Abstract
Background
Apoptosis plays a fundamental role in appropriate tissue development and function. Although expression of Bcl-2 has been reported during tooth and submandibular gland (SMG) development, the physiological role Bcl-2 plays during these processes has not been addressed. This study was performed to evaluate the impact of Bcl-2 expression on the formation and properties of tooth hard tissue, and saliva production.
Methods
Twenty-four mice (12 males and 12 females) were divided into three groups of eight (n=8): group A (Bcl-2 +/+), group B (Bcl-2 +/−), and group C (Bcl-2 −/−) and subjected to micro-CT analyses. The mineral content of first molars was analyzed by X-Ray diffraction (XRD) and scanning electron microscopy (SEM) color dot map. The surface microhardness was determined by Vickers test on labial surfaces of incisors. Saliva was collected from different groups of mice after subcutaneous injection of pilocarpine.
Results
Samples from Bcl-2 −/− mice showed significantly smaller micro-CT values, lower and poor crystallinity of hydroxyapatite (HA), and lowest surface micro hardness. SMG from Bcl-2 −/− mice showed remarkable reduction in size, consistent with reduced saliva accumulation.
Conclusions
The absence of Bcl-2 expression in SMG did not affect the expression of other Bcl-2 family members. Thus, Bcl-2 expression influence on the formation and properties of tooth hard tissue, and saliva accumulation.
Significance
Bcl-2 expression has a significant impact on the mineralogical content of enamel crystals of tooth structure. Lack of Bcl-2 expression led to impaired production of enamel ACP crystals.
Keywords: Bcl-2, Hydroxyapatite, Micro hardness, Mineralogy, Transgenic mice, XRD
1. Introduction
Programmed cell death (PCD) or apoptosis is a physiological process by which a cell terminates its life [1, 2]. The apoptotic process, in addition to determining cell fate, also plays an important role in various cellular and tissue developmental stages including the formation of tooth structure [3]. The pro-apoptotic Bcl-2 family members maintain the integrity of mitochondria and regulate apoptosis. Bcl-2 family members include anti-apoptotic members Bcl-2, Bcl-w, Bcl-XL, A1 and Mcl-1, as well as pro-apoptotic members Bak, Bax, Bad, Bid, Bok, BNIP3, Blk, Bik and Bim proteins [4]. Among these proteins, Bcl-2 and Bax are prominent molecules with antagonistic effects on survival. The Bcl-2/Bax ratio has a determining effect on cell survival such that the overexpression of Bcl-2 leads to increased cell survival, while Bax overexpression causes apoptosis [5].
In the developing tooth, Bcl-2 is detected in the enamel organ, ameloblasts, odontoblasts, and subodontoblastic layers [6, 7]. During the enamel formation and maturation about 50% of the ameloblast cells undergo apoptosis [8]. Irregular patterns of apoptosis can result in dysmorphogenesis and dental diseases including hypodontia, oligodontia, agenesis, and even periodontal disease [9]. In contrast to studies that mainly focused on the role of Bcl-2 expression after development, LeBrun et al. demonstrated that Bcl-2 is expressed in stem cell populations, and tissues derived from endocrine and neural cells [10]. Thus, Bcl-2 may be involved in tissue morphogenesis contributing to formation of cell condensations, which are committed to create more differentiated structures such as salivary glands [10]. After maturation of tooth structure and salivary gland the expression of Bcl-2 family members declines such that limited expression of Bcl-2 is observed following birth [11], but it has been shown to play a critical role in dental tissue regeneration [12]. Although, Bcl-2 expression in salivary gland cells with basal cell differentiation is shown to be effective in formation of pleomorphic adenomas [13], the role Bcl-2 expression plays in the development and function of salivary glands remains unknown.
Appropriate development of salivary glands and production of saliva play crucial roles in tooth health and function. Salivary glands are one of the most important exocrine glands with critical roles in tooth homeostasis by secreting saliva [14]. Saliva has multiple functions inside the oral cavity including lubrication, digestion, taste, pH buffering, vocalization, debridement/lavage, antibacterial, antifungal, anti-viral, and immunological activity [15]. It also maintains the teeth post-eruptive maturation of enamel, and remineralization of tooth structures after demineralization by acid diffusion [16]. Unfortunately, the impact of Bcl-2 expression on these activities remains largely unknown. Here we investigated the impact of Bcl-2 expression in tooth morphogenesis and physical properties, as well as in salivary gland function. The effect lack of Bcl-2 has on the formation and properties of tooth hard tissue and saliva production were evaluated.
2. Materials and Methods
2.1. Tooth Analysis and Sample Preparations
All animal care and procedures were in agreement with the Principles of Laboratory Animal Care and approved by the Institutional Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health. Twenty-four mice (twelve males and twelve females) were arranged in three groups (n=8, 4 males and 4 females) including: 1) Eight wild types that were used as the control group (Bcl-2 +/+); 2) Eight heterozygous mice (Bcl-2 +/−); and 3) eight homozygous mice with germline deletion of Bcl-2 (Bcl-2 −/−). The generation and screening of the Bcl-2 transgenic mice have been previously described [17]. We maintained a breeding colony of Bcl-2 +/− mice, and the progeny with the desired genotype used for this study. The evaluation of samples was performed on postnatal day 21.
2.2. The Micro-CT Analysis
The micro-CT analysis of experimental groups was performed by anatomic micro-CT scanning. Animals were sacrificed, the heads were wrapped in dry gauze, and scanned on the micro-CT unit using 3D micro-CT (Inveon, Siemens, Malvern, PA) with a 20 μm voxel size. All scans were visually evaluated by a dental clinician in a masked manner.
