Abstract
Objective
ATP‐binding‐cassette transporter A7(ABCA7) is suggested to be involved in lipid transport as well as in phagocytosis of amyloid‐β in the brain. We tested the hypothesis that a common genetic variant in ABCA7 is associated with dementia, ischemic heart disease, ischemic cerebrovascular disease, and with lipid levels in the general population, independent of the common apolipoprotein E(APOE) genotype.
Methods
For this purpose, we genotyped a common genetic variant in ABCA7, identified in genome‐wide‐association‐studies of Alzheimer's disease, in 104,258 individuals from the Danish general population, and also meta‐analyzed our results with publicly available consortia data.
Results
Multifactorially adjusted hazard ratios for Alzheimer's disease were 1.07 (95% confidence interval:0.93–1.23) and 1.72 (1.24–2.40) for GA and AA versus GG genotype. Results were similar after APOE genotype adjustment and when only APOE ɛ33 carriers were studied. Including 178,304 individuals, the meta‐analyzed odds ratio for Alzheimer's disease per one allele ABCA7 rs4147929 increase was 1.15 (1.12–1.18). ABCA7 genotype was not convincingly associated with vascular dementia, ischemic heart disease, ischemic cerebrovascular disease, or with lipid levels. Including 288,563 individuals, meta‐analyzed odds ratios for ischemic heart disease per one allele ABCA7 rs4147929 increase was 1.01 (0.99–1.03).
Interpretation
A common genetic variant in ABCA7 was associated with high risk of Alzheimer's disease independent of APOE genotype. The lack of association with vascular dementia, ischemic heart disease, ischemic cerebrovascular disease, and with lipid levels suggests that ABCA7 is not important for atherosclerosis. Thus, our findings support the suggested role of ABCA7 in Alzheimer's disease pathology and phagocytic clearance of amyloid‐β in the brain.
Introduction
Forty‐seven million people are currently living with Alzheimer's disease or other types of dementia, a number that is estimated to increase globally to 131 million in 2050.1 Recent demographic studies have shown a decrease in incidences in developed countries most likely due to improved treatment and prevention of vascular risk factors.2, 3, 4 Genome‐wide association studies (GWAS) have revealed novel molecular pathways, among these the ATP‐binding‐cassette transporter A7 (ABCA7),5, 6 which may be involved in both transmembrane lipid transport and phagocytosis of amyloid‐β in the brain.7, 8, 9, 10, 11, 12, 13, 14 Following these GWAS findings, rare loss‐of‐function variants in ABCA7 were reported to confer risk of Alzheimer's disease in case–control studies in Icelandic and Belgian populations.15, 16 Whether common genetic variation in ABCA7 confers risk of dementia, ischemic heart‐ and cerebrovascular disease in the general population, and whether potential associations are independent of the strong Alzheimer's disease apolipoprotein E (APOE) ɛ4 risk allele remains to be determined.
Alzheimer's disease is believed to be driven by the production and deposition of neurotoxic sticky amyloid‐β peptides which aggregate into senile plaques in the brain.17, 18, 19, 20 ABCA7 is highly expressed in brain tissue,9, 21, 22 most abundant in microglia and oligodendrocytes.7, 23 Previous studies comparing ABCA7 knock‐out mice with wild‐type mice or J20 amyloidogenic mice demonstrate that ABCA7 is involved in clearance of amyloid‐β in the brain through phagocytosis.12, 24 Furthermore, early studies on ABCA7 explored lipid efflux capacities and suggested that ABCA7 contributes to phospholipid and cholesterol transport.8, 9, 10, 21, 25, 26, 27 Since ABCA7 may be implicated in both Alzheimer's disease pathology and lipid metabolism, studying the impact of common genetic variation in ABCA7 for risk of Alzheimer's disease and other types of dementia, lipid metabolism, ischemic heart disease and ischemic cerebrovascular disease is of interest.
We tested the hypothesis that a common genetic variant in ABCA7 is associated with dementia, ischemic heart disease, ischemic cerebrovascular disease and with lipid levels in the general population, independent of the common APOE genotype. For this purpose, we genotyped the rs4147929 genetic variant in ABCA7, previously identified as a GWAS top hit for Alzheimer's disease,5 as well as APOE genotypes (rs7412 and rs429358) in a prospective study of the general population totaling 104,258 individuals. Finally, in meta‐analyses we included consortia data from the International Genomics of Alzheimer's Projects (IGAP) including 74,046 individuals and the CARDIoGRAMplusC4D including 184,305 individuals.
Subjects and Methods
Studies were approved by institutional review boards and Danish ethical committees, and conducted according to the Declaration of Helsinki. Written informed consent was obtained from all participants. All participants were white and of Danish descent. There was no overlap of individuals between studies.
Participants
Our study included participants from two similar prospective studies of the Danish general population in Denmark, the Copenhagen General Population Study (CGPS) and the Copenhagen City Heart Study (CCHS). Combining these two studies yielded a total of 104,258 participants.
