Abstract
Introduction
Despite recent diagnostic and therapeutic improvements, pancreas cancer remains one of the highly lethal cancers. The extracellular matrix (ECM) is a physiological barrier that limits the spread of cancer cells into surrounding tissues and distant organs. Disintegrin and metalloprotease with thrombospondin motifs (ADAMTS) is a family of 19 proteases, which is involved in various biological processes such as ECM remodelling and anti-angiogenesis.
Aim
To investigate the expression of ADAMTS1, 8, 9, and 18 proteinases in pancreas adenocarcinoma and its nodal metastasis.
Material and methods
The immunostaining status of ADAMTS1, 8, 9, and 18 were investigated in formalin-fixed paraffin-embedded samples of 25 patients who underwent pancreaticoduodenectomy for an adenocarcinoma located at the head of the pancreas.
Results
In semi-quantitive grading pathologically, ADAMTS1, 8, 9, and 18 were found to be highly stained in all cancerous pancreas samples compared with normal pancreas. In addition, the immune positivity of ADAMTS1, 9, and 18 was found to be higher in metastatic lymph nodes than in non-metastatic lymph tissue. Tumour size was correlated with ADAMTS9 and 18 expressions in cancerous pancreas.
Conclusions
According to the data obtained from the study, we suggest that these four ADAMTSs may have significant roles in the tumorigenesis and nodal spread of pancreas adenocarcinoma.
Keywords: ADAMTS, immunohistochemistry, pancreas cancer
Introduction
Pancreas cancer is one of the most lethal malignancies in the world [1]. Unfortunately, only 15–20% of the patients are suitable for surgery at the time of diagnosis, due to nonspecific symptoms and signs [2]. Current treatment modalities including surgery and radiochemotherapy have limited effectiveness, and hence surveys of the patients are quite poor with a ratio of 5% in metastatic disease and 20% in localised disease [3]. Therefore, development of alternative and effective therapeutic approaches is a necessity. In this context, the genetic or molecular basis of cancer has become one of the promising foci in recent studies.
As is well known, extracellular matrix (ECM) provides mechanical support to the cells and plays crucial roles in many physiological processes such as embryogenesis, cell migration, interaction between cells, haemostasis, wound repair, and apoptosis [4, 5]. Structural and functional disorders of the ECM may result in pathological conditions such as cancer. The ADAMTS family, as a relatively new group of ECM proteases, is involved in carcinogenesis and metastasis [6, 7]. Among all ADAMTSs, ADAMTS1, 8, 9, and 18 are known as aggrecanases. Additionally, ADAMTS1, 8, and 9 have inhibitory roles in angiogenesis, which is an essential step in tumuoral proliferation [7, 8]. ADAMTS18 is also considered as a tumour suppressor gene.
There are few reports on the expression of ADAMTSs in pancreas cancer.
Aim
We aimed to determine the expression status of ADAMTS1, 8, 9, and 18 in pancreas adenocarcinoma, by using immunohistochemistry.
Material and methods
Patients and paraffin-embedded preparates
Twenty-five patients (13 males and 12 females) who underwent surgery for pancreatic ductal adenocarcinoma in the Department of General Surgery, Turgut Özal University, Ankara, Turkey were included in this study. Resectability criteria of the tumours were as follows: no arterial (celiac axis, superior mesenteric artery, or common hepatic artery) and/or venous (superior mesenteric vein or portal vein) tumour contact. Curative pancreaticoduodenectomy (Whipple procedure) was performed in all cases. Of 25 patients, one had stage 2 tumour and 24 had stage 3 tumour, according to the current TNM classification (American Joint Committee on Cancer, 2010). Immunohistochemical analysis of ADAMTS1, ADAMTS8, ADAMTS9, and ADAMTS18 was performed by using paraffin-embedded samples of the cases. Patients’ noncancerous pancreatic tissues and non-metastatic lymph node tissues were used as control. The immunohistochemistry was evaluated by at least two trained pathologists. Written informed consent was obtained from all patients, and the study protocol was approved by the Medical Ethics Committee of Turgut Özal University, Faculty of Medicine, Turkey (Permit Number, date: 99950669/176, 21/03/2014).
