Abstract
We recently demonstrated a fundamental role for cystathionine-γ lyase (CSE)–derived hydrogen sulfide (H2S) in the cardioprotective effect of the centrally acting drug moxonidine in diabetic rats. Whether a downregulated CSE/H2S system in the rostral ventrolateral medulla (RVLM) underlies neuronal oxidative stress and sympathoexcitation in diabetes has not been investigated. Along with addressing this question, we tested the hypothesis that moxonidine prevents the diabetes-evoked neurochemical effects by restoring CSE/H2S function within its major site of action, the RVLM. Ex vivo studies were performed on RVLM tissues of streptozotocin (55 mg/kg, i.p.) diabetic rats treated daily for 3 weeks with moxonidine (2 or 6 mg/kg; gavage), H2S donor sodium hydrosulfide (NaHS) (3.4 mg/kg, i.p.), CSE inhibitor DL-propargylglycine (DLP) (37.5 mg/kg, i.p.), a combination of DLP with moxonidine, or their vehicle. Moxonidine alleviated RVLM oxidative stress, neuronal injury, and increased tyrosine hydroxylase immunoreactivity (sympathoexcitation) by restoring CSE expression/activity as well as heme oxygenase-1 (HO-1) expression. A pivotal role for H2S in moxonidine-evoked neuroprotection is supported by the following: 1) NaHS replicated the moxonidine-evoked neuroprotection, and the restoration of RVLM HO-1 expression in diabetic rats; and 2) DLP abolished moxonidine-evoked neuroprotection in diabetic rats, and caused RVLM neurotoxicity, reminiscent of a diabetes-evoked neuronal phenotype, in healthy rats. These findings suggest a novel role for RVLM CSE/H2S/HO-1 in moxonidine-evoked neuroprotection and sympathoinhibition, and as a therapeutic target for developing new drugs for alleviating diabetes-evoked RVLM neurotoxicity and cardiovascular anomalies.
Introduction
Diabetes mellitus, a metabolic disorder, is associated with oxidative stress (Yan et al., 2014) as a result of reactive oxygen species (ROS) overproduction and reduction in antioxidant defense mechanisms (Ceretta et al., 2012; Fouda and Abdel-Rahman, 2017). The brain is more sensitive to oxidative stress, which affects gene expression and multiple cell functions (Giacco and Brownlee, 2010), due to its high oxygen consumption rate, plentiful lipid content, and relatively limited antioxidant mechanisms (Abdel Moneim, 2015). Similar to nitric oxide and heme oxygenase–derived carbon monoxide, hydrogen sulfide (H2S) protects against diabetes-induced oxidative stress and cardiovascular complications (El-Sayed et al., 2016; van den Born et al., 2016); however, a mechanistic role for H2S regulation in diabetes-evoked neurotoxicity remains unknown.
Gasotransmitters modulate the interaction between the central cardiovascular regulation and metabolic disorders such as diabetes mellitus (Szczepanska-Sadowska et al., 2010), and affect many brain regions such as the hippocampus, paraventricular nucleus, dorsal motor nucleus of the vagus, hypothalamus, and rostral ventrolateral medulla (RVLM) (Biessels et al., 2002; Szczepanska-Sadowska et al., 2010). The RVLM regulates sympathetic tone and blood pressure (Pilowsky and Goodchild, 2002; Madden and Sved, 2003), and RVLM oxidative stress increases sympathetic activity (Konno et al., 2012). While high glucose-evoked neuronal oxidative stress (Bahniwal et al., 2017) might contribute to the neurotoxicity, sympathoexcitation, and cardiovascular anomalies associated with diabetes, a definitive role for a dysfunctional cystathionine-γ lyase (CSE)/H2S system in the RVLM of diabetic rats has not been investigated. Moreover, it should be noted that carbon monoxide and H2S-synthesizing enzymes colocalize in discrete brain areas (Ruginsk et al., 2015). Also, the H2S-dependent induction of heme oxygenase-1 (HO-1) in macrophages via the extracellular signal–regulated protein kinase 1 and 2 pathways (Oh et al., 2006) infers similar crosstalk in other cell types. It remains unknown if a deficit in the H2S/HO-1 signaling underlies diabetes-induced neurotoxicity.
We have recently shown that moxonidine conferred cardioprotection by reversing the CSE/H2S dysfunction in the heart of diabetic rats (El-Sayed et al., 2016). Notably, moxonidine, a well-known centrally acting imidazoline I1 receptor agonist, improves cardiac function in hypertensive rats (Mukaddam-Daher et al., 2009). Also, H2S modulates RVLM neuronal activity, which plays a vital role in hemodynamic control (Guo et al., 2011). Whether moxonidine protects RVLM neurons in diabetes and the mechanism of this neuroprotection have not been investigated.
The first objective of the current study was to ascertain a possible role for a deficit in CSE/H2S in diabetes-evoked RVLM neurotoxicity and sympathoexcitation. Afterward, we hypothesized that moxonidine mitigates the diabetes-induced RVLM neurotoxicity and sympathoexcitation by preserving neuronal CSE/H2S/HO-1 signaling.
Materials and Methods
Animals.
Male Wistar rats (225–250 g; Charles River Laboratories, Raleigh, NC) were used and allowed free access to water and Purina chow (St. Louis, MO). Rats were retained on a balanced light-dark cycle and the temperature was kept at 22°C ± 1°C. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (2011) and approved by the institutional animal care and use committee https://www.pittsburgh.va.gov/Research/docs/Guide_8thEdition.pdf.
Experimental Groups.