The Mineralogical Evaluations
According to Kim et al. [18] the components of five biological calcium phosphates including hydroxyapatite (HA), β-tricalcium phosphate (TCP), octacalcium phosphate (OCP), amorphous calcium phosphate (ACP), and dicalcium phosphate dehydrate (DCPD) were evaluated in the first molar of each mouse. Two left quadrants of mandible from each group of mice were selected randomly, most of the soft tissue was removed physically, and the mandibular bone was vertically grooved on buccal and lingual side, surfaced with diamond disk (KG Sorensen, 7015; Barueri -SP, Brasil) and a low-speed hand piece without entering the periradicular space, and then spilt longitudinally with a chisel to gently isolate the molar teeth. Samples were washed with ethanol and phosphate buffer saline (PBS; pH 7.4). The roots of the first molars were then sectioned 3 mm below the cemento-enamel junction and fixed on a metal slab, placed in 10% formalin and subjected to X-Ray Diffraction (XRD). The XRD analysis was performed as previously described by us [19] using a X-Ray diffractometer with Cu Ka radiation (40 kV, 30 mA). The operation was carried out in a step-scanning mode over a theta-2 range of 5–80 degrees with a step width of 0.01 and a count time of 10 seconds per step. In addition, samples in each group of mandible were randomly selected, and prepared for electron microscopy. This part of the study was similar to that previously reported by us [20]. Dot map was employed to determine the inorganic trace element distributions as previously described [20].
2.3. The Surface Microhardness Evaluations
The surface microhardness was evaluated as previously described by us [21]. Briefly, eight incisors from each group of mice were isolated and, after debridement of soft tissues by root scaling instruments, were stored in PBS at room temperature for less than 72 h. Samples were individually embedded in acrylic resin and the enamel of labial surfaces was prepared for microhardness measurements. The superficial enamel surface of teeth was flattened and polished with water cooled carborundum discs 1,200 grit silicon carbide paper in order to remove about 200 μm enamel. The surface to be tested was fitted with a Vicker’s diamond microhardness tester (Buehler Ltd, Lake Bluff, IL) and a 200N load was used to make indentations in the enamel surface. The indentations were separated by 100 μm from each other on the labial surface. The loaded diamond was allowed to rest on the surface for 15 seconds. The indentations were carefully evaluated by an optical microscope and the average lengths of two diagonals were used to measure the microhardness value (MHV).
2.4. Salivary Gland Analysis and Saliva Collection
Saliva collection was performed according to methods presented by Marmary et al. [22]. Briefly, mice were anesthetized and placed in a holder for easier handling, and then were given pilocarpine 80 mg/kg in 0.1 mL subcutaneously. The saliva was collected in two 5-min intervals using a simple suction device. The animal’s nose remained unobstructed for unhindered respiration. According to Lin et al. [23] tubing was used to collect the saliva from the oral cavity, while the nose was allowed to remain free for unhindered respiration. Saliva collection was performed every 15–30 sec for 10 min and accumulated on ice. The investigator was masked to the mice genotype for saliva collections.
2.5. Ribonucleic Acid Purification
The SMG from three mice were homogenized in 1 mL of Trizol (Life Technologies, Carlsbad, CA). First, 0.2 mL of chloroform was added to each sample, followed by vortexing for 20 sec, and incubated at room temperature for 2–3 min. Samples were centrifuged at 10,000 xg for 18 min at 4°C and the aqueous phase (top) was transferred to a new tube. An equal volume of 100% RNase-free ethanol was added to each sample. Samples were loaded onto an RNeasy column (Qiagen, Valencia, CA) and centrifuged for 30 sec at 8,000 xg. The flow-through was discarded and 700 μL of Buffer RW1 added to the column and centrifuged for 30 sec at 8,000 xg. Samples were washed twice with 500 μL of buffer RPE. The columns were transferred to a new 1.5 mL collection tube and 40 μL of RNase-free water was added directly onto the column membrane and incubated for 1–2 min at room temperature. RNA was eluted by centrifuging for 1 min at 8,000 xg.
2.6. Complimentary Deoxyribonucleic Acid Synthesis
Complimentary deoxyribonucleic acid (cDNA) synthesis was performed using 1 μg of total RNA and Sprint™ RT Complete-Double PrePrimed kit (Clontech; Mountain View, CA) as recommended by the supplier.
2.7. Quantitative Polymerase Chain Reaction Analysis
One μl of each cDNA (diluted 1:10) was used as template in qPCR assays, and performed in triplicates on Mastercycler Realplex (Eppendorf; Hauppauge, NY) using the SYBR qPCR Premix (Clontech) and specific primers (Table 1). Amplification parameters were as follows: 95°C for 2 min; 40 cycles of amplification (95°C for 15 sec, 60°C for 40 sec); dissociation curve step (95°C for 15 sec, 60°C for 15 sec, 95°C for 15 sec). The linear regression line for ng of DNA was determined from relative fluorescent units (RFU) at a threshold fluorescence value (Ct) to gene targets from tissue extracts and normalized by the simultaneous amplification of RpL13A (a housekeeping gene) for all samples. Mean and standard deviation for all experiments were calculated after normalization to RpL13A.
Table 1.
List of primers used in this study.
| Primer | Sequence (5′–3′) |
|---|---|
| Bad-forward | GGAGCAACATTCATCAGCAG |
| Bad-reverse | TACGAACTGTGGCGACTCC |
| Bak1-forward | CCACATCTGGAGCAGAGTCA |
| Bak1-reverse | TGTCCAGATGCCATTTTTCA |
| Bax-forward | CCAAGAAGCTGAGCGAGTGTCT |
| Bax-reverse | AGCTCCATATTGCTGTCCAGTTC |
| Bcl2-forward | GGAGAGCGTCAACAGGGAGA |
| Bcl2-reverse | CAGCCAGGAGAAATCAAACAGAG |
| Bcl-XL-forward | CCTTGGATCCAGGAGAACG |
| Bcl-XL-reverse | CAGGAACCAGCGGTTGAA |
| Bim-EL-forward | AGTGTGACAGAGAAGGTGGACAATT |
| Bim-EL-reverse | GGGATTACCTTGCGGTTCTGT |
| Mcl1-forward | TTCCTTTACTGTTGGCGTGTT |
| Mcl1-reverse | AAAATGGCCAGTGAAGAGCA |
| RpL13A-forward | TCTCAAGGTTGTTCGGCTGAA |
| RpL13A-reverse | GCCAGACGCCCCAGGTA |
2.8. Statistical Analysis
Statistical analysis was carried out using SPSS software, version 19.0 (SPSS Inc., Chicago, IL). Data were submitted to one-way analysis of variance (ANOVA) and the comparisons between groups were performed by using Post Hoc Tukey and Kruskal Wallis tests at a level of significance (P < 0.05).
