Abstract
Background
Combined treatment with intravitreal anti‐vascular endothelial growth factor (anti‐VEGF) and verteporfin photodynamic therapy (PDT) is widely used for patients with polypoidal choroidal vasculopathy (PCV), although clinical evidence regarding the therapeutic efficacy and safety of such treatment remains lacking.
Design/Methods
We performed a meta‐analysis of previously reported studies comparing combination treatment, PDT monotherapy, and anti‐VEGF monotherapy. Primary outcome measures included changes in best‐corrected visual acuity (BCVA) and central retinal thickness (CRT). The proportion of patients with polyp regression was regarded as the secondary outcome measure.
Results
Twenty studies (three RCTs and 19 retrospective studies) involving 1,178 patients with PCV were selected. Significant differences in the proportion of patients with polyps were observed between the PDT and anti‐VEGF monotherapy groups at 3 and ≥6 months (P < .00001; and P = .0001, respectively). Significantly greater reductions in CRT were observed in the anti‐VEGF than in the PDT group at the 3‐month follow‐up (P = .04). Significantly greater improvements in BCVA were observed in the combined therapy group than in the PDT monotherapy group at 3, 6, 12, and 24 months (P = .03; P = .005; P = .02; and P < .00001, respectively). Combined treatment also resulted in significantly greater improvements in BCVA than monotherapy with anti‐VEGF at 6 and 24 months (P = .001; P < .00001, respectively), and significantly greater polyp regression than that observed following anti‐VEGF treatment at 3 and ≥6 months (P < .00001; P < .0001, respectively).
Conclusions
Combined therapy involving anti‐VEGF agents and PDT may be more effective in improving long‐term outcomes for patients with PCV than monotherapy.
Keywords: combination therapy, intravitreal anti‐VEGF, meta‐analysis, photodynamic therapy, polypoidal choroidal vasculopathy
1. INTRODUCTION
Polypoidal choroidal vasculopathy (PCV), first identified and reported by Yannuzzi et al1 is a clinically distinct choroidal abnormality with a high prevalence in Asian populations.2 PCV is characterised by an abnormal branching vascular network (BVN) and polypoid dilations observed via indocyanine green angiography (ICGA), which is the gold standard for diagnosing PCV.3 The main therapeutic strategies for PCV vary according to the clinical pathological manifestations of the disease and include intravitreal anti‐vascular endothelial growth factor (VEGF) agents, verteporfin photodynamic therapy (PDT), and combined therapy with anti‐VEGF and PDT.
Currently, PDT is widely regarded as effective in the treatment of PCV due to the regression of polyp‐like dilations in spite of the relative lack of visual improvement within 2 years.4 Wong et al5 also reported that the visual outcome in eyes with PCV was stable until 2 years, while the outcome at 3 years worsened, particularly in eyes that experienced recurrence.
Anti‐VEGF agents such as bevacizumab, ranibizumab, and aflibercept are recombinant humanised monoclonal antibodies that have been reported to improve visual functioning by restoring normal retinal thickness and reducing elevated levels of VEGF.6 However, research has suggested that anti‐VEGF therapy is less effective in ensuring the regression of polyps and that the risk of subretinal haemorrhage increases following PDT monotherapy.7, 8
Considering the advantages and disadvantages of PDT monotherapy and anti‐VEGF monotherapy,7, 9 combined treatment with anti‐VEGF agents and PDT may result in both regression of polyps and anti‐angiogenesis. However, there is currently no consensus regarding the appropriate treatment for PCV.
Several meta‐analyses10, 11, 12 to date have compared various treatments for PCV, although these studies have not been comprehensive. For example, Tang et al11 analysed only those studies involving ranibizumab, while Wang et al12 failed to compare anti‐VEGF monotherapy to PDT monotherapy. To evaluate the best therapeutic strategy for PCV, we performed a comprehensive meta‐analysis to compare the efficacy of PDT monotherapy, monotherapy with intravitreal anti‐VEGF agents, and combined treatment with PDT and anti‐VEGF agents. Therapeutic effect estimates were determined based on the means and standard deviations (SD) of changes in best‐corrected visual acuity (BCVA), relative to baseline, using the logarithm of the minimum angle of resolution (logMAR); reductions in central retinal thickness (CRT) at follow‐up points; and the proportion of patients exhibiting polyp regression following treatment. We also evaluated the occurrence of ocular adverse events, including subretinal haemorrhage, which are essential in determining the most appropriate course of treatment for PCV.
