Abstract
Objectives
We investigated the effects of deep brain stimulation (DBS) or lesions of the ventral intermediate nucleus (Vim) of the thalamus for spinocerebellar ataxia (SCA) and examined the pathophysiological role of neuronal activity of the Vim underlying ataxia.
Methods
Five patients with SCA with cortical atrophy (ages 60‐69 years; 2 sporadic and three familial SCA) and five patients with essential tremor (ET) (ages 57–71 years) were treated with Vim surgery. Intraoperatively, we recorded neuronal activity from single neurons in the Vim thalamus while patients were at rest and compared the physiological properties of those neurons between patients with SCA and those with ET.
Results
Postsurgery mean scores for the Fahn–Tolosa–Marin Tremor Scale were improved from 78 to 44 in SCA patients and from 54 to 21 in ET patients. Stronger stimulation was necessary to optimize outcomes in SCA as compared to ET patients. We analyzed 68 Vim neurons in SCA and 60 Vim neurons in ET. Mean discharge rates, burst characteristics, and oscillatory activity were similar for both patient groups, however, we observed that the ratio of cells responding to passive manipulation was significantly smaller (P = 0.0001) in SCA (22%) than in ET (71%).
Interpretation
Thalamic surgery led to a significant improvement in tremor in SCA patients. One potential mechanism underlying ataxia in SCA may be disruption of cerebellar sensory feedback, which modulates motor commands in the cerebello‐thalamo‐cortical network.
Introduction
Cerebellar dysfunction has been linked to a number of clinical motor signs, including dyssynergia, dysmetria, dysdiadochokinesia, and dysarthria as well as ataxia of stance and gait.1 Disturbances of limb movements in cerebellar disease include increased reaction time, dysmetria, slowed initial movement, action tremor, inability to maintain constant force, hypotonia, and a disturbed time interval between movement components.2 Spinocerebellar ataxias (SCAs) are degenerative disorders with genetic heterogeneity involving the cerebellum and connected regions and are characterized by abnormal eye movements and ataxia. Some patients with SCA show a vigorous action tremor intractable to medical therapy. There is a long history of functional neurosurgery targeting the ventral intermediate nucleus (Vim) of the thalamus for treatment of tremor, most often for essential tremor (ET).3, 4 ET is not associated with overt ataxia, but a subset of ET was disclosed to involve a pathological abnormality of the cerebellum.5 The mode of tremor is similar, dominantly intention‐type action tremor, and the mechanism of tremor is estimated to share the same anatomical structures in SCA and ET.5, 6 Recent experiences of deep brain stimulation (DBS) of Vim showed favorable effects on tremor in SCA in single case studies,7, 8, 9, 10 which includes our previous case report.8
We performed Vim DBS to alleviate action tremor in five SCA patients and compared the effects with those in ET to evaluate efficacy of Vim DBS on tremor in SCA. We also recorded Vim neuronal activity during physiological mapping to delineate the border of the Vim with the sensory nucleus ventralis caudalis, Vc. The Vim is located at the midpoint of the cerebello‐thalamo‐cortical projection and is a key structure for generation of tremor. We analyzed discharge properties of the Vim and compared the patterns of thalamic neuronal activities between SCA and ET patients. The principal difference of the symptom between SCA with tremor and ET was ataxia. As the major receiving site of cerebellar output is the Vim, it is reasonable to hypothesize that differences in Vim neuronal activity between SCA with tremor and ET would reflect the alteration in cerebellar output signals responsible for ataxia.
A principle function of the cerebellum is to coordinate the movement of different body parts; it may also participate in certain cognitive and other higher brain functions.11, 12 Sensory signals and motor commands converge at the cerebellum, which is hypothesized to play a role in comparing the motor command with sensory information during movement and stance and, ultimately, sending corrective commands to refine movement and posture to the cerebral cortex as well as brainstem structures.12, 13 The major cerebellar output is derived from the deep cerebellar nuclei whose activity is modulated by input from Purkinje cells. Input from Purkinje cells encompasses coding strategies to refine motor commands14, 15 including, for example, a rate code16 and a time code.17 Hence, altered neuronal activities of the Vim in SCA could reflect the disrupted coding of cerebellar output that underlie the altered movement associated with these disorders, that is, ataxia and tremor.
Materials and Methods
Subjects
Five patients with SCA (3 males and 2 females) and five with ET (3 males and 2 females) underwent neurosurgery targeting the Vim at Shinshu University Hospital and Aizawa Hospital. The surgeries were one unilateral Vim DBS and four bilateral DBS in the patients with SCA and two unilateral Vim coagulation and three bilateral Vim DBS in those with ET. No patient had undergone previous stereotactic neurosurgery, with the exception of one patient with ET who underwent left gamma knife thalamotomy; neuronal recording from this side of the patient was not included in the neuronal analysis. The age of the patients with SCA ranged from 62 to 69 years (mean and SD, 64.5 ± 3.1 years) and that of the patients with ET ranged from 57 to 71 years (64.3 ± 5.7 years). Disease duration ranged from 2 to 13 years (9.5 ± 5.2 years) in the patients with SCA and 5 to 20 years (11.5 ± 6.2 years) in the patients with ET. The diagnoses of SCA were sporadic cortical cerebellar atrophy in two patients, SCA6 in two and SCA31 in one. All patients had medication‐intractable severe action tremor. Tremor frequency ranged from 2.5 to 3.3 Hz (3.0 ± 0.4 Hz) in the patients with SCA and 3.8–6.0 Hz (5.0 ± 1.0 Hz) in the patients with ET. Tremor of both SCA and ET was intention‐type, but the proximal parts of the upper extremity were more strongly involved in SCA than in ET. None of the patients had parkinsonian signs, for example, rigidity, bradykinesia, or freezing of gait. All patients with SCA showed mild to moderate dysarthria, limb ataxia, and gait ataxia, and could not walk without assistance. No patient with ET showed dysarthria, limb ataxia or gait disturbance, except for Case 10 who showed mild gait ataxia. Clinical details are summarized in Table 1. Brain MRI revealed mild to moderate atrophy of the cerebellar hemisphere, but no atrophy of the brainstem and cerebrum in all patients with SCA. No patients with ET showed atrophy of the cerebellum or brainstem. Tremor was evaluated using the Fahn–Tolosa–Marin Tremor Scale18 before surgery and 3 months after surgery. Ataxia was not evaluated because functional disability from ataxia could not be isolated from that due to tremor.
Table 1.