3. Results
3.1. Micro-CT Analysis
We previously reported that Bcl-2 −/− mice are smaller in stature, have smaller external ears and immature facial features compared to Bcl-2 +/+ or Bcl-2 +/− mice [17]. The anatomic micro-CT scanning of head samples showed dramatic differences between the experimental groups. The Bcl-2 −/− mice had smaller head, maxilla and mandible compared with Bcl-2 +/− and Bcl-2 +/+ mice (Fig. 1). However, the apparent organization and macrostructure of teeth were similar. Thus, Bcl-2 expression has a significant influence on overall growth and development of the evaluated structures as previously reported [17].
Fig. 1.
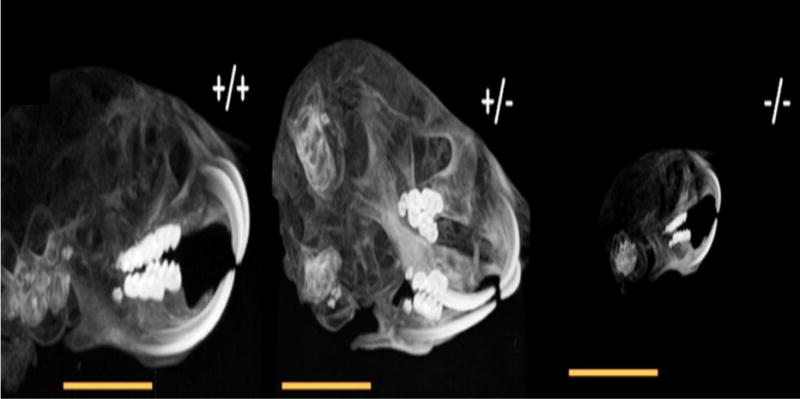
The anatomic micro-CT scanning of Bcl-2 +/+, Bcl-2 +/−, and Bcl-2 −/− mice. Bcl-2 −/− mice showed significantly smaller size of head, maxilla, and mandible compared with other two groups.
3.2. XRD Analysis
Appropriate apoptosis of ameloblasts is essential for proper mineralization of developing tooth [24]. Given the important role of Bcl-2 in regulation of apoptosis, we next ask whether Bcl-2 expression impacts tooth mineralization. The XRD analysis of the five biological calcium phosphates in first molars from mice with indicated genotype is shown in Figure 2A. The primary pattern of the biological calcium phosphate is the HA. However, ß-TCP and ACP were detected in small amounts. The XRD peaks in Bcl-2 −/− mice were wider than other groups, which suggested lower crystallinity of HA in the molars of Bcl-2 −/− mice. The main differences between XRD pattern of Bcl-2 −/− mice and their littermates were in the range of 2θ=10–15° and 2θ=45–55°. In fact, in the first range for Bcl-2 −/− mice XRD pattern, there was no peak, which is perhaps due to ACP in molars of Bcl-2 −/− mice. In the second range the observed peaks were not as sharp compared with the peaks in Bcl-2 +/− and Bcl-2 +/+ mice. This could be attributed to poor crystallinity of HA in Bcl-2 −/− mice (Fig. 2A).
Fig.2.
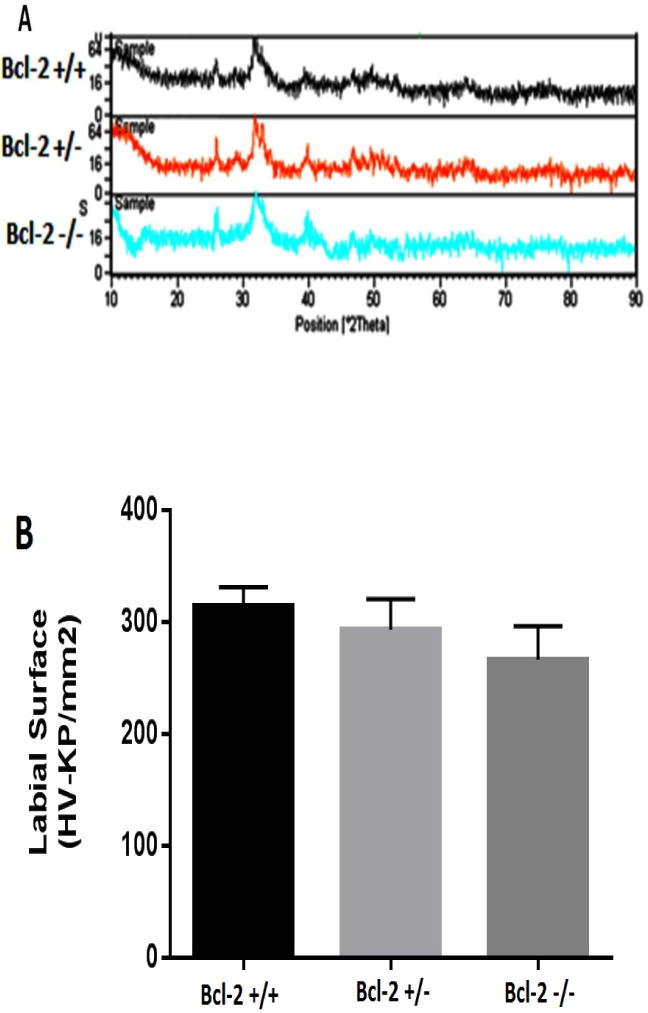
Bcl-2 expression affects tooth mineralization and hardness. A) XRD patterns of crown part of first molar tooth in Bcl-2 +/+, Bcl-2 +/−, and Bcl-2 −/− mice. Significant differences in mineral content were observed in Bcl-2 −/− mice compared with other two groups. Please note the absence of the peak in the first range of samples from Bcl-2 −/− mice. In addition, in the second range of samples from Bcl-2 −/− mice the peaks are not as sharp as peaks in samples from Bcl-2 +/+ and Bcl-2 +/− mice. (B) The surface microhardness values in Bcl-2 +/+, Bcl-2 +/−, and Bcl-2 −/− mice was determined as described in Methods. Bcl-2−/− mice showed significantly lower amount of surface hardness.
