Abstract
Objectives
To assess the patients’ perspective about factors associated with stent choice.
Background
Drug eluting stents (DES) markedly reduce the risk of repeat percutaneous coronary intervention (PCI), but necessitate a longer duration of dual anti-platelet therapy (DAPT) as compared with bare metal stents (BMS). Thus, understanding patients’ perspective about factors associated with stent choice is paramount.
Methods
Patients undergoing angiography rated, on a 10-point scale, the importance (1=not important, 10=most important) of avoiding repeat revascularization and avoiding the following potential DAPT drawbacks: bleeding/bruising, more pills/day, medication costs and delaying elective surgery. The factor, or group of factors, that was rated highest by each patient was identified.
Results
Among 311 patients, repeat revascularization was the single most important consideration to 14.4% of patients, while 20.6% considered avoiding one of the DAPT drawbacks as most important. Most patients (65%) considered avoiding at least one DAPT drawback as important as avoiding repeat revascularization. In no subgroup of patients did more than a quarter of patients prefer avoiding repeat revascularization above all other concerns. Among those undergoing PCI, more than three-quarters received a DES, regardless of their stated preferences (DES use among those most valuing DES benefits, avoiding DAPT drawbacks, or both equally were 78.7%, 86.2% and 85.6%, respectively, p=0.56).
Conclusion
Most patients reported that avoiding DAPT drawbacks was as important as avoiding repeat revascularization. Eliciting patient preferences regarding stent type can enhance shared decision-making and allow physicians to better tailor stent choice to patients’ goals and values.
Trial Registration
Developing and Testing a Personalized Evidence-based Shared Decision-making Tool for Stent Selection (DECIDE-PCI). ClinicalTrials.gov Identifier: NCT02046902. URL: https://clinicaltrials.gov/ct2/show/NCT02046902
Keywords: Patients’ preferences, shared decision-making, percutaneous coronary intervention, dual anti-platelet therapy
Introduction
Coronary Artery Disease (CAD) affects 15 million Americans and percutaneous coronary intervention (PCI) is performed more than 500,000 times/year in the U.S. alone.(1) While PCI procedures can be performed with bare metal (BMS) or drug eluting stents (DES), over 80% of PCI procedures in the U.S. are performed with DES.(2,3) The principal advantage of DES (over BMS) is the decreased risk of restenosis and lower likelihood of needing to undergo a repeat revascularization procedure.(4-8) Importantly, the benefits of DES in preventing repeat revascularization procedures are highly dependent on an individual patient’s risk for restenosis, with the number needed to treat to avoid one target vessel revascularization (TVR) ranging from 6 to 130, depending on individual patient characteristics. (9,10) The use of DES also necessitates treatment with dual-antiplatelet therapy (DAPT) for at least six months to reduce the risk of stent thrombosis,(11) as compared with as little as one month of DAPT with BMS.(12) This is important because there are drawbacks to prolonged DAPT therapy(13), including higher risks of major and minor bleeding(14),(15), the need to take additional medication every day, increased medication cost, and the need to delay future procedures and surgeries.(12)
Given the competing benefits and drawbacks of DES, it is somewhat surprising that DES are used in the vast majority of PCI cases, regardless of patients’ individualized restenosis risks.(2,16) While it is possible that patients value avoiding a repeat procedure much more than the drawbacks of prolonged DAPT therapy, this has never been studied. In contrast, it may be possible that stent selection is driven more by physicians’ preference to avoid TVR, rather than by actively engaging patients in discussing stent choices and eliciting their preferences. In fact, studies have documented that although 90% of patients express a desire to participate in treatment decisions, only 31% of patients recall discussing stent options with their physicians(17) and over 70% of patients report that the ‘doctor alone’ made the decision about which stent was used.(18) Whether the observed practice of preferentially using DES, regardless of the likelihood of benefit, is logical requires additional insight into patients’ preferences of the benefits of avoiding repeat revascularization, as compared with the drawbacks of DAPT. We thus designed a prospective study to survey patients prior to coronary angiography to investigate the relative importance of avoiding repeat revascularization, as compared with the potential drawbacks associated with prolonged DAPT. Understanding patients’ perceptions of these alternative considerations can either reinforce current practice patterns or underscore the need for better shared medical decision-making with respect to stent selection.