The brains used in the present study (n = 5/group) were obtained from treated and untreated diabetic and control rats used in our recent study (El-Sayed et al., 2016). Briefly, male rats were fasted overnight (16 hours), treated with a freshly prepared single dose of streptozotocin (STZ) (55 mg/kg, i.p.) in 0.1 M citrate buffer (pH 4) or the buffer (control), and drinking water was substituted with 5% dextrose for STZ-treated rats. Four weeks following diabetes induction (STZ injection), the rats were treated daily for 3 weeks with one of the following regimens or the vehicle (by the same route of administration): 1) H2S donor, sodium hydrosulfide (NaHS) (3.4 mg/kg per day, i.p.); 2) CSE inhibitor, DL-propargylglycine (DLP) (37.5 mg/kg per day, i.p.); 3) moxonidine (2 or 6 mg/kg per day, gavage); and 4) a combination of moxonidine (6 mg/kg) and DLP. At the end of the cardiovascular studies (El-Sayed et al., 2016), 7 weeks after STZ injection, the animals were euthanized by overdose of pentobarbital, brains were collected, flash frozen in 2-methylbutane (Sigma-Aldrich, St Louis, MO) in dry ice, and stored at −80°C until processed for the neurochemical studies detailed subsequently.
Neurochemical Studies.
The RVLM was anatomically identified with the coordinates −12.6 to −11.8 mm relative to bregma (Ibrahim and Abdel-Rahman, 2015), and used for the neurochemical measurements described subsequently. For histochemical measurements, three coronal sections containing the RVLM (14 μm; approximately 0.05 mm) were cut at −24°C with a microtome cryostat (HM 505 E; Microm International GmbH, Walldorf, Germany). The remaining RVLM tissue was collected with a 0.75 mm punch instrument (Stoelting Co., Wood Dale, IL), homogenized with phosphate-buffered saline (PBS) (for ROS measurement; 50 mM, pH 7.4) or with lysis buffer (for western blot analysis).
Quantification of Neurodegeneration (Fluorojade-C Staining).
Modified protocols for immunofluorescence used in our previous studies (Wang and Abdel-Rahman, 2005) were used for staining degenerated neurons with a fluorescent Nissl counterstain (Yang et al., 2015). A fluorojade C staining kit was used in accordance with the manufacturer’s instructions (TR-100-FJ; Biosensis, Thebarton, South Australia). Slides containing the brain sections were incubated in 0.06% potassium permanganate solution for 10 minutes followed by rinsing in distilled water for 2 minutes, and then incubated in fluorojade C solution (1:25) for 30 minutes. The slides were then washed and mounted on coverslips with Vecta-shield mounting medium (Vector Laboratories, Inc., Burlingame, CA). A Zeiss LSM 510 confocal microscope (Carl Zeiss, Inc., Thornwood, NY), and a blue (450–490 nm) excitation light was used for visualization of stained neurons and image acquisition (Yang et al., 2015). For quantification, the fluorescence intensity was measured in the RVLM using Zen Lite 2011 software (Carl Zeiss Microscopy GmbH, Germany) in brain sections from treatment and control groups (n = 5 brains/group).
RVLM Caspase-3 Expression.
The immunohistochemistry technique described in our previous studies (Wang and Abdel-Rahman, 2002; Nassar et al., 2012) was used for measuring RVLM caspase-3 expression. Briefly, RVLM sections were postfixed for 2 hours in 4% paraformaldehyde in Tris-buffered saline, and subsequently incubated in 0.3% H2O2 for 30 minutes to block endogenous peroxidase. Sections were then incubated with anti-caspase-3 polyclonal antibody (1:1000; Abcam, Cambridge, MA) overnight at 4°C using a modification of the avidin/biotin-complex method kit (Vector Laboratories, Inc.). For validation, control sections incubated only with the primary or secondary antibody showed no positive staining (data not shown). Neuronal profile counts, denoting the total number of caspase-3 immunoreactive neurons, were used for quantification of caspase-3 expression in an identical region (field = 0.125 mm2) of the RVLM of treated and control rats (n = 5). Positive profiles exhibited dark granular brown staining indicative of a 3,3-diaminobenzidine reaction product. The average per field count of positive neuronal profiles was then determined and subsequently converted into the number of profiles per unit area (square millimeter) for each rat brain (Marcus et al., 1998).
ROS Measurement.
Oxidative stress was measured using 2′,7′-dichlorofluorescein diacetate (DCFH-DA), a general detector of oxidative species (Rezq and Abdel-Rahman, 2016; Fouda and Abdel-Rahman, 2017). Briefly, a stock solution of DCFH-DA (20 mM; Molecular Probes, Grand Island, NY) was prepared in methanol and kept at −20°C protected from light. Punched RVLM tissues from treated and control groups were homogenized in PBS (50 mM, pH 7.4) and centrifuged at 14,000 rpm for 20 minutes at 4°C. Bio-Rad protein assay was used to quantify the proteins in the supernatant (Bio-Rad Laboratories, Hercules, CA). DCFH-DA stock solution was freshly diluted with PBS to prepare a 150 μM working solution. The reaction was initiated by adding 10 μl of RVLM homogenate supernatant in a 96-well plate to give a final concentration of 25 μM DCFH-DA to produce fluorescent 2′,7′-dichlorofluorescein (DCF) in the incubation medium at 37°C. Measurement of fluorescence intensity started 30 minutes later using a microplate fluorescence reader set at excitation 485 nm/emission 530 nm. The standard curve of DCF was constructed and the ROS level was determined as relative fluorescence units of generated DCF (Rezq and Abdel-Rahman, 2016).
Dihydroethidium (DHE) Staining for Superoxide Detection.
Following the recent recommendations of utilizing two or more different methods for ROS level measurements (Griendling et al., 2016), frozen brain sections containing the RVLM from treated and control rats (n = 5) were incubated with 10 µM DHE (Molecular Probes) at 37°C in the presence of 5% CO2 in a moist chamber for 30 minutes. The assay was validated using positive and negative controls. A Zeiss LSM 510 microscope was used for image visualization (Carl Zeiss, Inc.). Image J Software (National Institutes of Health, Bethesda, MD) was used for quantification and the changes in total fluorescence intensity, normalized to control, were calculated (Collin et al., 2007).
