Abstract
The conventional egg-grown influenza vaccines are trivalent. To test the feasibility of using multivalent influenza virus-like particles (VLPs) as an alternative influenza vaccine, we developed cell-derived influenza VLPs containing the hemagglutinin (HA) of the H1 subtype virus A/PR/8/34 or the H3 subtype virus A/Aichi/2/68. Mice immunized intramuscularly with bivalent influenza VLPs containing H1 and H3 HAs induced neutralizing activities against the homologous and closely related H1N1 strains A/PR/8/34 and A/WSN/33 as well as the H3N2 strains A/Aichi/2/68 and A/Hong Kong/68, but not the A/Philippines/2/82 strain isolated 14 years later. HA sequence and structure analysis indicated that antigenic distance could be a major factor in predicting cross-protection by VLP vaccines. The bivalent influenza VLP vaccine demonstrated advantages in broadening the protective immunity after lethal challenge infections when compared to a monovalent influenza VLP vaccine. High levels of the inflammatory cytokine IL-6 were observed in naïve or unprotected immunized mice but not in protected mice upon lethal challenge. These results indicate that multivalent influenza VLP vaccines can be an effective strategy for developing safe and alternative vaccine to control the spread of influenza viruses.
Keywords: Virus like particles, vaccine, influenza virus, H1N1, H3N2, protection
1. Introduction
Influenza virus is a causative agent for respiratory infection associated with widespread human diseases. Influenza viruses present in birds continue to be a source for a diverse combination of antigenic subtypes including 16 hemagglutinin (HA) and 9 neuraminidase (NA) and represent a large reservoir of novel antigens to which the human population is naïve [1, 2]. Chemically inactivated whole influenza A and B virus vaccines, or detergent-split virus vaccines have been extensively used in humans. The embryonated chicken egg is the major substrate for the preparation of these vaccines. Between pandemics, the HA and NA surface antigens of circulating viruses undergo progressive amino acid substitutions that can result in the evasion of previously acquired immunity. Therefore, influenza vaccines need to be updated annually. Vaccine strains are selected based on epidemiological and antigenic considerations of circulating human strains and their anticipated prevalence during the coming year. To obtain high yield vaccine viruses, the chosen strains are adapted to grow in embryonated eggs, or reassortant viruses are generated containing the glycoprotein (HA, NA) genes of current strains and genes for the internal proteins of A/PR/8/34 (H1N1) virus which confer high growth capacity in eggs [3].
The current egg-based system for influenza vaccine manufacture has drawbacks that include recent problems in vaccine supply in response to the influenza season, local or systemic allergic reactions to egg-derived vaccine components, and short duration of immune responses. Also, there are potential problems for growing pathogenic avian influenza virus in embryonated eggs because they sometimes kill the embryos to hamper virus production, and there are associated human safety concerns. In addition, diseases that affect chicken flocks due to an avian influenza virus outbreak could easily disrupt the supply of eggs for vaccine manufacturing. These factors as well as the requirement for biosafety level 3 or higher containment facilities for safe handling of pathogenic avian influenza viruses warrant the urgent need to develop a new influenza vaccine modality.
The non-infectious nature of virus-like particles (VLPs) and their lack of viral genomic material make them an attractive candidate vaccine, and will be particularly appropriate for the elderly and infant populations. VLP vaccines for viruses such as hepatitis B virus and human papillomavirus are safe for broad and repeated application [4–6]. In recent studies, immunization of mice with monovalent influenza VLPs reduced challenge virus replication and conferred protection against relatively low doses of viral challenges [7–11].
The conventional influenza vaccines are trivalent, containing two influenza A subtypes (H1N1 and H3N2) and one variant of influenza B virus. Previous studies demonstrated that monovalent influenza VLPs could be a promising alternative influenza vaccine [7–11]. However, the immune responses to multivalent influenza VLP vaccine have not been investigated despite its critical significance in evaluating the alternative influenza vaccine. In this study, we have investigated the protective efficacy of a bivalent influenza VLP vaccine after intramuscular immunization of mice in comparison with monovalent influenza VLP vaccines. Our results demonstrated that each HA component in the multivalent influenza VLP vaccine was highly immunogenic and that the protective immune responses induced by bivalent influenza VLPs were more broadly reactive and protective than the monovalent vaccine. Potential mechanisms for the cross-protective immunity by influenza VLPs and strategies to improve VLP vaccines are discussed.
2. Materials and Methods
2.1 Virus and cells
Influenza viruses, A/PR/8/1934 (H1N1, abbreviated as A/PR8), A/WSN/1933 (H1N1, A/WSN), A/Aichi/2/1968-x31 (a reassortant virus H3N2, A/Aichi), A/Hong Kong/1968 (H3N2, A/HK), and A/Philippines/2/1982 (H3N2, A/Philippines) were grown in 10-day old embryonated hen’s eggs and purified from allantoic fluid by using a discontinuous sucrose gradient (15%, 30% and 60%) layers. The purified virus was inactivated by mixing the virus with formalin at a final concentration of 1:4000 (v/v) as described [12, 13]. Inactivation of the virus was confirmed by plaque assay on confluent monolayer Madin-Darby canine kidney (MDCK) cells and inoculation of the virus into 10-day old embryonated hen’s eggs. For use in challenge experiments, mouse adapted A/PR8, A/WSN (H1N1), A/Aichi (H3N2), and A/Philippines (H3N2) were prepared as lung homogenates of infected mice. Spodoptera frugiperda Sf9 cells were maintained in suspension in serum free SF900II medium (GIBCO-BRL) at 27°C in spinner flasks at a speed of 70–80 rpm. Madin-Darby canine kidney (MDCK) cells were grown and maintained in Dulbecco’s modified Eagle’s medium (DMEM).