METHODS
Study design
This was a cross-sectional observational study conducted between May 2014 and May 2015. English or Spanish speaking patients undergoing coronary angiography with possible PCI for any non-emergent clinical indication at 2 Kansas City, Missouri hospitals (Saint Luke’s Mid America Heart Institute and Truman Medical Center) were eligible for inclusion. Patients who did not speak English or Spanish or who could not comply with the survey were excluded. A study coordinator surveyed patients prior to their procedure to assess the importance of different factors related to stent choice. The study was approved by the institutional review boards (IRB) at both institutions and all patients provided verbal informed consent.
Data collection
In addition to demographic and clinical data, the survey asked patients to consider the importance of 5 factors in stent choice; avoiding a repeat revascularization procedure, avoiding more pills/day, avoiding the increased costs of medications, avoiding bleeding/bruising and avoiding the possible need to delay/cancel elective surgeries for six months or longer after treatment (Appendix A). These factors were endorsed by the study team (which included two patient members on the Steering Committee) as being most relevant and important to patients. Patients rated how important each factor was to them on a 10-point scale that ranged from 1 (not at all important to avoid) to 10 (extremely important to avoid). The highest-ranked factor (or factors, when there was a tie) was considered the most important factor to the patient. Examples of the ranking method are shown in Appendix B.
Statistical methods
The primary study aim was to describe the factors that are most important to patients in choosing between BMS or DES, where avoiding a repeat revascularization procedure was considered to favor DES and avoiding drawbacks of DAPT therapy was considered to favor BMS. A secondary aim was to describe the differences in characteristics between patients who valued DES benefits (avoiding repeat revascularization), patients who valued DAPT drawbacks and patients who valued both equally. Finally, we examined whether the stent type used among patients undergoing PCI differed between patients who valued DES benefits, patients who valued DAPT drawbacks and those who valued both equally. ANOVA was performed for normally distributed continuous variables; chi-square or Fisher’s exact tests were run for categorical variables. A 2-sided probability value of < 0.05 was considered statistically significant. All statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Cary North Carolina).
RESULTS
Of 336 patients eligible for enrollment, 317 patients (94.3%) agreed to participate. Complete data for patient preferences about factors related to stent choice were available for 311 (98.1%) of the participating patients. Information about the final stent placed was missing in 15 (4.7%) patients. Table 1 shows the baseline characteristics of the patient cohort. Thirty-four percent of patients were female and 18% were African American. Diabetes was present in 45% of patients, 80% had hypertension, 10% had peripheral artery disease and 43% of patients had undergone a prior PCI, 61% of which had been performed >1 year prior.
Table 1.
Patient Characteristics:
| Variables | Total=311 N (%) |
|---|---|
| Female sex | 76 (34) |
| African American race | 57 (18.3) |
| Hispanic ethnicity | 3 (1.2) |
| Final stent placed: | |
| DES | 152 (49) |
| BMS | 28 (9) |
| No stent | 118 (38) |
| Diabetes Mellitus | 132 (45) |
| Hypertension | 233 (80) |
| Peripheral artery disease | 29 (10) |
| Previous CABG | 54 (18.6) |
| Previous PCI | 126 (43.4) |
| Previous PCI timeframe: | |
| < 6 months | 26 (22.4) |
| ≥6 months | 90 (77.6) |
Abbreviations: DES: Drug eluting stent, BMS: Bare metal stent, CABG: Coronary artery bypass graft and PCI: Percutaneous coronary intervention.
We found that 14.4% of patients considered avoiding a repeat revascularization procedure as the single most important factor when deciding on a choice of stent, while 20.6% considered avoiding one of the DAPT drawbacks as most important (Figure 1). The remaining 65% of patients considered avoiding at least one of the drawbacks of DAPT as important as avoiding repeat revascularization. Figure 2 shows the percentage of patients who ranked each consideration as one of their top concerns.
Figure 1. Distribution of the most important considerations to patients in selecting a stent.

This is a pie chart showing the distribution of the most important considerations to patients regarding stent choice.
Figure 2. Distribution of DAPT drawbacks which patients ranked most important to avoid.