Western Blot Analysis.
The detection and quantification of the expression of CSE, HO-1, and tyrosine hydoxylase (TH) enzymes were followed as described in our previous studies (El-Sayed et al., 2016; Rezq and Abdel-Rahman, 2016; Fouda and Abdel-Rahman, 2017). Punched RVLM tissues were collected, as described previously, and homogenized with lysis buffer containing 20 mM Tris, Ph 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton x-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, and 1 µg/ml leuptin with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). After homogenization and centrifugation, a Bio-Rad protein assay system (Bio-Rad Laboratories) was used to quantify proteins in the supernatant. Twenty micrograms of each protein were applied per lane of 4%–12% SDS-PAGE gel (Invitrogen, Carlsbad, CA) and transfer was done using nitrocellulose membranes. Then, the proteins were revealed by immunoblotting using a 1:500 dilution of anti-CSE, TH, or HO-1 polyclonal antibodies along with 1:5000 dilution of anti-glyceraldehyde-3-phosphate dehydrogenase (for CSE and TH) or anti-β-actin (for HO-1) (Abcam) at 4°C overnight. Afterward, the membranes were washed and incubated for 60 minutes with mixture containing IRDye680-conjugated goat anti-mouse and IRDye800-conjugated goat anti-rabbit (1:15000; LI-COR Biosciences, Lincoln, NE). The identified proteins were visualized using an Odyssey Infrared Imager and analyzed with Odyssey application software version 5.2 (LI-COR Biosciences, Lincoln, NE). Data represent the mean values of the integrated density ratio of CSE, HO-1, or TH normalized to the corresponding housekeeping protein, glyceraldehyde-3-phosphate dehydrogenase, or β-actin, and expressed as percentage of nondiabetic control.
Measurement of RVLM H2S Synthesizing Activity.
The method described in our previous study (El-Sayed et al., 2016) was used. Punched RVLM tissues from different groups were homogenized in PBS (50 mM, pH 7.4), centrifuged, and then the protein in the supernatant was quantified using a Bio-Rad protein assay system (Bio-Rad Laboratories). We added 100 μl sample (200 μg protein) to 900 μl of the reaction mixture (100 mM potassium phosphate buffer pH 7.4, 10 mM L-cysteine, and 2 mM pyridoxal 5′-phosphate). Cryovial tubes (2 ml) containing 0.5 ml of 1% zinc acetate and a filter paper (1 × 1.5 cm) to increase the air-liquid contact were used to trap the released H2S gas. The bottles were flushed with nitrogen and sealed with parafilm double layers. We started the reaction by incubating the bottles in a shaking water bath (37°C) for 90 minutes. The reaction was stopped by adding 500 μl of 50% trichloroacetic acid; the bottles were sealed again and returned to the shaking water bath for another 60 minutes to ensure trapping of all generated H2S. The contents were then transferred into Eppendorff tubes, and mixed with 134 μl each of N,N-dimethyl-p-phenylene diamine sulfate (20 mM) and ferric chloride (30 mM) followed by 20-minute incubation at room temperature. Finally, the contents were transferred into a 96-well plate and read at 650 nm in a microplate reader. We calculated the H2S concentrations using a calibration curve constructed with NaHS solution in 50 mM potassium phosphate buffer, pH 6.8 (0–320 μM NaHS equivalent to 0–96 μM H2S). The H2S concentration was calculated as 30% of the NaHS concentration as previously reported (Velasco-Xolalpa et al., 2013; El-Sayed et al., 2016), and the RVLM H2S enzyme-synthesizing activity was expressed as nanomoles per milligrams protein per minutes.
Drugs.
The following drugs and chemicals were used in the present study. Moxonidine (American Custom Chemicals Corp., San Diego, CA), DLP (Chem-Impex International Inc., Wood Dale, IL), N,N-dimethyl-p-phenylenediamine sulfate (Acros Organics Thermo Fisher Scientific, Bridgewater, NJ), and acrylamide 40% (Fischer Scientific, Pittsburgh, PA). All other chemicals were purchased from Sigma-Aldrich.
Data Analysis and Statistics.
Data are expressed as mean ± S.E.M. Statistical analyses were conducted by one-way or repeated measures analysis of variance for multiple comparisons followed by Tukey’s post hoc test and Student’s t test using Prism 5.0 software (GraphPad Software Inc., San Diego, CA); P < 0.05 was considered significant.
Results
Moxonidine Mitigates STZ-Induced RVLM Neurodegeneration and Oxidative Stress.
Fluorojade C staining, indicative of neuronal injury (Chaparro et al., 2013), was used to determine the number of RVLM-damaged neurons. The RVLM of STZ-diabetic rats or DLP (CSE inhibitor)–treated nondiabetic rats exhibited approximately 2-fold higher number of damaged neurons compared with the nondiabetic (vehicle-treated) control group (Fig. 1). Moxonidine (dose dependantly) or NaHS (H2S donor) reduced (P < 0.05) the number of RVLM-damaged neurons in STZ-diabetic rats, and DLP abolished the neuroprotective effect of moxonidine (6 mg/kg) in STZ-treated rats (Fig. 1).
Fig. 1.
FluoroJade C (FJC) positive cells examined in the RVLM of rats showing neurodegeneration. (A–I) Representative images of FJC-positive cells in male rats treated with STZ (55 mg/kg, i.p. for 4 weeks) or its vehicle (buffer) receiving NaHS (H2S donor, 3.4 mg/kg per day, i.p, for 3 weeks after diabetes induction), DLP (CSE inhibitor, 37.5 mg/kg, i.p., for 3 weeks after diabetes induction), moxonidine (MOX) (2 or 6 mg/kg per day for 3 weeks after diabetes induction, gavage), a combination of MOX and DLP, or their vehicle (for 3 weeks after diabetes induction). (J) Group data showing the neurodegeneration expressed as the mean number of FJC-positive cells measured using National Institutes of Health ImageJ analysis of confocal images. Values are expressed as mean ± S.E.M. (n = 5 rats/group). *P < 0.05 vs. corresponding control/vehicle (Ctrl/Veh) values; #P < 0.05 vs. corresponding STZ/Veh values; $P < 0.05 vs. STZ/MOX-6 values.
