Abstract
This pilot study tested the independent and interactive effects of sleep and meal times, under identical sleep and feeding conditions, on insulin sensitivity (Si) in overweight adults. Participants underwent a 4-phase randomized crossover inpatient study differing in sleep time: normal (Ns: 0000–0800 h) or late (Ls: 0330–1130 h); and meal times: normal (Nm: 1, 5, 11, and 12.5 h after awakening) or late (Lm: 4.5, 8.5, 14.5, and 16 h after awakening). An insulin-modified frequently sampled i.v. glucose tolerance test, at scheduled breakfast time, and a meal tolerance test, at scheduled lunch time, were performed to assess Si after 3 d in each condition. Six participants were enrolled (4 men, 2 women; mean age 25.1 ± [SD] 3.9 y, body mass index [BMI] 29.2 ± 2.7 kg/m2); only one failed to complete her last study phase. There were no effects of sleep and meal times or sleep x meal time interaction on Si (all P>0.35), acute insulin response to i.v. glucose (all P>0.20) and disposition index (all P>0.60) after adjusting for sex and BMI. Meal tolerance test glucose and insulin area under the curve were lower during Nm (glucose P=0.11; insulin P=0.0088). There was a sleep x meal interaction and an effect of meal times on overnight glucose (P=0.0040 and 0.012, respectively) and insulin (P=0.0075 and 0.067, respectively). Sleep timing, without concomitant sleep restriction, does not adversely affect Si and glucose tolerance but meal times may be relevant for health. Our results should be confirmed in a larger sample.
Keywords: sleep timing, meal timing, glucose tolerance, insulin sensitivity, cortisol, melatonin
Introduction
Sleep has recently been highlighted as a behavior that is closely associated with health. Indeed, short sleep duration has been shown to be related to an increased risk of obesity, type 2 diabetes, hypertension, and cardiovascular disease 1. Other sleep disorders are also linked to such adverse metabolic consequences, namely obstructive sleep apnea 2 and insomnia 3. More recently, however, studies have noted that not only is sleep duration important in modulating cardio-metabolic risk factors but also the timing of the sleep period may have an impact on health. At the population level, epidemiological studies suggest that individuals who work night shift, and thus sleep during the daytime, have an increased risk of obesity, type 2 diabetes, and cardiovascular disease 4,5. Less drastic differences in sleep timing are also associated with increased risk of chronic disorders. For example, Facco et al. reported an increased risk of gestational diabetes in women with midpoint of sleep later than 5 AM compared to women with an earlier sleep midpoint 6 and social jetlag of >2 h has been associated with a 2-fold increased risk of metabolic syndrome and type 2 diabetes 7.
Studies have shown that inverting sleep with a 12-h flip increased 24-h postprandial glucose and insulin area under the curve (AUC) by 1.6 and 9%, respectively, compared to the usual sleep period during the night (2300-0800 h) 8. Two-hour postprandial glucose AUC was 12% higher when measured at a test meal in the biological night (2000 h) compared to morning (0800 h). However, in that study, total sleep duration was lower when sleep occurred during the day compared to night. To address this limitation, Leproult et al. 9 performed a sleep restriction study in which participants either slept at night (0030–0530 h) or during the day (0900–1400 h), introducing an 8.5 h delay in sleep. An i.v. glucose tolerance test was performed at 0900 h after 6 nights of this protocol. Insulin sensitivity (Si) was reduced in both conditions relative to 10-h baseline sleep, showing a sleep restriction effect on this risk factor. There was no change in the acute insulin response to glucose (AIRg), resulting in a reduction in the disposition index (DI). We are not aware of intervention studies of mild shifts in sleep timing that mimic the real-life situation of the large proportion of Americans suffering from social jetlag or late sleep timing. This is what we aim to achieve in this pilot study.
It is important to note that, in conditions when sleep time is delayed, food intake must also occur later in the day. Mice fed during the light cycle, at a time when they would not be active, have greater body fat than mice fed during the dark cycle 10 and have increased risk of metabolic syndrome 11. Scheer et al. 12 noted that glucose and insulin levels vary across the behavioral cycle independent of the circadian cycle. When meals are consumed later in the day, postprandial glucose AUC is increased relative to an earlier eating occasion 13. However, this may be due to differences in fasting duration prior to the test meal between conditions.