Immunohistochemistry
All experimental steps were performed in accordance to the protocols recommended for the anti-human ADAMTS1, 8, 9, and 18 polyclonal antibodies (Abcam). After being deparaffinised at 65°C in a heat chamber and rehydrated, sections were subjected to epitope retrieval in 10× EDTA buffer (pH 8.0) at 110°C for 30 min. Subsequently, the sections were exposed to 3% H2O2 for 20 min to bleach endogenous peroxidases and were rinsed three times with phosphate-buffered saline (PBS) for 10 min. Sections were respectively incubated with a rabbit anti-human ADAMTSs (all 1 : 250 in BSA) for 1 h at 37°C, washed three times in PBS, and incubated in a biotinylated goat secondary anti-mouse polyclonal antibody for 15 min at 37°C. Following being washed in PBS, the tissues were visualised with 3,3′-diaminobenzidine tetrahydrochloride (DAB chromogen, Abcam) and counterstained with haematoxylin. Finally, the sections were dehydrated in graded ethanol, immersed in xylene, and coverslipped. All images were acquired using a 40× objective and an microscope (Leica).
Evaluation and scoring of staining
Immunoreactivities of ADAMTS1, 8, 9, and 18 in all samples were evaluated using a well-established immunoreactivity scoring system (IRS) that takes into account both the percentage of positive cells and staining intensity [9]. All tissues were scored between 0 (no staining) and 12 (maximum staining) according to IRS (Table I). All ADAMTS expressions were scored by two pathologists blinded to clinical details for each case. In addition to intensity of staining, intra/extracellular distribution of staining (cytoplasm, nucleus, and surrounding stroma) was also evaluated. All the statistical analyses between immunostaining status of ADAMTSs and the clinicopathological parameters were performed by using the mean IRSs of ADAMTSs in cancerous and healthy tissues.
Table I.
Immunoreactivity scoring system (IRS)* [A]
| Percentage of positive cells | Staining intensity | IRS score | IRS classification |
|---|---|---|---|
| No positive cell (0) | No reaction (0) | Negative (0–1) | Negative (0) |
| < 10% positive cells (1) | Mild reaction (1) | Mild (2–3) | Positive, weak exp. (1) |
| 10–50% positive cells (2) | Moderate reaction (2) | Moderate (4–8) | Positive, intermediate exp. (2) |
| 51–80% positive cells (3) | Strong reaction (3) | Strong (9–12) | Positive, strong exp. (3) |
| > 80% positive cells (4) |
IRS score – percentage of positive cells × staining intensity, exp – expression.
Statistical analysis
All data were statistically analysed by using the statistical package for social sciences (SPSS 21.0 software, IL-Chicago, USA). Results of descriptive analysis were expressed as the mean ± SD and/or number (percentage) for variables. The expression profiles (immunoreactivity scores) of ADAMTS1, 8, 9, and 18 between cancerous and non-cancerous tissues, and the relationship between ADAMTS1, 8, 9, and 18 immunostaining status and the pathological features of the tumour were assessed by using Pearson χ2, Fisher’s Exact Test, and Spearman’s correlation test. The significance level was accepted as p < 0.05.
Results
Demographic data of the patients and tumour characteristics are summarised in Table II. Complications related to surgery were observed in 6 (24%) patients (3 with wound infection, 2 with pancreatic fistula from pancreaticoenterostomy, and 1 with intra-abdominal haemorrhage). No patient died within the postoperative period of 1 month. Neoadjuvant therapy was not given to any patient; however, 20 of 25 patients received adjuvant chemoradiation (5-fluorouracil or gemcitabine-based chemoradiation). Five patients did not receive any adjuvant therapy due to the poor health status. All patients were followed-up regularly. The median overall survival was 22 months (8–43), and only 4 (16%) patients survived over 3 years after surgery.
Table II.
Patient demographics and tumour characteristics (N = 25)
| Parameters | Results |
|---|---|
| Age [years] | 58 to 79 years, mean: 67 ±6.56 |
| Gender: | |
| Male | 13 (52%) |
| Female | 12 (48%) |
| Localisation of tumour | Head of pancreas (25, 100%) |
| Type of surgery | Whipple procedure (25, 100%) |
| Histopathology | Adenocarcinoma (25, 100%) |
| Tumour size [mm] | 15 to 36 (mean: 28.68 ±4.68) |
| Number of totally lymph node | 20 to 75 (mean: 38.52 ±19.96) |
| Number of metastatic lymph node | 0 to 9 (mean: 4.96 ±2.01) |
| Tumour stage | Stage 2 (1, 4%), Stage 3 (96%) |
| Tumour differentiation grade: | |
| Well-differentiated | 9 (36%) |
| Moderate-differentiated | 16 (64%) |
| Venous invasion positivity | 12 (48%) |
| Lymphatic invasion positivity | 24 (96%) |
| Perineurial invasion positivity | 25 (100%) |
Age, tumour size, number of totally lymph node, and number of metastatic lymph node were presented as mean ± SD (range); other variables were presented as n (%).