Similar to the fluorojade findings, the number of RVLM caspase-3 immunoreactive neurons was higher (P < 0.05) in STZ-diabetic and DLP-treated nondiabetic rats compared with the nondiabetic control group (Fig. 2). Moxonidine (dose dependently) or NaHS (H2S donor) reduced (P < 0.05) the number of RVLM caspase-3 immunoreactive neurons in STZ-treated rats, and the neuroprotective effect of moxonidine (6 mg/kg) in STZ-treated rats was diminished by DLP (CSE inhibitor) coadministration (Fig. 2).
Fig. 2.
Immunohistochemical detection of caspase-3 examined in the RVLM of rats. (A–I) Representative images of caspase-3 expression in male rats treated with STZ (55 mg/kg, i.p., for 4 weeks) or its vehicle (buffer) receiving NaHS (H2S donor for 3 weeks after diabetes induction; 3.4 mg/kg per day), DLP (CSE inhibitor, 37.5 mg/kg, i.p., for 3 weeks after diabetes induction), moxonidine (MOX) (2 or 6 mg/kg per day for 3 weeks after diabetes induction, gavage), a combination of MOX and DLP, or their vehicle (for 3 weeks after diabetes induction). (J) Group data showing the mean number of caspase-3 expression measured using National Institutes of Health ImageJ analysis of confocal images. Values are expressed as mean ± S.E.M. (n = 5 rats/group). *P < 0.05 vs. corresponding control/vehicle (Ctrl/Veh) values; #P < 0.05 vs. corresponding STZ/Veh values; $P < 0.05 vs. STZ/MOX-6 values.
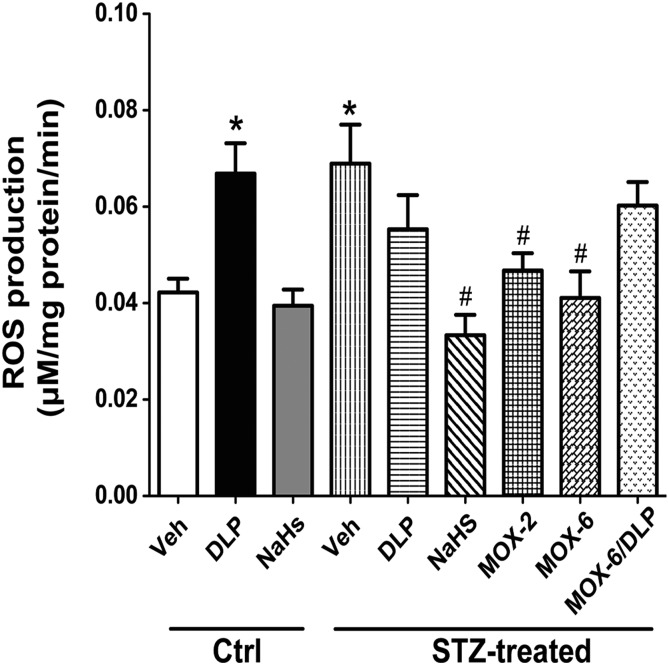
DCF kinetics (Fig. 3) and DHE fluorescence staining intensity (Fig. 4) showed that STZ-diabetic rats or DLP-treated nondiabetic rats exhibited higher (P < 0.05) ROS levels compared with the nondiabetic control group. NaHS or moxonidine reversed the increase in ROS level in STZ-diabetic rats (Figs. 3 and 4), and DLP diminished the favorable effect of moxonidine (6 mg/kg) on RVLM redox status in STZ-diabetic rats (Figs. 3 and 4). Furthermore, NaHS attenuated the increased ROS level and neuronal damage in STZ-treated rats, but it had no effect in control rats (Figs. 1–4).
Fig. 3.
The DCF biochemical assay of the generation of ROS showing the slopes (regression coefficients) of the regression lines representing the rate of ROS production in the RVLM of male rats treated with STZ (55 mg/kg, i.p., for 4 weeks) or its vehicle (buffer) receiving NaHS (H2S donor for 3 weeks after diabetes induction; 3.4 mg/kg per day), DLP (CSE inhibitor, 37.5 mg/kg, i.p., for 3 weeks after diabetes induction), moxonidine (MOX) (2 or 6 mg/kg per day for 3 weeks after diabetes induction, gavage), a combination of MOX and DLP, or their vehicle (for 3 weeks after diabetes induction). Values are expressed as mean ± S.E.M. (n = 5 rats/group). *P < 0.05 vs. corresponding control/vehicle (Ctrl/Veh) values; #P < 0.05 vs. corresponding STZ/Veh values.
Fig. 4.
(A–I) Confocal images showing superoxide level indicated by DHE staining (red) in the RVLM of male rats treated with STZ (55 mg/kg, i.p., for 4 weeks) or its vehicle (buffer) receiving NaHS (H2S donor for 3 weeks after diabetes induction; 3.4 mg/kg per day), DLP (CSE inhibitor, 37.5 mg/kg, i.p., for 3 weeks after diabetes induction), moxonidine (MOX) (2 or 6 mg/kg per day for 3 weeks after diabetes induction; gavage), a combination of MOX and DLP, or their vehicle (for 3 weeks after diabetes induction). (J) Values are expressed as mean ± S.E.M. (n = 5 rats/group). *P < 0.05 vs. corresponding control/vehicle (Ctrl/Veh) values; #P < 0.05 vs. corresponding STZ/Veh values; $P < 0.05 vs. STZ/MOX-6 values.