The CGPS was initiated in 2003, and enrollment is on‐going.28, 29, 30 Participants were selected with the use of the Danish Civil Registration System to reflect the adult Danish population aged 20 to 100 + years. Data were obtained from a self‐administered questionnaire reviewed together with an investigator on the day of attendance, a physical examination, and from blood samples including DNA extraction. We included 94,214 consecutive participants in the current analyses. During follow‐up (which ended in November 2014), 1145 were diagnosed with dementia, of whom 613 had Alzheimer's disease, 9039 were diagnosed with ischemic heart disease, and 5,975 were diagnosed with cerebrovascular disease.
CCHS baseline examinations were performed between 1976 and 1978 with follow‐up examinations in 1981–1983, 1991–1994, and 2001–2003. Participants were recruited and examined as in the CGPS. DNA was available from the 1991–1994 or 2001–2003 examinations. We included 10,044 consecutive participants in the current analysis. During follow‐up (which ended in November 2014), 915 were diagnosed with dementia, of whom 349 had Alzheimer's disease, 2345 were diagnosed with ischemic heart disease, and 1363 were diagnosed with cerebrovascular disease.
Consortia data
International Genomics of Alzheimer's Project
IGAP is a large two‐stage study based upon GWAS on individuals of European ancestry.5 In stage 1, IGAP used genotyped and imputed data on 7,055,881 SNPs to meta‐analyze four previously published GWAS datasets consisting of 17,008 Alzheimer's disease cases and 37,154 controls (The European Alzheimer's disease Initiative – EADI the Alzheimer Disease Genetics Consortium – ADGC The Cohorts for Heart and Aging Research in Genomic Epidemiology consortium – CHARGE The Genetic and Environmental Risk in AD consortium – GERAD). In stage 2, 11,632 SNPs were genotyped and tested for association in an independent set of 8,572 Alzheimer's disease cases and 11,312 controls. Finally, a meta‐analysis was performed combining results from stages 1 and 2.
Coronary ARtery DIsease Genome wide Replication and Meta‐analysis plus The Coronary Artery Disease (C4D) Genetics and 1000‐Genomes‐based GWAS
CARDIoGRAMplusC4D 1000 Genomes‐based GWAS is a meta‐analysis of GWAS studies mainly of European descent using the 1000 Genomes phase 1 v3 training set with 38 million variants for imputing. The study interrogated 9.4 million variants and involved 60,801 ischemic heart disease cases and 123,504 controls from 48 studies.31
Clinical endpoints
Information on births, deaths, emigrations, and immigrations was collected from the national Danish Civil Registration System. Information on diagnoses of dementia (International Classifications of Diseases (ICD) 8 290; and ICD10 F00, F01, F03, G30), ischemic heart disease (ICD8 410‐414 and ICD10 I20‐I25) and cerebrovascular disease (ICD8 431‐438 and ICD10 I60‐I69, G45) was drawn from the national Danish Patient Registry and the national Danish Causes of Death Registry. The national Danish Patient Registry has information on all patient contacts with all clinical somatic and psychiatric hospital departments in Denmark since 1977, including emergency wards and outpatient clinics from 1995. The national Danish Causes of Death Registry contains data on the causes of all deaths in Denmark, as reported by hospitals and general practitioners.28, 29, 30
Alzheimer's disease was ICD8 code 290.10 and ICD10 codes F00 and G30. Vascular dementia was ICD10 F01 and unspecified dementia was ICD8 290.18 and ICD10 F03. All dementia included Alzheimer's disease, vascular dementia, and unspecified dementia. The quality of these registry based dementia diagnoses has previously been validated,32 and was further validated by the well‐known association between the APOE ε4 allele and Alzheimer's disease and all dementia in the present cohorts.30
Ischemic heart disease (ICD8 code 410‐414 and ICD10 code I20‐I25) was defined as myocardial infarction or characteristic symptoms of stable angina pectoris.33 The quality of the myocardial infarction diagnosis has previously been validated.34
For cerebrovascular disease, validation of ICD codes has been described previously.35 In brief, information on diagnosis of cerebrovascular disease (ICD8 code 431–438 and ICD10 code I60‐I69, G45), including ischemic cerebrovascular disease (transitory ischemic attacks, amaurosis fugax, and ischemic stroke) and hemorrhagic stroke was validated by trained physicians using the WHO definition of cerebrovascular disease.35
Follow‐up time began at the establishment of the national Danish Patient Registry (January 1st, 1977) or on the birthday of the participant, whichever came later. Follow‐up ended at occurrence of event, death (n = 9906), emigration (n = 486) or on November 10th 2014 (last update of registry), whichever came first. Mean follow‐up was 37.0 years (range = 0–38 years) for dementia, 36.2 years (range = 0–38 years) for ischemic heart disease and 36.6 years (range = 0–38 years) for cerebrovascular disease. Because of the complete Danish registries, no individuals were lost to follow‐up.
Genotyping
An ABI PRISM 7900HT Sequence Detection System (Applied Biosystems Inc, Foster City, CA, USA) and TaqMan‐based assays were used to genotype ABCA7 (rs4147929) and APOE (rs7412 and rs429358). Variation at rs7412 and rs429358 defines six common APOE genotypes (ɛ22, ɛ32, ɛ42, ɛ33, ɛ43, and ɛ44), as previously described.30 APOE genotype was available for 100,426 individuals.