3.3. Surface Microhardness Analysis
The aberrant mineralization observed in the absence of Bcl-2 expression suggest alterations in tooth surface hardness. We next examined the surface hardness of teeth from wild type and mutant mice. The surface microhardness evaluations of the incisors from the experimental groups showed significant differences (Fig. 2B). The mean ± standard deviations of the surface microhardness of enamel in Bcl-2 +/+, Bcl-2 +/−, and Bcl-2 −/− mice were 313.9±16.9, 292.9 ±27.4, and 266.3±29.8, respectively. A significant difference was only noted between Bcl-2 +/+ and Bcl-2 −/− mice (P = 0.007), while the microhardness values were not significantly different between Bcl-2 +/+ and Bcl-2 +/− mice (P = 0.293), or Bcl-2 +/− and Bcl-2 −/− mice (P = 0.151) (Fig. 2B).
3.4. Saliva Accumulation
Appropriate salivary gland function and production of saliva plays a key role in tooth mineralization. We next determined whether the expression of Bcl-2 affects salivary gland formation and production of saliva. Fig 3A shows the gross anatomical examination of salivary glands in Bcl-2 +/+ and Bcl-2−/− mice. We observed that the salivary glands including SMG were significantly smaller in Bcl-2−/− mice compared with Bcl-2 +/+. To determine the consequences of these morphological appearances on salivary gland function, we determined the rate of saliva production in these mice. The mean and standard deviations of the amounts of saliva collected from the male samples in Bcl-2 +/+, Bcl-2 +/−, and Bcl-2 −/− mice were 0.978±0.0536, 1.035±0.205, 0.328±0.0796 mL, respectively. Before doing the ANOVA test, the normality of variance of the dependent group was analyzed first. Considering the significant differences at (x = 0.05) and (P = 5.284, df 2, 18, P = 0.016), the assumption of equality of variances were not accepted. We then used the Kruskal Wallis test, the results at (x = 0.05) showed significant differences (P = 0.001) (Fig. 3B).
Fig. 3.
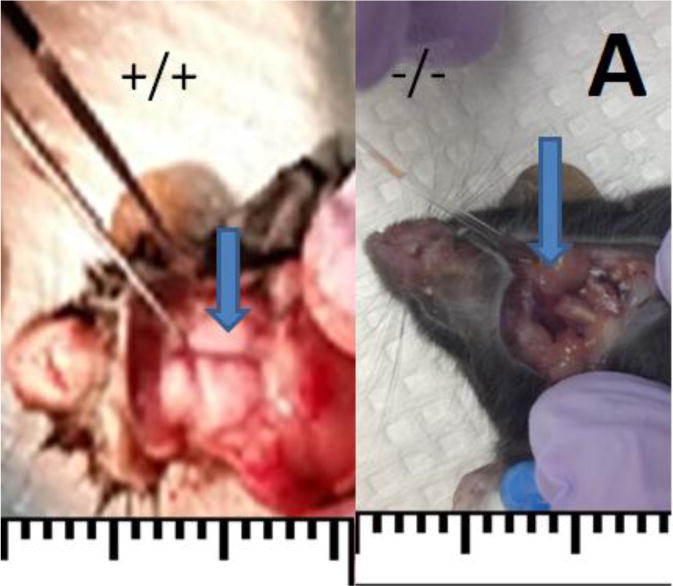
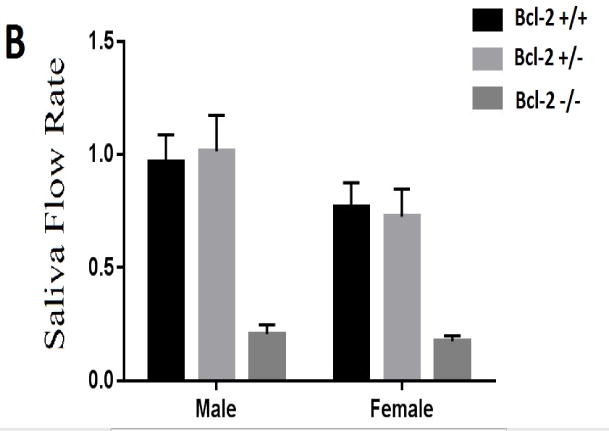
The morphological and functional examination of salivary glands. (A) Bcl-2 +/+ mice showed salivary gland in normal size (blue arrow), while Bcl-2 −/− mice showed a dramatic reduction in size (blue arrow). (B) The amounts of saliva secreted in various groups were determined as detailed in Methods. The mean and standard deviations of the saliva volume in Bcl-2 +/+, Bcl-2 +/−, and Bcl-2 −/− mice from samples from male and female mice showing significantly lower amount of saliva accumulation in Bcl-2 −/− mice.
The mean and standard deviations of the rate of saliva collected from the female samples in Bcl-2 +/+, Bcl-2 +/−, and Bcl-2 −/− mice were 0.85 ± 0.040, 0.691 ± 0.233, 0.112 ± 0.077 mL, respectively. Before performing the ANOVA test, the normality of variance of the dependent group was analyzed first. Considering the significant differences at (x=0.05) and (P =4.647, df 2, 18 P = 0.024), the assumption of equality of variances were not accepted. Using Kruskal Wallis test, the result showed at (x=0.05) significant differences (P = 0.001; Fig. 3B).