It was clearly revealed that pancreas adenocarcinoma expressed ADAMTS1, 8, 9, and 18 in all samples, with different IRS scores. The mean IRS scores of four ADAMTS proteins in cancerous and non-cancerous tissues are presented in Table III.
Table III.
Mean IRS scores of ADAMTS1, 8, 9, and 18 in cancerous and non-cancerous tissues
| Variable | ADAMTS1 | ADAMTS8 | ADAMTS9 | ADAMTS18 | ||||
|---|---|---|---|---|---|---|---|---|
| IRS | P-value | IRS | P-value | IRS | P-value | IRS | P-value | |
| NCPT | 1.32 ±1.4 (0–6) |
< 0.001 | 0.56 ±0.8 (0–3) |
< 0.001 | 0.7 ±0.8 (0–3) | < 0.001 | 1 ±0.9 (0–3) |
< 0.001 |
| CPT | 5.68 ±2.8 (2–12) |
3.1 ±1.5 (1–6) |
5.24 ±2.2 (2–9) | 6.5 ±3.4 (2–12) |
||||
| NMLN | 0.84 ±0.8 (0–3) |
< 0.001 | 1.6 ±1.5 (0–6) |
0.500 | 0.36 ±0.7 (0–2) | < 0.001 | 0.92 ±1.0 (0–3) |
< 0.001 |
| MLN | 5.4 ±3.3 (0–12) |
2.3 ±1.7 (0–8) |
5 ±2.4 (2–9) |
6.28 ±2.9 (2–12) |
||||
IRS scores are presented as mean ± SD (minimum-maximum). NCPT – non-cancerous pancreatic tissue, CPT – cancerous pancreatic tissue, NMLN – non-metastatic lymph node, MLN – metastatic lymph node.
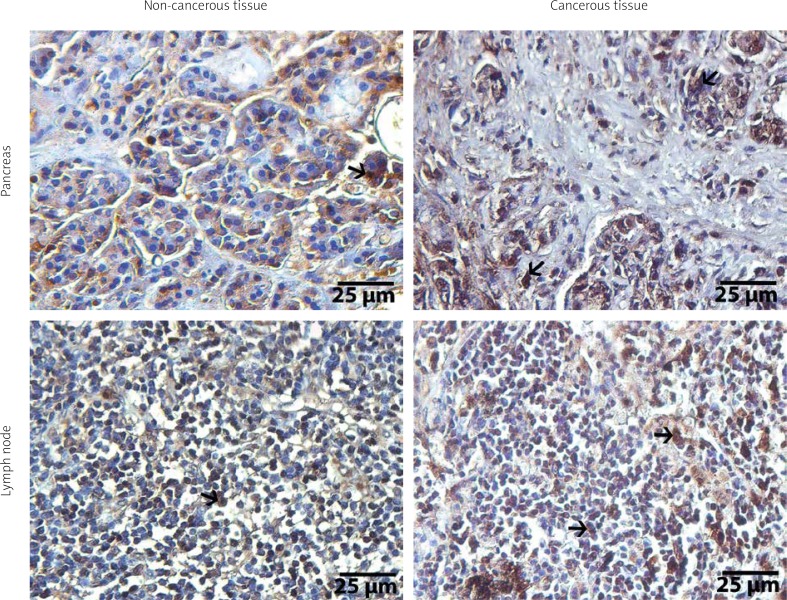
ADAMTS1 staining was observed in the cytoplasm and surrounding stromal tissues of the cancer cells, and it was statistically increased in the cancerous exocrine pancreas tissue compared with exocrine areas of normal pancreas tissue (p < 0.001). In addition, ADAMTS1 immunostaining was higher in the metastatic lymph node compared with healthy lymph node (p < 0.001) (Figure 1). The expression of ADAMTS1 in pancreatic cancerous tissue was found to be inversely correlated with vascular invasion (rh0 = –0.413, p = 0.040). However, no relationship was found between the expression of ADAMTS1 in the cancerous pancreatic tissue or metastatic lymph node and the other clinicopathological characteristics (p > 0.05).