Laboratory analyses
Plasma total cholesterol, high‐density lipoprotein (HDL) cholesterol, triglycerides, apolipoprotein B and AI were measured using standard hospital assays (Boehringer Mannheim GmbH, Mannheim, Germany; Konelab, Thermo Fischer Scientific, Waltheim, MA, USA). Low‐density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation36 when plasma triglycerides were ≤4 mmol/L (≤352 mg/dL), and otherwise measured directly (Konelab). ApoE was measured as previously described.30, 37
Other covariates
Body mass index was measured weight in kilograms divided by measured height in meters squared. Hypertension was a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, and/or use of antihypertensive medication. Diabetes mellitus was self‐reported disease, use of insulin or oral hypoglycemic agents, and/or non‐fasting plasma glucose levels of more than 11 mmol/L (>198 mg/dL). Smoking, alcohol consumption, physical activity, postmenopausal status and hormonal replacement therapy in women, use of lipid‐lowering therapy, as well as education, were all self‐reported and dichotomized. Smoking was current smoking. High alcohol consumption was >14/21 units of alcohol per week for women/men (1 unit alcohol~12 g). Physical inactivity was ≤4 h weekly of light physical exercise in leisure time. Lipid‐lowering therapy was mainly statins (yes/no). Low educational level was <8 years in school.
Statistical analysis
Stata/SE (Stata‐Corp, College Station, TX, USA) version 14.0 was used. Missing data on continuous covariates (0.21–1.16%) were imputed from age, sex, and the most related continuous parameters; however, results reported where similar without imputed data.
Cumulative incidences of Alzheimer's disease and all dementia were plotted against age and rs4147929 genotype, using the method of Fine‐Gray,38 to account for death and emigration as competing events. Cox proportional hazards regression models with age as time scale, left truncation (delayed entry), and censoring at death were used to estimate hazard ratios for Alzheimer's disease and all dementia as a function of rs4147929 genotype. Cox regression models were multifactorially adjusted for age (as time scale), sex, body mass index, hypertension, smoking, alcohol intake, physical inactivity, postmenopausal status, and hormonal replacement therapy in women, lipid‐lowering therapy and educational level. Furthermore adjustments were for APOE genotype or total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides. In sensitivity analyses we analyzed APOE ε33 genotype individuals separately.
Meta‐analyses were conducted using the user‐written metan command from Stata/S.E. v14.0 to estimate fixed and random effects odds ratios from regression coefficients and standard errors for the genetic variant.
Results
Baseline characteristics of the 104,627 study participants stratified by genotype are shown in Table 1. Risk factors were equally distributed among genotypes (Table 1). Rs4147929 genotype frequencies were 71.0%, 26.5%, and 2.5% for GG, GA, and AA, respectively, and did not deviate from the Hardy–Weinberg expectation (P = 0.93). All results are for the CGPS and CCHS combined unless otherwise stated.
Table 1.
Characteristics of study participants in the Copenhagen General Population Study and the Copenhagen City Heart Study combined as a function of ABCA7 rs4147929 genotype
| Characteristics | GG genotype | GA genotype | AA genotype |
|---|---|---|---|
| No. of individuals, % | 74,039 (71.0) | 27,656 (26.5) | 2,563 (2.5) |
| Age, years | 58 (48–67) | 58 (48–67) | 58 (47–67) |
| Female, % | 55 | 56 | 56 |
| Total cholesterol, mmol/L | 5.6 (4.9–6.3) | 5.6 (4.9–6.3) | 5.5 (4.8–6.3) |
| LDL cholesterol, mmol/L | 3.2 (2.6–3.9) | 3.2 (2.6–3.9) | 3.2 (2.6–3.8) |
| HDL cholesterol, mmol/L | 1.6 (1.2–1.9) | 1.6 (1.3–1.9) | 1.6 (1.3–1.9) |
| Triglycerides, mmol/L | 1.4 (1.0–2.0) | 1.4 (1.0–2.1) | 1.4 (0.9–2.0) |
| Body mass index, kg/m2 | 26 (23–28) | 26 (23–28) | 25 (23–28) |
| Hypertension, % | 59 | 59 | 58 |
| Diabetes mellitus, % | 4 | 4 | 3 |
| Smoking, % | 20 | 21 | 20 |
| High alcohol consumption, % | 17 | 17 | 17 |
| Physical inactivity, % | 50 | 50 | 49 |
| Post‐menopausal, %a | 67 | 67 | 67 |
| Hormonal replacement, %a | 16 | 17 | 17 |
| Lipid‐lowering therapy, % | 11 | 11 | 11 |
| Education <8 years, % | 12 | 12 | 12 |
HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Values are median (interquartile range) or percentage (%), and are from the day of enrollment (2003 and onward for the Copenhagen General Population Study, and from 1991 to 1994 or 2001 to 2003 for the Copenhagen City Heart Study). Hypertension was defined as use of antihypertensive medication, systolic blood pressure ≥140 mmHg, and/or diastolic blood pressure ≥90 mmHg. Diabetes mellitus was defined as self‐reported disease, use of insulin or oral hypoglycemic agents, and/or non‐fasting plasma glucose level >11 mmol/L (>198 mg/dL). Smoking was defined as current smoking. Alcohol consumption was defined as >14/21 units per week for women/men (1unit = 12 g alcohol, equivalent to 1 glass of wine or 1 beer [33 cl]). Physical inactivity was defined as ≤4 h per week of light physical activity in leisure time. Women reported menopausal status and use of hormonal replacement therapy. Lipid‐lowering therapy was primarily statins (yes/no), and low education was <8 years of education.