Moxonidine or NaHS Restores CSE, HO-1, and TH in the RVLM of Diabetic Rats.
Western blot analysis showed an increase (P < 0.05) in TH (Fig. 5A) and reductions (P < 0.05) in CSE (Fig. 5B) and HO-1 (Fig. 5C) expressions in the RVLM of STZ-diabetic rats compared with nondiabetic control rats. NaHS or moxonidine reversed these STZ-evoked effects and restored the protein levels of these enzymes to nondiabetic control levels (Fig. 5). Except for DLP-evoked reduction (P < 0.05) in CSE, NaHS or DLP had no effect on the expression level of these proteins in the RVLM of nondiabetic control rats (Fig. 5). However, DLP coadministration prevented the restoration of RVLM CSE, HO-1, and TH levels caused by moxonidine (6 mg/kg) in STZ-diabetic rats (Fig. 5). Finally, CSE activity was substantially (P < 0.05) reduced in the RVLM of STZ-diabetic rats and DLP-treated nondiabetic rats (Fig. 6). NaHS or moxonidine (6 mg/kg) reversed the reduction in CSE activity in the RVLM of STZ-diabetic rats, and concurrent DLP administration prevented the favorable effect of moxonidine on RVLM CSE activity (Fig. 6).
Fig. 5.
Western blot analyses showing the protein expression in the RVLM of male rats treated with STZ (55 mg/kg, i.p., for 4 weeks) or its vehicle (buffer) receiving NaHS (H2S donor for 3 weeks after diabetes induction; 3.4 mg/kg per day), DLP (CSE inhibitor; 37.5 mg/kg, i.p., for 3 weeks after diabetes induction), moxonidine (MOX) (2 or 6 mg/kg per day for 3 weeks after diabetes induction, gavage), a combination of MOX and DLP, or their vehicle (for 3 weeks after diabetes induction). (A) TH ratio to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein (housekeeping protein) and western bands depicting the protein expression are shown below the bar graphs. (B) CSE ratio to GAPDH protein (housekeeping protein) and western bands depicting the protein expression are shown below the bar graphs. (C) HO-1 ratio to β-actin protein (housekeeping protein) and western bands depicting the protein expression are shown below the bar graphs. Values are expressed as mean ± S.E.M. (n = 5 rats/group). *P < 0.05 vs. corresponding control/vehicle (Ctrl/Veh) values; #P < 0.05 vs. corresponding STZ/Veh values; $P < 0.05 vs. STZ/MOX-6 values.
Fig. 6.
H2S synthesizing enzyme activity in the RVLM of male rats treated with STZ (55 mg/kg, i.p., for 4 weeks) or its vehicle (buffer) receiving NaHS (H2S donor for 3 weeks after diabetes induction; 3.4 mg/kg per day), DLP (CSE inhibitor, 37.5 mg/kg, i.p., for 3 weeks after diabetes induction), moxonidine (MOX) (2 or 6 mg/kg per day for 3 weeks after diabetes induction, gavage), a combination of MOX and DLP, or their vehicle (for 3 weeks after diabetes induction). Values are expressed as mean ± S.E.M. (n = 5 rats/group). *P < 0.05 vs. corresponding control/vehicle (Ctrl/Veh) values; #P < 0.05 vs. corresponding STZ/Veh values; $P < 0.05 vs. STZ/MOX-6 values.
Discussion
The present study is the first to discern a physiologic neuroprotective role for H2S in a major cardiovascular regulating nucleus, the RVLM. Our findings also suggest H2S-dependent neuroprotective effect for moxonidine (I1 agonist) against diabetes-induced RVLM neuronal injury, oxidative stress, and sympathoexcitation. The main findings that support our conclusions are the following: 1) NaHS (H2S donor) or moxonidine mitigated the diabetes-induced RVLM neuronal injury, apoptosis, and oxidative stress-linked sympathoexcitation; 2) either intervention reversed the diabetes-induced reductions in CSE activity and in CSE and HO-1 expressions in the RVLM; and 3) CSE inhibition (DLP) reproduced a diabetic RVLM phentotype in nondiabetic rats and nullified the favorable RVLM neuroprotective effects of moxonidine. Together, these findings implicate CSE/H2S in moxonidine-evoked alleviation of diabetes-evoked neurotoxicity.
Our recent study raised important questions about the mechanism of the sympathoexcitation, which was associated with hypertension and autonomic dysregulation in diabetic rats (El-Sayed et al., 2016). Here, we addressed this question by testing the hypothesis that RVLM oxidative stress/neurotoxicity plays a pivotal role in these diabetes-evoked effects for the following reasons. First, the impaired glycemic control associated with diabetes activates RVLM neurons (Oshima et al., 2017), although this evidence was obtained in vitro and the mechanisms of this effect remain unknown. Second, whether the inhibition of CSE-derived H2S, which contributes to diabetes-evoked cardiac and autonomic dysfunction in vivo (El-Sayed et al., 2016), occurs and accounts for similar effects in the RVLM has not been investigated. To address these questions, we conducted detailed studies on the RVLM tissues obtained from diabetic and control rats used in our recent study (El-Sayed et al., 2016).
As an important foundation, our current study showed that STZ-diabetic rats exhibited RVLM injury as indicated by the number of degenerated neurons identified by fluorojade C staining (Fig. 1), and by increased neuronal apoptosis (Fig. 2). While this new finding replicates neurotoxicity in other brain nuclei of the same model (Wang et al., 2014), the mechanism of such neurotoxicity has not been investigated.