Figure 1.
Immunohistochemical illustrations of pancreas and lymph node tissues of ADAMTS1. Arrows show positive staining areas, streptavidine-peroxidase. Scale bar = 25 μm
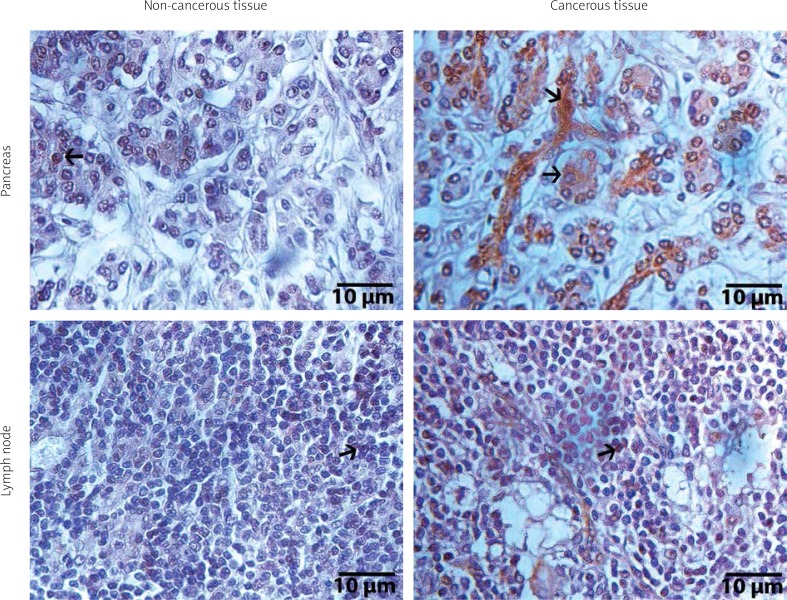
Immunohistochemical staining revealed that cytoplasmic expression of ADAMTS8 was higher in the cancerous exocrine pancreas tissue than in normal exocrine pancreas tissue (p < 0.001). Contrary to the other three ADAMTSs, immune-positivity of ADAMTS8 in the metastatic lymph node was not significantly different in comparison to healthy lymph node tissue (p = 0.500) (Figure 2). Similarly to ADAMTS1, there were no significant differences between ADAMTS8 expression in cancerous pancreatic tissue and the clinicopathological features (p > 0.05).
Figure 2.
Immunohistochemical illustrations of pancreas and lymph node tissues of ADAMTS8. Arrows show positive staining areas, streptavidine-peroxidase. Scale bar = 10 μm
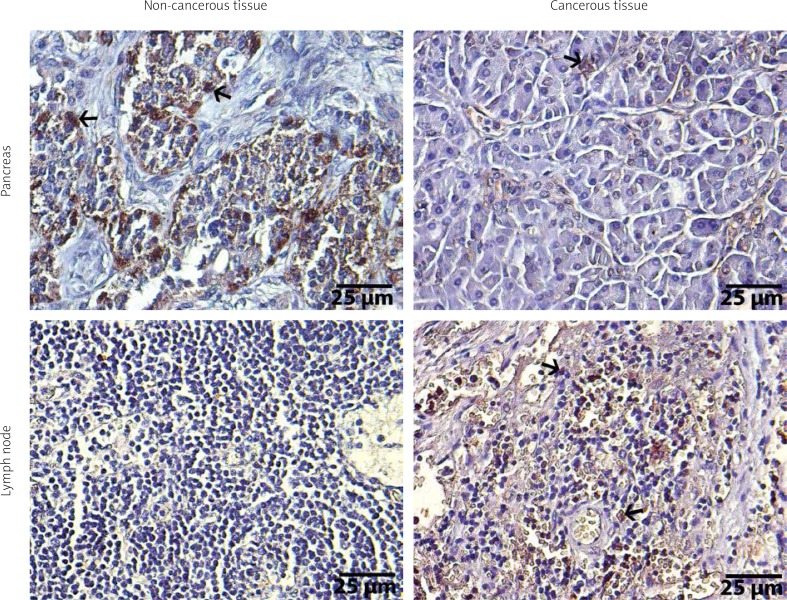
Higher cytoplasmic expression of ADAMTS9 in cancerous pancreas tissues (p < 0.001) and metastatic lymph nodes (p < 0.001) than in normal pancreas and lymph node tissues were found (Figure 3). ADAMTS9 expression in cancerous pancreas showed positive correlation with tumour size (rh0 = 0.610, p < 0.001); however, no relationship was found in terms of the other clinicopathological parameters (p > 0.05).