2. MATERIALS AND METHODS
Reporting of the study conforms to PRISMA statement and the broader EQUATOR guidelines.13
2.1. Literature search
Five databases (PubMed, EMBASE, Web of Science, Medline and Cochrane Library) were last searched for relevant studies on December 31, 2016. The following terms were used and adapted for the searches in each database: (“polypoidal choroidal vasculopathy” OR PCV) AND (“vascular endothelial growth factors” OR “anti‐VEGF” OR “angiogenesis inhibitors” OR ranibizumab OR lucentis OR “RhuFab V2” OR bevacizumab OR avastin OR aflibercept OR VEGF‐Trap) AND (“photodynamic therapy” OR PDT). There was no restriction on language or study design. When titles and/or abstracts fit the index words, the full article was retrieved.
2.2. Inclusion and exclusion criteria
The following inclusion criteria were used for the present meta‐analysis: (i) comparative study design; (ii) comparison of anti‐VEGF+PDT vs PDT treatment, or anti‐VEGF vs PDT treatment, for PCV; (iii) population consisting of treatment‐naive patients with PCV of any sex and race; and (iv) report of at least one of the following outcomes: best‐corrected visual acuity (BCVA), central retinal thickness (CRT), proportion of patients who achieved complete regression of polyps, and incidence of subretinal haemorrhage. Reports with incomplete text, full text without raw data, review articles, case reports, meeting abstracts, and duplicate publications were excluded, along with those including patients who had received previous treatment for PCV. Patients whose initial therapeutic strategy had changed were also excluded. For cases in which articles reported results associated with the same trial, the most recent report was included, although additional data could be obtained from previous reports.
2.3. Data extraction and quality assessment
Data regarding the following study characteristics were extracted from all included studies: (i) basic information: name of first author, the year of publication, location of the study, and trial design; (ii) patient information: age, gender, duration of follow‐up; (iii) treatment details: intervention groups (including sample number); and (iv) outcomes: means and standard deviations (SDs) of BCAV and CRT after treatment at a specific follow‐up period, the number of patients who achieved complete regression of polyps, and the number of patients with subretinal haemorrhage. Data not reported in articles was obtained from ClinicalTrials.gov when necessary, if available.
We also evaluated the methodological quality of included RCTs, which were analysed for bias using the Cochrane Risk of Bias Tool,14 while the methodological quality of retrospective studies was assessed using the modified Newcastle‐Ottawa scale,15 which consists of three factors: patient selection, comparability of the study groups, and outcome assessment. A score of 0‐9 stars is allocated to each study, except RCTs. RCTs and retrospective studies achieving seven or more stars are considered to be of high quality.
2.4. Evaluation indicators
Indicators of treatment efficacy included mean changes in BCVA and CRT, as well as the extent of polyp regression from baseline at different follow‐up points. Safety indicators included the incidence of subretinal haemorrhage following treatment.
2.5. Statistical analysis
Statistical analysis was performed using Review Manager 5.3.5 software (Cochrane Collaboration). In the present meta‐analysis, continuous data (eg, BCVA) were expressed as means and standard deviations, and weighted mean differences (WMD) were calculated. Dichotomous data (eg, number of events) were evaluated via odds ratios (OR). Continuous outcomes were reported as mean differences with a 95% confidence interval (CI), while dichotomous outcomes were reported as risk ratios with 95% CI. A P value <.05 was considered statistically significant. A Chi‐square test (P value) and the I 2 statistic were used to quantify the statistical heterogeneity among studies. If no heterogeneity among studies was observed (P > .1 or I 2 <50%), the fixed‐effects model was used for the analysis; otherwise, the random‐effects model was used. Forest plots were used to display the summary weighted estimates, while funnel plots were used to assess publication bias.
3. RESULTS
3.1. Search results
A total of 235 studies were initially identified using the index words, of which 92 were excluded as duplicate studies, 25 were excluded as reviews, 13 were excluded because of incomplete text, and 79 were excluded based on the titles and abstracts. The remaining 26 studies were retrieved for full‐text review. Among them, two articles were excluded as case reports, and two articles were excluded because they included additional treatment strategies for PCV. A final total of 22 studies16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 were included in the present meta‐analysis. The study selection process is shown in Figure 1.