Demographic data of the subjects
| Case | Age/Sex | Diagnosis | Action tremor | Tremor frequency | Ataxia | Gait | Parkinsonian signs | Record side |
|---|---|---|---|---|---|---|---|---|
| SCA | ||||||||
| 1 | 69/m | Sporadic | Severe | 3.2 Hz | Severe | Unable | ‐ | Lt |
| 2 | 64/m | SCA 6 | Severe | 3.3 Hz | Moderate | With assistance | ‐ | Lt |
| 3 | 63/f | SCA 31 | Severe | 2.5 Hz | Severe | Unable | ‐ | Lt |
| 4 | 62/f | SCA 6 | Severe | 3.0 Hz | Severe | Unable | ‐ | Lt |
| 5 | 60/m | Sporadic | Severe | 4.3 Hz | Severe | With assistance | ‐ | Rt |
| ET | ||||||||
| 6 | 71/m | Sporadic | Moderate | 3.8 Hz | None | Normal | ‐ | Rt |
| 7 | 57/m | Familial | Severe | 6.0 Hz | None | Normal | ‐ | Rt |
| 8 | 65/f | Familial | Severe | 5.5 Hz | None | Normal | ‐ | Lt |
| 9 | 64/m | Sporadic | Severe | 4.8 Hz | None | Normal | ‐ | Lt |
| 10 | 71/f | Sporadic | Severe | 4.8 Hz | Mild | Normal | ‐ | Lt |
SCA, spinocerebellar ataxia; ET, essential tremor.
The study was approved by the ethics committee of Shinshu University Hospital and Aizawa Hospital for research on human subjects according to the Helsinki declaration. All patients were fully informed on the procedure and the purpose of neuronal recording and gave their consent to all aspects of the study.
Surgical procedures
We performed coagulation of the Vim or implantation of a quadripolar electrode (DBS 3387; Medtronic, Inc., Minneapolis) into the Vim (surgery frame devices SBD‐02 and RFC‐11; Tokai Rika Inc., Japan) with microelectrode guidance under local anesthesia. No sedation was used in surgery. All medications were withheld overnight, and the period of time from the last medication to thalamic recordings was longer than 15 h. Glass‐coated elgiloy microelectrodes with an impedance of 0.4–1.0 MΩ at 1000 Hz were used for single‐cell recording. Neuronal activity recorded by the microelectrode was displayed on an oscilloscope and converted to sound by audio speakers. Recording tracks proceeded from anterodorsal to posteroventral at an angle of 20–30° from vertical and lateral to medial at 20°angle from the sagittal plane. The borders of the Vim were estimated as follows: the anterior border of the principal somatosensory nucleus of the thalamus, the Vc, on the oblique sagittal plane of the electrode track was defined physiologically and fit to the atlas maps19. The lateral distance to the midline was measured on an intraoperative CT scan; the borders of the Vim were estimated on the atlas maps based on the Vc border. A few days after DBS surgery, permanent pulse generators (Kinetra; Medtronic, Inc.) and connection leads were implanted subcutaneously under general anesthesia. 10–30 sec samples of spontaneous single‐cell activity from the thalamus were recorded with a filter band pass of 300–10,000 Hz during mapping and stored on digital audio tape for subsequent off‐line analysis.
Stimulation setting
To determine the optimal DBS setting for each patient a monopolar review was performed. Each contact was tested initially using a 60 μsec pulse width over frequencies from 80 Hz to 130 Hz with the pulse generator serving as the anode. Voltage was increased until tremor was abolished or continuous side effects were elicited. Once the optimal contact was determined, pulse width, frequency, and voltage were adjusted further to achieve the best reduction in tremor without side effects. In the case that the threshold for side effects was lower than the threshold for reduction in tremor, bipolar stimulation was used. Double‐contact stimulation (two contacts serving as the cathode) was applied for those cases where single‐contact stimulation was not adequate for reducing tremor.
Data analysis
The software for analysis of neuronal activity was developed using a C compiler running on DOS and Matlab scripts on Windows for off‐line analysis by the authors (TH and AM). The neuronal potentials were digitized at a sampling rate of 50 kHz with 12‐bit vertical resolution, discriminated by a template‐matching algorithm and converted into timestamps using a sampling rate of 1 kHz. The following physiological characteristics were determined for each cell; mean discharge rate, oscillatory activity, burstiness, and sensory responses to passive manipulation of peripheral body parts. The firing properties from five patients with SCA were compared with those from five patients with ET.
Burst detection
Burst activity of each neuron was examined using the modified Poisson surprise algorithm.19 This algorithm identifies bursts as low probability events assuming that the spike times within a train of action potentials have a Poisson distribution. The minimum interspike interval for detecting bursts in all three phases was set to half the average interspike interval. The minimum number of spikes in a burst was set as two and the maximum as ten.20 Any group of spikes, defined as a burst by the algorithm, with a P ≤ 0.05 (surprise index ≥ 3.0) was considered significant. For each unit, the frequency of bursting (bursts/min), mean intraburst rate (IBR, spikes/s), and the percent of spikes within bursts were calculated.
Oscillatory discharges
Power spectral density (PSD) was calculated by applying the Welch method21 to nonoverlapping 1 sec windows of spike counts in 1 msec time bins and averaging the resulting PSD estimates. No difference in the two groups was observed, irrespective of the bin width used (1, 5, or 10 msec). The Hanning window was applied to the windowed data prior to the discrete Fourier transform (DFT). The threshold for detection of significant peaks was set as the 95% confidence interval of the mean power, Bonferroni corrected for multiple comparisons (i.e., 5 bands); a spectral peak was considered significant if two consecutive bins were greater than the threshold. The ~150 Hz frequency range was divided into bands: ≤3 Hz, 3–8 Hz, 8–30 Hz, 30–58 Hz, 65–150 Hz; the left side limits of each band were excluded from the band. Distortion of the spectra due to the refractoriness of the cell and changes in firing rate during the recording interval, if present, were corrected using the local‐shuffling method described by Rivlin‐Etzion et al.22 The corrected spectrum is the ratio of the original and normalizing spectrum. The normalizing spectrum was also calculated from the same spike train, but after shuffling spikes with interspike intervals less than 1 s; the shuffling was repeated 100 times and the normalizing spectrum is the average of the power spectral density calculated from the shuffled spikes.
Somatosensory responses
The response of neurons to passive manipulations of the extremities and orofacial structures was examined. Passive movements of the ankle, knee, hip, wrist, elbow, shoulder, and jaw joints were systematically tested. A positive response was defined as an increase in the intensity of the audio signal of a Vim neuron corresponding to manipulation by two examiners. Responses to active movement were not examined.