2.2 Preparation of influenza VLPs
For generation of recombinant baculoviruses (rBVs), cDNAs encoding A/Aichi H3 HA, A/PR8 H1 HA, and M1 were cloned into the pFastBac plasmid, a BV transfer vector (Invitrogen). Recombinant Bacmid baculovirus DNAs (rAcNPV) containing Aichi HA or PR8 HA were isolated from transformed DH10Bac cells and were used to transfect Sf9 insect cells following the manufacturer’s instructions (Invitrogen). For Western blot analysis to determine the expression of M1 and HA, infected cells were dissolved in sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) sample buffer (50 mM Tris, 3% β-mercaptoethanol, 2% SDS, 10% glycerol), separated by SDS-PAGE, and then probed with mouse anti-M1 antibody (1:4000, Serotec) and sera from A/PR8 or A/Aichi virus infected mice (1:1000). The virus titer was determined with a Fast Plax titration kit according to the manufacturer’s instructions (Novagen, Madison, WI).
To produce VLPs containing influenza M1 and HA, Sf9 cells were co-infected with rBVs expressing HA and M1 at MOI of 4 and 2, respectively. Culture supernatants were harvested at 3 days post-infection, cleared by low speed centrifugation (2,000 × g for 20 min at 4°C) to remove cells, and VLPs in the supernatants were pelleted by ultracentrifugation (100,000 × g for 60 min). The sedimented particles were resuspended in PBS at 4°C overnight and further purified through a 20–30–60% discontinuous sucrose gradient at 100,000 × g for 1 hr at 4°C. The VLP bands were collected and analyzed by western blot probed with anti-M1 antibody and mouse anti-PR8 (or anti-Aichi) sera for detecting HA. The contents of HA in VLPs were determined by hemagglutination activity [14] in comparison with that of inactivated virus at the same concentration (1 mg/ml) and confirmed by quantitative western blot of VLPs and inactivated virus (1 to 5 µg protein per well). The hemagglutination assay gave a 3.1 ratio of inactivated virus to VLPs and also western blot analysis resulted in the similar ratio (3:1 ratio of inactivated virus to VLPs).
2.3 Immunization and challenge
Female inbred BALB/c mice (Charles River) aged 6 to 8 weeks were used. Mice (24 mice per group) were intramuscularly immunized with 10 µg of VLPs (approximately 0.95 µg HA) two times (week 0, and 3) in 50µl of PBS. For virus challenge, isoflurane-anesthetized mice were intranasally infected with 2000 PFU of A/PR8 virus (10× the 50% lethal dose, LD50), 2000 PFU A/WSN virus (10× LD50), 3.5 × 106 PFU A/Aichi virus (5× LD50) or 2000 PFU A/Philippines virus (10× LD50) in 50 µl of phosphate-buffered saline (PBS) per mouse at week 4 after the final immunization. Influenza A/Aichi virus has relatively low pathogenicity in mice and high challenge doses were used in previous studies [15, 16]. A/Aichi/2/68-x31virus, a reassortant virus is further reduced in pathogenicity, and thus, higher doses were required to cause mortality in mice. For measurement of immune response parameters, six mice from each group were sacrificed prior to challenge or at day 4 post-challenge. Mice were observed daily to monitor changes in body weight and to record mortality (more than 25 % loss in body weight). We followed an approved Emory IACUC protocol for this study.
2.4 Evaluation of humoral immune responses
Blood samples were collected by retro-orbital plexus puncture before immunization and at 2 weeks post-immunization. Influenza virus specific antibodies were determined in sera by enzyme-linked immunosorbent assay (ELISA) as described previously [11]. As coating antigens to measure virus specific antibodies, egg-grown inactivated influenza viruses (A/PR8, A/WSN, A/Aichi, or A/Philippines) were coated onto 96-well microtiter plates (Nunc Life Technologies, Rochester, NY.) with 100 µl in coating buffer (0.1 M sodium carbonate, pH 9.5, 4 µg inactivated virus per ml) at 4 overnight.
2.5 Lung viral titers, virus neutralization and cytokine assays
The whole lung extracts prepared as homogenates using frosted glass slides were centrifuged at 1,000 RPM for 10 min to collect supernatants. Lung viral titers and neutralization assays were performed using MDCK cells as previously described [11]. Cytokine ELISA was performed as described previously [11, 17]. Ready-Set-Go IL-6 and IFN-γ kits (eBioscience, San Diego, CA) were used for detecting cytokine levels in lung extracts following the manufacturer’s procedures.
2.6. Statistics
All parameters were recorded for individuals within all groups. Statistical comparisons of data were carried out using the ANOVA and Npar1-way Kruskal–Wallis test of the PC-SAS system. A value of P < 0.05 was considered significant.
