Abstract
Purpose:Differentiated thyroid cancer (DTC) accounts for approximately 95% of thyroid carcinomas. In the metastatic RAI-refractory disease, chemotherapy has very limited efficacy and is associated with substantial toxicity. With increasing knowledge of the molecular pathogenesis of DTC, novel targeted therapies have been developed. Lenvatinib is a tyrosine kinase inhibitor (TKI) with promising clinical activity based on the randomized phase III SELECT trial. In Switzerland, a Named Patient Program (NPP) was installed to bridge the time gap to Swissmedic approval. Here, we report the results from the Swiss Lenvatinib NPP including patients with metastatic RAI-refractory DTC.
Methods:Main inclusion criteria for the Swiss NPP were RAI-refractory DTC, documented disease progression, Eastern Cooperative Oncology Group (ECOG) performance status 0-3. The number of previous therapies was not limited. The Swiss Lenvatinib NPP was initiated in June 2014 and was closed in October 2015 with the approval of the drug.
Results:Between June 2014 and October 2015, 13 patients with a median age of 72 years have been enrolled. Most patients (69%) had at least one prior systemic therapy, mainly sorafenib. 31% of patients showed a PR and 31% SD. Median progression free survival was 7.2 months and the median overall survival was 22.7 months. Dose reduction due to adverse events was necessary in 7 patients (53%). At the time of analysis 6 patients (47%) were still on treatment with a median time on treatment of 9.98 months.
Conclusions:Our results show that lenvatinib has reasonable clinical activity in unselected patients with RAI-refractory thyroid cancer with nearly two-third of patients showing clinical benefit. The toxicity profile of lenvatinib is manageable.
Keywords: differentiated thyroid cancer, radioiodine refractory, tyrosine kinase inhibitors, lenvatinib, sorafenib.
Introduction
Differentiated thyroid cancer (DTC) accounts for approximately 95% of thyroid carcinomas and arises from aberrant follicular cells. It is histologically classified as either papillary, follicular (including Hurthle cell), or poorly differentiated. DTC is the most common endocrine malignancy, and its incidence from 3,6 per 100'000 in 1973 to 8,7 per 1000'000 in 2002 in the United States 1. Although most patients with this disease have an excellent prognosis, some suffer from an aggressive, refractory form. Generally, localized DTC is effectively treated by surgery, radioactive iodine (RAI), and suppressive thyroid hormone therapy 2. Up to one quarter of patients with DTC develop distant metastases and two third of patients with distant metastases will eventually become RAI-refractory 3, 4. Until recently the only systemic therapy approved by the American Food and Drug Administration (FDA) for these patients was doxorubicin 5. However, doxorubicin alone or in combination with other cytotoxic agents such as cisplatin has very limited efficacy and is associated with substantial toxicity including cardiac and hematologic adverse events. With increasing knowledge of the molecular pathogenesis of DTC, novel targeted therapies have been developed. Clinical trials investigated tyrosine kinase inhibitors (TKIs) interacting with the main signalling cascades important in the pathogenesis of DTC. Sorafenib is an orally active inhibitor of VEGF receptor - 3 and Raf kinases. In the randomized phase III DECISION trial a total of 417 patients with locally advanced or metastatic RAI-refractory DTC and disease progression within the past 14 months were randomly assigned to receive 400 mg of oral sorafenib twice daily or matching placebo6. Patients receiving placebo were allowed to receive open-label sorafenib upon disease progression. Sorafenib extended the median progression-free survival (PFS) from 5.8 to 10.8 months (Hazard ratio (HR) 0.59, 95% confidence interval (CI) 0.45-0.76, p<0.0001). Overall survival (OS) was statistically not different most likely due to the high rate (71,4%) of cross-over from placebo to sorafenib at disease progression. Based on these data, sorafenib is licenced for treatment of RAI-refractory DTC in many countries worldwide.