Statistical analysis
The statistic package, SYSTAT13 was used for all analyses. The tremor rating scales before and after DBS surgery were tested using the Wilcoxon signed‐rank test. Differences in tremor ratings between SCA and ET were tested using the Mann–Whitney test. The Mann–Whitney test also was used to assess the main effect of condition (i.e., ET vs. SCA) on the neuronal discharge parameters. The PSD counts were compared using the Yates’ corrected chi‐squared test. The PSD estimation was done using the Welch method with one second windows and a Hanning window of the same length. The 95% confidence interval, with Bonferroni correction applied to the omnibus alpha of 0.05 was used to detect significant peaks. The Yates’ corrected chi‐squared test and Mann–Whitney test were used for comparison of somatosensory responses. Difference was accepted when the P < 0.05 (in two‐way).
Results
Effects of thalamic surgery on tremor
An example of the effect of Vim DBS on tremor in SCA Case 1 is presented in Figure 1. Coarse action and postural tremor was strongly suppressed by Vim DBS (bipolar stimulation: 160 Hz, 90 μsec pulses, 3.0 V for the left Vim and 3.5 V for the right Vim). Although limb and truncal ataxia remained, when DBS was turned on the patient was able to eat using a fork, write large letters, and walk a few steps with assistance, actions that were impossible without DBS. The means of the scores for the Fahn–Tolosa–Marin Tremor Scale (maximum score, 144) in five SCA patients and five ET patients are presented in Figure 2. The mean total scores were improved from 78 (before surgery) to 44 (after surgery, DBS on) in SCA patients and from 54 to 21 in ET patients, (Wilcoxon signed‐rank test, P = 0.043 in SCA and P = 0.041 in ET). The mean scores of severity of tremor improved from 25 to 13 in SCA and from 20 to 8 in ET (P = 0.043 in SCA and P = 0.043 in ET, those of severity of hand writing improved from 31 to 17 in SCA and from 21 to 9 in ET (P = 0.042 in SCA and P = 0.043 in ET), and those of functional disability improved from 21 to 14 in SCA and from 13 to 4 in ET (P = 0.043 in SCA and P = 0.043 in ET). The functional disability during DBS on was composed of disability due to residual tremor and ataxia in SCA. The degrees of improvement in the subscales were compared between SCA and ET, and there was no significant difference in the changes in the subscores or the total score (Fig. 2C). Based on neurological examination, improvement persisted for the follow‐up period, mean 5.6 years in SCA patients and 7.4 years in ET patients. Improvement of ataxic symptoms other than tremor by Vim DBS appeared to be subtle or not recognized in all SCA patients during the follow‐up period.
Figure 1.
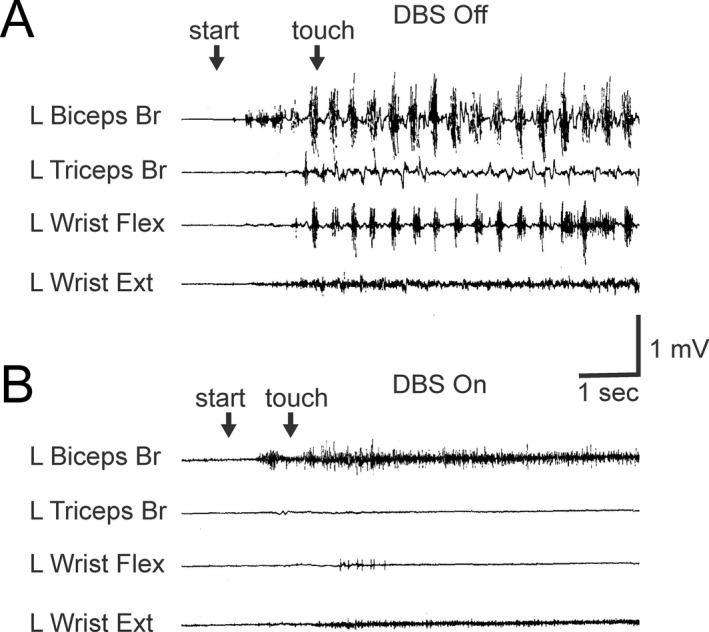
Effect of Vim DBS on action and postural tremor in a patient with SCA. Electromyograms from the left upper limb recorded under thalamic stimulation on and off in a patient with sporadic SCA (Case SCA1). 3.2 Hz tremor appeared when the patient raised the left arm (“start”) and persisted after touching the finger to the nose (“touch”) under stimulation off (A), but the tremor did not appear under stimulation on (B).
Figure 2.
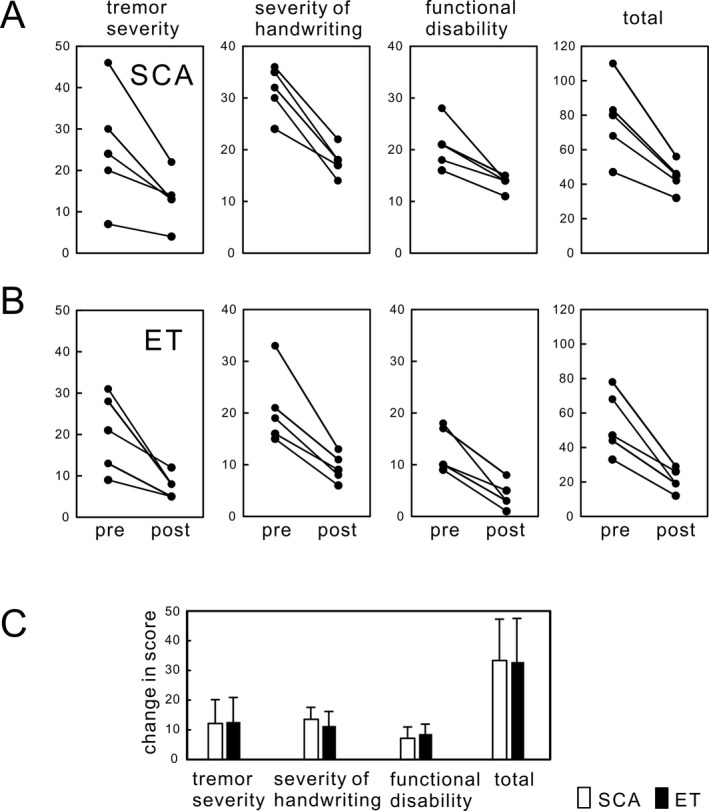
Improvement of tremor severity and function with Vim DBS. The means of the scores for Fahn–Tolosa–Marin Tremor Scale17 (maximum score, 144) are presented. The mean total scores, scores of severity of tremor, and those of functional disability were improved in both SCA (A) and ET patients (B). The scores of severity of handwriting and functional disability in SCA stayed higher than those in ET even during DBS on, because functional disability by ataxia did not improve in SCA. (C) Degrees of improvement in the subscales of tremor show no difference in changes in the scores in all subscores and total score between SCA and ET, suggesting similar improvement of tremor. Bars represent means and SDs.