3.5. Quantitative Polymerase Chain Reaction Analysis
Next we determined whether Bcl-2-deficiency altered the expression of other Bcl-2 family members. Figure 4A–G, shows the relative expression of Bcl-2, Bcl-XL, Mcl 1, Bad, Bax, Bak1, and Bim-EL. The PCR analysis in Bcl-2 −/− samples showed lack of Bcl-2 expression as expected (Fig. 4A). The decrease in Bcl-2 expression was not compensated by the increased expression of other pro-survival Bcl-2 family members including Bcl-XL or Mcl-1. Furthermore, no increase in expression of pro-apoptotic Bcl-2 family members including Bax, Bak1, Bad, and Bim-EL were observed (Fig. 4B–G).
Fig. 4.
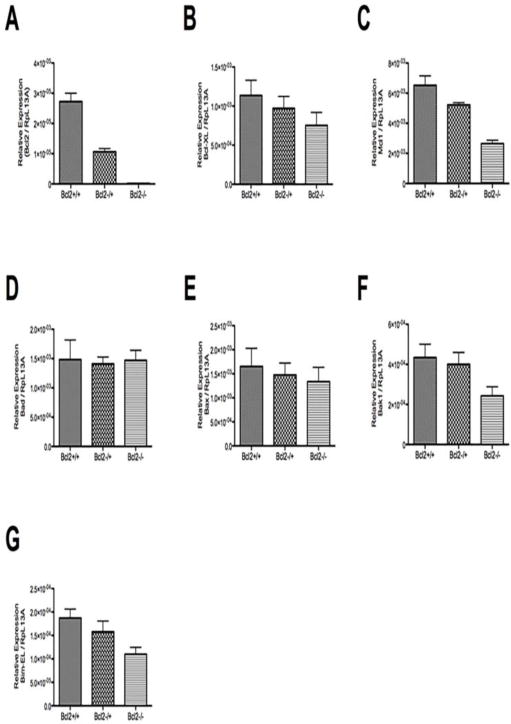
Expression of Bcl-2 family members. The relative expression of Bcl-2 (A), Bcl-XL (B), Mcl 1 (C), Bad (D), Bax (E), Bak1(F), and Bim-EL (G) was determined by real time qPCR as detailed in Methods using specific primers (Table 1). Please note no significant increase in expression of other Bcl-2 pro-survival family members. The expression of Bcl-2 pro-apoptotic family members were also not significantly affected in the absence of Bcl-2.
3.7. SEM analysis
To further investigate the morphological and mineralogical alterations affected by Bcl-2 expression, we performed SEM analysis of the mandible. The primary evaluations showed different occlusal pattern between Bcl-2+/+ and Bcl-2−/− mice (Fig. 5A). The SEM color dot map in Bcl-2 −/− mice varied from Bcl-2 +/+ mice, particularly for minerals including fluoride and strontium. In Bcl-2 +/+ mice the fluoride content was higher with detectable strontium levels (Fig. 5B), while samples from Bcl-2 −/− mice had lower fluoride content and no detectable strontium (Fig. 5C). We next determined calcium levels and showed that the calcium level peaks in samples from Bcl-2 −/− mice were not as large as those from Bcl-2 +/+ mice (Fig. 5B). Thus, Bcl2 −/− mice have poor levels of calcium in their tooth enamel.
Fig. 5.
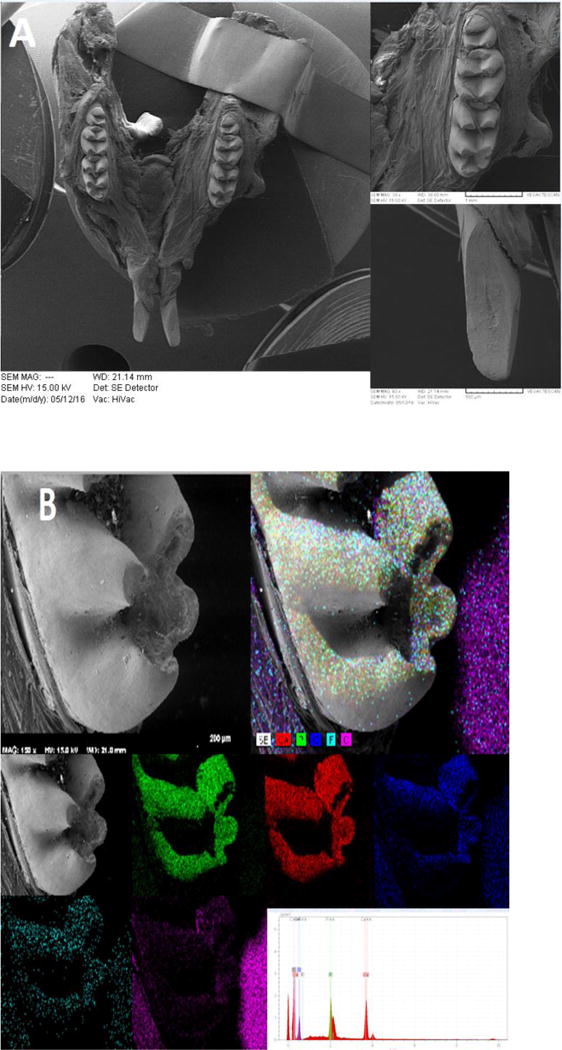
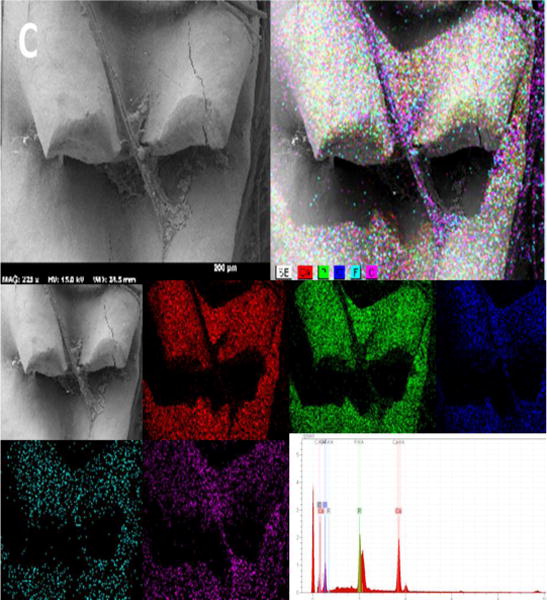
Scanning electron micrographs of mice mandibles by using secondary and back-scattered electron (BSE) detector. (A) The SEM image of Bcl-2 +/+ mice showing the normal structure of mice mandible (left), molar (upper right) and incisor (lower right) teeth. (B) The SEM color dot map image of Bcl-2 +/+ showing the presence of strontium and higher levels of calcium and fluoride. (C) The SEM color dot map image of Bcl-2 −/− showed no strontium and lower levels of calcium and fluoride ions.