In order to address the limitations of prior studies, we conducted a randomized, crossover pilot study consisting of 4-phases, in which sleep and meal times were fixed and food intake controlled. The main objective of our investigation was to determine the effects of sleep and meal times, independent of sleep duration and energy intake, on Si. We hypothesized that sleeping and eating late in the day would lead to an adverse metabolic profile relative to earlier sleep and meal times.
Materials and methods
Participants
Six men and women, 20–49 y of age with a body mass index between 25–34.9 kg/m2, were recruited to participate in this study. Recruitment procedures were identical to those previously employed in our lab 14 and involved 2 wk of sleep monitoring with actigraphy. Only those with habitual sleep 7–9 h/night and an intermediate chronotype, based on the Ostberg Morningness-Eveningness questionnaire, were enrolled. Individuals were required to habitually consume a meal within 1 h of awakening to be eligible. Those with sleep, eating, or other psychological disorders were excluded. The study was approved by the institutional review boards of Columbia University Medical Center and New York University Langone Medical Center and registered on Clinicaltrials.gov (#NCT02347020). All participants provided informed consent prior to enrolling in the study.
Experimental design
Once enrolled, participants were randomly assigned one of 24 study phase combinations generated from a randomization schedule. The 4 study phases included either normal or late sleep and normal or late meals (Table 1), which were separated by a washout period of 3 wk to ensure testing was done in the phase of the menstrual cycle in women. Sleep and meal times for the normal sleep/normal meals (Ns/Nm) phase were based on data from a convenience sample 15. Bedtimes for the late sleep (Ls) phases were based on bedtimes for late sleepers from that same study 15 and were delayed by 3.5 h relative to Ns. However, wake times were later than those of that sample to ensure equal time in bed in each phase. Meal times for the late meal (Lm) phases were delayed by an equal amount of time as the sleep delay (3.5 h). For each study phase, participants were required to become inpatients in the Columbia University Medical Center Irving Institute for Clinical and Translational Research for 5 nights. During their stay, participants consumed a fixed diet, designed to maintain their body weight, as estimated using the Mifflin-St. Jeor equation 16. Energy intake was distributed as follows: 25% at breakfast, 30% at lunch, 35% at dinner, and 10% at post-dinner snack. When snack was scheduled at bedtime, it was provided 10 min prior to scheduled bedtime. Participants were required to consume all foods provided and nothing else during this period.
Table 1.
Timing of sleep and meals in each study phase.
| Normal sleep/Normal meals | Normal sleep/Late meals | Late sleep/Normal meals | Late sleep/Late meals | |
|---|---|---|---|---|
| Sleep, h | 0000–0800 | 0000–0800 | 0330–1130 | 0330–1130 |
| Breakfast1 | 1/0900 | 4.5/1230 | 1/1230 | 4.5/1600 |
| Lunch | 5/1300 | 8.5/1630 | 5/1630 | 8.5/2000 |
| Dinner | 11/1900 | 14.5/2230 | 11/2230 | 14.5/0200 |
| Snack | 12.5/2030 | 16/0000 | 12.5/0000 | 16/0330 |
Hours from wake up time/military clock time (h).
Inpatient rooms in the Clinical Research Resource were equipped with noise-reducing black out curtains. Participants were kept in normal indoor room lighting (~200 lux at the level of the eye) during wakefulness and complete darkness during sleep. Sleep was monitored by actigraphy nightly and by polysomnography (PSG) on night 3 only. PSG recordings (Embletta MPR PG/ST+ Proxy, Natus Neurology, Middleton, WI) included frontal, central, and occipital electroencephalogram, electrooculogram, electromyogram (chin and left and right anterior tibialis), pulse oximetry, respiratory effort (rib cage and abdominal excursion), nasal airflow (nasal pressure cannula), and electrocardiogram. Recordings were visually scored in 30 s epochs according to standard criteria 17. All participants had an apnea-hypopnea index <5 events/h. The first night recording also included EMGs of the left and right anterior tibialis for screening of periodic limb movements (PLM) in sleep, and all participants showed a PLM index <15 events/h.