In women only.
ABCA7 rs4147929 and risk of Alzheimer's disease, vascular dementia, unspecified dementia, and all dementia
Cumulative incidences of Alzheimer's disease and all dementia as a function of age and ABCA7 rs4147929 genotype increased stepwise from GG to GA to AA (P = 0.02 and P = 0.07, respectively) (Fig. 1). Multifactorially adjusted hazard ratios for Alzheimer's disease were 1.07 (confidence interval 95% 0.93–1.23) for GA versus GG and 1.72 (1.24–2.40) for AA versus GG (Fig. 2). Results were similar after further adjustment for APOE genotype (middle panel), or when analyses were performed exclusively in APOE ε33 carriers (right panel). No associations between rs4147929 and vascular dementia, or unspecified dementia were observed.
Figure 1.
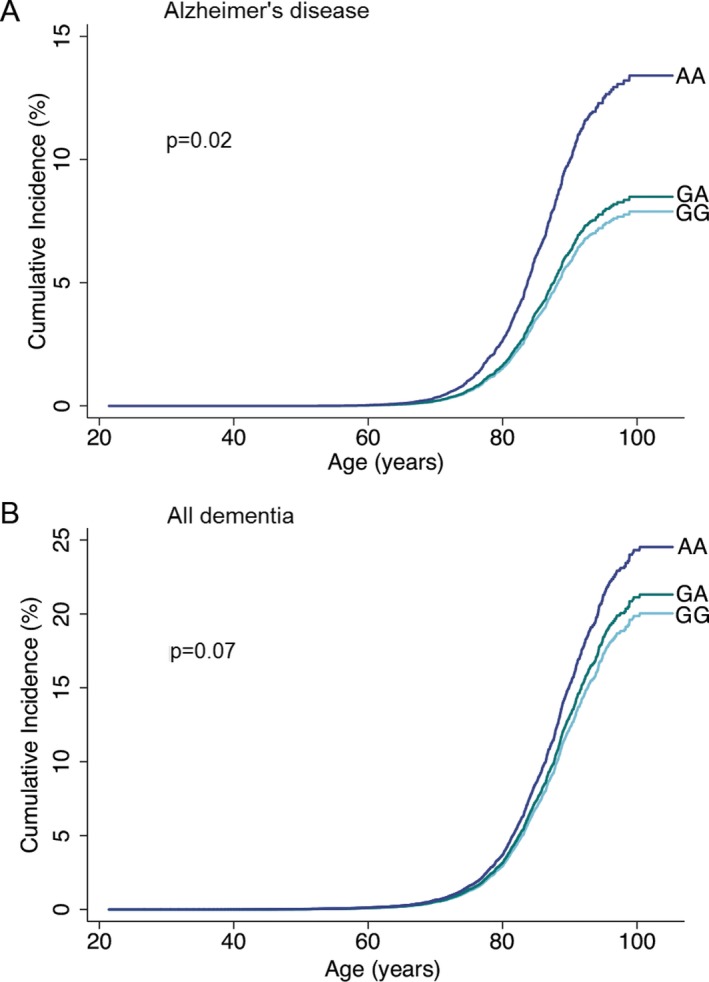
Cumulative incidences of Alzheimer's disease (A) and all dementia (B) as a function of age and ABCA7 rs4147929 genotype. Fine‐Gray models, allowing for death and emigration as competing events, were used. P‐values were calculated using the “stcrreg” command in Stata.
Figure 2.

Risk of Alzheimer's disease, vascular dementia, unspecified dementia and all dementia as a function of ABCA7 rs4147929 genotype. Analyses for Alzheimer's disease, vascular, unspecified, and all dementia included 104,258 individuals. Hazard ratios were multifactorially adjusted for age, sex, body mass index, hypertension, diabetes mellitus, smoking, alcohol consumption, physical inactivity, menopausal status and hormonal replacement therapy (only women), lipid‐lowering therapy, and education (left panel). Hazard ratios were further adjusted for APOE genotype (100,427 participants) (middle panel). Analysis of individuals with APOE ε33 genotype included 55,925 participants (right panel). N=number; CI=confidence interval.
Meta‐analysis of ABCA7 rs4147929 and risk of Alzheimer's disease
Meta‐analysis included the two present cohorts of the general Danish population and stage 1 and 2 from IGAP, the latter including meta‐analyzed data from 15 individual studies.5 For ABCA7 rs4147929 including 178,624 individuals, fixed‐ and random‐effects meta‐analyzed odds ratios for Alzheimer's disease per one allele increase were 1.15 (1.12–1.18) and 1.15 (1.12–1.18) (I 2 = 0.0%; P for heterogeneity = 0.95) (Fig. 3).
Figure 3.
