SUMMARY
Activation-induced cytidine deaminase (AID) initiates both class switch recombination (CSR) and somatic hypermutation (SHM) in antibody diversification. Mechanisms of AID targeting and catalysis remain elusive despite its critical immunological roles and off-target effects in tumorigenesis. Here, we produced active human AID and revealed its preferred recognition and deamination of structured substrates. G-quadruplex (G4)-containing substrates mimicking the mammalian immunoglobulin switch regions are particularly good AID substrates in vitro. By solving crystal structures of maltose binding protein (MBP)-fused AID alone and in complex with deoxycytidine monophosphate, we surprisingly identify a bifurcated substrate-binding surface that explains structured substrate recognition by capturing two adjacent single-stranded overhangs simultaneously. Moreover, G4 substrates induce cooperative AID oligomerization. Structure-based mutations that disrupt bifurcated substrate recognition or oligomerization both compromise CSR in splenic B cells. Collectively, our data implicate intrinsic preference of AID for structured substrates and uncover the importance of G4 recognition and oligomerization of AID in CSR.
In Brief
Qiao et al. demonstrated that structured substrates, like G4 and branched DNA, are preferred AID targets in vitro. A bifurcated substrate-binding surface in AID structure supports structured-substrate recognition. G4 substrates mimicking the Ig S regions also induce cooperative AID oligomerization. Disrupting structured-substrate recognition or AID oligomerization both compromise CSR.

INTRODUCTION
Antibody diversification is a central process in adaptive immunity that produces antigen-specific, high-affinity antibodies to combat millions of antigens. In B cells, immunoglobulin (Ig) genes undergo two DNA-alteration events to enhance the specificity and functionality of antibodies: somatic hypermutation (SHM) and class switch recombination (CSR). Until now, activation-induced cytidine deaminase (AID) is the single enzyme that is known to initiate both SHM and CSR. AID belongs to the APOBEC cytidine deaminase family. It converts deoxycytidine into deoxyuridine on single-stranded DNA (ssDNA) substrates in vitro and in vivo but does not show detectable activity on RNA substrates (Bransteitter et al., 2003). Although AID exhibits sequence similarity to APOBEC cytidine deaminases, its critical function in antibody diversification, especially in CSR, cannot be substituted by APOBEC proteins.
Across the genome, AID predominantly mutates immunoglobulin genes at the variable (V) and switch (S) regions. In SHM, V region sequencing data showed that a WRCH (W = A/T, R = A/G, and H = A/C/T) hotspot motif exhibits higher AID-induced mutation probability than average, by about 2- to 4-fold (Larijani et al., 2005; Rogozin and Diaz, 2004). In CSR, mammalian S regions lie between sets of constant region exons and are enriched in the AGCT sequence, a palindromic example of WRCH. Deamination at both strands of DNA may contribute to double-strand breaks (DSBs) by co-opted repair pathways (Han et al., 2011; Yeap et al., 2015). Joining of AID-initiated DSBs replaces the IgM heavy chain constant region with that of other isotypes to achieve class switching (Matthews et al., 2014a). Patients with AID mutations produce mainly low-affinity IgM antibodies with impairment in CSR and/or SHM, a syndrome known as hyper-IgM immunodeficiency (Xu et al., 2012). AID off-target mutations as well as the subsequent DSBs and chromosomal translocations often promote tumorigenesis, in particular for many types of leukemia and lymphoma (Xu et al., 2012).
The mechanism of AID targeting has been a long-standing mystery. Genome sequencing data showed that AID mutates Ig regions at a 10−4–10−3 per base, per generation frequency (McKean et al., 1984; Rajewsky et al., 1987), which is a million-fold higher than the 10−9 genomic basal mutation frequency. Numerous models of AID targeting have been proposed, which both propel the field forward and leave remaining questions unanswered. Chromatin accessibility and transcription that provides ssDNA substrates for AID to act is a known prerequisite, but it does not explain AID targeting specificity to limited genomic regions. Convergently transcribed regions in certain super-enhancer loci appear to correlate with enhanced AID off-target mutations (Meng et al., 2014), but it is not clear whether Ig regions undergo convergent transcription, and not all super-enhancer loci with convergent transcription are AID targets (Qian et al., 2014). The originally proposed hotspot model (Pham et al., 2003) also does not appear sufficient, as ssDNA substrates containing hotspot motifs do not show advantage in recruiting AID in vitro (Larijani et al., 2007), and hotspot motif distribution in the genome does not correlate with the AID off-targeting spectrum in vivo (Duke et al., 2013). In AID recruiter models, various proteins, including replication protein A (RPA) (Chaudhuri et al., 2004), Spt5 (Pavri et al., 2010), and 14-3-3 (Xu et al., 2010), have been proposed in guiding AID to genes. However, the genome distribution of these recruiters is not unique to Ig regions. A more recent recruiter model proposed that transcribed G-repeat rich switch RNAs form G-quadruplex (G4) structures, bind AID, and guide AID to genes for CSR (Zheng et al., 2015). However, the mechanism of transfer of AID between G4 RNA and target DNA has not been addressed.
Here, we present a comprehensive biochemical and structural study on AID, which provides unexpected insights on AID targeting specificity. Through protein engineering, we produced a fully functional monomeric AID with intact activity in vitro and in cells. Using various forms of DNA, we found that structured substrates containing multiple ssDNA overhangs, like G4 and branched DNA, are preferred by AID in binding and deamination over linear ssDNA substrates in vitro. This observation may form the basis for the frequent targeting of AID to mammalian Ig switch regions containing high density of G-repeat sequences. We determined the crystal structures of maltose binding protein (MBP)-fused AID and its complex with cytidine (C), deoxycytidine (dC), and deoxycytidine monophosphate (dCMP). These structures not only explain the discrimination between DNA and RNA in AID catalysis but also reveal a bifurcated substrate-binding surface, which strongly supports that one AID recognizes two adjacent ssDNA overhangs from one structured substrate to achieve high affinity. In addition, we observed that G4 structured substrates induce AID cooperative oligomerization, which may promote clustered mutations in Ig S regions. Structure-guided mutagenesis revealed that both the bifurcated substrate binding surface and the putative oligomerization interface are essential for CSR, elucidating recognition of structured substrates as an important AID-targeting mechanism to Ig S regions.
RESULTS
Active AID Monomer from Protein Engineering
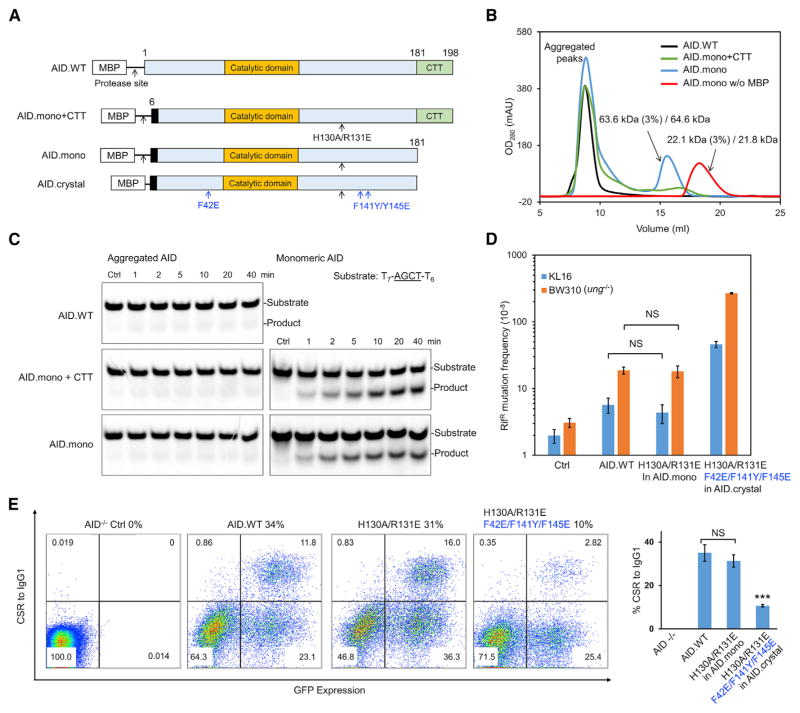
Although endogenous AID extracted from B cells exhibited nearmonomer molecular mass (Chaudhuri et al., 2003), previously published data (Larijani et al., 2007) and our results showed largely heterogeneous and poorly active aggregates of recombinant wild-type (WT) AID (Figures 1A, 1B, S1A, and S1B). To obtain physiologically relevant recombinant AID for functional elucidation, we performed rounds of protein engineering. We found that the His-MBP-fused double-mutant H130A/R131E produced a low amount of monomeric AID, and further N-and C-terminal tail (CTT) truncations improved the monomer yield, resulting in constructs that we named AID.mono+CTT and AID.mono, respectively (Figures 1A, 1B, S1A, and S1B). Both monomeric and aggregated AID fractions were purified and compared in deamination assays, revealing that monomeric AID with and without CTT exhibited much higher deamination activity on ssDNA than aggregated fractions of WT AID, AID.mono+CTT, and AID.mono (Figure 1C). Compared to previously reported AID turnover rates (~10 fmol/min/μg; King et al., 2015), the activities of monomeric AID.mono+CTT and AID.mono were roughly 1,000 fold higher (~104 fmol/min/μg). These data suggest that aggregation renders AID largely inactive. Consistent with the lack of sequence conservation of H130 and R131 among AID species (Figure S1C), the H130A/R131E mutant behaved as WT in mutation frequency by an SHM-mimic rifampicin resistance (RifR) assay (Petersen-Mahrt et al., 2002; Figure 1D) and fully rescued CSR when reconstituted into AID-deficient ex vivo CSR-activated splenic B cells (Figures 1E and S1F). Notably, all AID constructs tested in RifR and CSR assays contain only the internal mutations, with no fusion tag, and no truncations of the nuclear transportation signals at the N or C terminus. Because AID.mono+CTT and AID.mono both showed robust deamination activity and the His-MBP tag did not affect this activity (Figures S1D and S1E), we mainly used the His-MBP-fused AID.mono with higher yield in subsequent biochemical characterizations.
Figure 1. Active Monomeric AID from Protein Engineering.
(A) A schematic diagram of construct design with indicated mutation sites. More details are shown in Figure S1A.
(B) Gel filtration chromatography of WT AID, AID.mono+CTT, AID.mono, and AID.mono after MBP removal. The measured molecular masses by multi-angle light scattering (MALS) and the theoretical molecular masses are shown.
(C) In vitro deamination assay showing that both monomeric AID.mono and AID.mono+CTT had much higher activity than aggregated AID on ssDNA. Experiments used 0.1 μM AID and 1 μM DNA.
(D) Rifampicin resistance (RifR) assay (Petersen-Mahrt et al., 2002) in E. coli KL16 and its uracil-DNA glycosylase (UDG)-deficient derivative BW310 (ung−/−), showing mutation frequencies by AID.WT and AID with mutations in AID.mono and AID.crystal. UDG removes the uracil base as a first step in base-excision repair following cytidine deamination, and its deficiency enhances AID-mediated mutation frequency. Data are represented as mean ± SD from 12 independent measurements.