During the night from d 3 to d 4, blood samples were taken from an i.v. line via a port through the wall. Sampling started at 2200 h and continued at hourly intervals until 0300 h and every 2 h until the start of the insulin-modified frequently-sampled i.v. glucose tolerance test (FSIVGTT). The FSIVGTT was performed at scheduled breakfast time for each phase and a meal tolerance test (MTT) was done at the scheduled lunch time, per protocol (Table 1). Test times were 0900 h and 1300 h for the FSIVGTT and MTT, respectively, during Ns/Nm; 1230 h and 1630 h during Ns/Lm and Ls/Nm; and 1600 h and 2000 h during Ls/Lm. For the FSIVGTT, 4 basal blood samples were obtained prior to i.v. dextrose administration over one minute (0.03 mg 25% dextrose/kg body weight). Insulin (0.025 u/kg body weight) was injected i.v. 20 min later and blood samples were collected until 180 min from glucose infusion, as previously described 18. The MINMOD Millenium 2003 program (v. 5.16, Bergman, USC) was used to determine Si, AIRg, and DI (product of Si and AIRg, which reflects beta cell ability to compensate for peripheral insulin resistance; low DI predicts type 2 diabetes in epidemiological studies 19,20). For the MTT, a pre-meal blood sample was obtained, followed by consumption of a liquid meal (Boost, Nestle Nutritionals, Fremont, MI), providing 30% of the participant’s estimated energy requirements. Blood samples were obtained at 15, 30, 45, 60, 90, 120, and 180 min postprandially. The Matsuda index derived from the MTT parameters was used as a second measure of Si 21.
Glucose, insulin, and cortisol concentrations were analyzed by oxidase methods and radioimmunoassays (EMD Millipore, Billerica, MA) using standard protocols 22. Plasma melatonin obtained during the overnight sampling period was analyzed using ELISA (GenWay Biotech, San Diego, CA). Assays were conducted in the Hormone and Metabolite Core Laboratory of the New York Obesity Nutrition Research Center (Columbia University Medical Center, New York, NY).
2.3 Statistical analyses
Linear mixed model analyses were performed with insulin, glucose, Si, AIRg, DI, and cortisol as outcomes. Each of these analyses was done separately for FSIVGTT, MTT, and overnight blood draws. Overnight melatonin and cortisol were analyzed using repeated-measures ANOVA. Sleep and meal times, as well as their interaction, were used as independent variables. Additionally, for MTT and FSIVGTT, blood-draw (clock) time was used as an independent variable by categorizing it as morning (0600–1200 h), afternoon (1200–1800 h), evening (1800–2400 h) and after midnight (2400-0600 h). Note that, only two of these levels were present for the MTT, and hence blood-draw time has two categories for the MTT analyses: afternoon and evening. Subject was used as a random effect. Glucose and insulin AUC were calculated during the MTT. The Matsuda Index was calculated using fasting, 30, 60, 90, and 120 min time points for all participants except one for whom the 90-min sample was not available for one phase. Data for all 4 phases were analyzed in the same way. Each participant received 30% of their estimated energy requirements from a liquid meal replacement at the MTT. The glucose content of the meal was thus personalized for each participant based on the quantity consumed at the test meal. This was identical for all phases for each participant.
Repeated measure ANOVA and regression analysis (mixed-model) were used for average cortisol concentration (between 2200 and 0700 h), peak cortisol concentration and peak time. The independent variables were Sleep, Meal and their interaction. Subject was used as random effect. Data from the Matsuda Index, melatonin, and average cortisol concentration (2200-0700) were not normally distributed and were transformed using log2, after which data passed the normality test. One unit increase in log2 transformed variable corresponds to doubling of the original variable.
Total sleep duration, sleep efficiency, sleep onset latency, number of awakenings, and wake after sleep onset, assessed by polysomnography were also used as outcomes to perform repeated measure ANOVA and liner mixed-model regression analysis with subject as random effect. Sleep and meal times, as well as their interaction, were used as independent variables.
Phase order was included in all models and removed, as it was a not a significant factor. Data are presented as means ± SD. A p-value < 0.05 was considered as statistically significant.
3. Results
Of the 6 participants (4 men, 2 women) who enrolled in this study, 5 completed all 4 phases and one woman completed 3 of the 4 phases. Mean age and body mass index of the study participants were 25.1 ± 3.9 y and 29.2 ± 2.7 kg/m2, respectively. Two participants self-identified as non-Hispanic Black, 3 as non-Hispanic White, and one as Hispanic Black.