4. Discussion
The current study investigated the impact of Bcl-2 expression on tooth development and its properties including mineralogy and surface microhardness of enamel in mice. Mice share structural, functional and genetic traits with humans [25]. Moreover, powerful molecular and genetic tools developed in the past two decades make mice an ideal animal model to study complex traits [25, 26]. Previous studies indicated that tooth structure development, morphogenesis, maturation of enamel, and also regeneration of dentin-pulp complex could be influenced by the expression of Bcl-2 [8, 9, 12].
Teeth and bones are composed of inorganic calcium phosphate minerals which are approximated by hydroxyapatite and matrix proteins. However, the crystals of hydroxyapatite in teeth and bones are different from geological hydroxyapatite, which is referred to as “bioapatite” that plays a critical role in biological functions of teeth and bones [27]. The mineral content of tooth dentin structure correlates with its mechanical properties such as hardness and modulus of elasticity (MOE) [28]. In the present study the mineral content of five biological calcium phosphates including hydroxyapatite, β-tricalcium phosphate, octacalcium phosphate, amorphous calcium phosphate, and dicalcium phosphate dehydrate in enamel [18], and the surface microhardness of teeth in Bcl-2 −/− mice, were adversely affected.
Bcl-2 expression, beside its central role in apoptosis, is an effective modulator of various developmental processes including epithelium and endothelium branching morphogenesis in lung and kidney. Previous studies have also demonstrated that the Bcl-2 deficient mice exhibit accelerated cell death in the spleen, thymus, and kidney [17]. Here we demonstrated a role for Bcl-2 expression in tooth development and function. The micro-CT analysis of experimental groups showed that the Bcl-2 −/− mice have undergone a general symmetrical reduction in size and volume in all tissues of head and maxillary and mandibular arches. Thus, establishing and supporting an important role for Bcl-2 expression in appropriate tissue development and function.
To assess the development and formation of tooth hard tissues, five growth parameters for determining the shape and morphology of crown enamel including a) appositional growth rate, b) duration of appositional growth (on the cusp tip), c) ameloblast extension rate, d) duration of ameloblast cells extension, and e) spreading rate of appositional termination are considered. Appositional growth begins with formation of ACP ribbons, which later converts to hydroxyapatite crystallites as it elongates [29]. The results of present study are consistent with this outcome as in the XRD pattern of samples from Bcl-2 −/− mice, where in the first range there was no peak related to ACP formation. This observation can be explained by low and poor crystallinity of HA components in the second range of XRD patterns in samples from Bcl-2 −/− mice. Thus, lack of Bcl-2 interferes with the ameloblast cell extension resulting in immature enamel formation as a result of decreased HA crystallites in the tooth structure. In SEM color dot map analysis, similar results were noted. The SEM images from Bcl-2 −/− mice showed lower fluoride and calcium content with no strontium. These results can attribute to the lower crystallinity of HA in enamel structure of the molars from Bcl-2 −/− mice, which was consistent with XRD results.
The maturation of enamel structure after secretion is due to the maturation of enamel crystals that harden the tooth structure [30]. Bcl-2 −/− mice presented reduction on tooth surface microhardness, which can be attributed to the immaturity of hydroxyapatite crystals. This outcome can be attributed to the immaturity of hydroxyapatite crystals in samples from Bcl-2 −/− mice. As it was also evident in the XRD pattern of samples from Bcl-2 −/− mice, where the peaks related to HA were not as sharp as peaks in the other two groups. This indicates the lower and poor crystallinity of HA in enamel structure of samples from Bcl-2 −/− mice. These changes in enamel structure can explain the lower values of surface microhardness in samples from Bcl-2 −/− mice.
We also determined the impact of Bcl-2 expression on the saliva accumulation. For more than 50 years, the SMG has been used as a model organ culture due to its classic branching morphogenesis [31]. In the final developmental stage of SMG in mice, known as “terminal bud stage”, the epithelial cells on the periphery undergo proliferation while the central cells undergo apoptosis to form the ducts and acini structures [14]. Since we have previously shown that Bcl-2 expression is essential for proper differentiation of renal epithelial and mesenchymal cells and branching morphogenesis during kidney development [17, 32–34], we anticipated that the SMG morphogenesis might be influenced by lack of Bcl-2. Our results demonstrated that lack of Bcl-2 expression impacted salivary gland morphogenesis and saliva production. These results can be explained by the changes observed in the size of the SMG, which is remarkably decreased in samples from Bcl-2 −/− mice compared with Bcl-2 +/+ mice.