(E) CSR rescue of AID-deficient splenic B cells ex vivo by AID.WT and by AID with mutations in AID.mono and AID.crystal. Percent (%) CSR to IgG1 is the ratio between GFP+/IgG1+ cells (upper right quadrant) and total GFP+ cells (the two right quadrants). Data are represented as mean ± SD from three to six independent measurements.
See also Figure S1.
Linear and G4 Structured Substrates from Ig S Regions
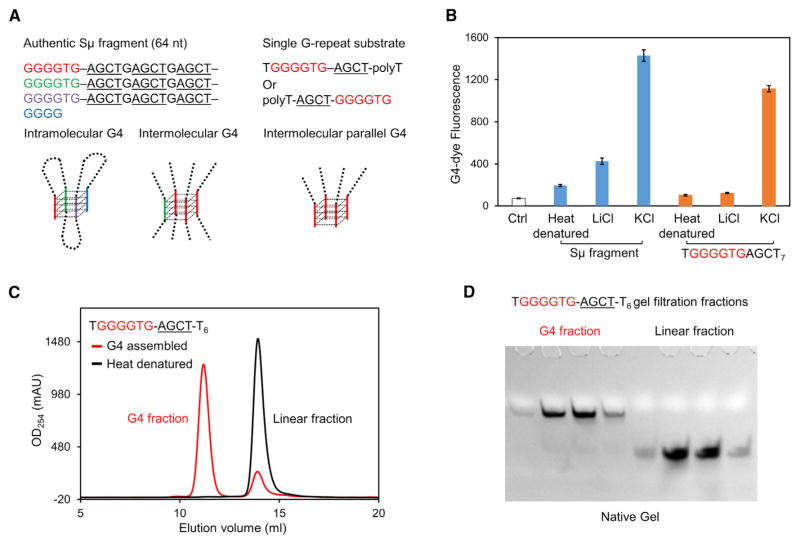
Genome sequencing has shown that the mammalian Ig S regions contain abundant tandem G repeats interspersed by AGCT hotspots and are heavily targeted by AID during CSR (Figure S2A; Yu et al., 2003). It has been proposed that, during transcription, G4 structures form on the G-repeat non-template strand and contribute to R-loop stability (Duquette et al., 2004). Consistently, we found that authentic non-template Sμ fragment (64 nt) and a single G-repeat substrate both spontaneously assembled into G4 structures (Figures 2A and 2B). The G4 assembly was validated by the fluorescence enhancement of a G4-specific dye and the disruptive effect of LiCl (Figure 2B; Bardin and Leroy, 2008). Gel electrophoresis showed that the 64-nt Sμ fragment formed heterogeneous oligomers, likely representing mixed inter- and intramolecular G4s (Figure S2B). Differently, the single G-repeat substrate formed homogeneous intermolecular G4 that could be separated from linear ssDNA by size-exclusion chromatography and gel electrophoresis (Figures 2C, 2D, and S2B). Dimethyl sulfate protection footprinting indicated that all Gs in the GGGGTG motif were involved in the G4 assembly (Figure S2C; Sun and Hurley, 2010). Circular dichroism (CD) spectroscopy showed that the G-repeat substrates were predominantly parallel, instead of anti-parallel, G4 structures (Figure S2D; Vorlíčková et al., 2012). Notably, the purified linear and G4 fractions of the single G-repeat substrate share the identical DNA sequence and only differ in structure. The same size-exclusion purification procedure was used in preparing other linear and G4 structured substrates containing a single G repeat for in vitro studies.
Figure 2. Linear and G4 Structured Substrates.
(A) Two types of AID substrates: one with multiple G-repeats and hotspots as in a mouse Sμ fragment and the other with a single G repeat and hotspot. The potential ability of these substrates to form either intramolecular or intermolecular G4 structure is illustrated.
(B) G4 structure formation confirmed by the G4-specific dye N-methyl mesoporphyrin IX (NMM). LiCl is known to inhibit G4 structures. In total, 10 μM DNA and 20 μM NMM were used in each experiment. Heat denaturation was done by 95°C incubation for 10 min followed by flash cooling to eliminate DNA structures. Data are represented as mean ± SD from three independent measurements.
(C) Separation of intermolecular G4 and linear fractions of single G-repeat substrate using a Superdex 75 gel filtration column.
(D) Native gel showing the clear separation of linear and G4 structured substrate from Superdex 75 gel filtration purification in (C).
See also Figure S2.
AID, but Not APOBECs, Prefers Binding and Deaminating G4 Structured Substrates over Linear Substrates
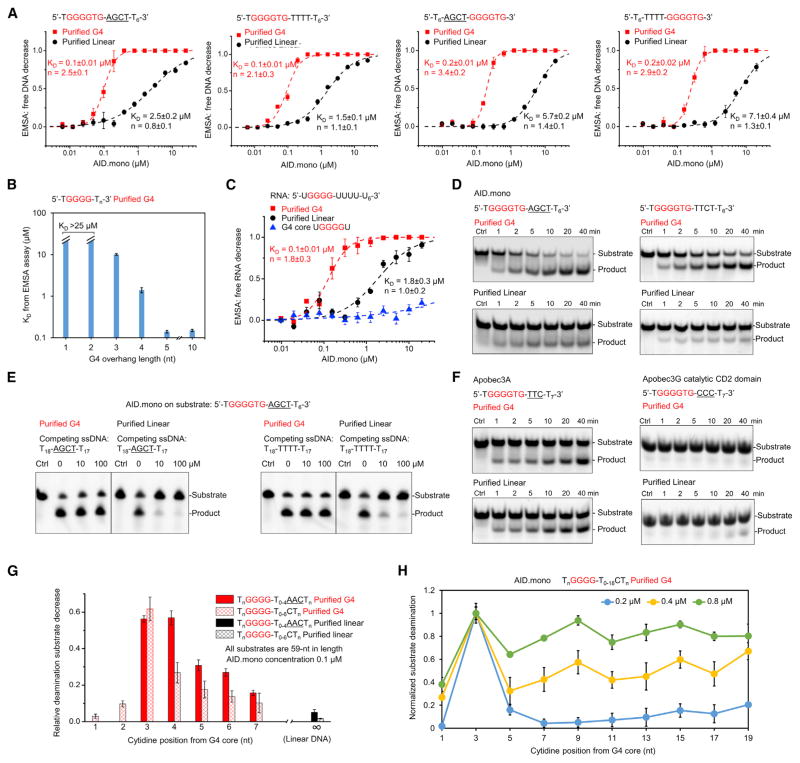
Despite the identical DNA sequence, the purified linear and G4 substrates exhibited distinct behavior in AID binding and deamination assays. The G4 structured substrates displayed ~10-fold higher AID binding affinity (KD = 0.1–0.2 μM) than the linear substrates of the same sequence (KD = 1.5–7.1 μM; Figure 3A). The difference is irrespective of the hotspot or the direction of the ssDNA overhang relative to the G-repeat sequence (Figure 3A). By dissecting the G4 substrate structure, we found that AID did not bind to the core structure but rather required at least 5-nt single-stranded overhangs for optimal interaction (Figure 3B). Previously, binding of AID to switch region RNA G4 transcripts has been observed (Zheng et al., 2015). Interestingly, we found that the binding of AID to RNA G4 is equal to DNA G4, with similar affinities and requirement for single-stranded overhangs (Figure 3C). Therefore, AID appears to recognize single-stranded overhangs adjacent to a G4 core, with little dependence on their sequence, orientation, and whether they are DNA or RNA.
Figure 3. AID Preferentially Binds and Deaminates G4 Structured Substrates.
(A) Electrophoretic mobility shift assay (EMSA) curves showing the significantly higher AID binding affinity of G4 fractions than linear fractions with identical primary sequences.
(B) KD calculated by EMSA showing that ssDNA overhangs in G4 substrates are required for AID binding. Affinities increased with overhang length and plateaued at 5 nt.
(C) EMSA showing that AID binds to RNA G4 similarly as to DNA G4.
(D) In vitro deamination assays showing that AID has a higher deamination activity on G4 substrates than on linear substrates, with or without hotspots. Experiments used 0.1 μM AID and 1 μM DNA.
(E) Competition assays showing that excess linear substrates did not compete with G4 substrates. Experiments used 1 μM AID, 1 μM substrate DNA, and up to 100 μM competitor ssDNA. Reaction time was 10 min.
(F) In vitro deamination assay showing that Apobec3A and 3G did not exhibit G4 preference. Experiments used 0.1 μM APOBEC protein and 1 μM substrate DNA.
(G) In vitro deamination assays showing that the peak activity of AID appears when the substrate nucleotide is at the third position 3′ to the G4 core. Experiments used 0.1 μM AID and 1 μM DNA. Reaction time was 10 min.
(H) In vitro deamination assays showing that AID oligomerization causes clustered mutations. Experiments used 0.2 μM DNA and 0.2, 0.4, or 0.8 μM AID.mono. Reaction time was 2 min.
Data in (A)–(C), (G), and (H) are represented as mean ± SD from three independent measurements. See also Figure S3.
Consistently, in vitro deamination assays showed that AID.mono with and without CTT exhibited more robust activity on the G4 structured substrates than the linear substrates, despite the identical DNA sequence (Figures 3D and S3A). The preference remained irrespective whether the substrates contain a hotspot (AGCT) or cold spot (TTCT; Figures 3D and S3A). Remarkably, in a competition assay, linear ssDNA with or without hotspots did not affect G4 structured substrate deamination even at 100-fold molar excess but severely inhibited linear substrate deamination at 10-fold molar excess (Figure 3E). In comparison, two AID homologs APOBEC3A and APOBEC3G did not show a catalytic preference for G4 structured substrate (Figure 3F), suggesting that the G4 structure preference may be a unique feature for AID specific functions, like CSR.
Location-Dependent Deamination by AID on G4 Structured Substrate
To probe how AID performs catalysis on G4 structured substrates, we designed a series of G4 substrates with a hotspot (AACT) or a cold spot (TTCT) located at different positions of an ssDNA overhang. We found that the peak deamination activity was achieved when the target deoxycytidine was placed at the third position 3′ to the G4 core (Figures 3G and S3B). AID-mediated deamination steadily decayed when the deoxycytidine was moved away from the third position, albeit still significantly higher than that for the linear substrate (Figure 3G). Peak activities at the third position were similar between hotspot- and cold-spot-containing substrates, suggesting that the G4 structure might override the hotspot preference in AID targeting. In positions away from the third position, deamination activity on hotspot substrates roughly doubled that on cold spot substrates, recapitulating the observed hotspot preference in vitro and in cells (Larijani et al., 2005; Pham et al., 2003). Notably, in Ig S regions, a deoxycytidine often exists exactly at the third position from the G-repeat motif (GGGGTG; Figure S2A), an ideal site for AID deamination, as suggested by our results.