3.1 Sleep
Total sleep duration, sleep efficiency, sleep onset latency, number of awakenings, and wake after sleep onset, assessed by polysomnography, were not different between phases (Table 2). Similarly, sleep and meal times, and their interaction, did not affect sleep stages.
Table 2.
Sleep and insulin sensitivity parameters under normal and late sleep and meal timing conditions.
| Variables | Normal sleep/Normal meals | Normal sleep/Late meals | Late sleep/Normal meals | Late sleep/Late meals |
|---|---|---|---|---|
| Polysomnography data | ||||
| Total sleep time, min | 430.5 ± 18.7 | 411.9 ± 46.8 | 413.2 ± 41.1 | 417.2 ± 34.8 |
| Sleep efficiency, % | 89.7 ± 3.9 | 85.8 ± 9.7 | 86.0 ± 8.5 | 86.8 ± 7.2 |
| Sleep onset latency, min | 15.4 ± 8.1 | 21.7 ± 9.7 | 18.9 ± 22.3 | 11.7 ± 3.8 |
| Stage 1 sleep, min | 22.6 ± 3.7 | 19.0 ± 1.2 | 20.9 ± 4.7 | 22.9 ± 3.8 |
| Stage 2 sleep, min | 248.2 ± 55.6 | 213.5 ± 63.4 | 222.9 ± 61.8 | 218.4 ± 56.0 |
| Slow wave sleep, min | 79.2 ± 65.5 | 108.2 ± 32.1 | 95.3 ± 52.2 | 92.2 ± 40.7 |
| Rapid eye movement sleep, min | 80.4 ± 13.3 | 71.3 ±19.2 | 74.1 ± 31.3 | 83.7 ± 23.0 |
| Frequently sampled i.v. glucose tolerance test data | ||||
| Acute insulin response to glucose | 4.38 ± 2.86 | 5.76 ± 3.01 | 4.20 ± 2.99 | 5.35 ± 3.96 |
| Disposition index | 3.41 ± 2.80 | 2.22 ± 1.60 | 2.60 ± 1.47 | 2.22 ± 1.31 |
| Insulin sensitivity | 3.99 ± 1.96 | 2.97 ± 1.67 | 3.60 ± 1.76 | 3.14 ± 0.96 |
Data are means ± SD, n = 6.
3.2 Insulin-modified frequently sampled i.v. glucose tolerance test
There was no effect of sleep time on AIRg, DI, or Si (all P > 0.90) (Table 2). The effect of meal time was also not significant: AIRg (P = 0.20), DI (P = 0.93), Si (P = 0.66). Finally, there was no sleep x meal time interaction on any of the variables (all P > 0.60).
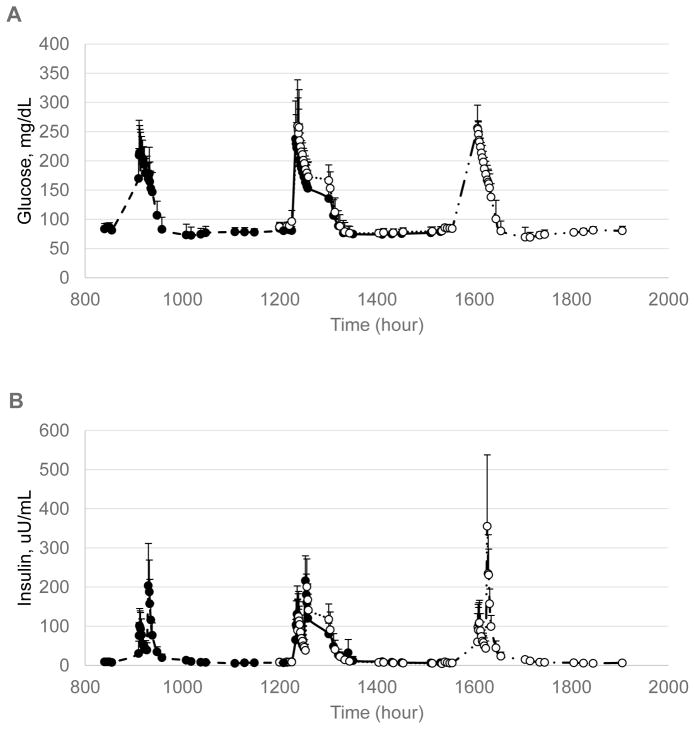
Glucose and insulin results during the FSIVGTT revealed no main effect of sleep time on glucose (P = 0.19) or insulin (P = 0.16) (Figure 1). Likewise, there was no effect of meal time on glucose concentrations (P = 0.90) although there was a trend for an effect on insulin concentrations (P = 0.072). Late meals tended to be associated with higher insulin concentrations. There were meal x sleep time interactions on glucose (P = 0.024) and insulin (P = 0.05).