Another parameter tested in this study was the accumulation of saliva in various samples, as the secretion of saliva has tremendous effects on tooth and oral tissue health [15, 16, 35]. Downregulation of Bcl-2 is associated with destruction of glandular tissue and decreased secretory function in Sjögren’s syndrome [36]. In the present study we also did not detect any developed and mature duct or formed acini in samples from Bcl-2 −/− mice compared with samples from Bcl-2 +/+ or Bcl-2 +/− mice (not shown). In addition, the outcomes of saliva accumulation showed a significant decrease in the amount of saliva in Bcl-2 −/− mice. The reduction of saliva levels was consistent with the reduction in size of SMG in Bcl-2 −/− mice. Thus, expression of Bcl-2 is essential for proper development and function of salivary glands, and remains a subject of future investigation in our laboratory.
We also showed that the decreased Bcl-2 expression in Bcl-2 +/− and Bcl-2 −/− SMG samples was not compensated by increased expression of other pro-survival Bcl-2 family members. These results are consistent with the previous studies demonstrating contemporaneous co-expression of Bcl-2, Bcl-XL, Bax and Bak proteins during tooth germ development [11]. In addition, the level of Bcl-2 expression was greater than Bax, Bak, and Bcl-XL. The reduced expression of Bcl-2 has adverse effect on developmental and functional properties of maxillofacial and tooth. Thus, expression of Bcl-2 is essential for normal development and function of salivary glands and saliva production. Collectively our studies demonstrate a significant role for Bcl-2 expression in tooth and salivary gland development and function. In addition, these defects may contribute to insufficient nutritional intake, the small size, and poor health of the mice deficient in Bcl-2.
5. Conclusions
According to the tested parameters, outcomes, and limitations of present study the following conclusions can be drawn:
Bcl-2 expression has a significant impact on the mineralogical content of enamel crystals of tooth structure. Lack of Bcl-2 expression led to impaired production of enamel ACP crystals.
The surface microhardness was influenced by Bcl-2 expression, as the germline deletion of Bcl-2 reduced the surface microhardness of enamel.
The germline deletion of Bcl-2 resulted in structural changes in SMG function, which results in reduction of saliva production in oral cavity.
The lack of Bcl-2 expression was not compensated by significant changes in the expression of other pro- and anti-apoptotic Bcl-2 family members in SMG.
Highlights.
Lack of Bcl-2 expression led to impaired production of enamel ACP crystals.
Germline deletion of Bcl-2 reduced the surface microhardness of enamel.
Bcl-2 expression is vitally important for SMG function and saliva production.
Lack of Bcl-2 expression was not compensated by expression of other Bcl-2 family members.
Acknowledgments
The authors express special thanks to Regenerative Group of Dr. H Afsar Lajevardi Research Cluster. NS is supported by an unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology, Retina Research Foundation, P30 EY016665, P30 CA014520, EPA 83573701, and EY022883. This publication is dedicated to the memory of Dr. H Afsar Lajevardi, a legendry Pediatrician (1953–2015) who passed away during this project. We will never forget Dr. H Afsar Lajevardi’s kindness and support.
Abbreviations
- SMG
Submandibular gland
- QPCR
Quantitative Polymerase Chain Reaction Analysis
- SEM
Scanning Electron Microscope
- HA
Hydroxyapatite
- XRD
X-Ray diffraction
- MHV
Microhardness Value
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions:
MAS: designed and conducted experiment, analyzed/interpreted data, wrote and proofed/revised the manuscript. AA: designed experiment, analyzed/interpreted data, wrote and proofed/revised the manuscript. ZG: conducted experiment, analyzed/interpreted data, wrote and proofed/revised the manuscript. CMS: designed and conducted experiments, analyzed/interpreted data, wrote and proofed/revised the manuscript. NS: designed experiment, analyzed/interpreted data, wrote and proofed/revised the manuscript. CMS is supported by RRF Daniel M. Albert Chair.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 2.NunÄez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 3.Smith C, Warshawsky H. Quantitative analysis of cell turnover in the enamel organ of the rat incisor. Evidence for ameloblast death immediately after enamel matrix secretion. The Anatomical Record. 1977;187:63–97. doi: 10.1002/ar.1091870106. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 5.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- 6.Zhang W, Ju J, Gronowicz G. Odontoblast‐targeted Bcl‐2 overexpression impairs dentin formation. J Cell Biochem. 2010;111:425–432. doi: 10.1002/jcb.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matalova E, Svandova E, Tucker AS. Apoptotic signaling in mouse odontogenesis. OMICS. 2012;16:60–70. doi: 10.1089/omi.2011.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph B, Savage N, Young W, Waters M. Insulin-like growth factor-I receptor in the cell biology of the ameloblast: an immunohistochemical study on the rat incisor. Epithelial Cell Biol. 1993;3:47–53. [PubMed] [Google Scholar]
- 9.Fleischmannova J, Matalova E, Sharpe P, Misek I, Radlanski R. Formation of the tooth-bone interface. J Dent Res. 2010;89:108–115. doi: 10.1177/0022034509355440. [DOI] [PubMed] [Google Scholar]
- 10.LeBrun DP, Warnke RA, Cleary ML. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. The American journal of pathology. 1993;142:743. [PMC free article] [PubMed] [Google Scholar]