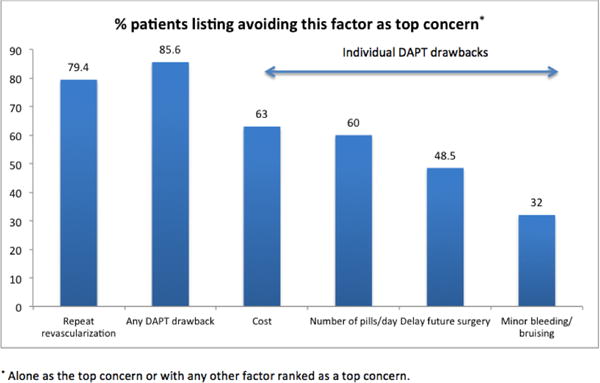
This is a bar graph showing the distribution of patients who listed avoiding different DAPT drawbacks as most important to avoid.
Table 2 shows the characteristics of patients based on what they most valued. Although statistically statistical differences in patient preferences were observed according to gender, race and elapsed time since prior PCI, only a minority of patients in all subgroups valued avoidance of repeat revascularization as the single most important factor in stent selection. Among the 60.6% of patients who subsequently underwent PCI, 85% were treated with DES, and no difference in DES use was observed among patients who most valued avoiding repeat revascularization, who most valued avoiding DAPT drawbacks or who valued both equally (78.7% vs 86.2% vs 85.6%%, respectively, p=0.56) (Figure 3).
Table 2.
Patient characteristics stratified by factors most important to patients.
| Valued DES Benefits (n=45) |
Valued BMS Benefits (n=64) |
Valued Both Equally (n=202) |
P value* | |
|---|---|---|---|---|
| Gender | ||||
| Female | 8 (10.5) | 12 (15.8) | 56 (73.7) | 0.041 |
| Male | 34 (23) | 28 (18.9) | 86 (58.1) | |
| Race | ||||
| African American | 2 (3.5) | 14 (24.6) | 41 (71.9) | 0.032 |
| White | 37 (16.5) | 46 (20.5) | 141 (62.9) | |
| Diabetes mellitus | ||||
| Yes | 15 (11.4) | 36 (27.3) | 81 (61.4) | 0.055 |
| No | 30 (16.7) | 28 (15.7) | 121 (67.6) | |
| Hypertension | ||||
| Yes | 32 (13.7) | 45 (19.3) | 156 (67) | 0.193 |
| No | 13 (16.6) | 19 (24.4) | 46 (59) | |
| PAD | ||||
| Yes | 6 (20.7) | 3 (10.3) | 20 (69) | 0.246 |
| No | 39 (13.9) | 61 (21.6) | 182 (64.5) | |
| Previous CABG | ||||
| Yes | 10 (18.5) | 12 (22.2) | 32 (59.3) | 0.483 |
| No | 35 (13.6) | 52 (20.2) | 170 (66.2) | |
| Previous PCI | ||||
| Yes | 20 (15.9) | 27 (21.4) | 79 (62.7) | 0.652 |
| No | 25 (13.5) | 37 (20) | 123 (66.5) | |
| PCI Timeframe: | 0.023 | |||
| <6 months | 1 (3.8) | 6 (23.1) | 19 (73.1) | |
| ≥6 months | 17 (18.8) | 18 (20) | 55 (61.2) |
Categorical variables compared using chi-square or Fisher’s exact test.
Abbreviations: PAD: Peripheral artery disease, CABG: Coronary artery bypass graft, PCI: Percutaneous coronary intervention.
Figure 3. Final type of stent placed (among patients who received stent).
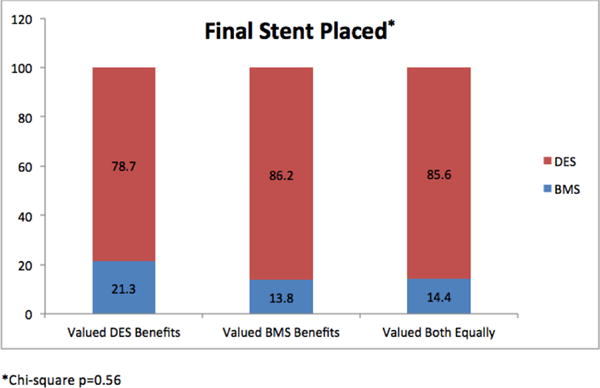
This is a bar graph showing the final type of stent placed stratified by what patients valued the most.