Stimulation parameters
The best locations of the DBS contacts and the coagulation lesions in all patients are illustrated in Figure 3. The common finding was that at least one cathodal contact was located in the lower and posterior quadrant of the Vim in most patients, (7 of 9 DBS leads in SCA and 5 of 7 DBS leads in ET). The lateral distance of the best contacts was 12–16 mm to the midline. The coagulation areas were located in the central area of the Vim in the thalamotomy cases.
Figure 3.
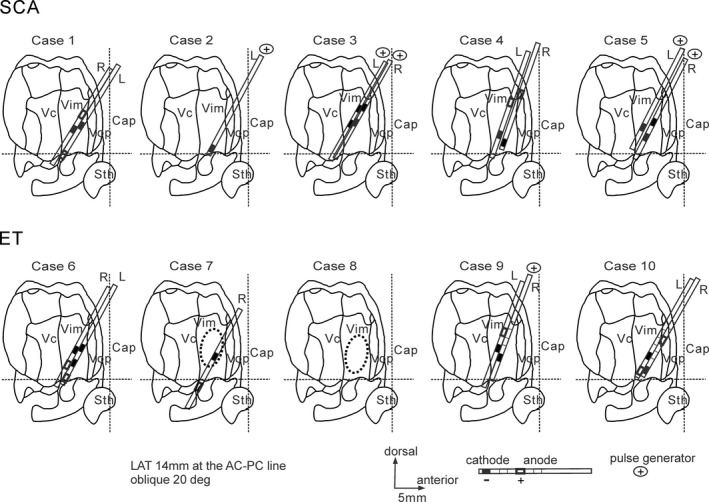
The best locations of the clinically defined therapeutic DBS contacts and the coagulation lesions presented on the parasagittal section with an oblique dorsolateral to medioventral angle of approximately 20 degrees (adapted from the Shaltenbrand and Bailey Atlas18). The common finding was that at least one cathodal contact was located in the lower and posterior quadrant in the sagittal section of the Vim in most patients in SCA and ET. The lateral distance of the best contacts was 12–16 mm from the midline. The coagulation areas were located in the central area of the Vim in the thalamotomy cases. The horizontal broken lines are the anterior commissure–posterior commissure lines (AC‐PC lines), and the vertical broken lines are the mid AC‐PC lines. Cap, capsule; Sth, subthalamic nucleus; Vc, nucleus ventrocaudalis; Vim, nucleus ventrointermedius; Vop, nucleus ventrooralis posterior.
Stimulation was started after surgery with monopolar stimulation with the stimulation frequency of 130 Hz and pulse width of 60 μsec. Therapeutic impedance was checked under the monopolar setting with 1.5 V, 210 μsec and 30 Hz by the programmer (N'Vision model 8840, Medtronic, Inc., USA) and confirmed to be within 500–2000 Ω. The stimulation parameters for the best improvement were as follows (Table 2): five of nine stimulations (56%) used two contacts as cathode in SCA, whereas two of seven stimulations (29%) used two contacts in ET; eight of the nine stimulations (89%) used pulse width > 60 μsec in SCA, whereas four of the seven stimulations (57%) used pulse width > 60 μsec in ET; three of the nine patients (33%) used frequency > 130 Hz in SCA, whereas all seven ET patients were optimized at 130 Hz; eight of nine stimulations (89%) used voltage > 3.0 V in SCA, whereas three of seven stimulations (43%) used voltage > 3.0 V in ET.
Table 2.
DBS stimulation setting for the best improvement
| No. of cathodal contacts | Pulse width | Frequency | Voltage | ||
|---|---|---|---|---|---|
| Monopolar stim | Bipolar stim | ||||
| SCA | 2: 1 contact | 2: 1 contact | 1: 60 μsec | 6: ≤130 Hz | 1: <3.0 V |
| (9 leads) | 3: 2 contacts | 2: 2 contacts | 8: 60 μsec ≤ | 3: 130 Hz < | 8: 3.0 V ≤ |
| ET | 1: 1 contact | 4: 1 contact | 3: 60 μsec | 7: ≤130 Hz | 4: <3.0 V |
| (7 leads) | 2: 2 contacts | 4: 60 μsec | |||
Vim neuronal discharges
We analyzed 68 Vim neurons in five SCA patients and 60 Vim neurons in five ET patients under awake, resting conditions (Fig. 4). These total include neurons with positive sensory responses, neurons with no sensory response, and those in which we did not test for sensory responses. Example raster plots representing Vim neuronal discharges and their corresponding autocorrelations are presented in Figure 5A. Overall, the mean discharge rate was slightly lower in SCA (16.2 ± 13.1 spikes/sec) than in ET (19.0 ± 13.0 spikes/sec), but the difference was not significant (P = 0.46) (Fig. 5B). We also calculated the mean discharge rates separately for those cells with a positive sensory response and those without a sensory response (Fig. 5B). The mean discharge rate was 19.3 spikes/sec for those with a sensory response and 17.4 spikes/sec for those without a sensory response in SCA, and 20.1 spikes/sec and 17.1 spikes/sec, respectively, in ET, no statistical difference was found (P = 0.44 for positive response neurons and P = 0.43 for negative response neurons). The intraburst discharge rates were not different between groups (109.2 ± 77.7 Hz in SCA and 133.0 ± 118.5 Hz in ET, P = 0.53) (Fig. 5C). The ratios of the burst period to the entire recording period were not different between groups (21.6 ± 14.8% in SCA and 25.3 ± 15.2% in ET, P = 0.44) (Fig. 5D). The burst frequencies were not different between groups (48.4 ± 49.6 bursts/min in SCA and 69.7 ± 89.2 bursts/min in ET, P = 0.48) (Fig. 5E). The percentage of cells with oscillatory discharges showed no significant difference across all frequency bands (Fig. 5F). There was, however, a significant difference (P = 0.0001) in the ratios of neurons that were responsive to passive manipulation in SCA (22%) vs. ET (71%) (Fig. 6A). The plots of the ratios of neurons responsive to passive manipulations for individual patients showed that the ratios for four of the five ET patients were higher than those for each of the SCA patients, and the difference was significant (P = 0.028) (Fig. 6B).