3. Results
3.1 Production of VLPs containing influenza M1 and H1 or H3 HA
Recombinant baculoviruses (rBVs) expressing influenza M1, H1 HA (A/PR/8/34, H1N1), or H3 HA (A/Aichi/2/68, H3N2) were generated using the pFastbac bacmid transfer vector containing the AcMNPV polyhedrin promoter and used to produce VLPs. HA and M1 containing VLPs were produced and released into the culture supernatants of insect cells co-infected with rBVs expressing M1 and HA as described [11]. Influenza VLPs produced in insect cells were purified by sucrose gradient ultracentrifugation and characterized by western blot. The presence of influenza M1 and PR8 H1 HA and Aichi H3 HA incorporated into particulate VLPs was confirmed (Fig. 1). The biological activity and content of HA incorporated into VLPs were determined by hemagglutination activities using chicken red blood cells and quantitative western blot assays for influenza A/PR8 and A/Aichi HA VLPs, and the corresponding influenza viruses. The HA titers were 3-fold higher in inactivated A/PR8 and A/Aichi viruses than those in the corresponding influenza VLPs. These results were consistent with the estimates obtained from the western blot analysis that showed approximately a 3:1 ratio. The HA content in influenza virus is estimated to be 29 % of the total protein [18]. Therefore, influenza VLPs contain approximately 0.95 µg per 10 µg of total VLP protein whereas inactivated virus contained 2.9 ug HA per 10 ug total protein. These results suggest that influenza VLPs contain functional HA although there is still room to further improve their quality.
Figure 1.
Production of influenza VLPs. Influenza VLPs were purified from the culture supernatants using sucrose gradient ultracentrifugation. A) Western blot analysis of A/PR8 HA VLPs (H1 VLPs). Blots for HA (top) and M1 (bottom) were probed using mouse anti-PR8 sera and purified mouse anti-M1 IgG antibody, respectively. Lanes 1, negative control (human immunodeficiency virus-like particles); 2, HA-negative M1 VLPs; 3, influenza VLPs containing PR8 HA and M1. B) Western blot analysis of A/Aichi HA VLPs (H3 VLPs). Rabbit or mouse anti-Aichi HA polyclonal antibodies were used to detect Aichi HA. Lanes 1, negative control (human immunodeficiency virus-like particles); 2, HA-negative M1 VLPs; 3, influenza VLPs containing Aichi HA and M1.
3.2 Humoral immune responses induced by multivalent or monovalent influenza VLPs
Current inactivated influenza vaccines are trivalent containing two influenza A subtypes (H1N1 and H3N2) and one strain of influenza B virus, and are administered to humans via parenteral immunization. In this study we examined the protective immune responses induced by multivalent influenza VLP vaccines. We immunized groups of mice intramuscularly with 10 µg of influenza VLPs containing 0.95 µg PR8 HA (H1 VLPs), or Aichi HA (H3 VLPs), or 20 µg of bivalent influenza VLPs (a mixture of H1 VLPs and H3 VLPs containing 0.95 µg of each HA, H1+H3 VLPs) at weeks 0 and 3.
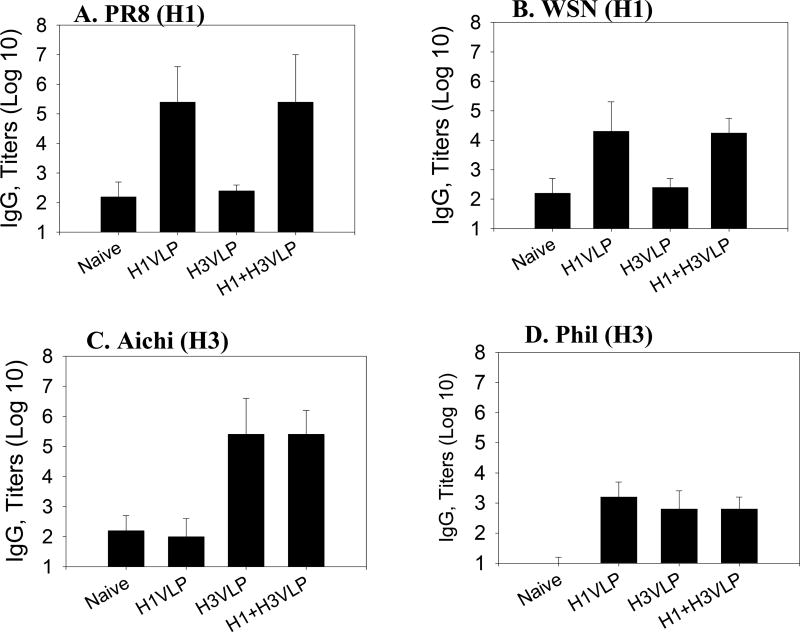
As a measure of humoral immune responses, serum antibody titers were determined by ELISA on plates coated with inactivated influenza virus (Fig. 2). Mice immunized intramuscularly with either monovalent H1 VLPs or H3 VLPs induced high levels of IgG antibodies specific to the corresponding homologous strains A/PR8 (H1N1) and A/Aichi (H3N2) respectively. The group of mice which received multivalent influenza VLPs also showed high IgG antibody levels specific to both viruses (Fig. 2A, C). In addition, immunization with bivalent influenza VLPs induced significant levels of antibodies binding to the heterologous strain A/WSN (H1N1) which were comparable to the monovalent H1 VLP group (Fig. 2B), although approximately 12 fold lower in titer compared to those binding to the homologous strain A/PR8. When a different H3N2 subtype strain (A/Philippines/2/82) was used as an ELISA coating antigen, we observed over 400-fold lower levels of antibodies cross reactive with this virus (Fig. 2D) indicating that there were no significant amounts of cross-reactive antibodies against A/Philippines even in groups of mice that received the A/Aichi HA component (H1+H3 or H3 VLP group). Overall, these data suggest that both H1 and H3 HA components in the multivalent influenza VLPs are immunogenic and that the bivalent influenza VLP vaccine resembles current influenza vaccines in inducing specific immune responses against either the homologous virus or closely related strains within the same subtype.
Figure 2.