DISCUSSION
The American Medical Association advised, in 2010, that when more than one clinically acceptable treatment is available, engaging patients in shared decision-making has the potential of improving the value of U.S. health care.(19) Similarly, the Institute of Medicine (IOM) includes patient-centered care as one of its 6 domains of quality.(20) In the setting of PCI, stent selection should be a preference-sensitive decision because of the offsetting benefits of DES and the drawbacks of prolonged DAPT. This study is the first to elicit and describe patients’ preferences regarding factors surrounding stent choice and provides a unique insight into whether or not the current use of DES (>80%) is aligned with patient preferences, or whether more effort to support shared decision-making is needed. We found that only 14.4% of patients undergoing coronary angiography with possible PCI were most concerned about having to return for repeat revascularization. Conversely, 20.6% of patients were most concerned about a DAPT drawback and the remaining 65% valued both the benefits of DES and the drawbacks of prolonged DAPT equally. We also found no association between patients’ value of DES benefits and DAPT drawbacks, with the actual stent received. Thus, the predominant use of DES in this study suggests that physicians are prioritizing the avoidance of repeat revascularization procedures, rather than engaging patients in shared decision-making so that treatment can more closely align with patients’ preferences. If shared decision making had occurred, one would expect patients who clearly valued DES benefits the most (14.4%) to receive DES and patients who clearly valued avoiding DAPT drawbacks the most (20.6%) to receive BMS. However, if even a small portion of patients who valued both equally (65%) received a BMS, the rate of DES use would be much lower than 85%. These data underscore the need for more systematically eliciting patient preferences for stent type prior to PCI.
While the best way to understand individual patients’ preferences is to ask them, we found some interesting associations between patient preferences and their clinical characteristics. Most interestingly, patients who valued avoiding DAPT drawbacks (more than they valued avoiding a repeat procedure) were more likely to have undergone a PCI within the past 6 months. Although we do not know what their preferences were at the time of their prior PCI, these are patients who have experienced a PCI procedure and the potential drawbacks of DAPT and preferred avoiding prolonged DAPT therapy more than the repeat procedure. Whether this group of patients had any DAPT-related complications that influenced their preferences is not known.
The lack of shared medical decision-making regarding stent choice, and the preferential use of DES in patients who prefer avoiding prolonged DAPT therapy, is congruent with previous reports. For example, a study of Medicare patients undergoing PCI reported that only 1 in 10 patients were offered alternatives to stenting and only 16% were asked about their treatment preferences.(17) In a prior 9-center study(18), less than a third of patients reported discussing stent options with their physicians prior to their PCI procedure. This lack of patient engagement in shared decision-making is concerning, since 90% of patients prefer to participate in treatment decisions.(18)
To align the stated goals of the American Medical Association and Institute of Medicine to honor patients’ preferences and for patients to participate in treatment decisions, a transformation in the process of stent selection is needed. One promising strategy would be to create shared decision-making tools for eliciting patient’s preferences regarding the benefits and drawbacks of DES to better engage patients in decision-making. Ideally, these tools would depict for patients their individualized risks of repeat revascularization with BMS and DES,(9) and their risks of bleeding or bruising with prolonged DAPT. Such a decision tool would provide the most accurate data with which patients can make decisions based upon their values. Once developed, it will be important to determine how best to implement these tools in routine clinical care so that patients can voice their preferences and physicians can have ready access to this information in tailoring their PCI approach. Moreover, if patients with a low likelihood of repeat revascularization choose BMS, this could translate into significant cost savings for the US health care system, lower complications from prolonged DAPT use and increased patient satisfaction. In fact, we have previously estimated an annual savings of $200M/year in the U.S., if just half of the patients at the lowest risk for TVR chose treatment with BMS2. Further research is needed to develop and implement shared decision-making tools and to study their effects on shared decision-making and stent choice.
Our findings should be interpreted in the context of the following potential limitations. Patients were surveyed prior to angiography and it is possible that patients may have responded differently in another setting, such as an outpatient clinic or after their procedure. However, this was a pragmatic time to elicit patients’ preferences, being just prior to the treatment decision being made, and it is unusual to have discussions about stent options long before the time of the procedure. Moreover, in this study, we did not require patients to identify a single most important factor in stent selection, enabling patients to independently assign values to each factor. Although requiring patients to choose which factor was most important may have altered their prioritization, our finding that over two-thirds of patients were concerned about both the risk of a repeat procedure and the complications of DAPT underscores the importance of engaging patients in shared decision-making as emphasized by a recent meta-analysis suggesting that decisions regarding the duration of DAPT therapy must take into account patients’ values and preferences.(21) It is also important to note that the updated current guidelines(22) recommend at least 6 months of DAPT for patients receiving DES and 12 months of DAPT for patients with acute coronary syndrome and we did not exclude patients with acute coronary syndrome from this study. Lastly, the extreme response bias is a potential limitation to the Likert scale, however, this bias was not seen in this particular study.