Figure 4.
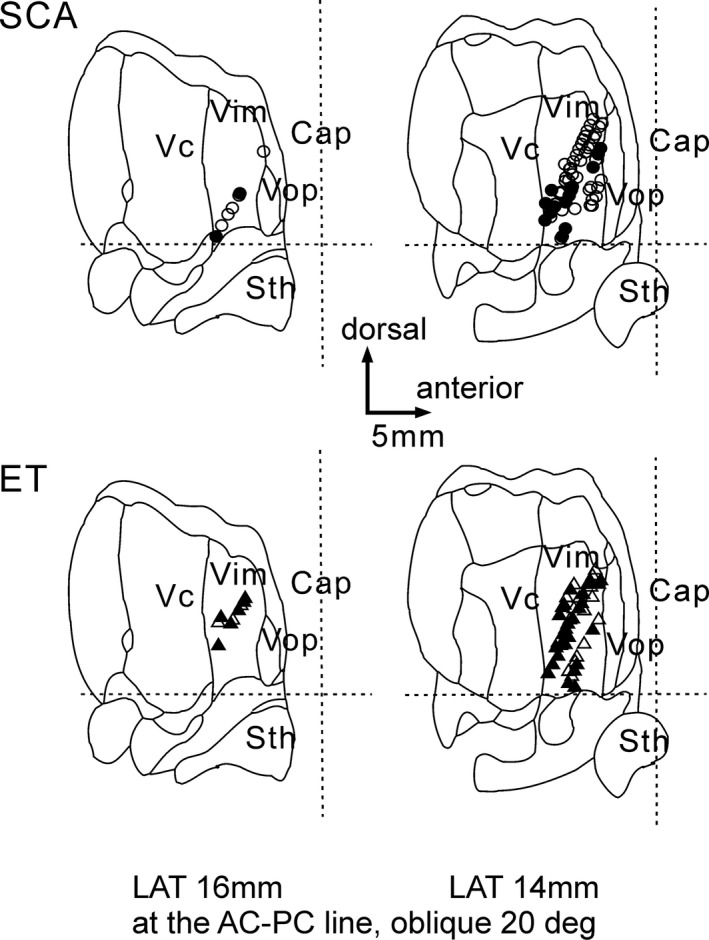
Location of neuronal recording. The positive sensory response was shown by filled circles and triangles. The recording areas were apparently no difference between SCA and ET. The horizontal broken lines are the AC‐PC lines, and the vertical broken lines are the mid AC‐PC lines. Cap, capsule; Sth, subthalamic nucleus; Vc, nucleus ventrocaudalis; Vim, nucleus ventrointermedius; Vop, nucleus ventrooralis posterior.
Figure 5.
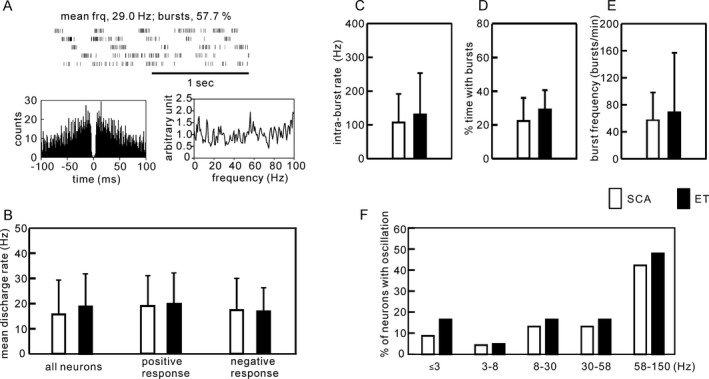
Comparison of the patterns of Vim neuronal discharges. Sixty‐eight Vim neurons in five SCA patients and 60 Vim neurons in five ET patients recorded under resting condition were analyzed. (A) An example of Vim neuronal discharges and the corresponding autocorrelogram and PSD. (B) The mean discharge rate of all neurons identified in Vim, neurons with positive sensory responses and neurons with negative sensory responses were not significantly different between SCA and ET. (C,D,E) The features of the bursting were not significantly different between groups. (F) The PSD analysis failed to reveal significant between‐group differences in any of the frequency bands analyzed. Bars represent means and SDs.
Figure 6.
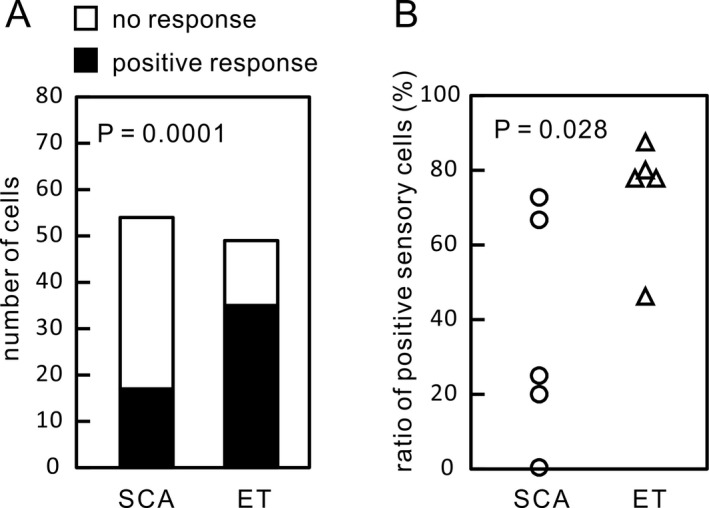
Comparison of sensory responses. (A) The ratio of neurons responsive to passive manipulations for all neurons analyzed was significantly lower in SCA than in ET. (B) The ratios of individual patients also show a significant difference between ET and SCA, irrespective of a small number of the patients.
Discussion
Effects of Vim DBS on Tremor Associated with SCA and ET
Vim DBS dramatically improved tremor in both SCA and ET, with the degree of the improvement in tremor severity and functional disability similar for both disorders. Although SCA patients were functionally disabled by both tremor and ataxia, DBS‐related abolishment of tremor alone led to a significant improvement in ADLs in the SCA patients as well as in ET patients. Thus, while Vim DBS may not significantly impact other cerebellar signs in SCA patients, for those with tremor it can yield marked benefit in functional disability. Historically, stereotactic surgery targeting the Vim, the cerebellar receiving zone of the thalamus, can significantly improve and in many cases abolish tremor in patients with ET.23, 24 This observation has provided compelling evidence in support of a role for the cerebellum in the pathophysiology of ET.25, 26 Even though the precise mechanism of tremor generation in SCA remains unclear, Vim DBS likely abolishes tremor in SCA by blocking tremor‐related oscillatory activity at the level of the Vim in the cerebello‐thalamo‐cortical pathway, similar to that in ET.