Because of the significant cooperativity observed in binding assays using G4 structured substrates (Hill coefficient n ≥ 2; Figure 3A), we suspected that AID-AID interaction might occur upon G4 binding. The hypothesis was confirmed by reconstituting AID.mono/G4 complex in vitro, which mainly eluted from the void position of a size-exclusion column with an apparent measured molecular mass of ~1.3 MDa (Figure S3C) and was observed as large oligomers under electron microscopy (Figure S3D). To discern the consequence of AID oligomerization, we designed a similar series of G4 substrates with the target C located at up to 18 nt away from the G4 core (Figure S3E), which showed a similar peak of deamination at the third position at a low AID concentration (Figure 3H, blue trace). However, when we increased the AID concentration to induce oligomerization, we observed that the cytidine sites away from the G4 core were more efficiently deaminated (Figure 3H, green and yellow traces), suggesting that AID oligomerization on G4 may spread the mutations to more distal sites. The peaks of deamination are separated by ~6 nt (Figure 3H), which is consistent with the 5-nt minimal ssDNA length for AID binding that we identified earlier (Figure 3B).
Structures of AID and Its Complex with Substrates
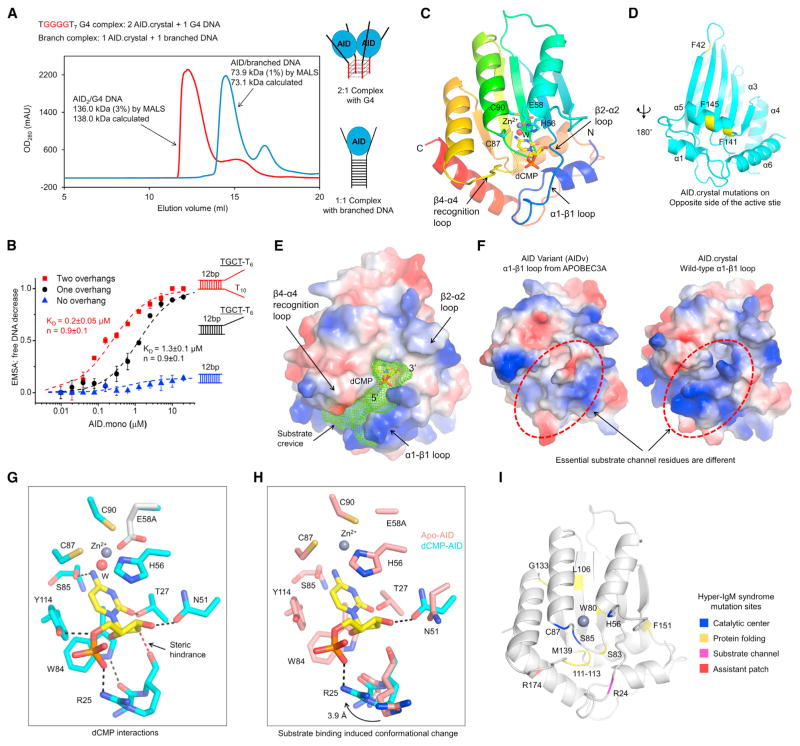
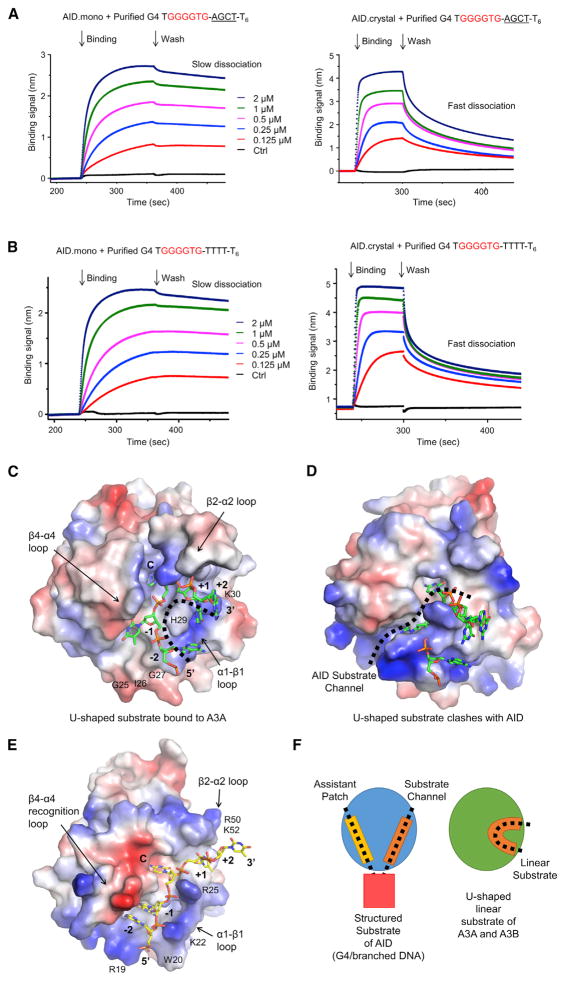
Because oligomerization of AID.mono upon binding to a G4 substrate resulted in a heterogeneous oligomeric complex, we screened additional mutations on surface hydrophobic residues that could disrupt AID oligomerization to facilitate crystallization. We found that the F42E/F141Y/F145E triple mutation plus shortening of the linker to the MBP tag rendered AID.mono entirely monomeric as measured by multi-angle light scattering (MALS) (Figures 1A and S4A). The new construct that we named AID.crystal maintained G4 preference in vitro (Figure S4B) and formed a homogeneous AID2/G4 complex without further oligomerization, as determined by MALS (Figure 4A). The stoichiometry of the complex suggested that each AID is capable of binding two ssDNA overhangs in the G4 substrate. Confirmatively, a designed branched substrate with only two ssDNA overhangs displayed a 1:1 interaction with AID.crystal (Figure 4A). Interestingly, AID.mono bound and deaminated better on the two-overhang branched substrate in comparison with a single-overhang substrate, but the binding did not show cooperativity (Hill coefficient n≈1.0; Figures 4B and S4C) as for G4 substrates (Figure 3A).
Figure 4. Structures of AID and Its Complex with dCMP.
(A) Gel filtration chromatography with in-line MALS showing that AID.crystal binds G4 DNA in 2:1 ratio and branched DNA in 1:1 ratio. Measured and calculated molecular masses are labeled.
(B) EMSA curves showing enhanced AID binding affinity for branched substrate with two overhangs (red), in comparison to that with one overhang (linear substrate, black) or no overhang (dsDNA, blue). Data are represented as mean ± SD from three independent measurements.
(C) Ribbon diagram of human AID in rainbow color showing the secondary structures and catalytic residues near active site Zn2+. W, water.
(D) Locations of F42, F141, and F145 mutated in AID.crystal on the face of the crystal structure opposite to the active site.
(E) Surface charge distribution of the AID/dCMP structure and pocket prediction revealed a substrate binding channel that passes through the active site (green mesh).
(F) Comparison between the AID-APOBEC3A hybrid AIDv (PDB: 5JJ4) and AID.crystal showed distinct surface charge distribution at the substrate channel.
(G) Substrate dCMP in AID (E58A) catalytic center showing the interactions with surrounding residues. The E58 side chain is taken from the WT Apo-AID structure.
(H) Alignment between Apo- and dCMP-bound AID structures showing the movement of R25 and N51 upon substrate recognition.
(I) Mutations associated with the hyper-IgM syndrome mapped to AID structure.
See also Figure S4 and Tables S1–S3.
The MBP-fused AID.crystal and its catalytically dead mutant E58A were crystallized in complex with G4 DNA or branched DNA but did not crystallize alone. However, despite confirmed presence of DNA in the crystals (Figure S4D), only fragmented DNA density was visible, which appeared to mediate crystal packing. Indeed, AID also co-crystallized with blunt-ended double- stranded DNA (dsDNA) that it did not bind in solution (Figure 4B); the dsDNA stacked in the crystal lattice and likely neutralized repulsion between highly positively charged AID with isoelectric point (PI) of ~9.0 (Figure S4E). Upon trying many different substrates (Table S1), we obtained in total seven structures of AID, alone and in complex with cytidine (C), 2′-deoxycytidine (dC), and 2′-deoxycytidine-5′-monophosphate (dCMP) at a highest resolution of 2.4 Å (Figures 4C; Table S2). Importantly, mutations in AID.crystal at residues F42, F141, and F145 all localize on the opposite side of the active site defined by the bound dCMP (Figure 4D).
In contrast to the recently reported crystal structure of an AID-APOBEC3A hybrid (AIDv) alone (Pham et al., 2016), our AID/dCMP complex captured using the E58A mutant revealed a deep substrate channel and the direction of ssDNA binding (Figure 4E). Because of the replacement of residues 7–36 of AID with those of APOBEC3A, the AIDv structure exhibits disrupted shape and charge distribution at the substrate channel (Figures 4F and S4F). The active site is comprised of the catalytic proton-donating residue E58 and the Zn2+ ion coordinated by H56, C87, C90, and usually a fourth ligand, e.g., a water in the dCMP complex or a cacodylic acid from an Apo-AID crystallization condition (Figures 4G and S4G). No significant conformational changes were observed at the active site between WT and E58A structures (Figure S4H).
Mechanisms of Cytosine Recognition and DNA/RNA Differentiation
The dCMP-defined substrate channel is mainly formed by the α1-β1, β2-α2, and the β4-α4 loops (Figure 4E), among which the β4-α4 loop was previously designated as the recognition loop important for hotspot specificity (Kohli et al., 2009; Wang et al., 2010). Although there are limited global conformational differences between structures of AID alone and its complexes, the side chain of F115 in the β4-α4 recognition loop is antiparallel to the conservedY114 in all complex structures (FigureS4I), whereas a stacking conformation between F115 and Y114 was also observed in Apo-AID structures (Figure S4I), suggesting that the flip of F115 may be induced or stabilized by substrate binding.
The cytosine is cradled by aromatic residues H56, W84, and Y114 (Figure 4G) and precisely positioned by interactions with surrounding residues. The atom N4 forms hydrogen bonds to the Zn2+-coordinating water and the carbonyl oxygen of S85, whereas the atom O2 hydrogen bonds with the hydroxyl of T27 (Figure 4G). If we superimpose the E58-containing Apo-AID structure, N4 also interacts with the side chain of E58 (Figure 4G). In contrast, the product uracil possesses an O4 instead of an N4 and cannot form the stabilizing hydrogen bonds, consistent with the lack of ligand density in AID co-crystallized with uridine (Table S1). The core arrangement of the AID active site is akin to that of human cytidine deaminase (CDA) despite a different structural fold (Figure S4J), with similar Zn2+ coordination and location of the catalytic Glu (Figure S4K). This structural observation suggests that AID uses a similar deamination mechanism, in which the E58 side chain interacts with N3 of the pyrimidine ring to facilitate nucleophilic attack at C4 by the Zn2+-activated water for deamination (Chaudhuri and Alt, 2004).