Figure 3.
Immunohistochemical illustrations of pancreas and lymph node tissues of ADAMTS9. Arrows show positive staining areas, streptavidine-peroxidase. Scale bar = 25 μm
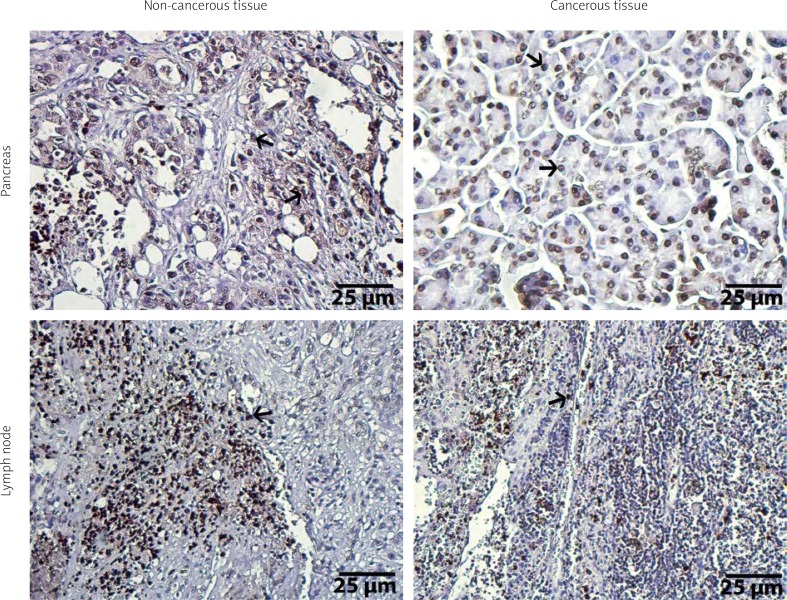
Finally, The immunostaining status of ADAMTS18 was statistically evaluated. Similar to other three ADAMTSs, ADAMTS18 clearly showed higher staining in cancerous pancreas (both in cytoplasm and nucleus of the cancer cells) compared with normal pancreatic tissue (p < 0.001). The immunostaining level of ADAMTS18 was also higher in metastatic lymph nodes in comparison to healthy lymph nodes (p < 0.001) (Figure 4). In addition, ADAMTS18 expression in cancerous pancreas showed positive correlation with tumour size (rh0 = 0.545, p < 0.001), similar to ADAMTS9. However, no relationship was found between ADAMTS18 expression both in cancerous pancreatic tissue/metastatic lymph node and the other clinical and pathological characteristics (p > 0.05).
Figure 4.
Immunohistochemical illustrations of pancreas and lymph node tissues of ADAMTS18. Arrows show positive staining areas, streptavidine-peroxidase. Scale bar = 25 μm
Discussion
It is well known that destruction of ECM is the first essential step in the progression of cancer [10]. In this respect, it has been suggested that ADAMTS proteinases play important roles in cancer invasion and metastasis [11]. To date, several ADAMTSs have been implicated in various benign and malignant diseases [12–17]. Among those, ADAMTS1 is the most investigated member of this protease family, and was identified as an epigenetically deregulated gene in colorectal tumorigenesis [18]. However, there are limited data on the relationship between ADAMTS proteases and pancreas carcinoma in the literature. In this study, we demonstrated the high expression status of ADAMTS1, 8, 9, and 18 in pancreas cancer immunohistochemically.