Our analysis of the clinically‐optimal stimulation parameters of Vim DBS in this study revealed that stronger stimulation was necessary to attain the best outcome in SCA as compared to ET. One potential explanation for this is the more proximal involvement and lower frequency of tremor in SCA. It has been reported that a larger lesion in Vim is required to alleviate tremor that is of a lower frequency and involves proximal body parts.27 One reason for this may be found in the somatotopic organization in Vim, which is similar in humans28 and nonhuman primates;29 the leg is represented in the most rostral and lateral regions, the orofacial structures in the most caudal and medial locations, and the fingers, forearm and proximal arm are located in between from medial to lateral and from caudal to rostral directions. Therefore, amelioration of tremor in SCA, which involves both the distal and proximal arm would need to include a larger area of the Vim as compared with that of tremor in ET, which may involve relatively more distal portions of the arm. Another potential explanation may lie in the filtering effects of DBS. DBS directly excites presynaptic terminals, excites the efferent axons and excites or inhibits neurons at downstream target sites through the synapses depending on the neurotransmitters.30, 31 Consequently, DBS at the stimulation site entrains neuronal discharge and abolishes oscillatory activity of tremor which is much slower than the stimulation frequency.30, 31 Furthermore, changes in the level of neurotransmitter in the synapse induced by DBS may modulate signal transmission in a frequency‐dependent fashion.32 Thus, “stronger” DBS may be necessary for alleviating tremor of relatively lower frequency depending on the filtering properties of the circuit affected by DBS.
Effects of vim DBS on ataxia
Neurological examination revealed that Vim DBS did not improve ataxia in our patients with SCA. Improvement of ataxia following Vim DBS has been reported in fragile X‐associated tremor/ataxia syndrome,33 however, the discrepancy may be ascribed to differences between the causal disorders, the method applied for assessing ataxia, or the small number of subjects. Dissociation of Vim surgery effects on tremor and ataxia in this study suggests that the neuronal group responsible for tremor mediation is predominantly restricted to the Vim, whereas sites for responsible for mediating ataxic motor signs may be located in wider areas within and outside of the thalamus. Fine assessment of ataxia is necessary to address this issue, but distinguishing between improvements of movements by improving ataxia versus improvement of tremor is difficult based on the clinical rating scales, which are not designed to address these differences. Whether Vim DBS improves ataxia or not will need further evaluation in SCA patients without tremor.
Although our assessment was based on clinical examination without quantitative evaluation, neither Vim DBS nor Vim lesions appeared to improve or worsen ataxia. Failure to induce ataxia after blocking the flow of information from the cerebellum to the cerebral cortex is difficult to explain if one hypothesizes that this circuitry plays an intimate role in cerebellar function. It has been reported that Vim thalamotomy can be accompanied by ataxia, but it is often mild and transient.34 DBS of Vim or the subthalamic area has also been reported to induce ataxia in some cases of ET.35 18F‐fluorodeoxyglucose positron emission tomography performed with and without active neurostimulation found metabolic changes induced by DBS that were consistent with antidromic activation of cerebello‐thalamic fibers,35 suggesting that disruption in activity in the cerebello‐thalamo‐cortical tract during Vim DBS lead to the development of ataxia. In patients with SCA during Vim DBS the ataxia did not get worse in our study. One potential explanation for this is that the remaining portions of the cerebello‐thalamo‐cortical tract may compensate for the region affected by lesions or stimulation. Another possible explanation is that other sites in the cerebellum or brainstem could also compensate particularly in patients with SCA where disruption in the cerebello‐thalamic pathway is chronic in nature, allowing more time for compensation to occur in other brain regions.
Difference in neuronal discharge patterns
The Vim is the apparent homolog of the nucleus ventralis posterior lateralis, pars oralis (VPLo) in primates.36 The VPLo is known to receive inputs through cerebellar, spinothalamic, and corticothalamic projections,37, 38 and it also receives disynaptic inhibition from the motor cortex via the thalamic reticular nucleus and thalamic interneurons.39 Neuronal discharge patterns in the Vim are thought to result from integration of these synaptic inputs. Previous studies revealed that pathological changes are confined to the cerebellar cortex in late cortical cerebellar atrophy,40 SCA6,41 and SCA31.42 Our SCA patients were genetically heterogenous but symptomatically homogenous. Based on these pathological findings and associated symptomatology, our SCA patients were considered free of degeneration in the spinal cord, cerebral cortex, and basal ganglia, therefore we hypothesize that changes in Vim neuronal discharge in our SCA patients mainly reflect changes in the activities of Purkinje cells and the output of the deep cerebellar nuclei.
A previous study using slice preparations of rat cerebellum showed that blockade of calcium currents, which was associated behaviorally with signs of cerebellar ataxia, was associated with high‐frequency bursting of deep cerebellar nuclei neurons.43 Another study using computational models of the deep cerebellar nuclei suggested that inhibition of small‐conductance calcium‐activated potassium channel, which could cause cerebellar ataxia in rats, resulted in an increased discharge rate and irregular activity in deep cerebellar neurons.44 However, the present results showed that the discharge rates, bursting, and oscillatory activity in Vim in SCA were not different from those in ET, suggesting that ataxia may occur independent of changes in rate, burst, or oscillatory activity in Vim. The present results are in contrast to the disrupting patterns of background neural activity underlying akinesia/bradykinesia of Parkinson's disease.45, 46 Coherent oscillation underlies movement‐related activities within the motor system including the basal ganglia and cerebellar systems in the normal condition.47 A number of studies have revealed that excessive synchrony of oscillations in the beta band, 8–30 Hz, and underactivity of oscillation in the gamma band, 60–90 Hz, within the basal ganglia system may underlie akinesia in Parkinson's disease.48 Excessive beta band oscillations are hypothesized to disrupt motor preparation, leading to delayed reaction times and akinesia/bradykinesia.48 Contrary to the findings in PD, the present results suggest that imbalance of neuronal oscillation across the alpha, beta, and gamma spectrums does not underlie cerebellar ataxia.