Figure 1.
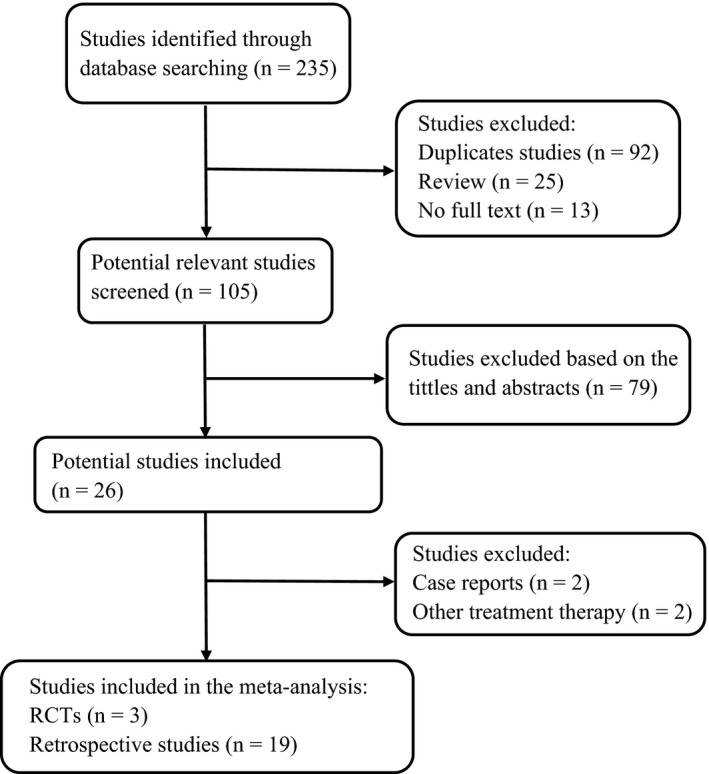
Flow diagram of literature screening
3.2. Characteristics of the eligible studies
The present meta‐analysis included 22 studies with a total of 1,178 patients with PCV. The basic characteristics of these studies are shown in Table 1. The mean age of patients across all studies ranged from 53 to 75.8 years, while study duration ranged from 1 to 36 months. Among the included studies were three RCTs16, 17, 18 and 19 retrospective comparative studies.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 Specific interventions and pro re nata (PRN) retreatment regimen used within each trial are listed in Table 1. All retrospective studies had received scores of at least seven stars and were considered to be of high quality.
Table 1.
Characteristics of the 22 trials
| Trials (Author, y) | Location | Design | Treatment Group (patients) | Age (y) Mean ± SD | Gender M/F | GLD (μm) Mean ± SD | Interventions | PRN retreatment regimen | Follow‐up (mo) |
|---|---|---|---|---|---|---|---|---|---|
| Koh15, 2012 | Singapore | RCT | IVR (n = 21) | 69.3 ± 8.27 | 15/6 | <5400 | PDT + IVR 0.5 mg | 1 + PRN PDT, 3 + PRN IVR | 1, 3, 6 |
| PDT (n = 21) | 62.2 ± 9.77 | 15/6 | <5400 | PDT 6 mg/m2 + sham | 1 + PRN PDT | ||||
| IVR + PDT (n = 19) | 63.8 ± 8.30 | 11/8 | <5400 | IVR 0.5 mg | 3 + PRN IVR | ||||
| Oishi16, 2013 | Japan | RCT | IVR (n = 46) | 75.4 ± 6.9 | 28/18 | 3347.4 ± 1288.3 | IVR 0.5 mg | 3 + PRN IVR | 3, 6, 12 |
| PDT (n = 47) | 75.8 ± 8.0 | 32/15 | 3051.1 ± 1177.7 | PDT 6 mg/m2 | 1 + PRN PDT | 24 | |||
| Lee17, 2013 | Korea | RCT | PDT (n = 8) | 66.33 ± 7.85 | 5/3 | NR | PDT 6 mg/m2 | NR | 1, 3 |
| IVR + PDT (n = 12) | 63.68 ± 8.78 | 11/1 | IVR 0.5 mg + PDT 6 mg/m2 | ||||||
| Lai18, 2008 | Hong Kong | Retro | IVB (n = 8) | 64.9 ± 7.6 | 6/2 | NR | IVB 1.25 mg | 3 + PRN IVB | 12 |
| IVB + PDT (n = 7) | 64.0 ± 7.1 | 5/2 | IVB 1.25 mg + PDT 6 mg/m2 | 3 + PRN IVB, 1 + PRN PDT | |||||
| Lee19, 2008 | Korea | Retro | IVB (n = 8) | 63 ± 7 | 3/1 | NR | IVB 1.25 mg | 1 + PRN IVB | 6 |
| IVB + PDT (n = 4) | 53 ± 7 | 8/0 | IVB 1.25 mg + PDT | 1 PDT, 1 + PRN IVB | |||||