- 11.Krajewski S, Hugger A, Krajewska M, Reed JC, Mai JK. Developmental expression patterns of Bcl-2, Bcl-x, Bax, and Bak in teeth. Cell Death Differ. 1998;5:408–415. doi: 10.1038/sj.cdd.4400360. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura C, Kimura K, Nakayama T, Toyoshima K, Terashita M. Primary and secondary induction of apoptosis in odontoblasts after cavity preparation of rat molars. J Dent Res. 2001;80:1530–1534. doi: 10.1177/00220345010800061001. [DOI] [PubMed] [Google Scholar]
- 13.Pammer J, Horvat R, Weninger W, Ulrich W. Expression of bcl-2 in salivary glands and salivary gland adenomas: A contribution to the reserve cell theory. Pathology-Research and Practice. 1995;191:35–41. doi: 10.1016/S0344-0338(11)80920-9. [DOI] [PubMed] [Google Scholar]
- 14.Tucker A. Semin Cell Dev Biol. Vol. 18. Elsevier; 2007. Salivary gland development; pp. 237–244. [DOI] [PubMed] [Google Scholar]
- 15.González-García M, Pérez-Ballestero R, Ding L, Duan L, Boise L, Thompson C, Nunez G. bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development. 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- 16.Lu QL, Poulsom R, Wong L, Hanby AM. BCL‐2 expression in adult and embryonic non‐haematopoietic tissues. The Journal of pathology. 1993;169:431–437. doi: 10.1002/path.1711690408. [DOI] [PubMed] [Google Scholar]
- 17.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 18.Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, Lim SY. Analysis of the inorganic component of autogenous tooth bone graft material. Journal of nanoscience and nanotechnology. 2011;11:7442–7445. doi: 10.1166/jnn.2011.4857. [DOI] [PubMed] [Google Scholar]
- 19.Saghiri MA, Asgar K, Gutmann JL, Garcia-Godoy F, Ahmadi K, Karamifar K, Asatorian A. Quintessence international. Vol. 43. Berlin, Germany: 2011. Effect of laser irradiation on root canal walls after final irrigation with 17% EDTA or BioPure MTAD: X-ray diffraction and SEM analysis; pp. e127–134. 1985. [PubMed] [Google Scholar]
- 20.Saghiri M, Asgar K, Lotfi M, Garcia-Godoy F. Nanomodification of mineral trioxide aggregate for enhanced physiochemical properties. Int Endod J. 2012;45:979–988. doi: 10.1111/j.1365-2591.2012.02056.x. [DOI] [PubMed] [Google Scholar]
- 21.Saghiri MA, Delvarani A, Mehrvarzfar P, Malganji G, Lotfi M, Dadresanfar B, Saghiri AM, Dadvand S. A study of the relation between erosion and microhardness of root canal dentin. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2009;108:e29–e34. doi: 10.1016/j.tripleo.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 22.Marmary Y, Fox PC, Baum BJ. Fluid secretion rates from mouse and rat parotid glands are markedly different following pilocarpine stimulation. Comparative Biochemistry and Physiology Part A: Physiology. 1987;88:307–310. doi: 10.1016/0300-9629(87)90488-9. [DOI] [PubMed] [Google Scholar]
- 23.Lin A, Johnson D, Wu Y, Wong G, Ebersole J, Yeh CK. Measuring short-term γ-irradiation effects on mouse salivary gland function using a new saliva collection device. Arch Oral Biol. 2001;46:1085–1089. doi: 10.1016/s0003-9969(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 24.Kondo S, Tamura Y, Bawden J, Tanase S. The immunohistochemical localization of Bax and Bcl-2 and their relation to apoptosis during amelogenesis in developing rat molars. Arch Oral Biol. 2001;46:557–568. doi: 10.1016/s0003-9969(00)00139-4. [DOI] [PubMed] [Google Scholar]
- 25.Saghiri MA, Sheibani N, Orangi J, Asatourian A. Validity and Variability of Animal Models Used in Dentistry. 2015 [Google Scholar]
- 26.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boskey AL. Mineralization of bones and teeth. Elements. 2007;3:385–391. [Google Scholar]
- 28.Angker L, Nockolds C, Swain MV, Kilpatrick N. Correlating the mechanical properties to the mineral content of carious dentine—a comparative study using an ultra-micro indentation system (UMIS) and SEM-BSE signals. Arch Oral Biol. 2004;49:369–378. doi: 10.1016/j.archoralbio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Michaelidis T, Sendtner M, Cooper J, Airaksinen M, Holtmann B, Meyer M, Thoenen H. Inactivation of bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development. Neuron. 1996;17:75–89. doi: 10.1016/s0896-6273(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 30.Simmer JP, Papagerakis P, Smith CE, Fisher DC, Rountrey AN, Zheng L, Hu JC. Regulation of dental enamel shape and hardness. J Dent Res. 2010;89:1024–1038. doi: 10.1177/0022034510375829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borghese E. Explantation experiments on the influence of the connective tissue capsule on the development of the epithelial part of the submandibular gland of Mus musculus. J Anat. 1950;84:303. [PMC free article] [PubMed] [Google Scholar]
- 32.Sheibani N, Scheef EA, Dimaio TA, Wang Y, Kondo S, Sorenson CM. Bcl-2 expression modulates cell adhesion and migration promoting branching of ureteric bud cells. J Cell Physiol. 2007;210:616–625. doi: 10.1002/jcp.20858. [DOI] [PubMed] [Google Scholar]
- 33.Kondo S, Oakes MG, Sorenson CM. Rescue of renal hypoplasia and cystic dysplasia in Bcl-2−/− mice expressing Bcl‐2 in ureteric bud derived epithelia. Dev Dyn. 2008;237:2450–2459. doi: 10.1002/dvdy.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorenson CM, Rogers SA, Korsmeyer SJ, Hammerman MR. Fulminant metanephric apoptosis and abnormal kidney development in bcl-2-deficient mice. American Journal of Physiology-Renal Physiology. 1995;268:F73–F81. doi: 10.1152/ajprenal.1995.268.1.F73. [DOI] [PubMed] [Google Scholar]
- 35.Mandel ID. The functions of saliva. J Dent Res. 1987;66:623–627. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- 36.Manganelli P, Quaini F, Andreoli A, Lagrasta C, Pilato F, Zuccarelli A, Monteverdi R, D’aversa C, Olivetti G. Quantitative analysis of apoptosis and bcl-2 in Sjogren’s syndrome. The Journal of rheumatology. 1997;24:1552–1557. [PubMed] [Google Scholar]
- 37.Saghiri MA, Saghiri AM. In Memoriam: Dr. Hajar Afsar Lajevardi MD, MSc, MS (1955–2015) Iran J Pediatr. 2017;27:e8093. doi: 10.5812/ijp.8093. [DOI] [Google Scholar]