Influenza virus specific total serum IgG antibody responses. Mice were intramuscularly immunized with monovalent influenza H1 (PR8) VLPs, H3 (Aichi) VLPs or bivalent influenza VLPs (H1+H3 VLPs) at 3 week intervals. Sera (2 weeks after the second immunization) were used to determine the total IgG antibodies specific to A) A/PR8, B) A/WSN, C) A/Aichi, and D) A/Phil (Philippines) antigens by ELISA. Optical densities were read at 450 nm (OD450), and IgG antibody titers in log10 are expressed as the highest dilution of serum having a mean optical density at 450 nm greater than the mean plus 2 standard deviations for similarly diluted naive serum samples, and error bars indicate standard deviation (SD). Naïve, unimmunized control; H1 VLP, H3 VLP, and H1+H3 VLP indicate groups of mice immunized with 10 µg of H1 (PR8) VLPs, 10 µg of H3 (Aichi) VLPs, and 20 µg of bivalent influenza VLPs, respectively.
3.3 Bivalent influenza VLPs induced a wider range of neutralizing activities
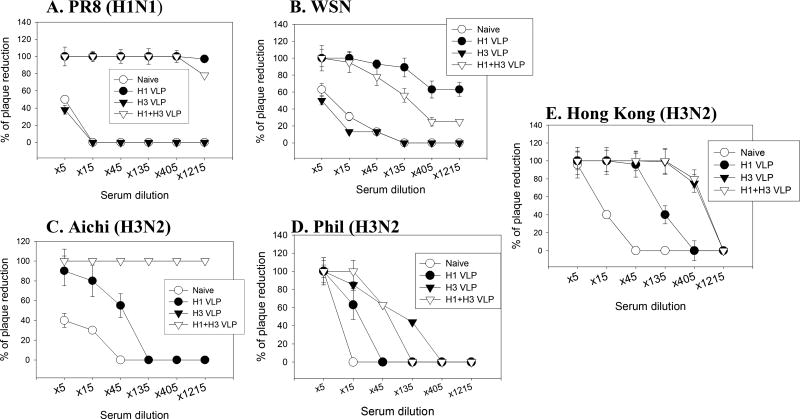
Assessment of virus neutralizing activities in immune sera is important since neutralizing titers can be a barometer for induction of protective immunity. As shown in Fig. 3, bivalent H1+H3 VLP and monovalent H1 VLP groups exhibited high neutralizing titers (over 1200 with 80 % plaque reduction) against the homologous strain A/PR8 which were not found in the monovalent H3 VLP group (Fig. 3A). Also, neutralizing titers of over 400 with 60 % plaque reduction against the heterologous strain A/WSN (H1N1) were observed with the bivalent H1+H3 VLP as in the monovalent H1 VLP group although titers were lower levels than those against the homologous strain A/PR8 (Fig. 3B). Mice immunized with bivalent H1+H3 VLPs as well as monovalent H3 VLPs showed similarly high neutralizing titers of over 1200 with 100 % plaque reduction against the homologous strain A/Aichi (H3N2), whereas the monovalent H1 VLP group did not show neutralizing activity against A/Aichi (H3N2) (Fig. 3C). Neutralizing activities against A/Philippines virus (H3N2) were not observed with any of immunized groups at meaningful levels (titers less than 100 with 50 % plaque reduction) (Fig. 3D). The multivalent H1+H3 VLP and monovalent H3 VLP group each showed neutralizing titers of over 400 against the heterologous H3N2 strain A/Hong Kong/1968 whereas monovalent H1 VLP did not (titers less than 100 with 50 % plaque reduction) (Fig. 3E). These results suggest that each component of the multivalent influenza VLPs can induce neutralizing activity against its counterpart virus or closely matched strains.
Figure 3.
Neutralization activity. Viral neutralizing antibody activities were determined using plaque assays in sera collected at 2 weeks after the second (final) immunization from mice immunized with 10µg VLPs. Serial dilutions of sera from individual mice were incubated with approximately 100 plaque forming unit of influenza virus for 1 hr at 37° C. The samples were then applied to monolayers of confluent MDCK cells, and a standard plaque reduction assay was performed. A) A/PR8 (H1N1), B) A/WSN (H1N1), C) A/Aichi (H3N2), D) A/Philippines (H3N2). E) A/Hong Kong (H3N2). Groups of mice are as described in Figure 2.
3.4 Bivalent influenza VLPs can expand the range of protective immunity
To determine whether vaccinated mice are protected against a lethal challenge, influenza VLP vaccinated mice were challenged with lethal doses of A/PR8 (H1N1), A/WSN (H1N1), A/Aichi (H3N2), or A/Philippines (H3N2) as shown in Fig. 4. Mice that received bivalent influenza (H1+H3) VLPs survived lethal virus challenges with A/PR8, A/WSN, or Aichi (H3N2). Mice immunized with monovalent H1 VLPs survived lethal virus challenges with A/PR8 as well as A/WSN, but did not survive a lethal virus challenge with A/Aichi or A/Philippines. Mice immunized with monovalent H3 VLPs survived lethal virus challenge with A/Aichi but not A/Philippines, A/PR8, or A/WSN (Fig. 4). Influenza VLP-immunized mice that were protected were very active and did not show decreases in body weight, indicating that the protected mice did not experience clinical illness (Fig. 5). In contrast, all naïve mice that received lethal doses of challenge viruses died of illness (Fig. 4 and 5). The pathogenicity of viruses is slightly different depending on the challenge strain, reflecting differences in timing of mortality. Most mice die on day 8 after A/PR8 infection whereas some mice showed delayed mortality after A/Philippines infection. A/WSN and A/Aichi showed similar timing of mortality at day 10 (Fig. 4). Also, we observed significant losses in body weight in influenza VLP-immunized mice that received lethal challenges with heterologous strains but were not protected. These results suggest that bivalent influenza VLPs can be utilized to expand the scope of protection.
