerative effects of bio-materials. It is accessible, simple and is unable to regenerate spontaneously because of the distance that the progenitor cells and blood vessels must travel from the healing margin to bridge the defect is great [13, 14]. In rat calvaria, the defect is 8 mm and is well suited for placement of particulate materials [15].
The purpose of this study was to evaluate qualitatively and quantitatively the ability of strontium ranelate to induce bone regeneration in rat critical-sized bone defects
Methods
Experimental design
The animal protocol was approved by the Ethical Committee of the National Research Centre, Cairo, Egypt. Forty-eight adult Sprague-Dawley rats (275 to 300 g) were randomly divided into six groups of eight animals each: The three test groups included Strontium 2.5 (SR), 5 mg and 10 mg that was dissolved in methylcellulose gel. The control groups included methylcellulose, simvastatin 1.2 mg and a negative control where the defect was left to heal without any intervention. In all group except the negative control group the gel was applied to the defect and at 44 days the groups were sacrificed and the bone defects were assessed histomorphometically to assess bone formation: Formulation of Strontium Ranelate (SR) gel A 4.0% (w/v) methylcellulose (4,000 cps) gel, (Sigma chemicals Co., St . Louis, MO), which served as the vehicle for SR was previously prepared, by adding the required amount of polymer to hot distilled water and cooling to gel at room temperature. Then 2.5, 5 and 10 mg of SR (EVA pharmaceuticals, Cairo, Egypt) was dissolved in 1 ml of methylcellulose [16].
Surgical Protocol
Animals were sedated using an intramuscular injection of two parts ketamine (100 mg/ml) Ketamar 100 (mg/ml) Amoun and one part xylazine (20 mg/ml) Xylazine Albrecht, Germany; Ceva, Germany; Serumber, Germany at a dosage of 0.2 ml/100 g, additional sedation was given if needed. Routine infiltration anaesthesia with 2% lidocaine and 1:100,000 epinephrine (Octacaine) was used at the surgical site.
An incision was made in the sagittal plane across the cranium, and a full-thickness flap was reflected, exposing the calvarial bone. A standardised, circular, transosseous defect, 8 mm in diameter, was created on the cranium with the use of a saline-cooled trephine drill (Biomet 3i, Palm Beach Gardens, USA). An 8 mm dental trephine was used to create a standardised, circular, transosseous defect, 8 mm in diameter, of the rat calvaria. The trephined calvarial bone was then carefully removed to avoid perforation of the dura mater.
To achieve standardisation 1 ml of the formula (either methylcellulose alone, with simvastatin or with strontium) was injected within the created defect.
In the first three groups, a 2.5, 5 and 10 mg/ml dose of SR added to an inert methylcellulose gel was inserted into the defect. The two positive control groups included a group were 5 mg of simvastatin dissolved in methylcellulose and a group of one ml of methylcellulose gel that was added to critical defect size. No material was added to the last group acting as a negative control, where the defect was left to heal without any intervention. The periosteum and skin were then closed and sutured with 4-0 Egysorb, (Taisier - med, Egypt). The animals were sacrificed after 44 days using CO2 asphyxiation.
Sample Preparation
The calvarium was removed, and the specimens were fixed in 10% formalin for 48 hours. Specimens were then transferred to the decalcifying solution (RDO Apex Engineering Products Corporation, IL - USA). After 72 hours, specimens were processed through ascending grades of alcohol, cleared in xylene and embedded in paraffin. Formalin-fixed, decalcified, paraffin-embedded tissue blocks were obtained. Paraffin sections of 5-micron thickness were prepared from each of the paraffin blocks with a longitudinal cut to show the defect with the surrounding bone healing. For routine histopathological examination, slides were stained with Hematoxylin & Eosin (H&E) stain. Assessment of new bone formation and morphometric analysis were performed on Masson trichrome-stained sections, where the mineralised bone was stained red, osteoid dark bluish green and collagen fibres greenish blue.