Figure 1.
Glucose (A) and insulin (B) concentrations during the FSIVGTT under late and normal sleep and meal timing conditions: Ns/Nm, hashed line/solid markers; Ns/Lm, solid line/solid markers; Ls/Nm, dotted line/open markers; Ls/Lm, mixed line/open markers. Measurements were obtained with glucose injection at the scheduled breakfast time (see Table 1). N=6 for all phases except Ls/Lm, n=5. There was a significant meal x sleep time interaction on both glucose (P = 0.024) and insulin (P = 0.050) concentrations.
3.3 Meal tolerance test
There was a trend for an effect of sleep time (P = 0.062) but not meal time (P = 0.17) or sleep x meal interaction (P = 0.22) on glucose concentrations during the MTT (Figure 2). There were no effects of sleep and meal times, and sleep x meal time interaction on insulin concentrations (all P > 0.35). For both glucose and insulin concentrations, tests performed in the evening hours, defined as 1800-0000 h, resulted in higher values (P < 0.01).
Figure 2.
Glucose (A) and insulin (B) concentrations during the MTT under late and normal sleep and meal timing conditions: Ns/Nm, hashed line/solid markers; Ns/Lm, solid line/solid markers; Ls/Nm, dotted line/open markers; Ls/Lm, mixed line/open markers. Measurements were obtained with glucose injection at the scheduled lunch time (see Table 1). N=6 for all phases except Ls/Lm, n=5. There was a significant meal x sleep time interaction on both glucose (P = 0.024) and insulin (P = 0.050) concentrations.
There was no effect of meal (P = 0.18), sleep (P = 0.27), or meal x sleep time interaction (P = 0.98) on the Matsuda index. Si, determined by the Matsuda index, was 5.67 ± 2.65 for Ns/Nm, 5.00 ± 2.69 for Ns/Lm, 4.72 ± 0.86 for Ls/Nm, and 3.87 ± 1.20 for Ls/Lm.
We calculated the total AUC for glucose and insulin. There was a slight trend for an effect of meal time on glucose AUC (P = 0.11) but no sleep (P = 0.743) or sleep x meal interaction (P = 0.18). The glucose AUC for Ns/Nm and Ls/Nm were 20560 ± 1650 and 21395 ± 1824 mg/dL * 180 min, respectively, compared to 22095 ± 3207 and 22009 ± 1754 mg/dL * 180 min for Ns/Lm and Ls/Lm, respectively. For insulin AUC, the effect of meal was significant (P = 0.0088) and, as for glucose AUC, there was no effect of sleep (P = 0.42) or sleep x meal interaction (P = 0.73). Insulin AUC for Ns/Nm and Ls/Nm were 8404 ± 4489 and 9816 ± 2966 μU/mL * 180 min, respectively, compared to 12534 ± 6407 and 13953 ± 7040 μU/dL * 180 min for Ns/Lm and Ls/Lm, respectively.
3.4 Overnight
Meal time influenced concentrations of glucose (P = 0.012) and insulin (P = 0.069) during the overnight hours. There was no effect of sleep time on glucose (P = 0.54) or insulin (P = 0.62). However, there was a sleep x meal interaction on glucose (P = 0.0046) and insulin (P = 0.0092) concentrations. The combination of Ns and Nm led to lower glucose and insulin concentrations whereas Nm effected higher concentrations.
3.5 Cortisol
Average cortisol concentrations between 2200 and 0700 h tended to be affected by meal time (P = 0.10) but not sleep time (P = 0.76) and there was no sleep x meal interaction (P = 0.96). Peak cortisol concentrations were not affect by meal (P = 0.23) or sleep times (P = 0.49) or their interaction (P = 0.29). However, the timing of the peak cortisol concentration shifted with sleep time, with earlier peak times when sleep timing was normal relative to late (effect of sleep time, P = 0.048). This timing of peak cortisol was not affect by meal time (P = 0.86).