The sole difference among the discharge patterns analyzed in this study was the decreased incidence of sensory responses of Vim neurons in SCA compared to ET. As the surgical approach and recording area were not different between SCA and ET, this difference cannot simply be attributed to different parts of the thalamus being explored. The sensory information on coordination is conveyed to the cerebellum through the direct and indirect spinocerebellar tracts and may have a differential effect on movement and posture.49 The spinocerebellar tracts are also involved in providing sensory feedback during movement.13 Purkinje cells receive afferent fibers originating from the brainstem and spinal cord11, 43 and project to the deep cerebellar nuclei and receive excitatory collateral inputs from mossy and climbing fibers.43 Dysfunction of the cerebellar neuron network by degeneration of Purkinje cells and their efferent systems may lead to the decreased sensory responses of the deep cerebellar nuclei and Vim. The cerebellum is argued to use both feedback and feedforward control systems. Feedback control transfers sensory information not only into corrective adjustment of ongoing movement, but also into adjustment of motor output from the cerebral cortex.13, 50 The present results suggest that sensory information through the cerebellar motor control system is disrupted, and failure in integration of sensory signals with modulatory motor control may be a critical component underlying the development of ataxia in SCA. As the patient number was limited in our study, future studies in human subjects or animals will be expected to confirm the present results.
Implication for treatment of ataxia
The present results demonstrate the possibility that recuperation from reduced somatosensory responsiveness in ataxia could be used in the treatment of patients with ataxia. Enforcement of sensory stimuli at peripheral sites through rehabilitation maneuvers may lead to restoration of sensory information and improvement in ataxia. Enforcement of sensory information through the cerebello‐thalamo‐cortical projection may be another possible strategy, which could be attained through peripheral or central stimulation or via medical intervention that would enhance sensory activity or sensory processing either in the cerebellum or via cerebello‐thalamo‐cortical projections.
Conclusions
DBS or lesions of the Vim improved tremor in SCA to a similar degree as ET and gave rise to significant functional improvement, in spite of the fact that there was no improvement in ataxia in SCA. Amelioration of tremor in SCAs tended to require higher voltage during DBS and could be due to the greater volumes of tissue that needed to be affected in patients with more proximal tremor. Analysis of Vim neuronal discharges revealed that discharge rate, bursts, and oscillations were not different between SCA and ET, but the ratio of cells that respond to sensory manipulation is significantly smaller in SCAs than in ET. The results on the Vim discharge patterns suggest that one potential mechanism underlying ataxia in SCAs may be disruption of the cerebello‐thalamo‐cortical sensory feedback control system.
Author Contributions
T.H., K.B.B., and J.L.V. were responsible for study concept, study design, and drafting the manuscript. T.H., A.M., K.Y., T.G., and T.Y. were responsible for acquisition and analysis of data.
Conflict of Interest
J.L.V. has received research funding from the NIH, Boston Scientific, Medtronic, and Abbott. He is also a consultant for Boston Scientific, Medtronic, InSightec, and Abbott and a scientific advisor for Surgical Information Systems. All other authors declare no conflict of interest.
Acknowledgments
The authors thank Masao Kudo for substantial assistance with intraoperative neuronal recording.
Funding Statement
This work was funded by Research Committee of the Ataxia grant ; Research on Policy Planning and Evaluation for Rare and Intractable Diseases grant ; Health and Labour Sciences Research Grants grant ; The Ministry of Health, Labour and Welfare, Japan grant .
References
- 1. Holmes G. The cerebellum of man. The Huglings Jackson memorial lecture. Brain 1939;62:1–30. [Google Scholar]
- 2. Diener HC, Dichgans J. Pathophysiology of cerebellar ataxia. Mov Disord 1992;7:95–109. [DOI] [PubMed] [Google Scholar]
- 3. Nagaseki Y, Shibazaki T, Hirai T, et al. Long‐term follow‐up results of selective VIM‐thalamotomy. J Neurosurg 1986;65:296–302. [DOI] [PubMed] [Google Scholar]
- 4. Schuuman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Eng J Med 2000;342:461–468. [DOI] [PubMed] [Google Scholar]
- 5. Benito‐León J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol 2006;2:666–678. [DOI] [PubMed] [Google Scholar]
- 6. Louis ED. Linking essential tremor to the cerebellum: neuropathological evidence. Cerebellum 2016;15:235–242. [DOI] [PubMed] [Google Scholar]
- 7. Pirker W, Back C, Gerschlager W, et al. Chronic thalamic stimulation in a patient with spinocerebellar ataxia 2. Mov Disord 2003;18:221–225. [DOI] [PubMed] [Google Scholar]
- 8. Shimojima Y, Hashimoto T, Kaneko K, et al. Thalamic Stimulation for Disabling Tremor in a Patient with spinocerebellar degeneration. Stereotact Funct Neurosurg 2005;83:131–134. [DOI] [PubMed] [Google Scholar]
- 9. Freund HJ, Barnikol UB, Nolte D, et al. Subthalamic‐thalamic DBS in a case with spinocerebellar ataxia type 2 and severe tremor ‐ a unusual clinical benefit. Mov Disord 2007;22:732–735. [DOI] [PubMed] [Google Scholar]
- 10. Oyama G, Thompson A, Foote KD, et al. Deep brain stimulation for tremor associated with underlying ataxia syndromes: a case series and discussion of issues. Tremor Other Hyperkinet Mov 2014;. https://doi.org/10.7916/D8542KQ5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ito M. The cerebellum. Brain for an implicit self. New Jersey: FT Press, 2012. [Google Scholar]