Lenvatinib is another TKI targeting VEGFR1-3, FGFR1-4, PDGFR-b, RET, and c-KIT. Phase II trials in advanced thyroid cancer documented response rates of up to 50% and provided the rationale for a subsequent randomized, placebo-controlled phase III trial 7, 8. The phase III randomized, double-blind, multicentre SELECT trial included 392 patients with RAI-refractory progressive DTC 9. Patients were randomized in a 2:1 fashion lenvatinib (24 mg daily) or matching placebo. The median PFS was 18.3 months with lenvatinib vs. 3.6 month in the placebo group (HR 0.21, 99% CI 0.14-0.31, p<0.001). The PFS benefit was statistically significant among all subgroups including patients previously treated with another TKI what was the case for 66 (25.3%) patients in the lenvatinib arm and 27 (20.6%) patients in the placebo arm. The clinical benefit was independent of distinct histological (i.e. papillary, poorly differentiated, follicular, and Hurthle cell) and molecular subtype (BRAF and RAS mutational status). In Switzerland, a Named Patient Program (NPP) was initiated to provide lenvatinib upon physician request on compassionate basis for eligible patients.
At this time, sorafenib was already approved and reimbursed in Switzerland. The question of sequencing both TKIs and their use in daily clinical practice outside clinical trials has yet not been investigated. Here, we report the results from the Swiss Lenvatinib NPP for patients with metastatic RAI-refractory DTC.
Patients and Methods
Main inclusion criteria for the Swiss NPP were RAI-refractory DTC, documented disease progression, Eastern Cooperative Oncology Group (ECOG) performance status 0-2. The number of previous therapies was not limited. Lenvatinib was provided by Eisai Pharma Switzerland. All patients received at least one dose. Therapy was initiated with a dose of 24 mg in all patients. Dose reduction upon toxicity assessment was at the discretion of the local investigator. Treatment was supposed to be continued until disease progression, clinical deterioration and/or unacceptable toxicities or until death. The Swiss Lenvatinib NPP was initiated in June 2014 and was closed in October 2015 with the approval of lenvatinib in Switzerland.
Patient- and tumor-specific data were obtained from the patients' medical records. Imaging studies were performed at the discretion of the treating physician. Patients were followed for disease progression and survival until December 2015.
Overall response rate (ORR) was defined according to Response Evaluation Criteria In Solid Tumors (RECIST) criteria version 1.110. If available we also report thyroglobulin levels before and under therapy. PFS was calculated from beginning of therapy to the first date of confirmed tumor progression, death or the date of last follow-up. Progression was defined as clinical and/or radiographic progression. OS was calculated from beginning of lenvatinib therapy of metastatic RAI-refractory disease to patients' death or date of last follow-up.
Objectives
The overall objective of this retrospective analysis was to evaluate the outcome of patients with metastatic DTC undergoing treatment with lenvatinib. The primary objective was to assess overall survival in patients with RAI-refractory DTC included in the Swiss Lenvatinib NPP. Secondary objectives included response rate, PFS and toxicity.
Ethical considerations
The respective local ethics committees approved implementation of the NPP. This retrospective data collection was approved by the ethics committee North-Western and Central Switzerland (EKNZ; 2015-364) as well as by the respective local ethics committees of participating centers.
Statistical methods
Numerical data are described with median, mean, standard deviation, quartiles and percentages as appropriate. PFS and OS were evaluated using Kaplan-Meier estimates with 95% confidence intervals (CI). PFS was defined as duration between the beginning of lenvatinib therapy until disease progression or death. OS was calculated from beginning of lenvatinib therapy of metastatic RAI-refractory disease to patients' death or date of last follow-up. All statistical analyses were performed with IBM SPSS Statistics software version 22 (IBM Corporation, New York, USA) and Excel Microsoft.
Results
Patients
Between June 2014 and October 2015, 13 patients have been enrolled in 8 different hospitals in Switzerland. From all these patient records were accessible and all 13 patients were considered for efficacy analysis. Patient- and tumor-specific characteristics are summarized in Table 1. Median age at the time of diagnosis was 61 years (Interquartile range (IQR) ±20.7) and at the time of inclusion in the NPP median age was 72 years (IQR ±16.8). All patients have previously been undergone by thyroidectomy and 11 patients (85%) had received postoperative radio-iodine therapy. At inclusion in the NPP, 12 patients (92%) had lung metastases and 9 patients (69%) had bone metastases. Median duration between RAI-refractory status and start of lenvatinib was 48 months.
Table 1.