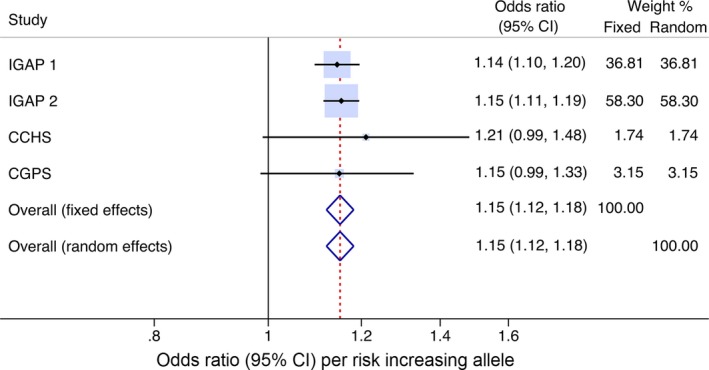
Meta‐analysis of ABCA7 rs4147929 and risk of Alzheimer's disease. Risk of Alzheimer's disease per risk increasing allele. Horizontal lines correspond to 95% confidence intervals by forest plots. Diamonds and broken vertical lines represent summary estimates. Confidence interval for the summary estimate corresponds to the width of the diamond. Squares correspond to the weight of the study in the meta‐analysis from the fixed effects model (left column). CI=confidence interval.
ABCA7 rs4147929 and risk of myocardial infarction, ischemic heart disease, ischemic stroke, ischemic cerebrovascular disease, hemorrhagic stroke, and cerebrovascular disease overall
Hazard ratios for ischemic heart disease were 1.01 (0.97–1.05) for rs4147929 GA versus GG genotype and 1.09 (0.97–1.23) for AA versus GG (Fig. 4). This association was similar after further adjustment for total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides (middle panel), or after adjustment for APOE genotype (right panel). A slightly stronger association was observed for myocardial infarction. ABCA7 rs4147929 genotype was not associated with ischemic cerebrovascular disease or other cerebrovascular disease subtypes.
Figure 4.
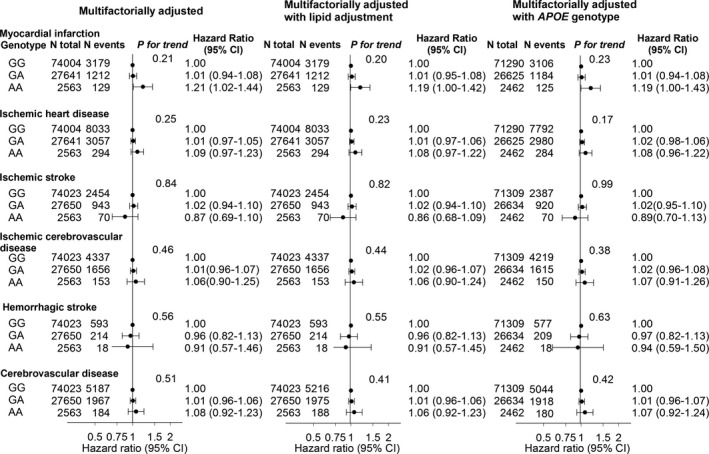
Risk of myocardial infarction, ischemic heart disease, ischemic stroke, ischemic cerebrovascular disease, hemorrhagic stroke, and all cerebrovascular disease as a function of ABCA7 rs4147929 genotype. Hazard ratios were multifactorially adjusted for age, sex, body mass index, hypertension, diabetes mellitus, smoking, alcohol consumption, physical inactivity, menopausal status and hormonal replacement therapy (only women), lipid‐lowering therapy, and education (left panel). Hazard ratios were further adjusted for lipid levels: total cholesterol, LDL and HDL cholesterol, and triglycerides (middle panel). Number of participants varied slightly (from 100,377 to 104,208) according to availability of data.
Meta‐analysis of ABCA7 rs4147929 and risk of ischemic heart disease
Meta‐analysis included the two present cohorts of the general Danish population and CARDIoGRAMplusC4D consortia data, the latter including data from 48 individual studies.39 For ABCA7 rs4147929 including 288,563 individuals, fixed and random‐effects meta‐analyzed odds ratios for ischemic heart disease per one allele increase were 1.01 (0.99–1.03) and 1.01 (0.99–1.03) (I 2 = 0.0%; P for heterogeneity = 0.42) (Fig. 5).
Figure 5.

Meta‐analysis of ABCA7 rs4147929 and risk of ischemic heart disease. Risk of ischemic heart disease per risk increasing allele. Horizontal lines correspond to 95% confidence intervals by forest plots. Diamonds and broken vertical lines represent summary estimates. Confidence interval for the summary estimate corresponds to the width of the diamond. Squares correspond to the weight of the study in the meta‐analysis from the fixed effects model (left column). CI=confidence interval.
Lipid, lipoprotein, and apolipoprotein levels as a function of ABCA7 rs4147929 genotype
The rs4147929 genotype was associated with a stepwise marginally lower level of apolipoprotein B from GG to GA to AA (P = 0.04) (Fig. 6). No other associations between rs4147929 genotype and lipid, lipoprotein and apolipoprotein levels were observed.
Figure 6.

Lipid, lipoprotein, and apolipoprotein levels as a function of ABCA7 rs4147929 genotype. Values are geometric mean±SEM for apolipoprotein E and triglyceride levels and otherwise mean±SEM. P‐values by Cuzick's test for trend. To convert cholesterol to mg/dL, divide by 0.0259; to convert triglycerides to mg/dL, divide by 0.0113. Abbreviations: SEM=standard error of the mean; HDL=high‐density lipoprotein; LDL=low‐density lipoprotein; Apo=apolipoprotein.