| Lim20, 2012 | Korea | Retro | IVB (n = 5) | 68.6 ± 7.2 | 5/0 | NR | IVB 1.25 mg | 3 + PRN IVB | 12 |
| IVB + PDT (n = 5) | 57.4 ± 7.2 | 3/2 | IVB 1.25 mg + PDT | 3 + PRN IVB, 1 + PRN PDT | |||||
| Gomi21, 2010 | Japan | Retro | PDT (n = 85) | 70.9 ± 6.8 | 68/17 | 2521 ± 996 | PDT | 1 + PRN PDT | 3, 6, 12 |
| IVB + PDT (n = 61) | 70.9 ± 7.1 | 44/16 | 2626 ± 1138 | IVB 1.25 mg + PDT | 1 + PRN IVB, 1 + PRN PDT | ||||
| Mitamura22, 2010 | Japan | Retro | IVB (n = 22) | 73.0 ± 8.9 | 17/5 | 3651 ± 1833 | IVB 1.25 mg | NR | 3 |
| PDT (n = 49) | 69.6 ± 7.8 | 42/7 | 3718 ± 1665 | PDT | |||||
| Kim23, 2011 | Korea | Retro | PDT (n = 19) | 65.9 ± 8.4 | 13/6 | 3626.3 ± 1334.6 | PDT 6 mg/m2 | 1 + PRN PDT | 1, 3, 6, 9 |
| IVB + PDT (n = 20) | 64.8 ± 8.3 | 15/5 | 3287.5 ± 1335.9 | IVB 1.25 mg + PDT 6 mg/m2 | 1 + PRN IVB, 1 + PRN PDT | 12 | |||
| Lai24, 2011 | Hong Kong | Retro | IVR (n = 7) | 64.6 ± 7.9 | 4/3 | 3610 ± 2240 | IVR 0.5 mg | 3 + PRN IVR | 3, 12 |
| PDT (n = 12) | 65.6 ± 11.0 | 10/2 | 2580 ± 707 | PDT 6 mg/m2 | 1 + PRN PDT | ||||
| IVR + PDT (n = 16) | 71.3 ± 11.8 | 8/8 | 3490 ± 1170 | IVR 0.5 mg + PDT 6 mg/m2 | 3 + PRN IVR, 1 + PRN PDT | ||||
| Maruko25, 2011 | Japan | Retro | PDT (n = 16) | 71.8 ± 10.4 | 12/4 | 3013 ± 1059 | PDT 6 mg/m2 | 1 PDT (no PRN) | 1, 3, 6 |
| IVR + PDT (n = 11) | 71.0 ± 9.8 | 6/5 | 2095 ± 1122 | IVR 0.5 mg + PDT 6 mg/m2 | 3 IVR, 1 PDT (no PRN) | ||||
| Rouvas26, 2011 | Greece | Retro | IVR (n = 16) | 66.5 | 4/6 | NR | IVR 0.5 mg | 3 + PRN IVR | 3, 6, 12 |
| PDT (n = 11) | 62.9 | 5/6 | PDT 6 mg/m2 | 1 + PRN PDT | |||||
| PDT + IVR (n = 9) | 64.67 | 4/5 | IVR 0.5 mg + PDT 6 mg/m2 | 3 + PRN IVR, 1 + PRN PDT | |||||
| Saito27, 2011 | Japan | Retro | IVR (n = 25) | 75.1 ± 6.4 | 21/4 | 5010 ± 2055 | IVR 0.5 mg | 3 + PRN IVR | 3, 6 |
| PDT (n = 34) | 74.8 ± 6.3 | 26/8 | 4549 ± 1692 | PDT | 1 + PRN PDT | ||||
| Song28, 2011 | Korea | Retro | IVR (n = 15) | 60.6 ± 10.7 | 6/9 | NR | IVR 0.5 mg | 1 + PRN IVR | 12 |
| PDT + IVR (n = 9) | 56.9 ± 12.1 | 0/9 | IVR 0.5 mg + PDT | 1 + PRN IVR, I + PRN PDT | |||||