Previous data showed that AID does not deaminate RNA substrates (Bransteitter et al., 2003). Particularly, replacing the target dC with C on an otherwise ssDNA substrate abolished AID-induced deamination (Nabel et al., 2013). Supporting this observation, we only captured significant electron density of dCMP, but not CMP, in the catalytic center (Figure S4L), in spite of similar binding behaviors of AID for DNA and RNA in vitro (Figures 3A and 3C). In the AID/dCMP complex, R25 interacts with the 5′-phosphate and Y114 interacts with the O5′, whereas N51 hydrogen bonds with the 3′-OH (Figure 4G). Compared to the Apo-AID structure, both R25 and N51 undergo significant side chain adjustments upon substrate binding (Figure 4H). Proximity of the carbonyl oxygen of R25 to the C2′ of the deoxyribose indicates a steric hindrance if the deoxyribose is replaced by a ribose (Figures 4G and 4H). Without the 5′-phosphate, the AID structure in complex with C or dC either showed different sugar position or much weaker density (Figures S4L and S4M), suggesting that the 5′-phosphate is essential for fixing dCMP orientation in the catalytic center for DNA/RNA differentiation. The interactions we observed in the complex structure explain many hyper-IgM syndrome mutations (Figure 4I; Table S3) and previously reported disruptive mutations, such as R25A/D, T27A, N51A, and Y114A (Basu et al., 2005; King et al., 2015; Shivarov et al., 2008).
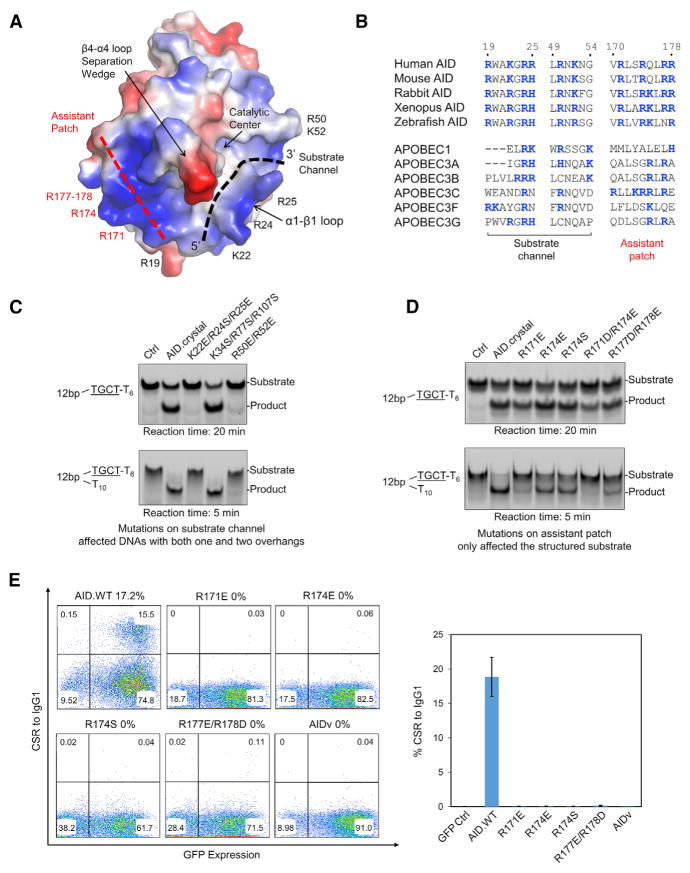
A Bifurcated Substrate-Binding Surface Explains G4 Preferences and Is Critical for CSR
As described earlier, our in vitro data suggested that one AID interacts with two ssDNA overhangs (Figure 4A). Supporting this observation, we found that, apart from the substrate channel, the AID structure contains an additional positively charged surface at helix α6, which we named the “assistant patch” (Figure 5A). Together with the substrate channel, the AID structure suggested a bifurcated substrate-binding surface wedged by a negatively charged β4-α4 loop (Figure 5A). This structural feature is surprisingly analogous to branched nucleic acid recognition by the T4 RNase H (Devos et al., 2007) and Cas9 (Jiang et al., 2016; Figures S5A and S5B). Thus, we propose a simultaneous recognition mechanism for two overhangs in structured substrates, such as G4 in AID targeting, in which one ssDNA overhang passes through the active site and an adjacent ssDNA binds at the assistant patch to enhance affinity. Sequence alignment shows that the positively charged residues in the bifurcated substrate-binding surface are highly conserved in AID across species, but this conservation is absent in APOBEC homologs (Figure 5B), explaining the lack of G4 preference in APOBEC3A and APOBEC3G (Figure 3F).
Figure 5. AID Structures Revealed a Bifurcated Recognition Site for Two ssDNA Overhangs.
(A) Surface charge distribution of AID structure revealed an additional positive patch away from the substrate-binding channel for recognition of an additional ssDNA.
(B) Sequence alignment among different species of AID as well as human APOBECs, showing that the unique basic residue distribution in the substrate channel and the assistant patch is not conserved in APOBECs.
(C) Mutations on positively charged residues in the substrate chain channel abolished deamination on substrates with either one or two ssDNA overhangs. The K34S/R77S/R107S mutant is a negative control on positively charged residues elsewhere.
(D) Mutations on positively charged residues on the assistant patch impaired deamination on the substrate with two overhangs, without significantly affecting AID activity on that with one overhang. Experiments in (C) and (D) used 0.1 μM AID and 1 μM DNA.
(E) Mutations on the assistant patch abolished CSR. The AIDv was also completely deficient in rescuing CSR. Notably, all AID constructs in this assay contain intact CTT, including AIDv. Data are represented as mean ± SD from three independent measurements.
See also Figure S5.
To validate the bifurcated substrate-binding surface, we mutated either the substrate channel or the assistant patch. For the substrate channel, the triple mutation on K22, R24, and R25 and the double mutation on R50 and R52 abolished AID deamination activity on DNA substrates with either one or two ssDNA overhangs (Figure 5C). As a control, a triple mutation on residues K34, R77, and R107, which are localized far away from the substrate-binding surface, did not affect AID activity (Figure 5C). For the assistant patch, mutations on R171, R174, R177, and R178 specifically compromised AID deamination activity on substrate with two ssDNA overhangs, without significantly altering AID activity on one ssDNA overhang substrate (Figure 5D), confirming its assistant role in recognizing structured substrates with multiple overhangs.
Remarkably, when reconstituted into AID-deficient splenic B cells, the assistant patch mutants showed completely abolished CSR activity (<1% of the WT; Figures 5E and S5C). Among the mutants, R174S was previously reported from patients with hyper-IgM syndrome (Durandy et al., 2006; Honjo et al., 2012; Table S3). Moreover, the AID-APOBEC3A hybrid AIDv (Pham et al., 2016) was also completely deficient in conducting CSR (Figures 5E and S1A), likely due to the disrupted substrate channel orientation (Figures 4F and S4F). Of note, all AID constructs tested in the CSR assay were with intact N-terminal nuclear localization sequence (NLS) and CTT, supporting that the functional deficiencies were solely contributed by the internal mutations. Collectively, we demonstrate that the integrity of the bifurcated substrate- binding surface, including both the substrate channel and the assistant patch, is essential for AID function in CSR.
Putative AID Oligomerization on G4 Contributes to CSR
Compared to AID.mono, the three additional mutations in AID.crystal (F42/F141/F145) localize on the opposite side of the proposed substrate-binding surface (Figure 4D). Interestingly, these mutations impaired CSR by ~60% in comparison to the WT without affecting AID deamination activity in vitro and in the SHM-mimic RifR assay (Figures 1D, 1E, and S4B), suggesting that CSR may require more AID properties than SHM. DNA binding assay showed that the oligomerization-deficient AID.crystal exhibited no cooperativity (Hill coefficient n ≈1.0; Figure S5D) or formed large oligomers (Figure S3D) upon G4 binding, in comparison to the highly cooperative AID.mono (Figure 3A). Additionally, AID.crystal exhibited much faster dissociation from G4 structured substrates than WT-like AID.mono as measured by bio-layer interferometry, irrespective of the DNA sequence (Figures 6A and 6B). Based on these data, we hypothesize that AID oligomerization upon G4 structure binding contributes to CSR by enhancing AID recruitment and accumulation to Ig S regions. It should be noted that AID.mono only cooperatively oligomerizes on G4, but not on a simpler structured substrate, such as branched DNA, that may be formed by local secondary structures (Figure 4B). The AID oligomerization may be further facilitated by the CTT (Mondal et al., 2016) and result in previously observed processivity (Pham et al., 2003).
Figure 6. AID Oligomerization and Structural Comparison with APOBECs.
(A) Bio-layer interferometry by Blitz showing the kinetics of a hotspot containing G4 substrate binding by AID.mono (left) and AID.crystal (right). Much faster dissociation was observed for AID.crystal in comparison with AID.mono.
(B) Bio-layer interferometry by Blitz showing the kinetics of non-hotspot G4 substrate binding by AID.mono (left) and AID.crystal (right). The much faster dissociation remained for AID.crystal.
(C) The U-shaped substrate-binding channel observed in A3A-ssDNA complex structure (PDB: 5SWW).
(D) AID surface with the U-shaped substrate from in A3A, showing steric clash.
(E) A structure model of AID in complex with the AGCTT ssDNA.
(F) A schematic diagram suggesting that a U-shaped substrate channel in A3A and A3B may not support structured substrate recognition.
See also Figure S6.
Comparison of Substrate Recognition by AID and APOBECs
Two recent papers reported crystal structures of APOBECs in complex with ssDNAs, including those of the non-catalytic APOBEC3G N-domain (A3G-N) (Xiao et al., 2016), APOBEC3A (A3A), and APOBEC3B containing a replaced α1-β1 loop from A3A (A3B-A chimera; Shi et al., 2017). Consistent with its lack of an active site, A3G-N interacts with ssDNA at a surface shifted from the analogous AID active site (Figure S6A). The A3A and A3B-A chimera structures displayed the same binding mode to U-shaped ssDNA (Figure 6C), among which the cognate C at the center binds similarly as dCMP in AID (Figure 6D). However, the remaining ssDNA clashes with the AID surface and does not follow the observed substrate channel of AID (Figures 6D and S6B).
To further validate the observed substrate channel in AID, we modeled the binding of an AGCTT hotspot substrate using the bound dCMP position as an anchor (Figure 6E). We found that the β4-α4 recognition loop stayed in the conformation observed in the dCMP complex structure (Figure S4I) and that conformation rearrangement is only required at the α1-β1 loop to accommodate the substrate at the −1 and −2 positions (Figures 6E and S6C). No gross conformational changes are needed for the superficial binding at +1 and +2 positions (Figure 6E). The modeling exercise therefore supports the observed substrate channel. We suspect that, if a U-shaped substrate channel exists in AID as in A3A and A3B, it will likely not be able to support simultaneous binding of another ssDNA at the assistant patch, whichmay be the reason for the difference between substrate recognition by AID and APOBECs (Figure 6F) and for the CSR deficiency of AIDv (Figure 5E).