Discussion
The principle findings of this study are that a common genetic variant in ABCA7 (rs4147929) is associated with increased risk of Alzheimer's disease, but not with atherosclerosis‐related diseases such as vascular dementia, ischemic heart disease, or ischemic cerebrovascular disease. Importantly, the association with Alzheimer's disease remained after adjustment for APOE genotype and when analyses were performed in APOE ɛ33 carriers alone. These novel findings were observed in 104,258 individuals from the general population.
To our knowledge, this is the first study to simultaneously assess risk of both dementia and atherosclerosis‐related diseases as a function of a common variant in ABCA7, and the first study to conclude that these associations are independent of APOE genotype. Common variation in ABCA7 was first reported to be associated with risk of Alzheimer's disease in a large genome‐wide association study.6 Subsequently rare loss‐of‐function mutations in ABCA7 were shown to be associated with Alzheimer's disease in Icelandic and Belgian populations.15, 16 Since Iceland has a modest, relatively isolated population, genetic variants are more likely to be passed down through generations than in larger mixed populations.40 Furthermore, analyses of rare mutations can be sensitive to bias because of population stratification and hidden relatedness.16 Therefore, such findings may not be applicable to other populations. This study suggests ABCA7 as a risk gene for Alzheimer's disease also in the general population.
The exact mechanism underlying our findings is unclear; however, experimental and human evidence suggests plausible explanations for the present data. The studies on loss‐of‐function mutations in ABCA7 suggest that loss of ABCA7 could be a potential pathogenic mechanism underlying Alzheimer's disease,15, 16 but also functional studies of a common missense variant in exon 33 (rs3752246, p.Gly1527Ala) support this.41 Rs3752246 was identified in a North American study,42 and is in high LD with the present rs4147929 variant (http://snipa.helmholtz-muenchen.de/snipa3/, r 2=0.97). The rs3752246 variant is located in a predicted post‐translational myristoylation site.41 Expression of human ABCA7 (hABCA7) carrying the rs3752246 risk allele led to increases in secreted amyloid‐β40 and amyloid‐β42 and β‐secretase activity in CHO‐ and HEK‐AβPPSwe cells, and molecular weight determination showed loss of post‐translational modification in the risk‐allele peptide spanning position 1527.41 Another common variant, rs3764650, was the first top significant hit reported in ABCA7.6 This variant is located in intron 13, and is not in strong LD with the present rs4147929 (http://snipa.helmholtz-muenchen.de/snipa3/, r 2 = 0.21), and therefore most likely does not contribute to the present findings.
Biologically, ABCA7 is important for microglial phagocytosis of amyloid‐β in the brain.11 Kim et al. found that ABCA7 knockout mice crossed with J20 amyloidogenic mice had double the amount of insoluble amyloid‐β concentration and plaque levels in the brain compared to J20 mice.24 The ability to phagocytose amyloid‐β‐oligomers in isolated microglia and macrophages was reduced in ABCA7 knockout mice compared to wild‐type mice.12 This study additionally showed that apoE concentrations in mice were not altered by ABCA7 loss, suggesting an apoE independent pathway for ABCA7. Finally, Sakae et al. reported that ABCA7 deficiency altered brain lipid profile and impaired memory in ABCA7 knock‐out mice.43 Since we do not find any association between ABCA7 and other types of dementia with vascular components, it is likely that the function of ABCA7 predominantly is through microglial amyloid‐β clearance within the brain.
ABCA7 function was initially reported to be related to cholesterol and phospholipid efflux, however, with conflicting results.10 Previous mice studies showed that ABCA7 was involved in apolipoprotein‐mediated phospholipid release, whereas results from studies on cholesterol release were less convincing.10 In our study, the risk of myocardial infarction was marginally increased for rs4147929 AA versus GG genotype, and for ischemic heart disease a similar trend was observed. For this reason, we tested the association between ischemic heart disease and ABCA7 rs4147929 genotype per risk increasing allele, in a meta‐analysis including data from the CARDIoGRAMplusC4D consortium. The meta‐analysis did not show any association between ABCA7 rs4147929 and risk of ischemic heart disease, in agreement with the lack of association between rs4147929 genotype and lipid and lipoprotein levels. This suggest that our findings for myocardial infarction represent a spurious observation; however, we naturally cannot totally exclude the possibility that our finding on myocardial infarction is true and may be due to other mechanisms than coronary artery atherosclerosis, such as small vessel disease of various pathological origin.