Baseline Characteristics
| All patients (N=13) | |
|---|---|
| Age at time of analysis (years) -Median (Range) | 72 (37-81) |
| Age at Diagnosis (years) -Median (Range) | 61 (30-77) |
| Duration from RAI-refractory disease up to the beginning of a therapy in (months) - Median (Range) | 48 (2-96) |
| Sites of Metastasis Median Number (Range) | 1 (0-4) |
| Lung | 12 |
| Bone | 9 |
| Lymph nodes | 4 |
| Soft tissue | 2 |
| Liver | 2 |
| Brain | 2 |
| Heart | 1 |
| Previous systemic treatment before Lenvatinib Median Number (Range) | 1 (0-4) |
| Sorafenib | 8 |
| Pazopanib | 1 |
| Vandetanib | 1 |
| Chemotherapy (Cisplatin/Doxorubicin or TCF) | 2 |
| None | 4 |
Previous therapy
Most patients had one prior systemic therapy. 8 patients (62%) were previously treated with sorafenib. 2 patients (15%) had a previous chemotherapy with docetaxel, cisplatin, 5-fluorouracil (TCF) and one patient (8%) was treated with cisplatin and doxorubicin. One patient previously treated with chemotherapy and sorafenib also received pazopanib and vandetanib. Another patient was pretreated with sorafenib and pazopanib. For 4 patients (23%) lenvatinib was the first systemic therapy.
Efficacy
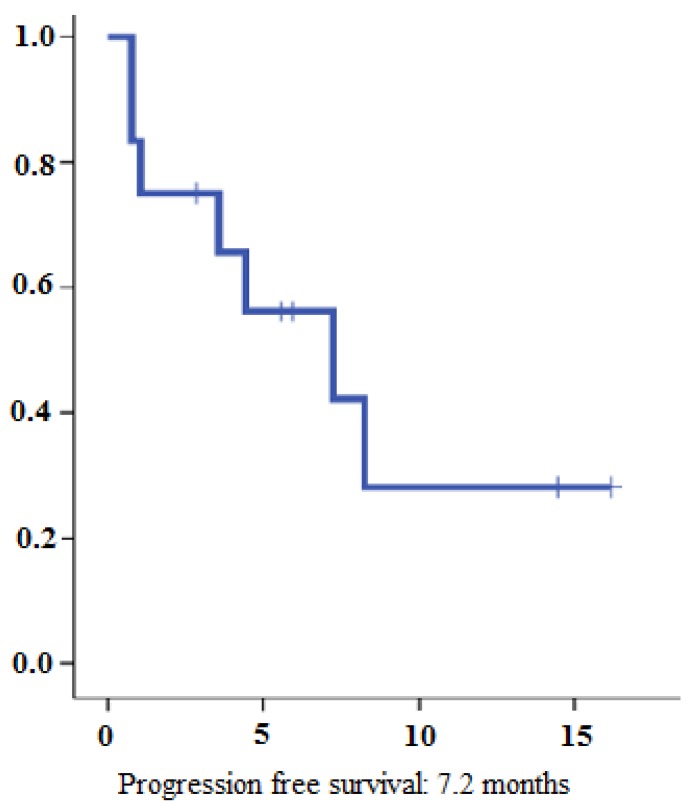
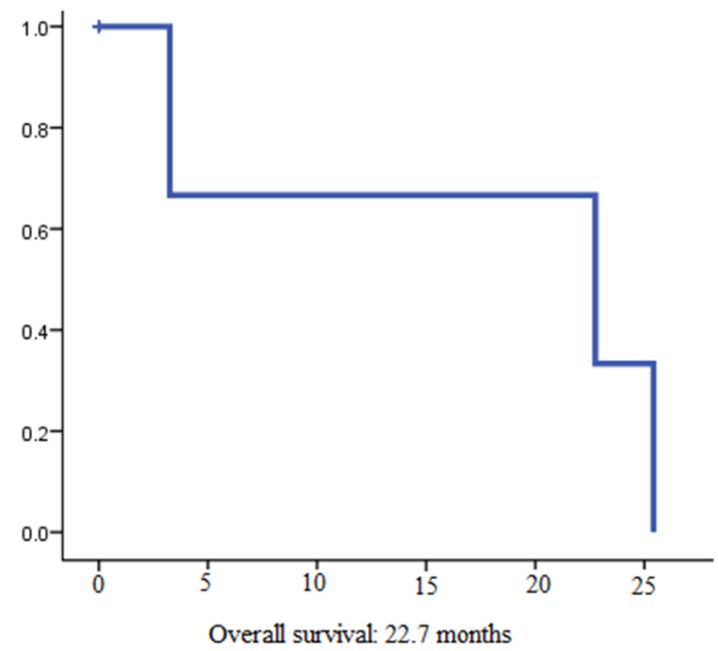
4 patients (31%) reached partial response (PR) and 4 additional patients (31%) stable disease (SD) (Table 2). In summary, disease control rate (DCR) was 62%. Out of 8 patients with disease control under lenvatinib therapy 4 had documented bone metastases at the time of treatment initiation. At the time of analysis, 7 patients (53%) had stopped treatment with lenvatinib. Two patients (15%) died due to disease progression and 3 patients (23%) stopped therapy due to drug toxicity. For two patients, the reason for treatment discontinuation was unclear. So far, the longest treatment duration was 18 months and is still receiving lenvatinib at a dose of 14 mg per day. Overall, the estimated median PFS was 7.2 months (95% CI, 0.78-13.67) (Figure 1). The estimated median OS was 22.7 months (95% CI 0-53.9) (Figure 2).