DISCUSSION
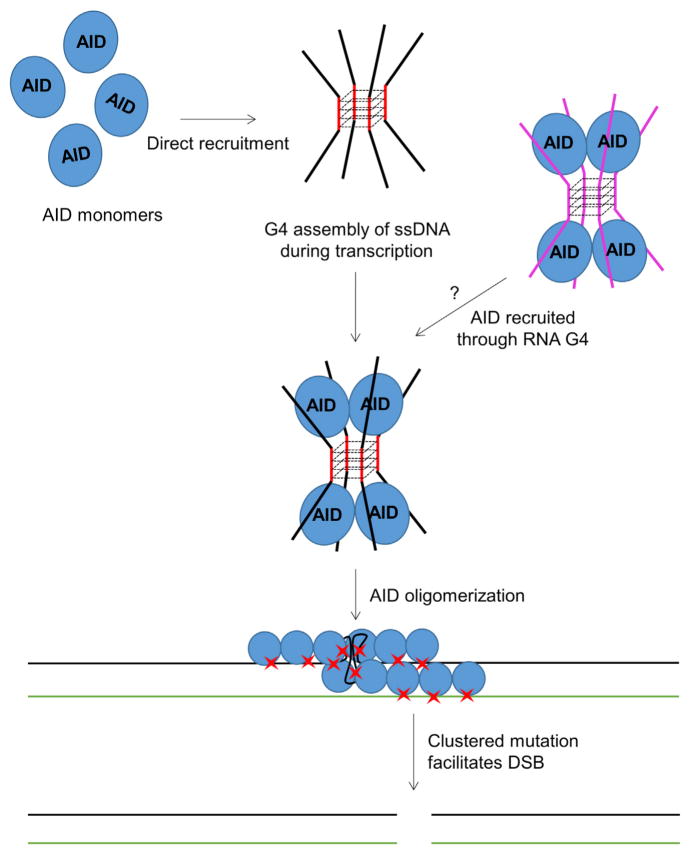
In this study, we combined biochemical, biophysical, and structural approaches to elucidate AID targeting mechanisms, especially in CSR. By using the fully functional AID.mono, we clearly demonstrated that G4 substrates mimicking the Ig S regions are preferred AID targets in vitro. Different from the previously proposed hotspot hypothesis, our data indicate that the AID preference for G4 substrates is predominantly due to their bundled ssDNA overhangs structure rather than any primary sequence motif. By solving structures of the MBP-fused AID.crystal alone and in complex with dCMP, we observed a bifurcated substrate-binding surface, which strongly supports a model of one AID recognizing two adjacent ssDNA overhangs in a structured substrate, such as G4, to achieve high affinity (Figure 7). Although AID can recruit either DNA or RNA in a sequence-independent manner, the positioning of dCMP in the active site suggests that only deoxycytidine, but not cytidine, can flip into the catalytic center to be deaminated.
Figure 7.
A Model of G4-Structure-Mediated AID Recruitment and Oligomerization that Create Mutation Clusters and DSB
Other than Ig S regions, it has been shown that G4 structures may be prevalent in other regions ofmammalian genomes (Chambers et al., 2015; Maizels and Gray, 2013). Especially, G-rich regions in AID off-target genes, like c-MYC and BCL6, have been proposed to be targeted by AID(Duquette et al., 2005, 2007). After inspecting many identified AID off-target genes, we found that these genes often contain G-repeat (GGG) enriched regions, particularly in their non-template strands, which may lead to G4 assembly during transcription and direct AID recruitment (Figure S6D; Table S4). However, more experimental data and genome sequence analysis may be required to establish the correlation between G-repeat motif distribution and AID targeting.
Additionally, our data suggest that G4 DNA-binding-induced cooperative AID oligomerization may contribute to its accumulation in Ig S regions, promoting high-density mutations, DSBs, and their joining during CSR (Figure 7). In comparison, SHM can be induced by CSR-deficient AID variants in cells (Hwang et al., 2015; Phamet al., 2016) and by APOBEC3 homologs during retroviral infection (Halemano et al., 2014), suggesting that mutations in Ig V regions may not require all the AID features employed in CSR. Because chromatin immunoprecipitation sequencing data showed that Ig V regions may not bind AID as stably as S regions (Matthews et al., 2014b), and the assistant patch mutant R174S also causes SHM deficiency (Durandy et al., 2006; Honjo et al., 2012), we speculate that Ig V regions may only transiently recruit AID using local secondary structures like branched DNA, which do not induce AID oligomerization and stable association.
Although our biochemical data and the bipartite DNA binding surface in AID structure strongly suggest a model of how AID recognizes structured substrates (Figure 6F), a definitive complex structure that contains fully characterized substrate conformation is still lacking, despite extensive efforts using both crystallography and electron microscopy (EM). Co-crystallization of purified AID/G4, AID/branched DNA, and AID/linear DNA complexes did not yield visible density for the substrates, likely due to crystal packing effects. Alternatively, we tried co-crystallization using 1- to 5-nt ssDNA fragments together with a 12-bp dsDNA (Table S1), but any ssDNA fragment longer than 1 nt did not reveal any density in the active site. Neither did soaking of various substrates into pre-formed AID crystals (Table S1). We further used EM on the AID/G4 complex, but the flexibility of the complex caused issues in the reconstruction (Figure S6E). Thus, the exact molecular basis for our model remains to be determined by future structural studies of AID/DNA complexes. Due to the close correlation of AID activity with B cell lymphoma and other types of cancers, the AID structures will also provide templates for potential therapeutic intervention against this important cytidine deaminase.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| APC Rat anti-Mouse IgG1 | BD Biosciences | 560089; RRID: AB_1645625 |
| Anti-AID | Chaudhuri et al., 2003 | N/A |
| Anti-GFP | MBL | Code # 598; RRID: AB_10597267 |
| Anti-CD40 | eBioscience | 16-0402-86 |
| Bacterial and Virus Strains | ||
| E.coli Bl21 (DE3) | Agilent Technologies | 200131 |
| E. coli KL16 | Coli Genetic Stock Center | CGSC# 4245 |
| E. coli BW310 | Coli Genetic Stock Center | CGSC# 6078 |
| GIBCO Sf9 cells | Thermo Scientific | 11496-015 |
| DH10Bac | Thermo Scientific | 10361-012 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| SYBR Gold Nucleic Acid Gel Stain | Thermo Scientific | S-11494 |
| 40% Acrylamide/Bis Solution, 19:1 | Bio-Rad | 161-0144 |
| SIGMAFAST Protease Inhibitor Cocktail Tablets, EDTA-Free | Sigma-Aldrich | S8830-20TAB |
| Rifampicin | Sigma-Aldrich | R3501-250MG |
| Dimethyl sulfate | Sigma-Aldrich | D186309-100ML |
| Piperidine | Sigma-Aldrich | 411027-100ML |
| Cellfectin II Reagent | Invitrogen | Cat#10362-100 |
| Recombinant Mouse Interleukin-4/IL-4 | Novoprotein | Cat#CK15 |
| Lipopolysaccharides from Escherichia coliO111:B4 | Sigma Aldrich | L2630-100MG |
| Uracil-DNA Glycosylase | NEB | M0280S |
| 2′-deoxycytidine | Sigma-Aldrich | D3897-500MG |
| 2′-deoxycytidine 5′-monophosphate | Sigma-Aldrich | D7625-100MG |
| Cytidine | Sigma-Aldrich | C4654-1G |
| Cytidine 5′-monophosphate | Sigma-Aldrich | C1006-1G |
| Uranyl formate | Electron Microscopy Sciences | 22450 |
| PreScission protease | GE Healthcare | 27-0843-01 |
| LB Broth | RPI | L24066-5000.0 |
| HyClone SFX-Insect Media | Thermo Scientific | SH30278LS |
| Grace’s Insect Medium, Supplemented | Thermo Scientific | 11605-102 |
| Fetal Bovine Serum | Sigma-Aldrich | TMS-013-B |
| Critical Commercial Assays | ||
| EasySep Mouse B Cell Isolation Kit | STEMCELL Technologies | Cat#19854 |
| Deposited Data | ||
| AID.crystal, co-crystallized with G4 DNA | Protein Data Bank | PDB: 5W0Z |
| AID.crystal E58A, co-crystallized with Branched DNA and cacodylic acid | Protein Data Bank | PDB: 5W0R |
| AID.crystal E58A, co-crystallized with dsDNA and cytidine | Protein Data Bank | PDB: 5W1C |
| AID.crystal E58A, co-crystallized with dsDNA and dCMP | Protein Data Bank | PDB: 5W0U |
| Recombinant DNA | ||
| pGEX6P1 | GE Healthcare | Cat#28954648 |
| pTrc99A | DNA Resource Core | N/A |
| pFastBac | Thermo Scientific | 10584-027 |
| All synthesized DNA/RNA | Integrated DNA Technologies | N/A |
| Other | ||
| S1000 Thermal Cycler | Bio-Rad | S1000 |
| ChemiDoc MP imager | Bio-Rad | 1708280 |
| Image Lab Version 4.1 | Bio-Rad | N/A |
| Image Scanner FLA-9000 | Fujifilm | N/A |
| Multi Gauge Version 3.0 | Fujifilm | N/A |
| Origin | OriginLab Corporation | N/A |
| mini-DAWN TRISTAR | Wyatt Technology | N/A |
| Optilab DSP | Wyatt Technology | N/A |
| ASTRA V | Wyatt Technology | N/A |
| BLItz system with Streptavidin (SA) Biosensor | ForteBio | N/A |
| BLItz 1.1 | ForteBio | N/A |
| Tecnai G2 Spirit BioTWIN electron microscope | FEI | N/A |
| J-815 CD Spectropolarimeter | Jasco | N/A |
| Phenix | Adams et al., 2010 | N/A |
| CCP4 | Winn et al., 2011 | N/A |
| Coot | Emsley et al., 2010 | N/A |
| Pymol | Schrödinger | N/A |
| POCASA 1.1 | Yu et al., 2010 | N/A |
| YASARA | YASARA Biosciences | N/A |
| Integrative Genomics Viewer | Broad Institute | N/A |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Hao Wu (wu@crystal.harvard.edu).
METHOD DETAILS
Protein Engineering and Purification
Constructs of human AID (UniProt: Q9GZX7) were generated in the pFastBac vector (Thermo Fisher Scientific) and expressed in Sf9 insect cells for 48 hr using recombinant baculoviruses. To overcome AID aggregation in recombinant expression, we created numerous AID constructs and found that adding the maltose binding protein (MBP) solubility tag, modifying the N-terminal nuclear localization sequence (NLS) and removing the C-terminal tail (CTT) resulted in improved yield, but retained AID aggregation. By screening non-conserved AID surface residue mutations, we identified the AID mutant H130A/R131E. We named this construct AID.mono because it contained a distinct monomeric fraction in addition to the aggregated faction, in contrast to complete aggregation of wild-type (WT) AID. When necessary, the MBP-tag was removed by incubating with the PreScission Protease (GE Healthcare) at 1/100 ratio at 4°C overnight. Adding C-terminal tail back (AID.mono+CTT) decreased the monomer yield by 95%. In AID.crystal, the linker between AID and MBP was shortened to facilitate crystallization. The proteins were affinity-purified using amylose resin (New England Biolabs), followed by chromatography using Superdex 200 10/300 GL, monomer fraction was further purified by HiTrap Heparin HP (GE Healthcare) and another Superdex 200. Final gel filtration buffer contains 20 mM Bis-Tris at pH 6.8, 200 mM NaCl and 1 mM tris(2-carboxyethyl)phosphine) (TCEP).
Human Apobec3A (1–199) and APOBEC3G (197–380) was cloned into a pGEX6p-1 vector and expressed in Bl21 (DE3) strain. The N-GST fusion proteins were purified by Glutathione Sepharose 4B resin (GE Healthcare), and the tag was removed by on-column Precision Protease treatment. APOBEC proteins were further purified over a Superdex 200.