Histomorphometric Analysis
A quantitative study was obtained with the aid of Leica Qwin 500 LTD (Leica Microsystem Corporation, Cambridge, England) using the software Quin 500 (England). The slides were examined using power magnification X 100 for measurement of area percent of newly formed bone detected by Masson trichrome stain. The red stained areas were outlined and then measured in micrometre square in successive fields of the section and the total new bone formation in the specimen was then calculated automatically by the morphometry software.
Statistical Analysis
Numerical data were explored for normality by checking the data distribution and using Kolmogorov-Smirnov and Shapiro-Wilk tests. Data showed non-parametric distribution. Data were represented by median and range values. Kruskal - Wallis test was used to compare the different groups. Mann-Whitney U test with Bonferroni’s adjustment was used for pairwise comparisons between the groups. The significance level was set at P ≤ 0.05. Statistical analysis was performed with IBM® SPSS® Statistics Version 23 for Windows.
Results
Histopathological Results
Histopathological examination showed areas filled with red blood cells, inflammatory cells and fibroblastic proliferation with neovascularisation and granulation tissue formation as well as mild osteoblastic proliferation with osteoid tissue was noticed together with few foci of disorganised woven bone formation especially at the periphery of the defect. The centre showed fewer areas of osteoid & bone formation.
Strontium Treated Group
The 2.5 and 5 mg groups showed more attempts at bone formation with less inflammatory reaction as compared to the control group. Moderate fibroblastic proliferation was seen with marked osteoblastic cells proliferation. More foci of disorganised, woven bone were observed with the more collagenous extracellular matrix. The active bone formation was suggested as newly formed osteoid was observed, in the form of spicules rimmed by numerous osteoblasts as well as osteoid tissue having viable osteocytes within widened lacunae. Changes were seen extending from the margins down to the centre of the defect (Fig. 1).
Figure 1.
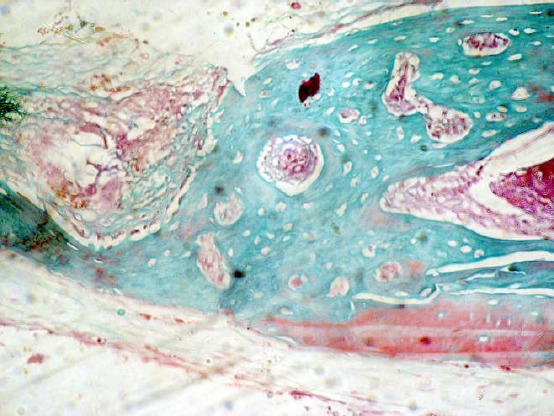
Photomicrograph of the methylcellulose group showing areas of osteoid formation and collagen without evidence of maturation (Masson trichrome, original magnification X 100)
The 10 mg group showed no osteoblastic proliferation at all. Large areas were filled with fibroblasts, and collagen fibres with heavy inflammatory cell infiltrate (Fig. 2).
Figure 2.
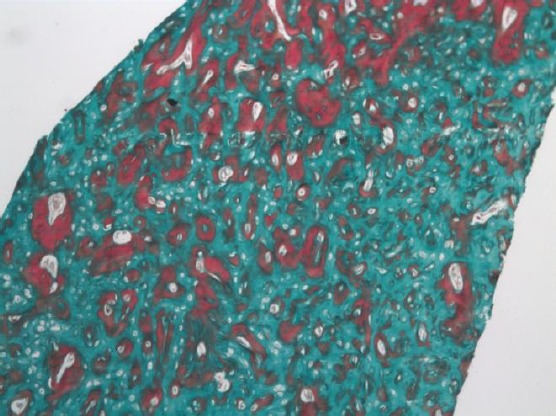
Photomicrograph of the 5mg strontium group showing areas of osteoid and woven bone formation with large marrow cavities (Masson trichrome, original magnification X 100)
Simvastatin Treated Group
The simvastatin group showed large, alternating areas of lamellar and woven bone formation with marked osteoblastic rimming and proliferation. No inflammatory cell infiltrate was detected. The new bone filled the margins of the defect till the centre (Fig. 3).