Strengths of this study are the large, well‐characterized, ethnically homogenous cohort of the general population with no individuals lost to follow‐up. Additional strengths are that risk of Alzheimer's disease per risk increasing allele in both CGPS and CCHS for ABCA7 rs4147929 were comparable to the estimates from IGAP stages 1 and 2, and that between study heterogeneity of the meta‐analysis estimate was 0.0%. Since the ABCA7 locus is on chromosome 19, it may be suspected that at least part of the ABCA7 association could be due to linkage disequilibrium with APOE. However, we now robustly show that the association between genetic variation in ABCA7 and risk of Alzheimer's disease is independent of the strong APOE ɛ4 allele. Furthermore, the quality of the Danish register‐ based dementia diagnosis has previously been validated.32 The core clinical criteria for Alzheimer's disease have been shown to provide very good diagnostic accuracy and utility in most patients,44 and the quality of Alzheimer's diagnoses was further validated by the presence of the well‐known association with the APOE ɛ4 allele in our two study cohorts.30 Additionally, characteristics of participants in the three genotype groups were similar, largely making confounding of the genetic variant unlikely, and thus supporting causality of the ABCA7 pathway. A potential limitation of our study may be that we only studied white individuals from an ethnically homogeneous population. For instance, a GWAS risk allele (rs115550680) in African Americans was prevalent in this ethnic group in contrast to non‐hispanic whites,45 and was in high LD with a 44‐base pair exonic deletion (rs142076058). This common ABCA7 deletion could represent an ethnic‐specific pathogenic alteration in Alzheimer's disease. The rs4147929 variant is, however, common in most ethnic groups (www.ensembl.org) and the present findings are therefore likely to be applicable to these ethnicities.
In summary, a common genetic variant in ABCA7 was associated with high risk of Alzheimer's disease independent of APOE genotype in the general population. The lack of association between the ABCA7 variant and vascular dementia, ischemic heart disease, ischemic cerebrovascular disease and lipid and lipoprotein levels suggests that ABCA7 is not likely to play a role in atherosclerosis. Our findings thus support the suggested role of ABCA7 in Alzheimer's disease pathology by phagocytic clearance of amyloid‐β in the brain.
Author Contributions
E.W.K, A.T.H, B.G.N, and R.F.S contributed to the conception and design of the study and to acquisition of data. Analysis of data, drafting the text and preparing the figures were performed by E.W.K and R.F.S.
Conflicts of Interest
Nothing to report.
Acknowledgments
We are indebted to staff and participants of the Copenhagen City Heart Study and the Copenhagen General Population Study for their important contributions to our study. We thank the International Genomics of Alzheimer's Project (IGAP) for providing summary results data for these analyses. The investigators within IGAP contributed to the design and implementation of IGAP and/or provided data but did not participate in analysis or writing of this report. Data for the meta‐analysis on ischemic heart disease have been contributed by CARDIoGRAMplusC4D investigators and have been downloaded from www.CARDIOGRAMPLUSC4D.ORG.
Funding Statement
This work was funded by Danish Council of Independent Research grant 6120‐0075B; Lundbeck Foundation grant ; Research Fund at the Capital Region of Denmark grant ; Danish Alzheimer Research Foundation grant ; M.L. Jørgensen & Gunnar Hansen's Fund grant .
References
- 1. World Health Organisation . 2015. The epidemiology and impact of dementia: current state and future trends. First WHO Minist. Conf. Glob. Action Against Dement. 1–4.
- 2. Matthews FE, Stephan BCM, Robinson L, et al. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun 2016;7:11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4. Satizabal CL, Beiser AS, Chouraki V, et al. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 2016;374:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lambert JC, Ibrahim‐Verbaas CA, Harold D, et al. Meta‐analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 2013;45:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet 2011;43:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaminski WE, Orsó E, Diederich W, et al. Identification of a novel human sterol‐sensitive ATP‐binding cassette transporter (ABCA7). Biochem Biophys Res Commun 2000;273:532–538. [DOI] [PubMed] [Google Scholar]
- 8. Abe‐Dohmae S, Ikeda Y, Matsuo M, et al. Human ABCA7 supports lipoprotein mediated release of cellular cholesterol and phospholipid to generate high density Lipoprotein. J Biol Chem 2004;279:604–611. [DOI] [PubMed] [Google Scholar]
- 9. Ikeda Y, Abe‐Dohmae S, Munehira Y, et al. Posttranscriptional regulation of human ABCA7 and its function for the apoA‐I‐dependent lipid release. Biochem Biophys Res Commun 2003;311:313–318. [DOI] [PubMed] [Google Scholar]
- 10. Abe‐Dohmae S, Ueda K, Yokoyama S. ABCA7, a molecule with unknown function. FEBS Lett 2006;580:1178–1182. [DOI] [PubMed] [Google Scholar]
- 11. Li H, Karl T, Garner B. Understanding the function of ABCA7 in Alzheimer's disease. Biochem Soc Trans 2015;43:920–923. [DOI] [PubMed] [Google Scholar]
- 12. Fu Y, Hsiao JHT, Paxinos G, et al. ABCA7 mediates phagocytic clearance of amyloid‐β in the brain. J. Alzheimer's Dis 2016;54:569–584. [DOI] [PubMed] [Google Scholar]
- 13. Jehle AW, Gardai SJ, Li S, et al. ATP‐binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol 2006;174:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iwamoto N, Abe‐Dohmae S, Sato R, Yokoyama S. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J Lipid Res 2006;47:1915–1927. [DOI] [PubMed] [Google Scholar]