In the literature, there is only one study on expression of ADAMTS1 and 8 in pancreatic cancers [19]. In that study, the authors examined ADAMTS1–8 m RNA expression in six pancreatic cell lines, and found increased ADAMTS1 expression together with very low levels of ADAMTS8 both in cancerous and non-cancerous pancreatic tissues. In accordance, we found that ADAMTS1 was expressed a little more than ADAMTS8 in cancerous pancreas and metastatic lymph nodes. The authors also showed that the expression level of ADAMTS1 was lower in pancreas cancer than in non-cancerous tissue. However, as one of the important results of that study, patients with higher levels of ADAMTS1 had worse survival rates than those with low level of ADAMTS1 due to severe lymph node metastasis and retroperitoneal invasion. This prometastatic property of ADAMTS1 is probably based on its role in degradation of ECM via cleaving some substrates such as syndecan-1 and glypican-1. These cell surface proteoglycans, members of the heparan sulphate proteoglycan family in the ECM, were identified to be overexpressed in pancreatic cancers [20, 21]. In our study, ADAMTS1 immunoreactivity was higher in pancreatic tumour cells compared with non-cancerous pancreatic tissue. Additionally, nuclear positivity of ADAMTS1 in non-metastatic lymph node was lower than in metastatic lymph node, and there was a negative correlation between ADAMTS1 expression in cancerous pancreas and vascular invasion of the tumour. All those findings suggested the potential anti-metastatic property of ADAMTS1 in pancreas cancer. There was also no relationship between ADAMTS1 expression and tumour size. In our opinion, these findings show that pancreas cancer cells abundantly express ADAMTS1, and this protease may have a suppressor role in the lymphatic spread, due to its anti-angiogenic effects. Interestingly, in a study by Liu et al. [22], the overexpression of full-length ADAMTS1 was found to promote tumour growth or metastasis; however, the ADAMTS1 fragments were shown to display antimetastatic activity.
Generally, ADAMTS8 is less expressed in cancerous tissues compared with ADAMTS1. This protease was shown to be down-regulated in various types of cancer, including breast, brain, and non-small cell lung cancers [23–26]. Porter et al. [24] found that high expression levels of ADAMTS8 together with low expression levels of ADAMTS15 were associated with poor prognosis in breast cancer. In another study, the expression of ADAMTS8 was found to be down-regulated in brain tumours [24]. Contrary to the study by Masui et al. [19], we found that positive immunostaining of ADAMTS8 in pancreatic cancerous tissue was higher than in adjacent normal pancreas in all paraffin samples. Although there was no significant difference between the staining levels of healthy and metastatic lymph tissues. ADAMTS8 was also not associated with tumour size or other pathological features. These data suggest that pancreatic cancer cells produce a large amount of ADAMTS8, and ADAMTS8 may have an inhibiting role in lymphatic metastasis, consistent with its angio-inhibitory property.
The third one, ADAMTS9, was identified as a tumour suppressive gene in several malignancies including nasopharyngeal and oesophageal cancers [27]. It is also a new member of anti-angiogenic ADAMTS family [7]. Additionally, in a study by Kleivi et al. [28], ADAMTS9 was found to be down-regulated in hepatic metastasis of colorectal cancers. In another study, expression of ADAMTS9 was investigated in three types of cancer including gastric, colorectal, and pancreatic cancers by using high-resolution melting analysis. The authors found that the frequency of ADAMTS9 methylation in those malignancies was significantly higher compared with noncancerous tissues [29]. In our study, higher cytoplasmic expression of ADAMTS9 was observed in cancerous pancreas tissues and metastatic lymph nodes than in normal pancreas and lymph node tissues. However, similar to other ADAMTSs, the expression of ADAMTS9 was not found to be associated with the clinicopathological characteristics.
Finally, the immunostaining status of ADAMTS18 was analysed in the present study. Normally, ADAMTS18 is expressed by endothelial cells in various organs such as brain, prostate, oesophagus, stomach, colon, and pancreas. ADAMTS18 is accepted as an aggrecanese [7], but this property is rather low and manifests itself at high concentrations of ADAMTS18 [30]. The other important function of ADAMTS18 is the maintenance of homeostasis via activation of platelet aggregation. This property is considered to be associated with inhibition of metastasis, probably through disruption of tumour emboli [6, 31]. The tumour suppressive effect of ADAMTS18 has also been shown in a recent study [32]. In addition, ADAMTS18 expression was determined to be reduced or totally silenced in multiple carcinoma cell lines [32]. The mutation and deletion of ADAMTS-18 gene in various tumours such as melanoma, colorectal cancer, and breast cancer were also reported [33–35]. However, there is limited data on the association between ADAMTS18 and pancreas adenocarcinoma. In a study, the frequency of ADAMTS18 methylation in pancreatic cancer was found to be significantly higher compared with adjacent noncancerous tissue. The authors also showed that ADAMTS-18 expression level was inversely correlated with methylation status, and there was no evidence between ADAMTS-18 methylation status and TNM staging of cancer [36]. In our study, ADAMTS18 was found to be highly expressed in tumoural tissue and metastatic lymph nodes compared with the noncancerous pancreatic and lymphatic tissues. However, ADAMTS18 expression level was not found to be correlated with the number of metastatic lymph nodes and the pathological features of the tumour including tumour size, grade, and venous, lymphatic, and perineurial invasions. In our opinion, strong staining status of ADAMTS18 in cancerous tissues may indicate its potential role in carcinogenesis, local invasion, and lymphatic spread of tumour cells. It should be also stated here that, to our knowledge, this is the first study regarding the immunohistochemical analysis of ADAMTS9 and ADAMTS18 in pancreas adenocarcinoma.