Figure 3.

Photomicrograph of the 10mg strontium group showing large areas of collagen fibres with no attempts at osteoid or bone formation (Masson trichrome, original magnification X 100)
Negative control Group
In the negative control groups, the limited bone repair was observed only at the margins of the defect, as recognised by the irregularly shaped bone trabeculae of newly formed mineralised bone and newly formed osteoid tissue. No bone formation or evidence of mineralization in the centre of the defect, where only a mature and well organized fibrous tissue was noticed.
Figure 4.
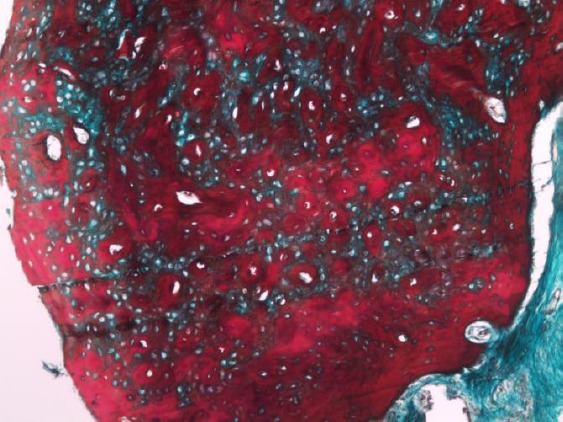
Photomicrograph of the simvastatin group showing large alternating areas of osteoid and woven bone formation (Masson trichrome, original magnification X 100)
Statistical Results
Descriptive statistics of area % values are presented in Table 1. There was a statistically significant difference between area % values of the different groups. Pair-wise comparisons between the groups revealed that there was no statistically significant difference between Strontium (2.5 mg), Strontium (5 mg) and Simvastatin with an as significant difference with Methylcellulose and Negative control.
Table 1.
Comparison between area % of the five groups
| Area % | Median | Minimum | Maximum | Rank | p-value |
|---|---|---|---|---|---|
| Strontium (2.5 mg) | 41.95 | 22.15 | 44.05 | a | ≤0.001* |
| Strontium (5 mg) | 39.89 | 19.56 | 57.25 | a | |
| Strontium (10 mg) | 30.19 | 16.09 | 46.96 | ab | |
| Simvastatin | 36.07 | 26.89 | 77.61 | a | |
| Methylcellulose | 23.12 | 11.01 | 30.48 | b | |
| Negative control | 20.70 | 10.50 | 23.70 | b |
Significant at P ≤ 0.05, Different letters in the same column are statistically significantly different
For the negative control group, the maximum bone area percentage was 23.7%, while that of methylcellulose was 30.48%, both were statistically different than the strontium and simvastatin groups., where the strontium groups showed a maximum area percentage of 44.05, 57.25 and 46.96% for the 2.5, 5 and 10 mg strontium respectively while that of simvastatin showed the higher area percentage with 77.61%.
Discussion
The study aimed to evaluate the bone regenerative potential of locally applied SR in different concentrations. The rat calvarial critical-sized defect is a well-established animal model for regenerative bone studies. The bone defect of critical size is unable to regenerate. Thus it is considered to be a suitable for the evaluation of osteoinductivity of various materials. Only materials that can induce bone regeneration show signs of bone formation throughout the entire critical defect [16, 17].
SR was used as it showed promising results in osteoporosis therapy, where it is known to increase in vitro osteoblasts’ differentiation from progenitors, as well as osteoblastic activity and survival. Furthermore, SR regulates osteoblast - induced osteoclastogenesis, and prevents bone resorption by decreasing osteoclastic differentiation and activity, while increasing their apoptosis [18].