| Lee29, 2012 | Taiwan | Retro | PDT (n = 33) | 65.6 ± 9.5 | 18/15 | 2832 ± 1092 | PDT | 1 + PRN IVR | 3, 6, 9, 12 |
| PDT + IVB (n = 36) | 67.8 ± 9.8 | 21/15 | 2619 ± 843 | IVB 1.25 mg + PDT | 1 + PRN IVR, I + PRN PDT | 15, 18, 24 | |||
| Inoue30, 2013 | Japan | Retro | IVR (n = 33) | 73.2 ± 7.5 | 19/14 | 4171 ± 2631 | IVR 0.5 mg | 1 + PRN IVR | 3, 6, 12 |
| PDT (n = 44) | 71.0 ± 7.8 | 30/14 | 3640 ± 2120 | PDT 50 J/cm2 | 1 + PRN PDT | 18, 24 | |||
| Kang31, 2013 | Korea | Retro | IVR (n = 23) | 68.05 ± 8.12 | NR | 2790.05 ± 871.50 | IVR 0.5 mg | 3 + PRN IVR | 3, 6, 12 |
| PDT (n = 19) | 66.21 ± 9.00 | 2810.87 ± 974.10 | PDT 6 mg/m2 | 1 + PRN PDT | 24 | ||||
| IVB + PDT (n = 44) | 70.00 ± 7.75 | 2815 ± 910.12 | IVB 0.5 mg + PDT 6 mg/m2 | 3 + PRN IVB, 1 + PRN PDT | |||||
| Saito32, 2013 | Japan | Retro | PDT (n = 32) | 75.0 ± 6.5 | 2759 | 4867 ± 1855 | PDT 6 mg/m2 | 1 + PRN PDT | 3, 6, 12 |
| IVR + PDT (n = 25) | 74.0 ± 8.6 | 17/8 | 4074 ± 1459 | IVR 0.5 mg + PDT 6 mg/m2 | 3 + PRN IVR, 1 + PRN PDT | 24 | |||
| Sakurada33, 2013 | Japan | Retro | PDT (n = 34) | 70.1 ± 7.1 | 25/9 | 2364 ± 716 | PDT 6 mg/m2 | 1 + PRN PDT | 3, 6, 12 |
| IVR + PDT (n = 24) | 73.2 ± 7.4 | 16/8 | 2039 ± 847 | IVR 0.5 mg + PDT 6 mg/m2 | 1 + PRN IVR, 1 + PRN PDT | 24 | |||
| Gomi34, 2015 | Japan | Retro | IVR (n = 35) | 73.8 ± 7.1 | NR | NR | IVR 0.5 mg | 3 + PRN IVR, PRN PDT | 3 |
| PDT + IVR (n = 37) | 73.6 ± 5.8 | IVR 0.5 mg + PDT | 3 + PRN IVR, 1 + PRN PDT | ||||||
| Kikushima35,2016 | Japan | Retro | IAI (n = 33) | 72.7 ± 8.5 | 25/8 | 2041 ± 1273 | IAI 2.0 mg | 3 + PRN IAI | 3, 6, 9, 12 |
| PDT + IAI (n = 33) | 73.4 ± 8.3 | 22/11 | 1692 ± 747 | IAI 2.0 mg + PDT 6 mg/m2 | 1 + PRN IAI, 1 + PRN PDT | ||||
| Sakai36,2016 | Japan | Retro | IVR (n = 20) | 75.3 ± 8.1 | 13/7 | 2937 ± 1040 | IVR 0.5 mg | 3 + PRN IVR | 6, 12, 18 |
| PDT + IVR (n = 25) | 72.6 ± 6.2 | 21/4 | 2800 ± 823 | IVR 0.5 mg + PDT 6 mg/m2 | 3 + PRN IVR, 1 + PRN PDT | 24, 30, 36 |
IVR, intravitreal ranibizumab; IVB, intravitreal bevacizumab; IAI, intravitreal aflibercept injection; PDT, photodynamic therapy; PRN, pro re nata.