Table 2.
Clinical Outcome
| All patients (N=13) | |
|---|---|
| Best response on lenvatinib (N) | |
| Complete response | 0 |
| Partial response | 4 |
| Stable disease | 4 |
| Progressive disease | 1 |
| not evaluable | 4 |
| Duration of lenvatinib treatment (months) - median (range) | 5 (1-18+) |
| Ongoing (N) | 6 |
| Terminated (N) | 7 |
Figure 1.
Progression-free survival for lenvatinib therapy
Figure 2.
Overall survival from beginning of lenvatinib therapy
Thyroglobulin levels
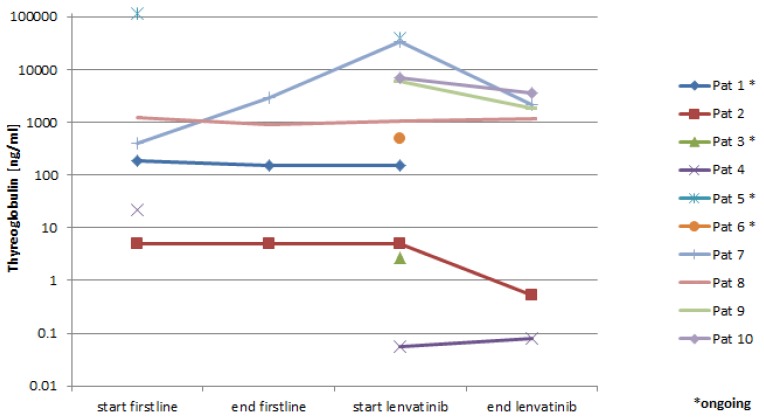
6 patients had serial determination of serum thyroglobulin levels. Five patients (83%) had a response and showed a decrease in thyroglobulin levels correlating with the radiologic response (Figure 3).
Figure 3.
Thyroglobulin levels in the serum.
Toxicity
In general, side effects were very common, 12 of 13 patients had at least one adverse event (AE). 7 patients (54%) had a grade 3 or 4 (G3/4) AEs. The most common AEs were fatigue (46%), diarrhoea (30%) and stomatitis (30%) (Table 3). The most common severe (G3/4) adverse events were fatigue, hypertension, acute pancreatitis, neutropenia, mucositis/stomatitis, and diarrhoea. There were no unexpected AEs when compared to the published literature. Generally, side effects were manageable; 7 patients (53%) had a dose reduction in the course of lenvatinib therapy (6 patients to 14 mg per day and one to 10 mg per day). All patients started with the recommended dose of 24 mg daily. Lenvatinib dose was reduced after a median treatment time of 162 days. 3 patients had to discontinue due to toxicity. Reasons for discontinuation in these 3 patients were proteinuria, oral ulcers, and acute pancreatitis.