3.6 Melatonin
Melatonin concentrations from the overnight sampling period showed a significant effect of sleep time (P = 0.003) but not meal time (P = 0.53) and there was no sleep x meal interaction (P = 0.42). The main effect of sleep time was due to a delay in the onset of melatonin secretion during the late vs. normal sleep condition (Figure 4).
Figure 4.
Melatonin concentrations under normal sleep (solid line) and late sleep (dashed line) conditions. Lights off in each condition is shown by arrows. N=5 for all phases.
Raw differences between normal and late sleep and between normal and late meal, for each outcome, are provided in supplemental online material. In this same document, we have also reported the corresponding effect sizes (the raw difference in the unit of standard deviation) for each outcome.
Discussion
This preliminary investigation is the first to assess the independent and additive effects of sleep and meal time, without alterations in sleep duration and quality and quantity and quality of food intake, on insulin sensitivity in adults. In this small group of overweight, young men and women, altering sleep and meal times to later in the day did not have an adverse effect on insulin sensitivity assessed by either Si derived from the FSIVGTT or by the Matsuda index. Glucose tolerance, in response to a liquid meal (MTT), was also unaffected by the timing of sleep and meals, despite a trend for lower glucose concentrations with Ns and lower glucose (trend) and insulin AUC with Nm. Not surprisingly, meal times influenced overnight concentrations of glucose and insulin and there was an interaction between sleep and meal times such that glucose and insulin concentrations were lowest during the Ns/Nm condition and highest in the Ls/Nm condition. Ns/Lm and Ls/Lm conditions yielded intermediate values.
Results of the MTT revealed no independent influence of sleep and meal times on glucose and insulin other than the well-known effect of circadian time on these concentrations. Indeed, when the test meal was provided later in the day, irrespective of sleep and meal times, glucose and insulin concentrations were higher than when the test was administered earlier in the day. This was also observed by Bandin et al. 13 in normal weight females when glucose tolerance at an early meal (1300 h) and late meal (1630 h) were compared. Our results show that this seems to be a reflection of the circadian rhythms of glucose and insulin rather than the result of the shift in meal schedule. Sleep schedule also did not affect glucose and insulin concentrations in response to the MTT. This can be compared directly from Ns/Nm and Ls/Nm phases in which the MTT was conducted at the same clock time. Glucose and insulin concentrations did not differ despite the 3.5 h delay in sleep timing between these two phases.
As expected, cortisol levels were lowest during nighttime and rose early morning to reach a peak shortly after awakening 12,23. The peak was delayed during Ls relative to Ns but was not different in magnitude from Ns phases. Similar results were observed by Rehman et al. when sleep was shifted by 8 h from nighttime to daytime 24. In that study, 24-h rhythm of cortisol was similar between sleep conditions. In the present investigation, shifting meal times had little influence on cortisol levels. Bandin et al. 13 also found no difference in nighttime cortisol when lunch was consumed early vs late but did find some differences in morning and afternoon values, with lower values being observed when the meal was consumed later. Our measurements did not extend beyond 0700 h for the Ns/Nm phase and we could not compare daytime and afternoon levels due to the FSIVGTT and MTT.
In our protocol, overnight secretion of melatonin was affected by sleep timing, with lower levels and a delay observed in Ls relative to Ns. This is expected to occur as a result of the longer duration of room light exposure during the night preceding bedtime during the Ls vs. Ns condition. Exposure to regular indoor room lighting in the hours preceding bedtime suppresses melatonin secretion and delays the timing of its nocturnal secretion onset 25. Ambient lighting in hours preceding bedtime was identical in all 4 phases, therefore an effect of sleep timing per se, independent of room lighting, is possible. Specifically, an imposed delay in the timing of sleep by 3 h for 3 d, similar to what was done here, was found to induce a significant phase delay in the nocturnal onset of melatonin when controlling for light by maintaining participants in dim (< 10 lux) light levels 26. These observations may have clinical relevance, since lower nighttime (0300 h) melatonin levels were seen in women with metabolic syndrome vs. controls 27. Future studies should make efforts to control for ambient light and posture when determining the effects of altered sleep and meal timing on melatonin secretion patterns.