Figure 4.
Protection of mice from lethal virus challenges expressed as percent (%) of survival. At week 4 after the final immunization, naïve and immunized mice were intranasally infected with a lethal dose of mouse-adapted A/PR8 (10× LD50), A/WSN (10× LD50), A/Aichi (5× LD50) or A/Philippines (H3N2) virus (10× LD50). Mice were monitored daily for 16 days to determine the percentage of mortality rates. A) A/PR8 virus challenge, B) A/WSN challenge, C) A/Aichi challenge, D) A/Philippines challenge. Groups of mice are as described in Figure 2.
Figure 5.
Body weight changes after virus challenge infections. Naïve or VLP immunized mice were intranasally infected with a lethal dose of mouse-adapted A/PR8 (10× LD50), A/WSN (10× LD50), A/Aichi (5× LD50) or A/Philippines (H3N2) virus (10× LD50). Mice were monitored daily for 16 days to determine the body weight changes as an indicator of morbidity. A) A/PR8 virus challenge, B) A/WSN challenge, C) A/Aichi challenge, D) A/Philippines challenge. Groups of mice are as described in Figure 2.
3.5 Bivalent influenza VLPs conferred complete inhibition of replication in lungs
Determining virus titers in lungs at an early time point can provide a more sensitive assay in assessing protection against challenge virus. Viral titers in lungs were analyzed to determine the replication of challenge virus (Fig. 6). No plaques were detected in lung homogenates of mice immunized with bivalent H1+H3 VLPs and challenged with A/PR8, while viral titers of over 7 log10/ml were observed in lungs from naïve and monovalent H3 VLP-immunized mice at day 4 post-challenge (Fig. 6A). When mice were immunized with H3 VLPs and challenged with the heterologous A/WSN virus no protection was observed, as both immunized and naïve mice displayed high titers of A/WSN in their lungs (Fig. 6B). However, the H1 VLP immunized group showed a 1000-fold reduction in viral titers. Interestingly, even greater protection appeared to be induced in the group that received the bivalent H1+H3 VLPs, as no virus was detected in the lungs of these animals. Similarly, bivalent H1+H3 VLP groups showed no viral titers in lung at day 4 post challenge with A/Aichi, in contrast to the high titers observed for naïve or H1 VLP immunized mice (Fig. 6C). No protection was apparent for any of the groups challenged with A/Philippines/2/82 (Fig. 6D). Overall, these results indicate that bivalent influenza (H1+H3) VLP vaccine can induce immune responses providing complete protection against a range of strains including homologous (A/PR8, A/Aichi) virus and a closely related heterologous strain (A/WSN).
Figure 6.
Lung viral titers. Lung samples from individual mice in each group (n=6) were collected on day 4 post-challenge with a lethal dose of mouse-adapted A) A/PR8 (H1N1), B) A/WSN (H1N1), C) A/Aichi (H3N2) or D) A/Philippines (H3N2) virus. Each lung sample was diluted to 1 ml with DMEM media. The titers are presented as PFU per ml.
3.6 An inverse correlation between protection and lung IL-6 levels
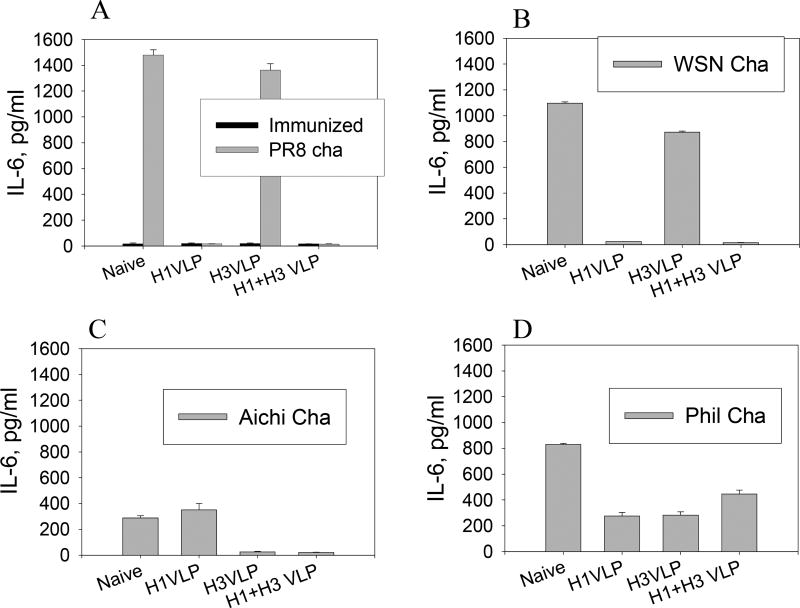
Levels of the inflammatory IL-6 cytokine were determined in lung extracts using a cytokine ELISA (Fig. 7). Despite slight variations depending on different influenza virus strains, high levels of IL-6 were observed in naive mice upon lethal infections indicating lung inflammatory responses probably due to high levels of viral replication. Influenza VLP immunized mice that were protected against challenge infection showed no induction of IL-6 cytokine production in lungs whereas non-protected groups showed high levels of lung IL-6. It is of interest to note that mice immunized with H1 VLPs also did not induce IL-6 cytokine after A/WSN challenge, although they showed 103 lung viral titers. This might be because these mice did not show any clinical symptoms indicating low or no inflammatory responses. Our results and others [19, 20] suggest that influenza viruses have differential capacities in inducing inflammatory responses depending on the strain and pathogenicity of the virus. High levels of the inflammatory cytokine IFN-γ were also found in the non-protected groups (data not shown), whereas IL-6 showed a better correlation. These results indicate that lung IL-6 can be a prognostic factor implying lung inflammation upon influenza virus infection and possibly can provide an alternative indicator for protective efficacy.