- 15. Steinberg S, Stefansson H, Jonsson T, et al. Loss‐of‐function variants in ABCA7 confer risk of Alzheimer's disease. Nat Genet 2015;47:445–447. [DOI] [PubMed] [Google Scholar]
- 16. Cuyvers E, De Roeck A, Van den Bossche T, et al. Mutations in ABCA7 in a Belgian cohort of Alzheimer's disease patients: a targeted resequencing study. Lancet Neurol. 2015;14:814–822. [DOI] [PubMed] [Google Scholar]
- 17. Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci 1991;12:383–388. [DOI] [PubMed] [Google Scholar]
- 18. Hardy JA, Higgins GA. Alzheimer's disease : the amyloid cascade hypothesis. Science 1992;256:184–185. [DOI] [PubMed] [Google Scholar]
- 19. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297:353–356. [DOI] [PubMed] [Google Scholar]
- 20. Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: time, space and “wingmen”. Nat Neurosci 2015;18:800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim WS, Fitzgerald ML, Kang K, et al. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem 2005;280:3989–3995. [DOI] [PubMed] [Google Scholar]
- 22. Tachikawa M, Watanabe M, Hori S, et al. Distinct spatio‐temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J Neurochem 2005;95:294–304. [DOI] [PubMed] [Google Scholar]
- 23. Kim WS, Guillemin GJ, Glaros EN, et al. Quantitation of ATP‐binding cassette subfamily‐A transporter gene expression in primary human brain cells. NeuroReport 2006;17:891–896. [DOI] [PubMed] [Google Scholar]
- 24. Kim WS, Li H, Ruberu K, et al. Deletion of Abca7 increases cerebral amyloid‐β accumulation in the J20 mouse model of Alzheimer's disease. J Neurosci 2013;33:4387–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayashi M, Abe‐Dohmae S, Okazaki M, et al. Heterogeneity of high density lipoprotein generated by ABCA1 and ABCA7. J Lipid Res 2005;46:1703–1711. [DOI] [PubMed] [Google Scholar]
- 26. Wang N, Lan D, Gerbod‐Giannone M, et al. ATP‐binding cassette transporter A7 (ABCA7) binds apolipoprotein A‐I and mediates cellular phospholipid but not cholesterol efflux. J Biol Chem 2003;278:42906–42912. [DOI] [PubMed] [Google Scholar]
- 27. Quazi F, Molday RS. Differential phospholipid substrates and directional transport by ATP‐binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease‐causing mutants. J Biol Chem 2013;288:34414–34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frikke‐Schmidt R, Nordestgaard BG, Stene MCA, et al. Association of loss‐of‐function mutations in the ABCA1 gene with high‐density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008;299:2524–2532. [DOI] [PubMed] [Google Scholar]
- 29. Jørgensen AB, Frikke‐Schmidt R, Nordestgaard BG, Tybjærg‐Hansen A. Loss‐of‐function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;60:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Rasmussen KL, Tybjærg‐Hansen A, Nordestgaard BG, Frikke‐Schmidt R. Plasma levels of apolipoprotein E and risk of dementia in the general population. Ann Neurol 2015;77:301–311. [DOI] [PubMed] [Google Scholar]
- 31. Nikpay M, Goel A, Won H‐H, et al. A comprehensive 1000 Genomes–based genome‐wide association meta‐analysis of coronary artery disease. Nat Genet 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phung TKT, Andersen BB, Høgh P, et al. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord 2007;24:220–228. [DOI] [PubMed] [Google Scholar]
- 33. Fox K, Alonso GMA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary ‐ the task force on the management of stable angina pectoris of the European Society of Cardiology. Eur Heart J 2006;27:1341–1381. [DOI] [PubMed] [Google Scholar]
- 34. Kamstrup PR, Tybjærg‐Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a). JAMA 2009;301:2331–2339. [DOI] [PubMed] [Google Scholar]
- 35. Nordestgaard LT, Tybjærg‐Hansen A, Nordestgaard BG, Frikke‐Schmidt R. Loss‐of‐function mutation in ABCA1 and risk of Alzheimer's disease and cerebrovascular disease. Alzheimer's Dement 2015;11:1430–1438. [DOI] [PubMed] [Google Scholar]
- 36. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 37. Rasmussen KL, Tybjærg‐Hansen A, Nordestgaard BG, Frikke‐Schmidt R. Plasma levels of apolipoprotein E and risk of ischemic heart disease in the general population. Atherosclerosis 2016;246:63–70. [DOI] [PubMed] [Google Scholar]
- 38. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 39. Schunkert H, König IR, Kathiresan S, et al. Large‐scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Circ Cardiovasc Genet 2011;43:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Helgason A, Nicholson G, Donnelly P. A reassessment of genetic diversity in icelanders: strong evidence from multiple loci for relative homogeneity caused by genetic drift. Ann Hum Genet 2003;67:281–297. [DOI] [PubMed] [Google Scholar]
- 41. Bamji‐Mirza M, Li Y, Najem D, et al. Genetic variations in ABCA7 can increase secreted levels of amyloid‐β40 and amyloid‐β42 peptides and ABCA7 transcription in cell culture models. J Alzheimers Dis 2016;53:875–892. [DOI] [PubMed] [Google Scholar]
- 42. Naj AC, Gyungah J, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late‐onset Alzheimers's disease. Nat Genet 2011;43:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sakae N, Liu C‐C, Shinohara M, et al. ABCA7 deficiency accelerates amyloid‐β generation and Alzheimer's neuronal pathology. J Neurosci 2016;36:3848–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKhann G, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐ Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cukier HN, Kunkle BW, Vardarajan BN, et al. ABCA7 frameshift deletion associated with Alzheimer disease in African Americans. Neurol Genet 2016;2:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]