Conclusions
ADAMTS1, 8, 9, and 18 have high expression levels in pancreas cancer and its nodal metastasis, suggesting their important roles in carcinogenesis and lymphatic metastasis. In addition, these findings may indicate that these proteases may be promising candidates for alternative treatment modalities in pancreas cancer. However, the results of the present study should be confirmed by large-scale studies using different experimental methods.
Acknowledgments
This study was supported by the Scientific and Technological Research Council of Turkey (TUBİTAK) (3001 Project no: 114S233) and the Foundation of Scientific Research of Turgut Ozal University, Ankara, Turkey (No: 005-10-2013).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Yuan SJ, Chen YT, et al. Preclinical evaluation of herpes simplex virus armed with granulocyte-macrophage colony-stimulating factor in pancreatic carcinoma. World J Gastroenterol. 2013;19:5138–43. doi: 10.3748/wjg.v19.i31.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang MC, Wong JM, Chang YT. Screening and early detection of pancreatic cancer in high risk population. World J Gastroenterol. 2014;20:2358–64. doi: 10.3748/wjg.v20.i9.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vong S, Kalluri R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer. 2011;2:1139–45. doi: 10.1177/1947601911423940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagstaff L, Kelwick R, Decock J, et al. The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front Biosci. 2011;16:1861–72. doi: 10.2741/3827. [DOI] [PubMed] [Google Scholar]
- 7.Demircan K, Akyol S, Armutcu F. A multi-functional gene family from arthritis to cancer: a disintegrin-like metalloproteinase with thrombospondin type-1 motif (ADAMTS) J Clin Anal Med. 2013;4:429–34. [Google Scholar]
- 8.Vàzquez F, Hastings G, Ortega MA, et al. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274:23349–57. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- 9.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–40. [PubMed] [Google Scholar]
- 10.Hart IR, Saini A. Biology of tumour metastasis. Lancet. 1992;339:1453–7. doi: 10.1016/0140-6736(92)92039-i. [DOI] [PubMed] [Google Scholar]
- 11.Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–5. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 12.Koo BH, Coe DM, Dixon LJ, et al. ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. Am J Pathol. 2010;176:1494–504. doi: 10.2353/ajpath.2010.090655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colige A, Sieron AL, Li SW, et al. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet. 1999;65:308–17. doi: 10.1086/302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai HM. Thrombotic thrombocytopenic purpura: a thrombotic disorder caused by ADAMTS13 deficiency. Hematol Oncol Clin North Am. 2007;21:609–32. doi: 10.1016/j.hoc.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K, Hirohata S, Murakami T, et al. Dynamic induction of ADAMTS1 gene in the early phase of acute myocardial infarction. J Biochem. 2004;136:439–46. doi: 10.1093/jb/mvh138. [DOI] [PubMed] [Google Scholar]
- 16.Uysal S, Unal ZN, Erdogan S, et al. Augmentation of ADAMTS9 gene expression by IL-1beta is reversed by NFkappaB and MAPK inhibitors, but not PI3 kinase inhibitors. Cell Biochem Funct. 2013;31:539–44. doi: 10.1002/cbf.2932. [DOI] [PubMed] [Google Scholar]
- 17.Filou S, Korpetinou A, Kyriakopoulou D, et al. ADAMTS expression in colorectal cancer. PLoS One. 2015;10:e0121209. doi: 10.1371/journal.pone.0121209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lind G, Kleivi K, Meling G, et al. ADAMTS1, CRABP1, and NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell Oncol. 2006;28:259–72. doi: 10.1155/2006/949506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masui T, Hosotani R, Tsuji S, et al. Expression of METH-1 and METH-2 in pancreatic cancer. Clin Cancer Res. 2001;7:3437–43. [PubMed] [Google Scholar]