The incorporation of strontium into mesoporous bioactive glass scaffolds was shown to be a viable way to stimulate the biological response of periodontal ligament cells as well as stimulate bone formation in bone defects in an osteoporotic rat model [19, 20].
Previous studies showed that 44 days represent adequate timing to analyse newly formed bone in rat calvarial defects [2]. The bone turnover rate of rats and the ability of fast healing make the evaluation of bone formation 6 to 8 weeks after implantation of a biomaterial appropriate. Thus no major differences are expected to be observed in bone formation even if prolonged healing time is allowed [16].
All defect sites exhibited bone formation, but there was a statistically significant difference in the amount of new bone formation between all groups, where the 2.5 SR group showed the highest median bone percentage, being 41.95 %, followed by the 5, and 10 mg SR demonstrating a median bone are percentage of 39.89%, and 30.19% respectively. Simvastatin showed a median bone percentage of 36.07%, while the methylcellulose and the negative control groups demonstrated the lowest median area percentage of 23.12 and 20.70 % respectively.
The histologic evaluation of the 2.5 and 5 mg groups showed more attempts at bone formation with less inflammatory reaction as compared to the control group. This could be attributed to the anti-inflammatory effect of SR that has been proposed by due to the antagonising effect of SR to NF-κB activation. Furthermore, in unpublished data by the same group, they proposed that SR may act as TNF inhibitor which could explain its anti-inflammatory properties [21].
Bone defect healing occurs naturally after a phase of bleeding and inflammation and terminates with the formation of woven bone which is then remodelled by osteoclasts and replaced by lamellar bone by osteoblasts [3].
The osteoblasts presence was marked in the 2.5 and 5 mg SR groups compared to the control group) which may be explained due to the fact that SR has the ability to stimulate PGE2 production and osteoblastic differentiation in marrow stromal cells, which is markedly affected by inhibition of COX-2 activity or disruption of COX-2 gene expression [22]. Furthermore, [23] concluded that SR promotes the replication of osteoblasts which could explain the marked osteoblastic proliferation in the 2.5 and 5 mg SR groups.
Woven osteoid bone and mineralised bone presence were marked in the SR group than by the control group. All these observations could be clearly to the dual effect of SR that promoted the bone formation on several levels and through various pathways.
In the negative control groups, the limited bone repair was observed only at the margins of the defect, as recognised by the irregularly shaped bone trabeculae of newly formed mineralised bone and newly formed osteoid tissue. The findings of no bone formation or evidence of mineralization in the center of the defect, where only a mature and well organized fibrous tissue was noticed, is consistent with the calvarial nonunion defects study of [17] where both the central and peripheral regions of the 8 mm calvarial defects were characterized by dense fibrous tissue repair and inactive fibroblasts.
Simvastatin was used in the present study as a positive control, to compare its effect on bone formation with that of SR, where former research demonstrated that local application of simvastatin in alveolar defects could promote bone regeneration [12]. In the present study large, areas of lamellar and woven bone formation with marked osteoblastic proliferation where demonstrated histologically, where the new bone filled the margins of the defect till the centre with no detected inflammatory cell infiltrates. The local application has been demonstrated anti-inflammatory effects as well, anabolic effects on bone and a stimulatory effect on vasculogenesis which was attributed to the increase in the expression of BMP-2, and vascular endothelial growth factor [24] which may explain the results of the present study. Our findings are also in line with a former research that demonstrated the ability of local simvastatin application to enhance healing of the bone defects even in the diabetic rat models [25].
In conclusion, the current study demonstrated that the local application of a single dose of SR (2.5 and 5 mg) could up-regulate the bone formation. The local application of SR may prove to be a cost-effective and safe method to stimulate bone formation. However more in vivo studies and different concentrations are recommended to define the optimal dose to equilibrate soft tissue inflammation and bone stimulation.