We had expected worse Si and glucose intolerance as a result of Ls and Lm. Studies have shown that later midpoint of sleep is associated with poor glycemic control in patients with type 2 diabetes 28. However, previous research in this field has been observational and participants self-report their sleep and meal times. Such self-reports demonstrate that later midpoint of sleep and higher proportion of energy consumed at dinner are related to poorer glycemic control. Interestingly, in that study, late chronotypes had a midpoint of sleep that was 4 h later than early chronotypes, similar to the difference in sleep timing produced in the current study. However, in our study, meal and sleep times did not influence Si but later test times for the MTT led to higher glucose and insulin concentrations. It is important to note that the quality of food intake was not taken into consideration in the study by Reutrakul et al. 28. In our intervention, sleep duration and food intake were identical in all conditions. It is possible that the impact of sleep and meal timing on glycemic control is contingent on the association of short duration, poor quality sleep and low quality of nutritional intakes. Indeed, individuals with a later chronotype have been shown to consume more fast foods and sugar-sweetened beverages and fewer fruits and vegetables than those with earlier bed and rise times 15,29,30. Rehman et al. 24 also suggested that changes in glucose and insulin concentrations throughout the day are likely more related to differences in the timing of food intake between conditions than the result of changes in sleep timing.
A major strength of the current study was the controlled sleep and meal schedule that ensured identical sleep duration and energy intakes between phases. The 4 phases also allowed for normal and late conditions for both sleep and meal times to disentangle the effects of those conditions on Si and glucose tolerance. Prior studies that assessed shifts in sleep timing invariably had corresponding shifts in meal times. One could therefore not readily determine whether changes in outcome variables were the result of the shift in sleep or meal times. The inclusion of Ns/Lm and Ls/Nm phases in the present study allowed us to examine the effects of shifts in sleep time with meals provided at identical clock times. Moreover, fasting duration from the last meal of the day to the first meal of the following day was identical in all 4 phases.
We acknowledge that our conclusions are limited by the small sample size and thus our power to detect differences between phases in the MTT was quite low (<30% for all variables). However, power was higher for the FSIVGTT-derived variables where power for AIRg, DI, and Si were 51, 24, and 99%, respectively. However, the exemplary completion rate (~96% of phases completed) and within subject design of the study provide some robustness to our findings. Also, we did not assess changes in endogenous circadian rhythms resulting from shifts in sleep schedule under controlled, unmasked conditions. Such determination would require a constant routine protocol which limits participant mobility (posture), food intake, and light exposure. However, this was not our intent. Our goal for this study was to mimic the real-life circumstances of individuals who voluntarily delay their sleep and meal times, as much as is reasonably feasible within the confines of an inpatient setting.
Finally, the results of this study show that, in the context of identical sleep duration and food intake, glucose tolerance is reduced at meals consumed later in the day but suggest that the timing of sleep has minimal influence. Shifting the sleep episode by 3.5 h, without concomitant changes in its duration and quality (sleep efficiency and stages) or in quality and quantity of food intake, does not affect insulin sensitivity or glucose tolerance. However, peak cortisol concentration occurs at later time when sleep is delayed. This, and repeatedly eating late in the day, may have adverse consequences for diabetes risk but this should be evaluated under longer interventions. Additional studies with larger sample size are needed to confirm these preliminary findings.
Supplementary Material
Figure 3.
Cortisol concentrations overnight under late and normal sleep and meal timing conditions: Ns/Nm, hashed line/solid markers; Ns/Lm, solid line/solid markers; Ls/Nm, dotted line/open markers; Ls/Lm, mixed line/open markers. N=5 for all phases. There was no effect of sleep timing, meal timing or meal x sleep time interaction on cortisol concentrations.
Acknowledgments
This study was funded by the National Institutes of Health grants R56HL0119945 (MPSO) and DK26687, and also in part by Columbia University’s CTSA grant UL1TR000040 from NCATS/NIH.
MPSO designed the study, analyzed and interpreted data, and wrote the manuscript. She takes full responsibility for the data contained in this report. TP and KK conducted the study, analyzed data, and contributed to writing the manuscript. AS performed sleep analyses and was involved in data interpretation and manuscript preparation. BL provided medical supervision for the study, assisted with data interpretation and manuscript preparation. AR performed statistical analysis of the data and assisted with data interpretation and manuscript preparation.