- 20.Conejo JR, Kleeff J, Koliopanos A, et al. Syndecan-1 expression is up-regulated in pancreatic but not in other gastrointestinal cancers. Int J Cancer. 2000;88:12–20. doi: 10.1002/1097-0215(20001001)88:1<12::aid-ijc3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Kleeff J, Ishiwata T, Kumbasar A, et al. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J Clin Investig. 1998;102:1662–73. doi: 10.1172/JCI4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Xu Y, Yu Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene. 2006;25:2452–67. doi: 10.1038/sj.onc.1209287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter S, Scott SD, Sassoon EM, et al. Dysregulated expression of adamalysin-thrombospondin genes in human breast carcinoma. Clin Cancer Res. 2004;10:2429–40. doi: 10.1158/1078-0432.ccr-0398-3. [DOI] [PubMed] [Google Scholar]
- 24.Dunn JR, Reed JE, du Plessis DG, et al. Expression of ADAMTS-8, a secreted protease with antiangiogenic properties, is downregulated in brain tumors. Br J Cancer. 2006;94:1186–93. doi: 10.1038/sj.bjc.6603006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocks N, Paulissen G, Quesada Calvo F, et al. Expression of a disintegrin and metalloprotease (ADAM and ADAMTS) enzymes in human non-small-cell lung carcinomas (NSCLC) Br J Cancer. 2006;94:724–30. doi: 10.1038/sj.bjc.6602990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter S, Span PN, Sweep FC, et al. ADAMTS8 and ADAMTS15 expression predicts survival in human breast carcinoma. Int J Cancer. 2006;118:1241–7. doi: 10.1002/ijc.21476. [DOI] [PubMed] [Google Scholar]
- 27.Lo PH, Lung HL, Cheung AK, et al. Extracellular protease ADAMTS9 suppresses esophageal and nasopharyngeal carcinoma tumor formation by inhibiting angiogenesis. Cancer Res. 2010;70:5567–76. doi: 10.1158/0008-5472.CAN-09-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleivi K, Lind G, Diep C, et al. Gene expression profiles of primary colorectal carcinomas, liver metastases, and carcinomatoses. Mol Cancer. 2007;6 doi: 10.1186/1476-4598-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, Shao Y, Zhang W, et al. High-resolution melting analysis of ADAMTS9 methylation levels in gastric, colorectal, and pancreatic cancers. Cancer Genet Cytogenet. 2010;196:38–44. doi: 10.1016/j.cancergencyto.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Zeng W, Corcoran C, Collins-Racie LA, et al. Glycosaminoglycanbinding properties and aggrecanase activities of truncated ADAMTSs: comparative analyses with ADAMTS-5, -9, -16 and -18. Biochim Biophys Acta. 2006;1760:517–24. doi: 10.1016/j.bbagen.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Nardi MA, Li YS, et al. C-terminal ADAMTS-18 fragment induces oxidative platelet fragmentation, dissolves platelet aggregates, and protects against carotid artery occlusion and cerebral stroke. Blood. 2009;113:6051–60. doi: 10.1182/blood-2008-07-170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin H, Wang X, Ying J, et al. Epigenetic identification of ADAMTS18 as a novel 16q23.1 tumor suppressor frequently silenced in esophageal, nasopharyngeal and multiple other carcinomas. Oncogene. 2007;26:7490–8. doi: 10.1038/sj.onc.1210559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei XM, Prickett TD, Viloria CG, et al. Mutational and functional analysis reveals ADAMTS18 metalloproteinase as a novel driver in melanoma. Mol Cancer Res. 2010;8:1513–25. doi: 10.1158/1541-7786.MCR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 35.Nordgard SH, Johansen FE, Alnaes GI, et al. Genome-wide analysis identifies 16q deletion associated with survival, molecular subtypes, mRNA expression, and germline haplotypes in breast cancer patients. Genes Chromosomes Cancer. 2008;47:680–96. doi: 10.1002/gcc.20569. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Zhang W, Shao Y, et al. High-resolution melting analysis of ADAMTS18 methylation levels in gastric, colorectal and pancreatic cancers. Med Oncol. 2010;27:998–1004. doi: 10.1007/s12032-009-9323-8. [DOI] [PubMed] [Google Scholar]