Ex Vivo CSR Assay
The AID constructs used in this assay contained point mutations in AID.mono (H130A/R131E), AID.crystal (H130A/R131E plus F42E/F141Y/F145E), assistant patch residues, or AIDv. The ex vivo CSR assay was performed as described previously (Cheng et al., 2009). Briefly, AID-deficient mouse splenic B cells were stimulated with IL-4 (Novoprotein) and anti-CD40 (eBioscience) to induce CSR to IgG1. One day after stimulation, WT or mutant AID together with GFP via an internal ribosome entry site (IRES) was retrovirally delivered into the cells. The percentage of GFP-positive cells that underwent switching to IgG1 was taken as the level of CSR rescue. The data for each mutant were either from 3 or 6 mice.
Rifampicin Resistance (RifR) Assay
WT and mutant AID were cloned into the pTrc99A vector (pTrc99A-AID) and the rifampicin resistance (RifR) assay was performed as previously described (Wang et al., 2009). Briefly, E. coli strain KL16 (Hfr [PO-45] relA1 spoT1 thi-1) and its UDG-deficient derivative BW310 bacteria transformed with pTrc99A-AID plasmids were grown overnight to saturation in LB medium supplemented with ampicillin (100 μg/ml) and isopropyl β-D-1-thiogalactopyranoside (IPTG, 1 mM), and plated on LB low-salt agar containing ampicillin (100 μg/ml) and rifampicin (50 μg/ml). Mutation frequency was measured by determining the median number of colony-forming cells that survived selection per 107 viable cells plated from 12 independent cultures. The identity of mutations was determined by sequencing the relevant section of rpoB (typically from 25 to 200 individual colonies) after PCR amplification using oligonucleotides 5′-TTGGCGAAATGGCGGAAAACC-3′ and 5′-CACCGACGGATACCACCTGCTG-3′) (synthesized by Integrated DNA Technologies).
In Vitro Deamination Assay
Each 10 μl reaction contained 0.1 or 1 μMAID, and1μMsubstrateDNA labeled by 6-carboxyfluorescein (FAM) at 5′ end (synthesized by Integrated DNA Technologies) in 20 mM HEPES at pH 7.5, 100 mM KCl and 1 mMDTT. Following incubation at 37°C for the indicated length of time, each reaction was raised to 95°C for 10 min and flash cooled to inactivate AID and resolve DNA structures. Sufficient amount of UDG (NewEngland Biolabs, 5 unit with each unit catalyzing 60 pmol/min at 37°C) was then added and the mixture was incubated at 37°C for 1h. Lastly, NaOH was added to a final concentration of 0.15 M, and treated at 95°C for 15 min to break abasic sites. Urea gel was used to separate the product fromthe substrate. Experiments in Figures 5Gand 5H used SYBRGold staining to save cost. Images were taken on Image Scanner FLA-9000 (Fujifilm) using excitation wavelength of 495 nm and emission wavelength of 519 nm. Quantification was performed using the Image Lab (Bio-Rad) and Multi Gauge (Fujifilm) software.
In Vitro EMSA
Up to 25 μMAID was titrated into 10 nM FAM labeled DNA followed by 10%–12% acrylamide:bisacrylamide (19:1) native gel to separate free DNA and the AID/DNA complex. Quantified free DNA amounts at different AID concentrations were used to calculate the dissociation constant (KD) and the Hill coefficient (n) of the AID/DNA interaction. 100 nM unlabeled TGGGGT1-5 and TGGGGT10 were used in experiments of Figure 3B to eliminate any effect from the fluorophore and its linker to DNA. SYBR Gold was used for staining. Images were taken on Image Scanner FLA-9000 (Fujifilm). The Multi Gauge (Fujifilm) software was used for quantification and the Origin software (OriginLab Corporation) was used in curve fitting.
G4 and Branched DNA Purification and Characterization
To generate mostly G4 structures, Sμ fragment and G-repeat DNAs were dissolved in 20 mM HEPES at pH 7.5, 100 mM KCl and 1 mM DTT, incubated at 95°C for 5 min and slowly cooled down to room temperature. In contrast, to generate mostly linear DNA, heat denaturation at 95°C for 10 min followed by flash cooling on ice was used to eliminate DNA structures. A Jasco J-815 Circular dichroism (CD) spectropolarimeter was used in determining the parallel and anti-parallel conformation of the G4 assembly (Vorlíčková et al., 2012). When preparing large amounts of G4 structures, up to 1M KCl was used. The size exclusion column Superdex 75 or 200 (GE Healthcare) was used to separate G4 and linear DNA fractions. Notably, the FAM fluorescence intensity was quenched significantly upon single G-repeat G4 assembly. DMS protection assay was performed as described (Sun and Hurley, 2010). Branched DNAs were generated by the similar annealing procedure without the further purification steps.
AID/DNA Complex Crystallization and Structure Determination
Gel filtration purified AID.crystal/G4 complex containing G4 DNA with the sequence of TGGGGTTTTTTT was crystallized using 0.26 M NaCl, 0.1 M MES at pH 6.0 and 12% PEG3350 at 25°C. Other AID complexes with G4 substrates including TGGGGTTTTT, TGGGGTTTTTT, TGGGGTTCTTTT, TGGGGTTCTTT, TGGGGAACTTT, TGGGGAAUTTT and TTTTTTGGGGGTTCTTT were also crystallized, which showed similar AID structures and no ordered DNA.
Gel filtration purified AID.crystal (WT and E58A)/branched complexes containing DNA with the sequences of GTTCAAGGCCA GAGCTTT and TTTTTCCTGGCCTTGAAC were crystallized using 0.05 M sodium cacodylate at pH 5.5, 20 mM MgCl2, 10 mM CaCl2, 10 mM spermidine and 5% PEG3350 at 16°C.
When using dsDNA as a crystallization chaperone, AID.crystal (E58A) was mixed with dsDNA with the sequences of GTTCAAGG CCAG and CTGGCCTTGAAC at 1:1 molar ratio, and crystallized either alone or with various substrate ligands such as 5′-dCMP, deoxycytidine, and cytidine (Sigma) at 20mM concentration, under 0.1MMES at pH 6.2, 3% PEG3350 and 10mMCaCl2 at 16°C. Many other short oligos were also tried in co-crystallization, but did not show ordered nucleotide density in the structures (Table S1).
The AID/G4 structure was solved by molecular replacement calculations in Phaser using the crystal structure of MBP (PDB: 3VD8) and APOBEC3C as the model (PDB: 3VOW) (Kitamura et al., 2012). Subsequent structures were determined by molecular replacement using the AID structure as a model. Phenix, CCP4 and Coot were used in model building and refinement (Adams et al., 2010; Emsley et al., 2010; Winn et al., 2011). The POCASA 1.1 server was used for pocket prediction (Yu et al., 2010). The YASARA server was used for energy minimization of the substrate manually docked to AID (YASARA Biosciences). Pymol was utilized for molecular visualization and structure display (Schrödinger).
Molecular Mass Measurement by Multi-Angle Light Scattering (MALS)
AID alone and AID-DNA complexes were reconstituted and purified as described above. The complex peak fractions containing ~0.2 mg protein were loaded onto a Superdex 200 gel filtration column coupled to a three-angle light scattering detector (mini-DAWN TRISTAR) and a refractive index detector (Optilab DSP) (Wyatt Technology). Data analysis was carried out using ASTRA V.
Electron Microscopy
AID and AID/DNA complex were applied to carbon-coated grids and negatively stained with1% uranyl formate (Electron Microscopy Sciences). Samples were examined in a Tecnai G2 Spirit BioTWIN electron microscope (FEI) at an accelerating voltage of 80 keV and a nominal magnification of × 49,000.
QUANTIFICATION AND STATISTICAL ANALYSES
In Vitro EMSA and Deamination Assay Quantification
Images were obtained by instruments described in Method Details. The band areas were boxed and backgrounds were subtracted. The band quantification results were averaged from three repeats and the error bars were shown as standard deviations.
Data Analysis in Flow Cytometry
Cell population was identified in FlowJo. Unpaired t test was used and two-tailed P value was calculated using GraphPad Prism6. The class switch recombination (CSR) results showed average from three biological replicates, with bars indicated SD.
DATA AND SOFTWARE AVAILABILITY
Accession Numbers
The accession numbers for the data reported in this paper are PDB: 5W0Z (AID.crystal, co-crystallized with G4 DNA), 5W0R (AID.crystal E58A, co-crystallized with Branched DNA and cacodylic acid), 5W1C (AID.crystal E58A, co-crystallized with dsDNA and cytidine), and 5W0U (AID.crystal E58A, co-crystallized with dsDNA and dCMP).
Supplementary Material
Figure S1. Active Monomeric AID from Protein Engineering, Related to Figure 1
(A) Sequence alignment of WT AID, AID.mono+CTT, AID.mono, AID.crystal and AIDv. Secondary structures and important residues are labeled.
(B) SDS-PAGE of gel filtration fractions of AID WT, AID.mono+CTT, and AID.mono, showing that AID.mono contained a most significant monomeric peak.
(C) Sequence alignment of AID showing that residues H130 and R131 are not conserved across species.
(D) Coomassie blue stained SDS-PAGE showing His-MBP tag removal of AID.mono.
(E) In vitro deamination assay showing that AID.mono exhibited similar activity with and without the MBP tag. Experiments used 0.1 μM AID and 1 μM DNA.
(F) Western blot showing the expression levels of AID WT and mutants used in ex vivo CSR assay.
Figure S2. Linear and G4 Structured Substrates, Related to Figure 2
(A) An example of mouse Sμ region sequence showing the pattern of G-repeats (red) and tandem AGCT hotspots (underlined).
(B) Native PAGE showing oligomer formation of the Sμ fragment and single G-repeat substrate. The intermolecular G4 band intensity for the single G-repeat substrate was quenched relative to the linear fraction band.
(C) Dimethyl sulfate (DMS) modification assay showed that guanines in GGGGTG motif are mostly protected by the G4 structure (lane 2). DMS methylates Gs except those in Hoogsteen bonding in G4.
(D) Circular dichroism (CD) spectrum of the G4 samples at 25°C. DNA samples were annealed in 100mM KCl as described in Methods. The positive signal around 270nm and negative signal around 240nm indicated predominant parallel conformation (anti-parallel G4 would show positive signal around 295nm and negative signal around 265nm).
Figure S3. AID preferentially binds and deaminates G4 structured substrates, Related to Figure 3
(A) In vitro deamination assay showing that AID.mono+CTT has higher activity on a G4 substrate than on a linear substrate, with and without hotspots. Experiments used 0.1 μM AID and 1 μM DNA.
(B) Sequences of G4 substrates used in Figure 3G.
(C) Gel filtration chromatography of AID.mono oligomerization in the presence of G4 substrate.
(D) EM images showing AID.mono but not AID.crystal formed oligomers upon G4 binding. 2 μM AID and 1 μM G4 DNA were used.
(E) Sequences of G4 substrates used in Figure 3H.
Figure S4. Structures of AID and its Complex with dCMP, Related to Figure 4
(A) Purification of AID.crystal showing the predominant monomer peak. Molecular mass was measured by MALS.
(B) In vitro deamination assay showing that AID.crystal has the same G4 preference. Experiments used 0.1 μM AID and 1 μM DNA.