We focused on neuronal oxidative stress as an underlying mechanism for the diabetes-evoked neuronal injury and sympathoexcitation based on current evidence in different model systems (Wang et al., 2014; Fouda and Abdel-Rahman, 2017; Oshima et al., 2017). In accordance with current guidelines (Griendling et al., 2016), we confirmed the diabetes-evoked increase in RVLM ROS by two different assays (DCF and DHE). Evidence suggests that the diabetes-evoked neuronal oxidative stress, observed here (Figs. 3 and 4) and in reported studies, could be caused by glucose autoxidation, endoplasmic reticulum stress, and impaired antioxidant defenses (Li et al., 2005; Correia et al., 2008) as well as the increased vulnerability of the brain to oxidative stress (Carvalho et al., 2012; Duarte et al., 2013).
The results of the present study and reported findings suggest a causal role for local oxidative stress in the diabetes-evoked sympathoexcitation (increased TH, see Fig. 5A) in the RVLM. Notably, TH in the RVLM reflects sympathetic activity (Guyenet, 2006) and oxidative stress induces sympathoexcitation in brain stem nuclei (Zimmerman and Davisson, 2004; Huang et al., 2006; Fujita et al., 2012). Furthermore, sympathoexcitation exacerbates neurodegeneration (Burke et al., 2004) and may contribute to cardiovascular anomalies in the same STZ-diabetic rats (El-Sayed et al., 2016) because the RVLM serves a pivotal role in blood pressure regulation (Pilowsky and Goodchild, 2002; Madden and Sved, 2003).
The present findings suggest a pivotal role for CSE/H2S downregulation (Fig. 5B) in diabetes-induced oxidative stress and the subsequent RVLM neurotoxicity (Figs. 1–5) given the antioxidant and anti-inflammatory actions of H2S (Mustafa et al., 2009). This premise is supported by the ability of CSE inhibition (DLP) to cause oxidative stress and to reproduce the diabetic phenotype in the RVLM of nondiabetic rats. Notably, the new finding that DLP reduced CSE protein levels in these nondiabetic rats (Fig. 5B) likely resulted from DLP-evoked oxidative stress (Figs. 3 and 4) via the inhibition of CSE catalytic activity (Fig. 6). This possibility is supported by the finding that H2O2-evoked oxidative stress suppressed CSE protein level in cultured cells (Manna et al., 2014), and by the inverse relationship between ROS and CSE expression in the RVLM (Figs. 3–5) and liver (Manna et al., 2014) of STZ-diabetic rats. These findings suggest an inhibitory role for oxidative stress on CSE protein expression, and identify CSE/H2S upregulation as a novel target for the alleviation of RVLM neurotoxicity in diabetes.
The results of the present study show that moxonidine inhibits sympathoexcitaion (Fig. 5A) and neuronal death (Figs. 1 and 2) in diabetic rats. These findings agree with neuroprotective effects of moxonidine against ischemic insults in neuronal cultures (Milhaud et al., 2000; Bakuridze et al., 2009) and against glutamate-evoked neurotoxicity (Keller and García-Sevilla, 2016). However, the mechanism of the neuroprotective effect of moxonidine was not investigated in the reported studies.
Our findings suggest a pivotal role for RVLM CSE/H2S upregulation in moxonidine-evoked neuroprotection and sympathoinhibition because these responses were tightly correlated in moxonidine-treated diabetic rats and were abolished in the presence of CSE inhibition (DLP). These findings are consistent with the neuroprotective effect of H2S and its antiapoptotic effect through increasing glutathione level and suppressing oxidative stress (Kimura and Kimura, 2004; Kimura et al., 2010; Mikami et al., 2016). These reported findings raised the possibility that H2S interacts with another antioxidant gaseous neuromodulator, HO-1.
We studied the role of HO-1 in our model system because it is expressed in the RVLM neurons (Mazza et al., 2001), exerts neuronal antioxidant and antiapoptotic effects (Spitz et al., 1987; Fouda and Abdel-Rahman, 2017; Kim et al., 2017), and mediates sympathoinhibition (Nassar et al., 2011). Our findings suggest H2S-dependent regulation of HO-1 in the RVLM contributes to the diabetes-evoked neurotoxicity and its alleviation by moxonidine for the following reasons. First, CSE/H2S inhibition in diabetic and healthy rats following DLP was associated with reduced RVLM HO-1 expression (Fig. 5C). Second, the H2S donor NaHS or moxonidine restored RVLM HO-1 expression in diabetic rats (Fig. 5C). Third, CSE inhibition (DLP) abolished the moxonidine-evoked restoration of HO-1 expression in diabetic rats (Fig. 5C).
The present findings provide two new pieces of evidence. First, CSE/H2S inhibition mediates neuronal injury, oxidative stress, and increased presympathetic neuronal activity in the RVLM in diabetic rats. Second, restoration of RVLM CSE-derived H2S mediates the sympathoinhibitory and neuroprotective actions of moxonidine in diabetes. The neuropathological consequences of diabetes and their reversal by moxonidine might explain the cardiovascular anomalies and their alleviation by moxonidine, respectively, in our previous in vivo study (El-Sayed et al., 2016). The findings also suggest that H2S confers neuroprotection and sympathoinhibition, at least partly, via HO-1, and highlight the RVLM CSE/HO-1 pathway as a viable target for developing novel therapeutics for alleviating the neurotoxicity and cardiovascular anomalies associated with diabetes.
Acknowledgments
We thank Kui Sun and Dr. Fanrong Yao for technical assistance.
Abbreviations
- CSE
cystathionine-γ lyase
- DCF
2′,7′-dichlorofluorescein
- DCFH-DA
DCFH-DA
- DHE
dihydroethidium
- DLP
DL-propargylglycine
- HO-1
heme oxygenase-1
- H2S
hydrogen sulfide
- NaHS
sodium hydrogen sulfide
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- RVLM
rostral ventrolateral medulla
- STZ
streptozotocin
- TH
tyrosine hydoxylase
Authorship Contributions
Participated in research design: Fouda, El-Sayed, Abdel-Rahman.
Conducted experiments: Fouda.
Performed data analysis: Fouda.
Wrote or contributed to the writing of the manuscript: Fouda, Abdel-Rahman.