Acknowledgement
The authors thank EVA Pharma Inc, Abdeen, Cairo, Egypt, for providing the Strontium Ranelate powder.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Intini G, Andreana S, Buhite RJ, Bobek L. A comparative analysis of bone formation induced by human demineralised freeze-dried bone and enamel matrix derivative in rat calvarial critical-size bone defects. J Periodontol. 2008;79:1217–24. doi: 10.1902/jop.2008.070435. https://doi.org/10.1902/jop.2008.070435 PMid:18597604. [DOI] [PubMed] [Google Scholar]
- 2.Thylin MR, McConnell JC, Schmid MJ, Reckling RR, Ojha J, et al. Effects of simvastatin gels on murine calvarial bone. J Periodontol. 2002;73:1141–8. doi: 10.1902/jop.2002.73.10.1141. https://doi.org/10.1902/jop.2002.73.10.1141 PMid:12416771. [DOI] [PubMed] [Google Scholar]
- 3.Francis PO, McPherson JC, Cuenin MF, Hokett SD, Peacock ME, et al. Evaluation of a novel alloplast for osseous regeneration in the rat calvarial model. J Periodontol. 2003;74:1023–31. doi: 10.1902/jop.2003.74.7.1023. https://doi.org/10.1902/jop.2003.74.7.1023 PMid:12931765. [DOI] [PubMed] [Google Scholar]
- 4.Puri K, Puri N. Local drug delivery agents as adjuncts to endodontic and periodontal therapy. J Med Life. 2013;6:414–9. PMid:24868252 PMCid:PMC4034307. [PMC free article] [PubMed] [Google Scholar]
- 5.Montero J, Manzano G, Albaladejo A. The role of topical simvastatin on bone regeneration:A systematic review. J Clin Exp Dent. 2014;6:286–90. doi: 10.4317/jced.51415. https://doi.org/10.4317/jced.51415 PMid:25136432 PMCid:PMC4134860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elavarasu S, Suthanthiran TK, Naveen D. Statins:A new era in local drug delivery. J Pharm Bioallied Sci. 2012;4:S248–51. doi: 10.4103/0975-7406.100225. https://doi.org/10.4103/0975-7406.100225 PMid:23066263 PMCid:PMC3467872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burlet N, Reginster J-Y. Strontium ranelate:the first dual acting treatment for postmenopausal osteoporosis. Clin Orthop Relat Res. 2006;443:55–60. doi: 10.1097/01.blo.0000200247.27253.e9. https://doi.org/10.1097/01.blo.0000200247.27253.e9 PMid:16462426. [DOI] [PubMed] [Google Scholar]
- 8.Hamdy NAT. Strontium ranelate improves bone microarchitecture in osteoporosis. Rheumatology. 2009;48:9–13. doi: 10.1093/rheumatology/kep274. https://doi.org/10.1093/rheumatology/kep274 PMid:19783592. [DOI] [PubMed] [Google Scholar]
- 9.Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering:recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. https://doi.org/10.1615/CritRevBiomedEng.v40.i5.10 PMid:23339648 PMCid:PMC3766369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Wei L, Wu C, Miron RJ. Periodontal regeneration using strontium-loaded mesoporous bioactive glass scaffolds in osteoporotic rats. PLoS One. 2014;9:e104527. doi: 10.1371/journal.pone.0104527. https://doi.org/10.1371/journal.pone.0104527 PMid:25116811 PMCid:PMC4130544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Er K, Polat ZA, Ozan F, Taşdemir T, Sezer U, et al. Cytotoxicity analysis of strontium ranelate on cultured human periodontal ligament fibroblasts:a preliminary report. J Formos Med Assoc. 2008;107:609–15. doi: 10.1016/S0929-6646(08)60178-3. https://doi.org/10.1016/S0929-6646(08)60178-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Yang JY, Zhang SY, Feng L, Ren J. Effects of simvastatin gel on bone regeneration in alveolar defects in miniature pigs. Chin Med J (Engl) 2011;124:3953–8. [PubMed] [Google Scholar]