Table 3.
Toxicity
| Total number of patients (N=13) | |||
|---|---|---|---|
| All grade, N | Grade 1-2, N | Grade 3-4, N | |
| Any AE | 12 | 3 | 9 |
| Diarrhea | 4 | 2 | 2 |
| Constipation | 3 | 2 | 1 |
| Fatigue | 6 | 4 | 2 |
| Stomatitis/Mucositis | 4 | 3 | 1 |
| Dyspnea | 1 | 1 | 0 |
| Dysphonia | 1 | 1 | 0 |
| Exanthema | 2 | 2 | 0 |
| Acute Pancreatitis | 1 | 0 | 1 |
| Hypertension | 2 | 1 | 1 |
| Anorexia | 3 | 2 | 1 |
| Nausea | 1 | 1 | 0 |
| Hand-Foot-Syndrome | 1 | 1 | 0 |
| Proteinuria | 1 | 0 | 1 |
| Haematotoxicity | 1 | 0 | 1 |
| Angina pectoris | 1 | 1 | 0 |
Discussion
In this retrospective analysis of the Swiss Lenvatinib NPP, we were able to collect comprehensive clinical data including sequential thyroglobulin levels from 13 unselected patients included in this program. Compared to the pivotal SELECT study 9 of lenvatinib in RAI-refractory DTC with a response rate of 64.8% and a median PFS time of 18.3 months, our results are clearly inferior. However, our patients had a more advanced disease setting compared to those in the SELECT trial. In the Swiss Lenvatinib NPP 69% of patients had bone metastases, a known negative prognostic factor. This rate was only 40% in the SELECT trial. In the SELECT trial only 25% of patients were previously treated with another systemic therapy for RAI-refractory disease. In our retrospective analysis patients had received up to 4 previous treatment lines including TKIs and chemotherapy. Most importantly, 62% of our patients had previous therapy with sorafenib. Nevertheless, 62% of patients treated with lenvatinib showed a clinical benefit and 31% had a documented response. The median PFS of 7.2 months is substantial for this advanced and heavily pretreated patient population.
Absolute thyroglobulin levels have been mainly studied as a prognostic factor for recurrence and survival in DTC 11, 12. The role of monitoring of thyroglobulin in patients with advanced DTC during treatment with anti-angiogenic drugs is not well established. In the DECISION trial changes in thyroglobulin concentrations correspond to radiographic response 6. Despite the small number of patients with consecutive thyroglobulin concentration measurement in our study, we also see a correlation to radiographic response supporting the hypothesis that serum thyroglobulin determination can be used on an individual basis to monitor treatment with TKIs.
More than 50% of patients needed a dose reduction, but could be treated over long time with a good toxicity management. One patient had an 18 months lenvatinib exposition and is still ongoing, although at a reduced dosage.
In a recent meta-analysis the relationship between toxicities and clinical benefits of lenvatinib and sorafenib in patients with DTC has been analysed 11. The toxicity rate was similar to our analysis. However, adverse events usually seem manageable and lenvatinib was associated with clinical benefit when considering adverse events. Another meta-analysis focussing on AEs of lenvatinib reported in the 14 currently published clinical trials reported involved a total number of 978 patients predominantly with thyroid cancer and renal cell carcinoma 12. The most frequent grade ≥3 treatment-related adverse events were thrombocytopenia, hypertension, peripheral edema and increased aspartate aminotransferase levels.
Current guidelines from the American Thyroid Association list lenvatinib as one possible systemic treatment option for RAI-refractory DTC 2. The knowledge of its toxicity profile should prompt considerable restraint in the use of kinase inhibitors (e.g. lenvatinib) in patients with stable or slowly progressive disease who likely have a disease-specific mortality that may also be low over the following years. Interestingly, the median time delay from initial diagnosis of DTC until inclusion in the Swiss Lenvatinib NPP was 11 years and the median time for diagnosis of RAI-refractory disease to start of lenvatinib was 4 years. This finding confirms the generally slow growing of the disease. Therefore, one of the most important questions in the treatment of RAI-refractory DTC is which patients are in need of a systemic therapy. These patients often survive for a long time, and in many cases, their quality of life is very good. For this reason, only patients with an advanced and progressive disease should undergo a treatment with targeted therapies. According to expert opinion 13, good candidates are patients with a RAI-refractory metastatic thyroid cancer with radiographic measurable lesions that have been in progression over the previous 12-14 months, as defined by RECIST criteria.