Footnotes
This work was supported by the National Institutes of Health [Grant 2R01 AA14441-10].
Dr. Mohamed Fouda is a visiting scholar from the Department of Pharmacology and Toxicology, Faculty of Pharmacy, Alexandria University, Egypt.
References
- Abdel Moneim AE. (2015) The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab Brain Dis 30:935–942. [DOI] [PubMed] [Google Scholar]
- Bahniwal M, Little JP, Klegeris A. (2017) High glucose enhances neurotoxicity and inflammatory cytokine secretion by stimulated human astrocytes. Curr Alzheimer Res 14:731–741. [DOI] [PubMed] [Google Scholar]
- Bakuridze K, Savli E, Gongadze N, Baş DB, Gepdiremen A. (2009) Protection in glutamate-induced neurotoxicity by imidazoline receptor agonist moxonidine. Int J Neurosci 119:1705–1717. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. (2002) Ageing and diabetes: implications for brain function. Eur J Pharmacol 441:1–14. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, et al. (2004) Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology 25:101–115. [DOI] [PubMed] [Google Scholar]
- Carvalho C, Cardoso S, Correia SC, Santos RX, Santos MS, Baldeiras I, Oliveira CR, Moreira PI. (2012) Metabolic alterations induced by sucrose intake and Alzheimer’s disease promote similar brain mitochondrial abnormalities. Diabetes 61:1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceretta LB, Réus GZ, Abelaira HM, Ribeiro KF, Zappellini G, Felisbino FF, Steckert AV, Dal-Pizzol F, Quevedo J. (2012) Increased oxidative stress and imbalance in antioxidant enzymes in the brains of alloxan-induced diabetic rats. Exp Diabetes Res 2012:302682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro RE, Quiroga C, Bosco G, Erasso D, Rubini A, Mangar D, Parmagnani A, Camporesi EM. (2013) Hippocampal cellular loss after brief hypotension. Springerplus 2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin B, Busseuil D, Zeller M, Perrin C, Barthez O, Duvillard L, Vergely C, Bardou M, Dumas M, Cottin Y, et al. (2007) Increased superoxide anion production is associated with early atherosclerosis and cardiovascular dysfunctions in a rabbit model. Mol Cell Biochem 294:225–235. [DOI] [PubMed] [Google Scholar]
- Correia S, Carvalho C, Santos MS, Seiça R, Oliveira CR, Moreira PI. (2008) Mechanisms of action of metformin in type 2 diabetes and associated complications: an overview. Mini Rev Med Chem 8:1343–1354. [DOI] [PubMed] [Google Scholar]
- Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, Plácido A, Santos MS, Oliveira CR, Moreira PI. (2013) Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim Biophys Acta 1832:527–541. [DOI] [PubMed] [Google Scholar]
- El-Sayed SS, Zakaria MN, Abdel-Ghany RH, Abdel-Rahman AA. (2016) Cystathionine-γ lyase-derived hydrogen sulfide mediates the cardiovascular protective effects of moxonidine in diabetic rats. Eur J Pharmacol 783:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouda MA, Abdel-Rahman AA. (2017) Endothelin confers protection against high glucose-induced neurotoxicity via alleviation of oxidative stress. J Pharmacol Exp Ther 361:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Ando K, Kawarazaki H, Kawarasaki C, Muraoka K, Ohtsu H, Shimizu H, Fujita T. (2012) Sympathoexcitation by brain oxidative stress mediates arterial pressure elevation in salt-induced chronic kidney disease. Hypertension 59:105–112. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, Bhatnagar A, American Heart Association Council on Basic Cardiovascular Sciences (2016) Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res 119:e39–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Jin S, Wang XL, Wang R, Xiao L, He RR, Wu YM. (2011) Hydrogen sulfide in the rostral ventrolateral medulla inhibits sympathetic vasomotor tone through ATP-sensitive K+ channels. J Pharmacol Exp Ther 338:458–465. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7:335–346. [DOI] [PubMed] [Google Scholar]
- Huang BS, Amin MS, Leenen FH. (2006) The central role of the brain in salt-sensitive hypertension. Curr Opin Cardiol 21:295–304. [DOI] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. (2015) A pivotal role for enhanced brainstem Orexin receptor 1 signaling in the central cannabinoid receptor 1-mediated pressor response in conscious rats. Brain Res 1622:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B, García-Sevilla JA. (2016) Inhibitory effects of imidazoline receptor ligands on basal and kainic acid-induced neurotoxic signalling in mice. J Psychopharmacol 30:875–886. [DOI] [PubMed] [Google Scholar]
- Kim S, Chin YW, Cho J. (2017) Protection of cultured cortical neurons by luteolin against oxidative damage through inhibition of apoptosis and induction of heme oxygenase-1. Biol Pharm Bull 40:256–265. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Goto Y, Kimura H. (2010) Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 12:1–13. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H. (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18:1165–1167. [DOI] [PubMed] [Google Scholar]
- Konno S, Hirooka Y, Kishi T, Sunagawa K. (2012) Sympathoinhibitory effects of telmisartan through the reduction of oxidative stress in the rostral ventrolateral medulla of obesity-induced hypertensive rats. J Hypertens 30:1992–1999. [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Sima AA. (2005) The role of impaired insulin/IGF action in primary diabetic encephalopathy. Brain Res 1037:12–24. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Sved AF. (2003) Rostral ventrolateral medulla C1 neurons and cardiovascular regulation. Cell Mol Neurobiol 23:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna P, Gungor N, McVie R, Jain SK. (2014) Decreased cystathionine-γ-lyase (CSE) activity in livers of type 1 diabetic rats and peripheral blood mononuclear cells (PBMC) of type 1 diabetic patients. J Biol Chem 289:11767–11778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DL, Strafaci JA, Miller DC, Masia S, Thomas CG, Rosman J, Hussain S, Freedman ML. (1998) Quantitative neuronal c-Fos and c-Jun expression in Alzheimer’s disease. Neurobiol Aging 19:393–400. [DOI] [PubMed] [Google Scholar]