Figure 7.
Pro-inflammatory cytokine IL-6 in lungs after virus challenge. Lung extracts were prepared at day 4 post-challenge. Lung samples from naive mice that received the same dose of lethal challenge infection are shown for comparison. IL-6 in lung extracts was determined by ELISA, and results shown are geometric mean values obtained from 6 mice at each time point. A) A/PR8 challenge. All groups of immunized mice prior to challenge infection showed only basal levels of IL-6. B) A/WSN challenge, C) A/Aichi challenge, D) A/Philippines challenge.
3.7 Comparative immune response between VLPs and inactivated virus
To compare directly the immunogenicity between VLPs and inactivated virus, mice were immunized with 10 µg of influenza VLPs containing A/PR8 HA (0.9 µg HA) or 10 µg (corresponding to 2.9 µg HA) of inactivated A/PR8 virus (Fig. 8). The levels of binding antibodies specific to A/PR8 virus were monitored for over 7 months. Mice intramuscularly (Fig. 8) or intranasally (data not shown) immunized with VLPs showed comparable antibody titers to those induced by immunization with inactivated virus, although the HA amount in VLPs was lower than that in the inactivated virus. As an extension of long-term protective immunity by influenza VLPs, we observed that mice immunized with monovalent A/PR8 VLPs or bivalent VLPs were completely protected against lethal challenge infection with A/PR8 virus even after 14 months post-immunization without any clinical symptoms (data not shown). These results further support the conclusion that VLP approaches can be developed as a promising alternative vaccine against influenza virus or other viral pathogens.
Figure 8.
Comparison of long-term antibody responses between VLPs and inactivated viruses. Mice were intramuscularly immunized 2 times with monovalent influenza H1 (PR8) VLPs and inactivated PR8 viruses at 3 weeks intervals (n=6). Groups of mice were immunized with influenza PR8 VLPs (VLP, 0.95µg HA) and PR8 inactivated viruses (Ina PR8, 2.9µg HA). Blood samples were collected individually at 2, 4, 8, 20 and 28 weeks after the 2nd immunization and total sera IgG antibody responses specific to influenza A/PR8 antigen were compared. Optical densities were read at 450 nm (OD450), and results are expressed as the arithmetic mean (OD450) and error bars indicate standard deviation (SD)
4. Discussion
In a previous study, we reported immune responses to monovalent influenza VLPs delivered via mucosal delivery [11]. Inactivated trivalent influenza virus vaccines are administered intramuscularly to humans. In this study, bivalent influenza VLPs containing different subtypes of HA (H1+H3) were administered to mice intramuscularly to parallel the conventional route of influenza vaccination for humans. The current results provide a proof-of-concept that multivalent influenza VLPs can be an alternative to seasonal (as well as pandemic) influenza vaccines. The multivalent influenza VLP vaccines induce binding and neutralizing antibody responses to the individual components included in the vaccine. The bivalent influenza VLP vaccines induced broader protective immunity. Bivalent influenza VLP vaccines are also likely to be more effective in inhibiting viral replication than the monovalent VLP vaccine. Finally, we now provide direct, experimental evidence to support the role of antigenic drift (but not the extent of amino acid homology) in evading cross-protection. That is, the protection was relatively specific to the homologous or heterologous strains intimately related to the vaccine strains as expected. Overall, the current study provides further evidence that influenza VLPs produced in insect cells can be a promising alternative approach to the egg substrate-based conventional influenza vaccine.
The protective efficacy of the current influenza vaccines depends principally on the induction of neutralizing antibodies targeted to the HA protein. We observed that H3 (A/Aichi) VLP immunization induced strain specific protective immunity but did not protect against the heterologous strain A/Philippines (H3N2) or the heterosubtypic strain A/PR8 (H1N1). However, promising prospects for the use of influenza VLP vaccines were provided by our demonstration that immunization with bivalent influenza VLPs (H1+H3 VLPs) can induce protective immunity and/or neutralizing activity against both strains (A/PR8, A/Aichi) included in the vaccine, as well as closely related heterologous strains (H1N1 A/WSN, H3N2 A/Hong Kong). Importantly, bivalent influenza VLP showed an improved protection against the heterologous A/WSN strain in terms of lung viral titers post-challenge compared to the monovalent H1 VLP vaccine, which indicates a potential advantage for the use of multivalent influenza VLP vaccine in broadening the cross-protection.