In conclusion, given the growing demand for engaging patients in medical decision-making, we report the first study describing patients’ perspectives of the relative importance of different factors related to stent choice. We found that most patients undergoing coronary angiography regarded avoiding one of the drawbacks of DAPT at least as important as avoiding TVR and only a minority regarded avoiding repeat revascularization as the single most important consideration when selecting a stent. Explicitly eliciting patients’ preferences is important to better tailor stent choices to the goals and values of individual patients undergoing PCI.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was partially funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CE-1304-6448). The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. The researchers of this study are independent from PCORI.
Disclosure of potential conflicts of interest:
Drs. Qintar, Shafiq and Pokharel were supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL110837. Dr. Chhatriwalla receives grant funding from Patient-Centered Outcomes Research Institute (PCORI) and has received travel reimbursement from Edwards Lifesciences, Abbott Vascular, Medtronic Inc. and St Jude Medical. Dr. Spertus receives grant funding from Patient-Centered Outcomes Research Institute (PCORI), Abbott Vascular, Lilly and an equity interest in Health Outcomes Sciences. He owns the copyright to the SAQ, KCCQ and PAQ.
Abbreviations
- PCI
percutaneous coronary intervention
- DES
Drug Eluting Stent
- BMS
Bare Metal Stent
- DAPT
dual anti-platelet therapy
- SDM
shared decision-making
Footnotes
Information on author access to data:
Authors of this manuscript had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The relevant de-identified patient level data are available on reasonable request from the authors.
Authors’ contributions:
Mohammed Qintar, MD: Analysis of data, interpretation of data, drafting and revisions of manuscript.
Adnan K. Chhatriwalla, MD: Conception and design of study, coordination of study, interpretation of data, review and revisions of manuscript.
Suzanne V. Arnold, MD MHA: Interpretation of data, revisions of manuscript.
Fengming Tang: Statistical analyses, revisions of manuscript.
Donna M. Buchanan, PhD: Interpretation of data, revisions of manuscript.
Ali Shafiq, MD: Interpretation of data, revisions of manuscript.
Yashashwi Pokhkarel, MD: Interpretation of data, revisions of manuscript.
Javed M. Ashraf, MD: Design of study, coordination of study, interpretation of data, revisions of manuscript.
John A. Spertus, MD MPH: Conception and design of study, coordination of study, interpretation of data, drafting and revisions of manuscript.
All authors have approved the final manuscript.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Amin AP, Spertus JA, Cohen DJ, Chhatriwalla A, Kennedy KF, Vilain K, Salisbury AC, Venkitachalam L, Lai SM, Mauri L, et al. Use of drug-eluting stents as a function of predicted benefit: clinical and economic implications of current practice. Arch Intern Med. 2012;172(15):1145–52. doi: 10.1001/archinternmed.2012.3093. [DOI] [PubMed] [Google Scholar]
- 3.Krone RJ, Shaw RE, Klein LW, Blankenship JC, Weintraub WS. Ad hoc percutaneous coronary interventions in patients with stable coronary artery disease–a study of prevalence, safety, and variation in use from the American College of Cardiology National Cardiovascular Data Registry (ACC-NCDR) Catheter Cardiovasc Interv. 2006;68(5):696–703. doi: 10.1002/ccd.20910. [DOI] [PubMed] [Google Scholar]
- 4.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 5.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 6.Weisz G, Leon MB, Holmes DR, Jr, Kereiakes DJ, Popma JJ, Teirstein PS, Cohen SA, Wang H, Cutlip DE, Moses JW. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) Trial. J Am Coll Cardiol. 2009;53(17):1488–97. doi: 10.1016/j.jacc.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 7.Ellis SG, Stone GW, Cox DA, Hermiller J, O’Shaughnessy C, Mann T, Turco M, Caputo R, Bergin PJ, Bowman TS, et al. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV-SR: Treatment of De Novo Coronary Disease Using a Single Paclitaxel-Eluting Stent) JACC Cardiovasc Interv. 2009;2(12):1248–59. doi: 10.1016/j.jcin.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Sarno G, Lagerqvist B, Frobert O, Nilsson J, Olivecrona G, Omerovic E, Saleh N, Venetzanos D, James S. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drug-eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR) Eur Heart J. 2012;33(5):606–13. doi: 10.1093/eurheartj/ehr479. [DOI] [PubMed] [Google Scholar]