(C) In vitro deamination assay showing that AID.mono has higher activity on a branched DNA substrate than on a linear substrate. Experiments used 0.1 μM AID and 1 μM DNA.
(D) Urea gel of dissolved crystals showing the presence of DNA.
(E) Stacked dsDNA (magenta and yellow) separates the positively charged AID molecules (cyan) in the AID/dsDNA crystal lattice, suggesting that the dsDNA functions as a “crystallization chaperone”. The 2Fo-Fc map at 1.0 σ (blue) is shown superimposed with dsDNA. The positively charged assistant patch at helix α6 lies close to the dsDNA in the crystal lattice.
(F) Structural alignment showed major difference at α1-β1 loop between AIDv and AID.crystal.
(G) Active site of an Apo-AID structure showing a cacodylate as the fourth ligand for Zn2+.
(H) Superimposed active sites of WT and E58A Apo-AID structures.
(I) Structural alignment showing the two conformations of the β4-α4 loop.
(J) Structural alignment showing different structure folding of AID and cytidine deaminase (CDA, PDB code1MQ0), but conserved Zn2+ positions (pink and cyan).
(K) Structural alignment showing similar catalytic center arrangement between AID and CDA. Residue E58 was positioned by alignment with the Apo-AID structure.
(L) Omit difference maps (green, 2.0 σ) showing the electron density for dCMP, CMP, C, and dC.
(M) Structural alignment of bound cytidine and dCMP showing the similar base position, but different sugar position.
Figure S5. AID Structure Revealed a Bifurcated Recognition Site for Two ssDNA Overhangs, Related to Figure 5
(A–B) Structures of T4 RNase H and Cas9 exhibit architectures of bifurcated DNA binding surface similar to AID.
(C) Western blot showing the expression levels of AID WT and assistant patch mutants used in ex vivo CSR assay. Mutant R171D/R174E has a much lower expression level and was not included in Figure 5E.
(D) An EMSA curve showing that the binding of AID.crystal to G4 substrate does not exhibit coorperativity (n ≈ 1), unlike AID.mono. Data are represented as mean ± SD from three independent measurements.
Figure S6. Conformational Change and Oligomerization in AID Substrate Recognition, Related to Figure 6 and Table S4
(A) Structure of APOBEC3G N-domain binding a poly-T ssDNA.
(B) Structural alignment showing that the loops surrounding the active sites are significantly different between AID and A3A.
(C) Modeling of AGCTT into the predicted substrate chain channel revealed a movement of the α1-β1 loop.
(D) The GGG frequency of G-rich regions in the non-template strand of AID off-target genes. Data calculated from genes and segments listed in Table S4.
(E) The 2D classification result from 4,584 negative strained AID.crystal4/G4 particles. The significant blurriness of the result suggests strong flexibility of the complex. Arrow: views with all four AID blobs.
Table S1. AID/Substrate Complex Co-crystallization Efforts, Related to Figure 4
Table S2. Crystallographic Statistics, Related to Figure 4
Table S3. AID mutations in hyper-IgM syndrome patients, Related to Figure 4
Highlights.
Structured substrates, such as G4 substrates, are preferred AID targets in vitro
A bifurcated substrate-binding surface supports structured-substrate recognition
G4 substrates induce AID oligomerization upon binding
Disrupting structured-substrate recognition or AID oligomerization compromises CSR
Acknowledgments
We thank Ermelinda Damko and Devendra Srivastava for their earlier work on this project; Ming Tian, Zhou Du, Leng Siew Yeap, Jiazhi Hu, and Junchao Dong for discussions; Yang Li for EM data analysis; Xia Xie for assistances in CSR assay; Rida Mourtada in Dr. Loren D. Walensky’s lab and Kelly Arnett in Harvard Medical School Center for Macromolecular Interactions for their assistance with CD spectroscopy; Sukumar Narayanasami and Surajit Banerjee of NE-CAT at the Advance Photon Source for their assistance on data collection; and Maria Ericsson and Louise Trakimas of Harvard Medical School EM facility for assistance on EM imaging. This work was supported by a Cancer Research Institute Irvington Postdoctoral Fellowship (to Q.Q.), a Lymphoma Research Foundation Fellowship (to F.-L.M.), NIH R01 AI077595 (to F.W.A.), and NIH F30 AI114179 (to J.K.H.). F.W.A. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Supplemental Information includes six figures and four tables can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2017.06.034.
AUTHOR CONTRIBUTIONS
H.W. supervised the project. Q.Q. and L.W. designed the experiments and performed protein engineering, in vitro assays, structure determination, and analysis. F.-L.M. performed ex vivo CSR assay, and F.W.A. supervised the effort. H.W. and Q.Q. wrote most of the manuscript with help from J.K.H. and F.W.A. for the introduction. All authors contributed to data analysis and critical interpretation of results and approved the manuscript.
References
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin C, Leroy JL. The formation pathway of tetramolecular G-quadruplexes. Nucleic Acids Res. 2008;36:477–488. doi: 10.1093/nar/gkm1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu U, Chaudhuri J, Alpert C, Dutt S, Ranganath S, Li G, Schrum JP, Manis JP, Alt FW. The AID antibody diversification enzyme is regulated by protein kinase A phosphorylation. Nature. 2005;438:508–511. doi: 10.1038/nature04255. [DOI] [PubMed] [Google Scholar]
- Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci USA. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers VS, Marsico G, Boutell JM, Di Antonio M, Smith GP, Balasubramanian S. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat Biotechnol. 2015;33:877–881. doi: 10.1038/nbt.3295. [DOI] [PubMed] [Google Scholar]
- Chaudhuri J, Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4:541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt FW. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- Chaudhuri J, Khuong C, Alt FW. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature. 2004;430:992–998. doi: 10.1038/nature02821. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Vuong BQ, Basu U, Franklin A, Schwer B, Astarita J, Phan RT, Datta A, Manis J, Alt FW, Chaudhuri J. Integrity of the AID serine-38 phosphorylation site is critical for class switch recombination and somatic hypermutation in mice. Proc Natl Acad Sci USA. 2009;106:2717–2722. doi: 10.1073/pnas.0812304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos JM, Tomanicek SJ, Jones CE, Nossal NG, Mueser TC. Crystal structure of bacteriophage T4 5′ nuclease in complex with a branched DNA reveals how flap endonuclease-1 family nucleases bind their substrates. J Biol Chem. 2007;282:31713–31724. doi: 10.1074/jbc.M703209200. [DOI] [PubMed] [Google Scholar]
- Duke JL, Liu M, Yaari G, Khalil AM, Tomayko MM, Shlomchik MJ, Schatz DG, Kleinstein SH. Multiple transcription factor binding sites predict AID targeting in non-Ig genes. J Immunol. 2013;190:3878–3888. doi: 10.4049/jimmunol.1202547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette ML, Handa P, Vincent JA, Taylor AF, Maizels N. Intracellular transcription of G-rich DNAs induces formation of G-loops, novel structures containing G4 DNA. Genes Dev. 2004;18:1618–1629. doi: 10.1101/gad.1200804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette ML, Pham P, Goodman MF, Maizels N. AID binds to transcription-induced structures in c-MYC that map to regions associated with translocation and hypermutation. Oncogene. 2005;24:5791–5798. doi: 10.1038/sj.onc.1208746. [DOI] [PubMed] [Google Scholar]
- Duquette ML, Huber MD, Maizels N. G-rich proto-oncogenes are targeted for genomic instability in B-cell lymphomas. Cancer Res. 2007;67:2586–2594. doi: 10.1158/0008-5472.CAN-06-2419. [DOI] [PubMed] [Google Scholar]
- Durandy A, Peron S, Taubenheim N, Fischer A. Activation-induced cytidine deaminase: structure-function relationship as based on the study of mutants. Hum Mutat. 2006;27:1185–1191. doi: 10.1002/humu.20414. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halemano K, Guo K, Heilman KJ, Barrett BS, Smith DS, Hasenkrug KJ, Santiago ML. Immunoglobulin somatic hypermutation by APOBEC3/Rfv3 during retroviral infection. Proc Natl Acad Sci USA. 2014;111:7759–7764. doi: 10.1073/pnas.1403361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Masani S, Yu K. Overlapping activation-induced cytidine deaminase hotspot motifs in Ig class-switch recombination. Proc Natl Acad Sci USA. 2011;108:11584–11589. doi: 10.1073/pnas.1018726108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T, Kobayashi M, Begum N, Kotani A, Sabouri S, Nagaoka H. The AID dilemma: infection, or cancer? Adv Cancer Res. 2012;113:1–44. doi: 10.1016/B978-0-12-394280-7.00001-4. [DOI] [PubMed] [Google Scholar]
- Hwang JK, Alt FW, Yeap LS. Related mechanisms of antibody somatic hypermutation and class switch recombination. Microbiol Spectr. 2015;3 doi: 10.1128/microbiolspec.MDNA3-0037-2014. MDNA3-0037-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Taylor DW, Chen JS, Kornfeld JE, Zhou K, Thompson AJ, Nogales E, Doudna JA. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science. 2016;351:867–871. doi: 10.1126/science.aad8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JJ, Manuel CA, Barrett CV, Raber S, Lucas H, Sutter P, Larijani M. Catalytic pocket inaccessibility of activation-induced cytidine deaminase is a safeguard against excessive mutagenic activity. Structure. 2015;23:615–627. doi: 10.1016/j.str.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Ode H, Nakashima M, Imahashi M, Naganawa Y, Kurosawa T, Yokomaku Y, Yamane T, Watanabe N, Suzuki A, et al. The APOBEC3C crystal structure and the interface for HIV-1 Vif binding. Nat Struct Mol Biol. 2012;19:1005–1010. doi: 10.1038/nsmb.2378. [DOI] [PubMed] [Google Scholar]
- Kohli RM, Abrams SR, Gajula KS, Maul RW, Gearhart PJ, Stivers JT. A portable hot spot recognition loop transfers sequence preferences from APOBEC family members to activation-induced cytidine deaminase. J Biol Chem. 2009;284:22898–22904. doi: 10.1074/jbc.M109.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larijani M, Frieder D, Basit W, Martin A. The mutation spectrum of purified AID is similar to the mutability index in Ramos cells and in ung(−/−)msh2(−/−) mice. Immunogenetics. 2005;56:840–845. doi: 10.1007/s00251-004-0748-0. [DOI] [PubMed] [Google Scholar]