Cross-protection among different subtypes is not usually expected by systemic immunization with inactivated virus or VLPs since the amino acid differences between subtypes of HA are often greater than 50%. The HA amino acid homology between A/PR8 and A/WSN (H1N1) is similar to that between A/Aichi and A/Philippines (H3N2) ranging from 90 to 92% identity. We observed cross protection against A/WSN in the mice immunized with PR8 HA VLPs whereas there was no cross-protection in the H3 VLP or bivalent (H1+H3) VLP-immunized mice against A/Philippines. To better understand the potential of cross-protection between strains, the sequences of Aichi HA and Philippines HA have been aligned (Table 1) and the positions that deviate are mapped on the three-dimensional structures of the Aichi and PR8 HAs (Fig. 9). For the H3 HA, nearly all differences are confined to well-recognized antigenic regions (antigenic sites A to E [21]). This is not surprising as these sites were identified based on antigenic drift, and the indicated changes have been selected due to immunity in the human population during 14 years between isolation of strains. By contrast, A/PR/8/34 and the parental strain of WSN virus, A/WS/33, differ by only one year in time of isolation from humans. The WSN virus was adapted for mouse neurovirulence from the human strain by multiple passages in culture, followed by several serial passages in mouse brain by intracerebral inoculation [22]. The structural locations of residues that differ between PR8 and WSN HAs shows that, while some differences are present in clearly defined antigenic regions, others are at positions less likely to be of antigenic significance, or at residues in the receptor-binding region as shown in Fig. 9. These may have been selected during adaptation to mouse brains for properties unrelated to antigenicity, or may in fact be spurious mutations that arose during the process. For example, some of the differences between these H1 strains are at positions that are buried in the interior of the molecule, or sites that are more membrane proximal than the loop homologous to the corresponding site C. These are less likely to be antigenically relevant [23]. Therefore, from a purely antigenic standpoint, A/WSN/33 and A/PR/8/34 are probably much more closely related to one another than A/Aichi/2/68 and A/Philippines/2/82, despite similar levels of sequence identity. In addition, three additional N-glycosylation motifs (Asn 44, 109 and 127, Table 1) are predicted from these amino acid differences in A/Philippines compared to A/Aichi, which may also modulate antigenicity. Carbohydrate masking of antigenic sites has been observed for HAs [24, 25].
Table 1.
Influenza HA amino acid Sequence comparison* (HA1 domain aa 43–315)
Sequences were obtained from the NCBI protein sequence database (PR8: A/PR/8/34, ABD77675; WSN: A/WSN/1933, ABF47955; Aichi: A/Aichi/2/1968, AAA43239; Phil: A/Philippines/2/1982, AAA18781, HK: A/Hong Kong/1968, ABQ97200). Within the same subtype, sequences are aligned (PR8 and WSN, Aichi and Phil). Dots indicate the identical amino acid within the comparing pair (PR8 versus WSN; Aichi versus Phil). Different amino acid residues are indicated.
Figure 9.
Locations of amino acid differences between H1 and H3 strains utilized here in the three-dimensional structure of HA. The left panel shows the structure of PR8 HA [Gamblin, 2004 #45]. The HA1 subunit is shown in gray and the HA2 in red. The green residues indicate the positions in the membrane distal portion of HA1 that differ between the PR8 and WSN strains used for these studies. The right panel depicts the structure of Aichi HA [Wilson, 1981 #266]. HA1 and HA2 subunits are in gray and red respectively. The colored residues show the positions that differ between the HAs of A/Aichi/2/68 and A/Philippines/2/82. They are colored differently to differentiate the five major antigenic regions that have been designated for H3 HAs [Wiley, 1987 #1851]. The positions of amino acid changes are located within the five antigenic sites labeled as A to E.
In contrast, we observed that immune sera of bivalent or H3 (A/Aichi) showed significant levels of cross neutralizing activities against A/Hong Kong/1968 (H3N2) that is the same year isolate as A/Aichi and has 98% homology of HA between these two strains (Table 1). A recent study using immune sera from the influenza VLP-vaccinated ferrets demonstrated an inverse correlation between the antigenic distance based on the year of virus isolation and the levels of hemagglutination inhibition titers [9]. Therefore, the pattern of HA amino acid changes as well as sequence homology may significantly affect the cross protection observed in influenza vaccination.
The recent outbreaks of highly pathogenic avian influenza viruses highlight the potential problems associated with the production of conventional influenza vaccines and emphasize the need for an effective alternative vaccine that does not rely on chicken egg substrates. In a recent comparative study of influenza VLPs (A/Fujian, H3N2) and recombinant soluble HA, VLPs induced over 10 fold higher serum titers than soluble HA when equal amounts of HA in soluble form and in VLPs were compared [9]. Also, this study demonstrated 2 fold higher hemagglutination inhibition titers induced by VLP immunization than by inactivated influenza virus. Also, our results suggest that influenza VLPs may have antigen sparing effects by 3 fold when compared to the inactivated whole virus vaccine (Fig. 8). The viral glycoproteins in VLPs are presented in a native conformation since VLPs are assembled by the normal process of budding on the cell surface, and are unmodified by fixatives. Antigens expressed in their native three-dimensional conformation can induce more effective antibody responses [9, 26]. We observed that SHIV (simian human immunodeficiency virus) VLPs produced in insect cells were effectively taken by dendritic cells which preferentially activated CD4 T and B cells [27]. Influenza VLP vaccines also have the potential to provide cross-protective immune responses as demonstrated by the current and previous studies [9, 11]. Therefore, our studies and others support the proof of concept that influenza VLPs can induce protective immunity that is comparable to the conventional influenza vaccine [9].
Overall, we show that vaccination with multivalent cocktails of VLPs can offer the similar type of protection conferred by the standard inactivated vaccines that are in current use, but the VLP approaches have the advantage that their production can circumvent the requirement for embryonated chicken eggs. The strategies involving VLPs are also very versatile. Thus far we have investigated the use of only two HA components in bivalent influenza VLPs, but it may be possible to increase the number of HA strains included in the multivalent VLP format to broaden the scope of neutralizing immunity, and we are investigating the limitations of this. In addition, the VLP approaches are amenable to inclusion of immunostimulatory molecules [27, 28] or other viral components such as NA or nucleoprotein, which may aid in expanding the narrow breadth of protection provided by current influenza vaccines.