- 13.Gomes PS1, Fernandes MH. Rodent models in bone-related research:the relevance of calvarial defects in the assessment of bone regeneration strategies. Lab Anim. 2011;45:14–24. doi: 10.1258/la.2010.010085. https://doi.org/10.1258/la.2010.010085 PMid:21156759. [DOI] [PubMed] [Google Scholar]
- 14.Choi J, Jung U, Kim C, Eom T, Kang E, et al. The effects of newly formed synthetic peptide on bone regeneration in rat calvarial defects. J Periodontal Implant Sci. 2010;40:11–8. doi: 10.5051/jpis.2010.40.1.11. https://doi.org/10.5051/jpis.2010.40.1.11 PMid:20498754;PMCid:PMC2872809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986:299–308. https://doi.org/10.1097/00003086-198604000-00036. [PubMed] [Google Scholar]
- 16.Intini G, Andreana S, Intini FE, Buhite RJ, Bobek LA. Calcium sulfate and platelet-rich plasma make a novel osteoinductive biomaterial for bone regeneration. J Transl Med. 2007;5:13. doi: 10.1186/1479-5876-5-13. https://doi.org/10.1186/1479-5876-5-13 PMid:17343737 PMCid:PMC1831762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz JP, Schwartz Z, Hollinger JO, Boyan BD. Characterization of rat calvarial nonunion defects. Acta Anat (Basel) 1990;138:185–92. doi: 10.1159/000146937. https://doi.org/10.1159/000146937. [DOI] [PubMed] [Google Scholar]
- 18.Fonseca JE, Brandi ML. Mechanism of action of strontium ranelate:what are the facts? Clin Cases Miner Bone Metab. 2010;7:17–8. PMid:22461285 PMCid:PMC2898000. [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Zhou Y, Lin C, Chang J, Xiao Y. Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering. Acta Biomater. 2012;8:3805–15. doi: 10.1016/j.actbio.2012.06.023. https://doi.org/10.1016/j.actbio.2012.06.023 PMid:22750735. [DOI] [PubMed] [Google Scholar]
- 20.Wei L, Ke J, Prasadam I, Miron RJ, Lin S, et al. A comparative study of Sr-incorporated mesoporous bioactive glass scaffolds for regeneration of osteopenic bone defects. Osteoporos Int. 2014;25:2089–96. doi: 10.1007/s00198-014-2735-0. https://doi.org/10.1007/s00198-014-2735-0 PMid:24807629. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi M, Weitzmann MN. The intact strontium ranelate complex stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol Cell Biochem. 2012;359:399–407. doi: 10.1007/s11010-011-1034-8. https://doi.org/10.1007/s11010-011-1034-8 PMid:21874315. [DOI] [PubMed] [Google Scholar]
- 22.Choudhary S, Halbout P, Alander C, Raisz L, Pilbeam C. Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells:involvement of prostaglandins. J Bone Miner Res. 2007;22:1002–10. doi: 10.1359/jbmr.070321. https://doi.org/10.1359/jbmr.070321 PMid:17371157. [DOI] [PubMed] [Google Scholar]
- 23.Caverzasio J. Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone. 2008;42:1131–6. doi: 10.1016/j.bone.2008.02.010. https://doi.org/10.1016/j.bone.2008.02.010 PMid:18378206. [DOI] [PubMed] [Google Scholar]
- 24.Maeda T, Kawane T, Horiuchi N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology. 2003;144:681–92. doi: 10.1210/en.2002-220682. https://doi.org/10.1210/en.2002-220682 PMid:12538631. [DOI] [PubMed] [Google Scholar]
- 25.Ezirganli Ş, Kazancioǧlu HO, Mihmanli A, Aydin MŞ, Sharifov R, et al. The effect of local simvastatin application on critical size defects in the diabetic rats. Clin Oral Implants Res. 2014;25:969–76. doi: 10.1111/clr.12177. https://doi.org/10.1111/clr.12177, PMid:23600677. [DOI] [PubMed] [Google Scholar]