Our study has several limitations. The retrospective design, the small sample size and the broad heterogeneity of the patient population are the most relevant factors. However, this is to our best knowledge the first report on real life experience with lenvatinib in patients with RAI-refractory DTC patients after the report of the randomized phase III SELECT trial 9. Therefore, we think that these data are of relevance with regard to the feasibility and the efficacy of lenvatinib in DTC patients. Furthermore, we show that the toxicity profile of this drug is generally manageable.
In conclusion, lenvatinib has clinically meaningful benefits in patients with RAI-refractory DTC. It is important to closely follow-up patients during therapy, to establish comprehensive toxicity management guidelines and to consider dose reduction in patients with higher-grade toxicities. Patients should be instructed to report any side effects as early as possible to allow a prompt management. Providing good information and good communication with doctors and/or dedicated nurses is the best way to help patients during this therapeutic program 14.
Acknowledgments
We thank Eisai for the support of this analysis with an unrestricted grant.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE. et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Freedman S, Brennan MF. et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg. 2003;197:191–7. doi: 10.1016/S1072-7515(03)00332-6. [DOI] [PubMed] [Google Scholar]
- 4.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP. et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–9. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb JA, Hill CS. Chemotherapy of thyroid cancer with adriamycin. Experience with 30 patients. N Engl J Med. 1974;290:193–7. doi: 10.1056/NEJM197401242900404. [DOI] [PubMed] [Google Scholar]
- 6.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L. et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet (London, England) 2014;384:319–28. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlumberger M, Jarzab B, Cabanillas ME, Robinson B, Pacini F, Ball DW. et al. A Phase II Trial of the Multitargeted Tyrosine Kinase Inhibitor Lenvatinib (E7080) in Advanced Medullary Thyroid Cancer. Clin Cancer Res. 2016;22:44–53. doi: 10.1158/1078-0432.CCR-15-1127. [DOI] [PubMed] [Google Scholar]
- 8.Cabanillas ME, Schlumberger M, Jarzab B, Martins RG, Pacini F, Robinson B. et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: A clinical outcomes and biomarker assessment. Cancer. 2015;121:2749–56. doi: 10.1002/cncr.29395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R. et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Malandrino P, Latina A, Marescalco S, Spadaro A, Regalbuto C, Fulco RA. et al. Risk-Adapted Management of Differentiated Thyroid Cancer Assessed by a Sensitive Measurement of Basal Serum Thyroglobulin. J Clin Endocrinol Metab. 2011;96:1703–1709. doi: 10.1210/jc.2010-2695. [DOI] [PubMed] [Google Scholar]
- 12.Brassard M, Borget I, Edet-Sanson A, Giraudet A-L, Mundler O, Toubeau M. et al. Long-Term Follow-Up of Patients with Papillary and Follicular Thyroid Cancer: A Prospective Study on 715 Patients. J Clin Endocrinol Metab. 2011;96:1352–1359. doi: 10.1210/jc.2010-2708. [DOI] [PubMed] [Google Scholar]
- 13.Ye X, Zhu Y, Cai J. Relationship between toxicities and clinical benefits of newly approved tyrosine kinase inhibitors in thyroid cancer: A meta-analysis of literature. J Cancer Res Ther. 2015;11:185. doi: 10.4103/0973-1482.168182. [DOI] [PubMed] [Google Scholar]
- 14.Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K. et al. Safety and efficacy profile of lenvatinib in cancer therapy: a systematic review and meta-analysis. Oncotarget. 2016;7:44545–44557. doi: 10.18632/oncotarget.10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F. et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2:356–358. doi: 10.1016/S2213-8587(13)70215-8. [DOI] [PubMed] [Google Scholar]
- 16.Worden F, Fassnacht M, Shi Y, Hadjieva T, Bonichon F, Gao M. et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr Relat Cancer. 2015;22:877–87. doi: 10.1530/ERC-15-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]