- Larijani M, Petrov AP, Kolenchenko O, Berru M, Krylov SN, Martin A. AID associates with single-stranded DNA with high affinity and a long complex half-life in a sequence-independent manner. Mol Cell Biol. 2007;27:20–30. doi: 10.1128/MCB.00824-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N, Gray LT. The G4 genome. PLoS Genet. 2013;9:e1003468. doi: 10.1371/journal.pgen.1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews AJ, Zheng S, DiMenna LJ, Chaudhuri J. Regulation of immunoglobulin class-switch recombination: choreography of noncoding transcription, targeted DNA deamination, and long-range DNA repair. Adv Immunol. 2014a;122:1–57. doi: 10.1016/B978-0-12-800267-4.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews AJ, Husain S, Chaudhuri J. Binding of AID to DNA does not correlate with mutator activity. J Immunol. 2014b;193:252–257. doi: 10.4049/jimmunol.1400433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean D, Huppi K, Bell M, Staudt L, Gerhard W, Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci USA. 1984;81:3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng FL, Du Z, Federation A, Hu J, Wang Q, Kieffer-Kwon KR, Meyers RM, Amor C, Wasserman CR, Neuberg D, et al. Convergent transcription at intragenic super-enhancers targets AID-initiated genomic instability. Cell. 2014;159:1538–1548. doi: 10.1016/j.cell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S, Begum NA, Hu W, Honjo T. Functional requirements of AID’s higher order structures and their interaction with RNA-binding proteins. Proc Natl Acad Sci USA. 2016;113:E1545–E1554. doi: 10.1073/pnas.1601678113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel CS, Lee JW, Wang LC, Kohli RM. Nucleic acid determinants for selective deamination of DNA over RNA by activation-induced deaminase. Proc Natl Acad Sci USA. 2013;110:14225–14230. doi: 10.1073/pnas.1306345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavri R, Gazumyan A, Jankovic M, Di Virgilio M, Klein I, Ansarah-Sobrinho C, Resch W, Yamane A, Reina San-Martin B, Barreto V, et al. Activation-induced cytidine deaminase targets DNA at sites of RNA polymerase II stalling by interaction with Spt5. Cell. 2010;143:122–133. doi: 10.1016/j.cell.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–103. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- Pham P, Bransteitter R, Petruska J, Goodman MF. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424:103–107. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- Pham P, Afif SA, Shimoda M, Maeda K, Sakaguchi N, Pedersen LC, Goodman MF. Structural analysis of the activation-induced deoxycytidine deaminase required in immunoglobulin diversification. DNA Repair (Amst) 2016;43:48–56. doi: 10.1016/j.dnarep.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Wang Q, Dose M, Pruett N, Kieffer-Kwon KR, Resch W, Liang G, Tang Z, Mathé E, Benner C, et al. B cell super-enhancers and regulatory clusters recruit AID tumorigenic activity. Cell. 2014;159:1524–1537. doi: 10.1016/j.cell.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K, Förster I, Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987;238:1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Rogozin IB, Diaz M. Cutting edge: DGYW/WRCH is a better predictor of mutability at G:C bases in Ig hypermutation than the widely accepted RGYW/WRCY motif and probably reflects a two-step activation-induced cytidine deaminase-triggered process. J Immunol. 2004;172:3382–3384. doi: 10.4049/jimmunol.172.6.3382. [DOI] [PubMed] [Google Scholar]
- Shi K, Carpenter MA, Banerjee S, Shaban NM, Kurahashi K, Salamango DJ, McCann JL, Starrett GJ, Duffy JV, Demir Ö, et al. Structural basis for targeted DNA cytosine deamination and mutagenesis by APOBEC3A and APOBEC3B. Nat Struct Mol Biol. 2017;24:131–139. doi: 10.1038/nsmb.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivarov V, Shinkura R, Honjo T. Dissociation of in vitro DNA deamination activity and physiological functions of AID mutants. Proc Natl Acad Sci USA. 2008;105:15866–15871. doi: 10.1073/pnas.0806641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Hurley LH. Biochemical techniques for the characterization of G-quadruplex structures: EMSA, DMS footprinting, and DNA polymerase stop assay. Methods Mol Biol. 2010;608:65–79. doi: 10.1007/978-1-59745-363-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlíčková M, Kejnovská I, Sagi J, Renčiuk D, Bednářová K, Motlová J, Kypr J. Circular dichroism and guanine quadruplexes. Methods. 2012;57:64–75. doi: 10.1016/j.ymeth.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang Z, Rada C, Neuberger MS. AID upmutants isolated using a high-throughput screen highlight the immunity/cancer balance limiting DNA deaminase activity. Nat Struct Mol Biol. 2009;16:769–776. doi: 10.1038/nsmb.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Rada C, Neuberger MS. Altering the spectrum of immunoglobulin V gene somatic hypermutation by modifying the active site of AID. J Exp Med. 2010;207:141–153. doi: 10.1084/jem.20092238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li SX, Yang H, Chen XS. Crystal structures of APOBEC3G N-domain alone and its complex with DNA. Nat Commun. 2016;7:12193. doi: 10.1038/ncomms12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Fulop Z, Wu G, Pone EJ, Zhang J, Mai T, Thomas LM, Al-Qahtani A, White CA, Park SR, et al. 14-3-3 adaptor proteins recruit AID to 5′-AGCT-3′-rich switch regions for class switch recombination. Nat Struct Mol Biol. 2010;17:1124–1135. doi: 10.1038/nsmb.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol. 2012;12:517–531. doi: 10.1038/nri3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap LS, Hwang JK, Du Z, Meyers RM, Meng FL, Jakubauskaitė A, Liu M, Mani V, Neuberg D, Kepler TB, et al. Sequence-intrinsic mechanisms that target AID mutational outcomes on antibody genes. Cell. 2015;163:1124–1137. doi: 10.1016/j.cell.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Chedin F, Hsieh CL, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhou Y, Tanaka I, Yao M. Roll: a new algorithm for the detection of protein pockets and cavities with a rolling probe sphere. Bioinformatics. 2010;26:46–52. doi: 10.1093/bioinformatics/btp599. [DOI] [PubMed] [Google Scholar]
- Zheng S, Vuong BQ, Vaidyanathan B, Lin JY, Huang FT, Chaudhuri J. Non-coding RNA generated following lariat debranching mediates targeting of AID to DNA. Cell. 2015;161:762–773. doi: 10.1016/j.cell.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Active Monomeric AID from Protein Engineering, Related to Figure 1
(A) Sequence alignment of WT AID, AID.mono+CTT, AID.mono, AID.crystal and AIDv. Secondary structures and important residues are labeled.
(B) SDS-PAGE of gel filtration fractions of AID WT, AID.mono+CTT, and AID.mono, showing that AID.mono contained a most significant monomeric peak.
(C) Sequence alignment of AID showing that residues H130 and R131 are not conserved across species.
(D) Coomassie blue stained SDS-PAGE showing His-MBP tag removal of AID.mono.
(E) In vitro deamination assay showing that AID.mono exhibited similar activity with and without the MBP tag. Experiments used 0.1 μM AID and 1 μM DNA.
(F) Western blot showing the expression levels of AID WT and mutants used in ex vivo CSR assay.
Figure S2. Linear and G4 Structured Substrates, Related to Figure 2
(A) An example of mouse Sμ region sequence showing the pattern of G-repeats (red) and tandem AGCT hotspots (underlined).
(B) Native PAGE showing oligomer formation of the Sμ fragment and single G-repeat substrate. The intermolecular G4 band intensity for the single G-repeat substrate was quenched relative to the linear fraction band.
(C) Dimethyl sulfate (DMS) modification assay showed that guanines in GGGGTG motif are mostly protected by the G4 structure (lane 2). DMS methylates Gs except those in Hoogsteen bonding in G4.
(D) Circular dichroism (CD) spectrum of the G4 samples at 25°C. DNA samples were annealed in 100mM KCl as described in Methods. The positive signal around 270nm and negative signal around 240nm indicated predominant parallel conformation (anti-parallel G4 would show positive signal around 295nm and negative signal around 265nm).
Figure S3. AID preferentially binds and deaminates G4 structured substrates, Related to Figure 3
(A) In vitro deamination assay showing that AID.mono+CTT has higher activity on a G4 substrate than on a linear substrate, with and without hotspots. Experiments used 0.1 μM AID and 1 μM DNA.
(B) Sequences of G4 substrates used in Figure 3G.
(C) Gel filtration chromatography of AID.mono oligomerization in the presence of G4 substrate.
(D) EM images showing AID.mono but not AID.crystal formed oligomers upon G4 binding. 2 μM AID and 1 μM G4 DNA were used.
(E) Sequences of G4 substrates used in Figure 3H.
Figure S4. Structures of AID and its Complex with dCMP, Related to Figure 4
(A) Purification of AID.crystal showing the predominant monomer peak. Molecular mass was measured by MALS.
(B) In vitro deamination assay showing that AID.crystal has the same G4 preference. Experiments used 0.1 μM AID and 1 μM DNA.
(C) In vitro deamination assay showing that AID.mono has higher activity on a branched DNA substrate than on a linear substrate. Experiments used 0.1 μM AID and 1 μM DNA.
(D) Urea gel of dissolved crystals showing the presence of DNA.
(E) Stacked dsDNA (magenta and yellow) separates the positively charged AID molecules (cyan) in the AID/dsDNA crystal lattice, suggesting that the dsDNA functions as a “crystallization chaperone”. The 2Fo-Fc map at 1.0 σ (blue) is shown superimposed with dsDNA. The positively charged assistant patch at helix α6 lies close to the dsDNA in the crystal lattice.
(F) Structural alignment showed major difference at α1-β1 loop between AIDv and AID.crystal.
(G) Active site of an Apo-AID structure showing a cacodylate as the fourth ligand for Zn2+.
(H) Superimposed active sites of WT and E58A Apo-AID structures.
(I) Structural alignment showing the two conformations of the β4-α4 loop.
(J) Structural alignment showing different structure folding of AID and cytidine deaminase (CDA, PDB code1MQ0), but conserved Zn2+ positions (pink and cyan).
(K) Structural alignment showing similar catalytic center arrangement between AID and CDA. Residue E58 was positioned by alignment with the Apo-AID structure.
(L) Omit difference maps (green, 2.0 σ) showing the electron density for dCMP, CMP, C, and dC.
(M) Structural alignment of bound cytidine and dCMP showing the similar base position, but different sugar position.
Figure S5. AID Structure Revealed a Bifurcated Recognition Site for Two ssDNA Overhangs, Related to Figure 5
(A–B) Structures of T4 RNase H and Cas9 exhibit architectures of bifurcated DNA binding surface similar to AID.
(C) Western blot showing the expression levels of AID WT and assistant patch mutants used in ex vivo CSR assay. Mutant R171D/R174E has a much lower expression level and was not included in Figure 5E.
(D) An EMSA curve showing that the binding of AID.crystal to G4 substrate does not exhibit coorperativity (n ≈ 1), unlike AID.mono. Data are represented as mean ± SD from three independent measurements.
Figure S6. Conformational Change and Oligomerization in AID Substrate Recognition, Related to Figure 6 and Table S4
(A) Structure of APOBEC3G N-domain binding a poly-T ssDNA.
(B) Structural alignment showing that the loops surrounding the active sites are significantly different between AID and A3A.
(C) Modeling of AGCTT into the predicted substrate chain channel revealed a movement of the α1-β1 loop.
(D) The GGG frequency of G-rich regions in the non-template strand of AID off-target genes. Data calculated from genes and segments listed in Table S4.
(E) The 2D classification result from 4,584 negative strained AID.crystal4/G4 particles. The significant blurriness of the result suggests strong flexibility of the complex. Arrow: views with all four AID blobs.
Table S1. AID/Substrate Complex Co-crystallization Efforts, Related to Figure 4
Table S2. Crystallographic Statistics, Related to Figure 4
Table S3. AID mutations in hyper-IgM syndrome patients, Related to Figure 4