- 9.Yeh RW, Normand SL, Wolf RE, Jones PG, Ho KK, Cohen DJ, Cutlip DE, Mauri L, Kugelmass AD, Amin AP, et al. Predicting the restenosis benefit of drug-eluting versus bare metal stents in percutaneous coronary intervention. Circulation. 2011;124(14):1557–64. doi: 10.1161/CIRCULATIONAHA.111.045229. [DOI] [PubMed] [Google Scholar]
- 10.Tu JV, Bowen J, Chiu M, Ko DT, Austin PC, He Y, Hopkins R, Tarride JE, Blackhouse G, Lazzam C, et al. Effectiveness and safety of drug-eluting stents in Ontario. N Engl J Med. 2007;357(14):1393–402. doi: 10.1056/NEJMoa071076. [DOI] [PubMed] [Google Scholar]
- 11.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;2016 doi: 10.1016/j.jtcvs.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Grines CL, Bonow RO, Casey DE, Jr, Gardner TJ, Lockhart PB, Moliterno DJ, O’Gara P, Whitlow P, American Heart A, American College of C, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007;115(6):813–8. doi: 10.1161/CIRCULATIONAHA.106.180944. [DOI] [PubMed] [Google Scholar]
- 13.Valgimigli M, Park SJ, Kim HS, Park KW, Park DW, Tricoci P, Ferrante G. Benefits and risks of long-term duration of dual antiplatelet therapy after drug-eluting stenting: a meta-analysis of randomized trials. Int J Cardiol. 2013;168(3):2579–87. doi: 10.1016/j.ijcard.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 15.Amin AP, Bachuwar A, Reid KJ, Chhatriwalla AK, Salisbury AC, Yeh RW, Kosiborod M, Wang TY, Alexander KP, Gosch K, et al. Nuisance bleeding with prolonged dual antiplatelet therapy after acute myocardial infarction and its impact on health status. J Am Coll Cardiol. 2013;61(21):2130–8. doi: 10.1016/j.jacc.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krone RJ, Shaw RE, Klein LW, Blankenship JC, Weintraub WS, American College of Cardiology - National Cardiovascular Data R Ad hoc percutaneous coronary interventions in patients with stable coronary artery disease–a study of prevalence, safety, and variation in use from the American College of Cardiology National Cardiovascular Data Registry (ACC-NCDR) Catheter Cardiovasc Interv. 2006;68(5):696–703. doi: 10.1002/ccd.20910. [DOI] [PubMed] [Google Scholar]
- 17.Fowler FJ, Jr, Gallagher PM, Bynum JP, Barry MJ, Lucas FL, Skinner JS. Decision-making process reported by Medicare patients who had coronary artery stenting or surgery for prostate cancer. J Gen Intern Med. 2012;27(8):911–6. doi: 10.1007/s11606-012-2009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spertus JA, Bach R, Bethea C, Chhatriwalla A, Curtis JP, Gialde E, Guerrero M, Gosch K, Jones PG, Kugelmass A, et al. Improving the process of informed consent for percutaneous coronary intervention: patient outcomes from the Patient Risk Information Services Manager (ePRISM) study. Am Heart J. 2015;169(2):234–241 e1. doi: 10.1016/j.ahj.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Medical Association. 2010 Strategic issues [database on the Internet] The Association (Getting the most for our health care dollars: Shared Decision Making) 2010 Available from http://www.allhealth.org/briefingmaterials/AMASharedDecisionMaking-1936.pdf.
- 20.Crossing the Quality Chasm: A New Health System for the 21st Century 2001
- 21.Spencer FA, Prasad M, Vandvik PO, Chetan D, Zhou Q, Guyatt G. Longer- Versus Shorter-Duration Dual-Antiplatelet Therapy After Drug-Eluting Stent Placement: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(2):118–26. doi: 10.7326/M15-0083. [DOI] [PubMed] [Google Scholar]
- 22.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2016;152(5):1243–1275. doi: 10.1016/j.jtcvs.2016.07.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