- 12. Jörntell H. Jan 1. Cerebellar physiology: links between microcircuitry properties and sensorimotor functions. J Physiol 2017;. https://doi.org/10.1113/JP272769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spann A, Jörntell H. Processing of multi‐dimensional sensorimotor information in the spinal and cerebellar neuronal circuitry: a new hypothesis. PLoS Comput Biol 2013;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R. Encoding of action by the Purkinje cells of the cerebellum. Nature 2015;526:439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sudhakar SK, Torben‐Nielsen B, Schullet ED. Cerebellar nuclear neurons use time and rate coding to transmit Purkinje neuron pauses. PLoS Comput Biol 2015;. https://doi.org/10.1371/journal.pcbi.1004641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armstrong BYDM, Edgley SA. Discharge of nucleus interpositus neurons during locomotion in the cat. J Physiol 1984;351:411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Person AL, Raman IM. Purkinje neuron synchrony elicits time‐locked spiking in the cerebellar nuclei. Nature 2012;481:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fahn S, Tolosa E, Marín C. Clinical rating scale for tremor In: J Jankovic, E Tolosa. eds. Parkinson's disease and movement disorders. pp. 225–234. Baltimore‐Munich: Urban & Schwarzenberg, 1988. [Google Scholar]
- 19. Shaltenbrand G, Bailey P. Introduction to stereotaxis with an atlas of the human brain. Stuttgart: Thieme, 1959. [Google Scholar]
- 20. Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 1985;53:926–939. [DOI] [PubMed] [Google Scholar]
- 21. Welch PD. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust 1967;15:70–73. [Google Scholar]
- 22. Rivlin‐Etzion M, Ritov Y, Heimer G, Bergman H, et al. Local shuffling of spike trains boosts the accuracy of spike train spectral analysis. J Neurophysiol 2006;95:3245–3256. [DOI] [PubMed] [Google Scholar]
- 23. Ohye C, Hirai T, Miyazaki M, et al. Vim thalamotomy for the treatment of various kinds of tremor. Appl Neurophysiol 1982;45:275–280. [DOI] [PubMed] [Google Scholar]
- 24. Benabid AL, Pollak P, Gervason C, et al. Long‐term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991;337:403–406. [DOI] [PubMed] [Google Scholar]
- 25. Deuschl G, Raethjen J, Lindemann M, Krack P. The pathophysiology of tremor. Muscle Nerve 2001;24:716–735. [DOI] [PubMed] [Google Scholar]
- 26. Filip P, Lungu OV, Manto MU, Bareš M. Linking essential tremor to the cerebellum: physiological evidence. Cerebellum 2016;15:774–780. [DOI] [PubMed] [Google Scholar]
- 27. Ceresa A, Quattrone A. Linking essential tremor to the cerebellum‐neuroimaging evidence. Cerebellum 2016;15:263–275. [DOI] [PubMed] [Google Scholar]
- 28. Hirai T, Miyazaki M, Nakajima H, et al. The correlation between tremor characteristics and the predicted volume of effective lesions in stereotactic nucleus ventralis intermedius thalamotomy. Brain 1983;106:1001–1018. [DOI] [PubMed] [Google Scholar]
- 29. Lenz FA, Dostrovsky JO, Tasker RR, et al. Single‐unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol 1988;59:299–316. [DOI] [PubMed] [Google Scholar]
- 30. Vitek JL, Ashe J, DeLong MR, Alexander GE. Physiologic properties and somatotopic organization of the motor thalamus. J Neurophysiol 1994;71:1498–1513. [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto T, Elder CM, Okun MS, et al. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 2003;23:1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ito HT, Schuman EM. Frequency‐dependent signal transmission and modulation by neuromodulators. Front Neurosci 2008;. https://doi.org/10.3389/neuro.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weiss D, Mielke C, Wächter T, et al. Long‐term outcome of deep brain stimulation in fragile X‐associated tremor/ataxia syndrome. Parkinson Relat Disord 2015;21:310–313. [DOI] [PubMed] [Google Scholar]
- 34. Benabid AL, Pollak P, Gao D, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg 1996;84:203–214. [DOI] [PubMed] [Google Scholar]
- 35. Reich MM, Brumberg J, Pozzi NG, et al. Progressive gait ataxia following deep brain stimulation for essential tremor: adverse effect or lack of efficacy? Brain 2016;139:2948–2956. [DOI] [PubMed] [Google Scholar]
- 36. Hirai T, Hones EG. A new percellation of the human thalamus on the basis histochemical staining. Brain Res Rev 1989;14:1–34. [DOI] [PubMed] [Google Scholar]
- 37. Asanuma C, Thach WT, Jones EG. Anatomical evidence for segregated focal groupings of efferent cells and their terminal ramifications in the cerebellothalamic pathway of the monkey. Brain Res Rev 1983;5:267–297. [DOI] [PubMed] [Google Scholar]
- 38. Ando N, Izawa Y, Shinoda Y. Relative contributions of thalamic reticular nucleus neurons and intrinsic interneurons to inhibition of thalamic neurons projecting to the motor cortex. J Physiol 1995;73:2470–2485. [DOI] [PubMed] [Google Scholar]
- 39. Tsuchiya K, Ozawa E, Saito F, et al. Neuropathology of late cortical cerebellar atrophy in Japan: distribution of cerebellar change on an autopsy case and review of Japanese cases. Eur Neurol 1994;34:253–262. [DOI] [PubMed] [Google Scholar]
- 40. Tsuchiya K, Ishikawa K, Watabiki S, et al. A clinical, genetic, neuropathological study in a Japanese family with SCA6 and a review of Japanese autopsy cases of autosomal dominant cortical cerebellar atrophy. J Neurol Sci 1998;160:54–59. [DOI] [PubMed] [Google Scholar]
- 41. Owada K, Ishikawa K, Toru S, et al. A clinical, genetic, and neuropathologic study in a family with 16q‐linked ADCA type III. Neurology 2005;65:629–632. [DOI] [PubMed] [Google Scholar]
- 42. Alviña K, Khodakhah K. Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by N‐type calcium channels in juvenile rats. J Physiol 2008;586:2523–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abbasi S, Abbasi A, Sarbaz Y, Shahabi P. Contribution of somatic dendritic SK channels in the firing rate of deep cerebellar nuclei: implication in cerebellar ataxia. Basic Clin Neurosci 2016;7:57–61. [PMC free article] [PubMed] [Google Scholar]
- 44. Brown P, Oliviero A, Mazzone P, et al. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci 2001;21:1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kühn AA, Tsui A, Aziz T, et al. Pathological synchronization in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol 2009;215:380–387. [DOI] [PubMed] [Google Scholar]
- 46. Marsden JF, Ashby P, Limousin‐Dowsey P, et al. Coherence between cerebellar thalamus, cortex and muscle in man. Brain 2000;123:1459–1470. [DOI] [PubMed] [Google Scholar]
- 47. Williams D, Tijssen M, van Bruggen G, , et al. Dopamine‐dependent changes in the functional connectivity between basal ganglia and cerebral cortex in human. Brain 2002;125:1558–1569. [DOI] [PubMed] [Google Scholar]
- 48. Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol 2005;116:2510–2519. [DOI] [PubMed] [Google Scholar]
- 49. Jiang J, Azim E, Ekorot CF, Alstermark B. Direct and indirect spino‐cerebellar pathways: shared ideas but different functions in motor control. Front Comp Neurosci 2015;9:75 https://doi.org/10.3389/fncom.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trend Cogn Sci 2000;1:423–431. [DOI] [PubMed] [Google Scholar]