None of the authors report any conflicts of interest related to this work.
Abbreviations
- AIRg
Acute insulin response to glucose
- AUC
Area under the curve
- DI
Disposition index
- FSIVGTT
Frequently-sampled i.v. glucose tolerance test
- Lm
Late meal
- Ls
Late sleep
- MTT
Meal tolerance test
- Nm
Normal meal
- Ns
Normal sleep
- Si
Insulin sensitivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.St-Onge M-P, Grandner MA, Brown D, et al. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health. Circulation. 2016 doi: 10.1161/CIR.0000000000000444. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis. 2013;229(2):489–495. doi: 10.1016/j.atherosclerosis.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60(4):929–935. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramin C, Devore EE, Wang W, Pierre-Paul J, Wegrzyn LR, Schernhammer ES. Night shift work at specific age ranges and chronic disease risk factors. Occup Environ Med. 2015;72(2):100–107. doi: 10.1136/oemed-2014-102292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang XS, Armstrong ME, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: the epidemiological evidence. Occupational medicine. 2011;61(2):78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Facco FL, Grobman WA, Reid KJ, et al. Objectively measured short sleep duration and later sleep midpoint in pregnancy are associated with a higher risk of gestational diabetes. Am J Obstet Gynecol. 2017 doi: 10.1016/j.ajog.2017.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koopman ADM, Rauh SP, van’t Riet E, et al. The Association between Social Jetlag, the Metabolic Syndrome, and Type 2 Diabetes Mellitus in the General Population: The New Hoorn Study. J Biol Rhythms. 2017 doi: 10.1177/0748730417713572. 748730417713572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris CJ, Yang JN, Garcia JI, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(17):E2225–2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17(11):2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherji A, Kobiita A, Damara M, et al. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(48):E6691–6698. doi: 10.1073/pnas.1519807112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandin C, Scheer FA, Luque AJ, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. International journal of obesity. 2015;39(5):828–833. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 14.St-Onge M-P, Roberts A, Chen J, Kelleman M, O’Keeffe M, Jones P. Short sleep duration increases energy intakes but does not change expenditure in normal weight individuals. The American journal of clinical nutrition. 2011;94(2):410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 16.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. The American journal of clinical nutrition. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and assorted events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. The American journal of physiology. 1979;236(6):E667–677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzo C, Wagenknecht LE, Rewers MJ, et al. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes care. 2010;33(9):2098–2103. doi: 10.2337/dc10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss R, Magge SN, Santoro N, et al. Glucose effectiveness in obese children: relation to degree of obesity and dysglycemia. Diabetes care. 2015;38(4):689–695. doi: 10.2337/dc14-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 22.St-Onge MP, O’Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35(11):1503–1510. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88(3):934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehman JU, Brismar K, Holmback U, Akerstedt T, Axelsson J. Sleeping during the day: effects on the 24-h patterns of IGF-binding protein 1, insulin, glucose, cortisol, and growth hormone. Eur J Endocrinol. 2010;163(3):383–390. doi: 10.1530/EJE-10-0297. [DOI] [PubMed] [Google Scholar]
- 25.Gooley JJ, Chamberlain K, Smith KA, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. The Journal of clinical endocrinology and metabolism. 2011;96(3):E463–472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordijn MC, Beersma DG, Korte HJ, van den Hoofdakker RH. Effects of light exposure and sleep displacement on dim light melatonin onset. J Sleep Res. 1999;8(3):163–174. doi: 10.1046/j.1365-2869.1999.00156.x. [DOI] [PubMed] [Google Scholar]
- 27.Corbalan-Tutau D, Madrid JA, Nicolas F, Garaulet M. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiology & behavior. 2014;123:231–235. doi: 10.1016/j.physbeh.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Reutrakul S, Hood MM, Crowley SJ, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes care. 2013;36(9):2523–2529. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleig D, Randler C. Association between chronotype and diet in adolescents based on food logs. Eating behaviors. 2009;10(2):115–118. doi: 10.1016/j.eatbeh.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Olds TS, Maher CA, Matricciani L. Sleep Duration or Bedtime? Exploring the Relationship between Sleep Habits and Weight Status and Activity Patterns. Sleep. 2011;34(10):1299–1307. doi: 10.5665/SLEEP.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.