- Mazza E, Thakkar-Varia S, Tozzi CA, Neubauer JA. (2001) Expression of heme oxygenase in the oxygen-sensing regions of the rostral ventrolateral medulla. J Appl Physiol (1985) 91:379–385. [DOI] [PubMed] [Google Scholar]
- Mikami Y, Kakizawa S, Yamazawa T. (2016) Essential roles of natural products and gaseous mediators on neuronal cell death or survival. Int J Mol Sci 17:1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhaud D, Fagni L, Bockaert J, Lafon-Cazal M. (2000) Imidazoline-induced neuroprotective effects result from blockade of NMDA receptor channels in neuronal cultures. Neuropharmacology 39:2244–2254. [DOI] [PubMed] [Google Scholar]
- Mukaddam-Daher S, Menaouar A, Paquette PA, Jankowski M, Gutkowska J, Gillis MA, Shi YF, Calderone A, Tardif JC. (2009) Hemodynamic and cardiac effects of chronic eprosartan and moxonidine therapy in stroke-prone spontaneously hypertensive rats. Hypertension 53:775–781. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Snyder SH. (2009) Signaling by gasotransmitters. Sci Signal 2:re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar NN, Abdelsalam RM, Abdel-Rahman AA, Abdallah DM. (2012) Possible involvement of oxidative stress and inflammatory mediators in the protective effects of the early preconditioning window against transient global ischemia in rats. Neurochem Res 37:614–621. [DOI] [PubMed] [Google Scholar]
- Nassar NN, Li G, Strat AL, Abdel-Rahman AA. (2011) Enhanced hemeoxygenase activity in the rostral ventrolateral medulla mediates exaggerated hemin-evoked hypotension in the spontaneously hypertensive rat. J Pharmacol Exp Ther 339:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, Chung HT. (2006) Hydrogen sulfide inhibits nitric oxide production and nuclear factor-κB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med 41:106–119. [DOI] [PubMed] [Google Scholar]
- Oshima N, Onimaru H, Matsubara H, Uchida T, Watanabe A, Imakiire T, Nishida Y, Kumagai H. (2017) Direct effects of glucose, insulin, GLP-1, and GIP on bulbospinal neurons in the rostral ventrolateral medulla in neonatal wistar rats. Neuroscience 344:74–88. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Goodchild AK. (2002) Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens 20:1675–1688. [DOI] [PubMed] [Google Scholar]
- Rezq S, Abdel-Rahman AA. (2016) Rostral ventrolateral medulla EP3 receptor mediates the sympathoexcitatory and pressor effects of prostaglandin E2 in conscious rats. J Pharmacol Exp Ther 359:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruginsk SG, Mecawi AS, da Silva MP, Reis WL, Coletti R, de Lima JB, Elias LL, Antunes-Rodrigues J. (2015) Gaseous modulators in the control of the hypothalamic neurohypophyseal system. Physiology (Bethesda) 30:127–138. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Dewey WC, Li GC. (1987) Hydrogen peroxide or heat shock induces resistance to hydrogen peroxide in Chinese hamster fibroblasts. J Cell Physiol 131:364–373. [DOI] [PubMed] [Google Scholar]
- Szczepanska-Sadowska E, Cudnoch-Jedrzejewska A, Ufnal M, Zera T. (2010) Brain and cardiovascular diseases: common neurogenic background of cardiovascular, metabolic and inflammatory diseases. J Physiol Pharmacol 61:509–521. [PubMed] [Google Scholar]
- van den Born JC, Hammes HP, Greffrath W, van Goor H, Hillebrands JL, DFG GRK International Research Training Group 1874 Diabetic Microvascular Complications (DIAMICOM) (2016) Gasotransmitters in vascular complications of diabetes. Diabetes 65:331–345. [DOI] [PubMed] [Google Scholar]
- Velasco-Xolalpa ME, Barragán-Iglesias P, Roa-Coria JE, Godínez-Chaparro B, Flores-Murrieta FJ, Torres-López JE, Araiza-Saldaña CI, Navarrete A, Rocha-González HI. (2013) Role of hydrogen sulfide in the pain processing of non-diabetic and diabetic rats. Neuroscience 250:786–797. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Yin J, Song YF, Zhang L, Ren YX, Wang DG, Gao LP, Jing YH. (2014) Brain aging and AD-like pathology in streptozotocin-induced diabetic rats. J Diabetes Res 2014:796840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Abdel-Rahman AA. (2002) Estrogen modulation of eNOS activity and its association with caveolin-3 and calmodulin in rat hearts. Am J Physiol Heart Circ Physiol 282:H2309–H2315. [DOI] [PubMed] [Google Scholar]
- Wang X, Abdel-Rahman AA. (2005) Effect of chronic ethanol administration on hepatic eNOS activity and its association with caveolin-1 and calmodulin in female rats. Am J Physiol Gastrointest Liver Physiol 289:G579–G585. [DOI] [PubMed] [Google Scholar]
- Yan YH, Chou CCK, Wang JS, Tung CL, Li YR, Lo K, Cheng TJ. (2014) Subchronic effects of inhaled ambient particulate matter on glucose homeostasis and target organ damage in a type 1 diabetic rat model. Toxicol Appl Pharmacol 281:211–220. [DOI] [PubMed] [Google Scholar]
- Yang LY, Chu YH, Tweedie D, Yu QS, Pick CG, Hoffer BJ, Greig NH, Wang JY. (2015) Post-trauma administration of the pifithrin-α oxygen analog improves histological and functional outcomes after experimental traumatic brain injury. Exp Neurol 269:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MC, Davisson RL. (2004) Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol 84:125–149. [DOI] [PubMed] [Google Scholar]