Acknowledgments
This work was supported by NIH/NIAID grant AI0680003 (R.W.C.). We thank Dr. Huan Nguyen for the mouse adapted influenza viruses A/PR/8/34 and A/Philippines/2/1982 strains and Dr. Yumiko Matsuoka for the influenza A/WSN/33 strain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992 Mar;56(1):152–79. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005 Mar;79(5):2814–22. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson JS, Nicolson C, Newman R, Major D, Dunleavy U, Wood JM. High growth reassortant influenza vaccine viruses: new approaches to their control. Biologicals. 1992 Sep;20(3):213–20. doi: 10.1016/s1045-1056(05)80040-5. [DOI] [PubMed] [Google Scholar]
- 4.Crum CP, Rivera MN. Vaccines for cervical cancer. Cancer J. 2003 Sep-Oct;9(5):368–76. doi: 10.1097/00130404-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001 Feb 21;93(4):284–92. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 6.Assad S, Francis A. Over a decade of experience with a yeast recombinant hepatitis B vaccine. Vaccine. 1999 Aug 20;18(1–2):57–67. doi: 10.1016/s0264-410x(99)00179-6. [DOI] [PubMed] [Google Scholar]
- 7.Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005 Dec 30;23(50):5751–9. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 8.Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18(1):244–51. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- 9.Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007 May 10;25(19):3871–8. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 10.Pushko P, Tumpey TM, Van Hoeven N, Belser JA, Robinson R, Nathan M, et al. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine. 2007 May 22;25(21):4283–90. doi: 10.1016/j.vaccine.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 11.Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007b Apr;81(7):3514–24. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novak M, Moldoveanu Z, Schafer DP, Mestecky J, Compans RW. Murine model for evaluation of protective immunity to influenza virus. Vaccine. 1993;11(1):55–60. doi: 10.1016/0264-410x(93)90339-y. [DOI] [PubMed] [Google Scholar]
- 13.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000 Jun;74(11):4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006 Aug 28;24(35–36):6110–9. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Weis KF, Chu Q, Erickson C, Endres R, Lively CR, et al. Epidermal powder immunization induces both cytotoxic T-lymphocyte and antibody responses to protein antigens of influenza and hepatitis B viruses. J Virol. 2001 Dec;75(23):11630–40. doi: 10.1128/JVI.75.23.11630-11640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B, Yao DF, Ohuchi M, Ide M, Yano M, Okumura Y, et al. Ambroxol suppresses influenza-virus proliferation in the mouse airway by increasing antiviral factor levels. Eur Respir J. 2002 May;19(5):952–8. doi: 10.1183/09031936.02.00253302. [DOI] [PubMed] [Google Scholar]
- 17.Quan FS, Matsumoto T, Lee JB, Timothy O, Kim TS, Joo KH, et al. Immunization with Trichinella spiralis Korean isolate larval excretory-secretory antigen induces protection and lymphocyte subset changes in rats. Immunol Invest. 2004 Feb;33(1):15–26. doi: 10.1081/imm-120027681. [DOI] [PubMed] [Google Scholar]
- 18.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001 Jun;75(11):5141–50. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tumpey TM, Lu X, Morken T, Zaki SR, Katz JM. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol. 2000 Jul;74(13):6105–16. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007 Mar;81(6):2736–44. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype) Cell. 1982 Dec;31(2 Pt 1):417–27. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 22.Francis T, Moore AE. A study of the neurotropic tendency in strains of the virus of epidemic influenza. J Exp Med. 1940;72:717–28. doi: 10.1084/jem.72.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knossow M, Skehel JJ. Variation and infectivity neutralization in influenza. Immunology. 2006 Sep;119(1):1–7. doi: 10.1111/j.1365-2567.2006.02421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skehel JJ, Stevens DJ, Daniels RS, Douglas AR, Knossow M, Wilson IA, et al. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1779–83. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe Y, Takashita E, Sugawara K, Matsuzaki Y, Muraki Y, Hongo S. Effect of the addition of oligosaccharides on the biological activities and antigenicity of influenza A/H3N2 virus hemagglutinin. J Virol. 2004 Sep;78(18):9605–11. doi: 10.1128/JVI.78.18.9605-9611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBurney SP, Young KR, Ross TM. Membrane embedded HIV-1 envelope on the surface of a virus-like particle elicits broader immune responses than soluble envelopes. Virology. 2007 Feb 20;358(2):334–46. doi: 10.1016/j.virol.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Sailaja G, Skountzou I, Quan FS, Compans RW, Kang SM. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology. 2007 Jun 5;362(2):331–41. doi: 10.1016/j.virol.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skountzou I, Quan FS, Gangadhara S, Ye L, Vzorov A, Selvaraj P, et al. Incorporation of Glycosylphosphatidylinositol-Anchored Granulocyte- Macrophage Colony-Stimulating Factor or CD40 Ligand Enhances Immunogenicity of Chimeric Simian Immunodeficiency Virus-Like Particles. J Virol. 2007 Feb;81(3):1083–94. doi: 10.1128/JVI.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamblin SJ, Haire LF, Russell RJ, Stevens DJ, Xiao B, Ha Y, et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 2004 Apr 19;303(5665):1838–42. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- 30.Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–73. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 31.Wiley DC, Skehel JJ. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annual review of biochemistry. 1987;56:365–94. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]