3.3. Comparison of intravitreal anti‐VEGF monotherapy and PDT monotherapy
As BCAV is an essential functional outcome measure, we considered changes in BCAV to be most important for evaluating treatment efficacy. The pooled results revealed no significant difference in BCVA change between the PDT group and anti‐VEGF group at 3, 6, 12, and 24 months in either RCTs or retrospective studies.
Central retinal thickness (CRT) was also evaluated to assess changes in retinal health following treatment (Figure 2). The pooled results revealed that anti‐VEGF agents significantly reduced CRT compared with PDT at 3 months (P = .04) with no heterogeneity (P = .85, I 2 = 0%), although no significant difference was observed at 6 months. Furthermore, our results indicated that there were significant differences in polyp regression between the two groups at 3 and 6 or more months (P < .00001; and P = .0001, respectively).
Figure 2.
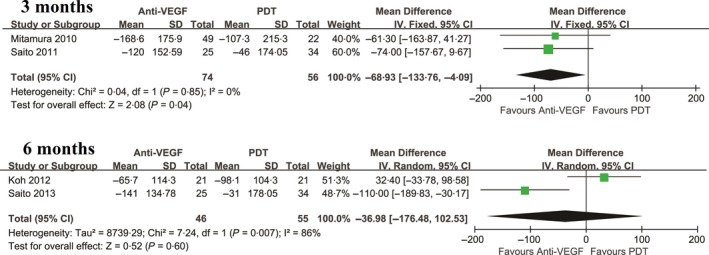
Mean change in CRT for the comparison of PDT and anti‐VEGF at 3,6 mo. SD, standard deviation; IV, inverse variance; CI, confidence interval. PDT: photodynamic therapy; PCV: polypoidal choroidal vasculopathy; anti‐VEGF: anti vascular endothelial growth factor
All results for the comparison of PDT monotherapy and anti‐VEGF monotherapy for the treatment of PCV are shown in Table 2.
Table 2.
Comparison of PDT monotherapy and anti‐VEGF monotherapy for PCV
| Outcome of interest | Studies (n) | WMD/OR (95% CI) | P | Study heterogeneity | ||
|---|---|---|---|---|---|---|
| χ2 | P | I 2 (%) | ||||
| Comparison of mean change in BCVA from baseline at follow‐up points (logMAR) | ||||||
| 3 mo | 7 | 0.01 (−0.07, 0.08) | .86 | 13.66 | .03 | 56 |
| 6 mo | 5 | 0.01 (−0.06, 0.09) | .75 | 8.42 | .08 | 52 |
| 12 mo | 5 | −0.01 (−0.13, 0.12) | .93 | 15.57 | .004 | 74 |
| 24 mo | 3 | 0.03 (−0.16, 0.22) | .77 | 10.49 | .005 | 81 |
| Comparison of mean change in CRT from baseline at follow‐up points | ||||||
| 3 mo | 2 | −68.93 (−133.76, −4.09) | .04 | 0.04 | .85 | 0 |
| 6 mo | 2 | −36.98 (−176.48, 102.53) | .60 | 7.24 | .007 | 86 |
| Comparison of the proportions of patients with polyp regression | ||||||
| 3 mo | 3 | 15.23 (5.77, 40.20) | <.00001 | 3.11 | .21 | 36 |
| ≥6 mo | 2 | 6.32 (2.44, 16.38) | .0001 | 0.00 | .98 | 0 |
PDT, photodynamic therapy; NA, not available; logMAR, logarithm of the minimal angle of resolution; CRT, central retinal thickness; PCV, polypoidal choroidal vasculopathy; anti‐VEGF; anti‐vascular endothelial growth factor; WMD, weighted mean difference; OR, odds ratio; CI, confidence interval; χ 2, chi‐square statistic; P, P‐value; I 2, I‐square heterogeneity statistic; Bold italic values indicate statistically significant results.
3.4. Comparison of combined treatment and PDT monotherapy
Combined treatment resulted in significantly greater improvements in BCVA than PDT monotherapy at 3, 6, 12, and 24‐months after treatment in patients with PCV (P = .03; P = .005; P = .02; and P < .0001, respectively) (Figure 3). In contrast, no significant difference in the proportion of patients exhibiting improvements in VA (at least three lines) was observed at any time point when pooled ORs were calculated.
Figure 3.
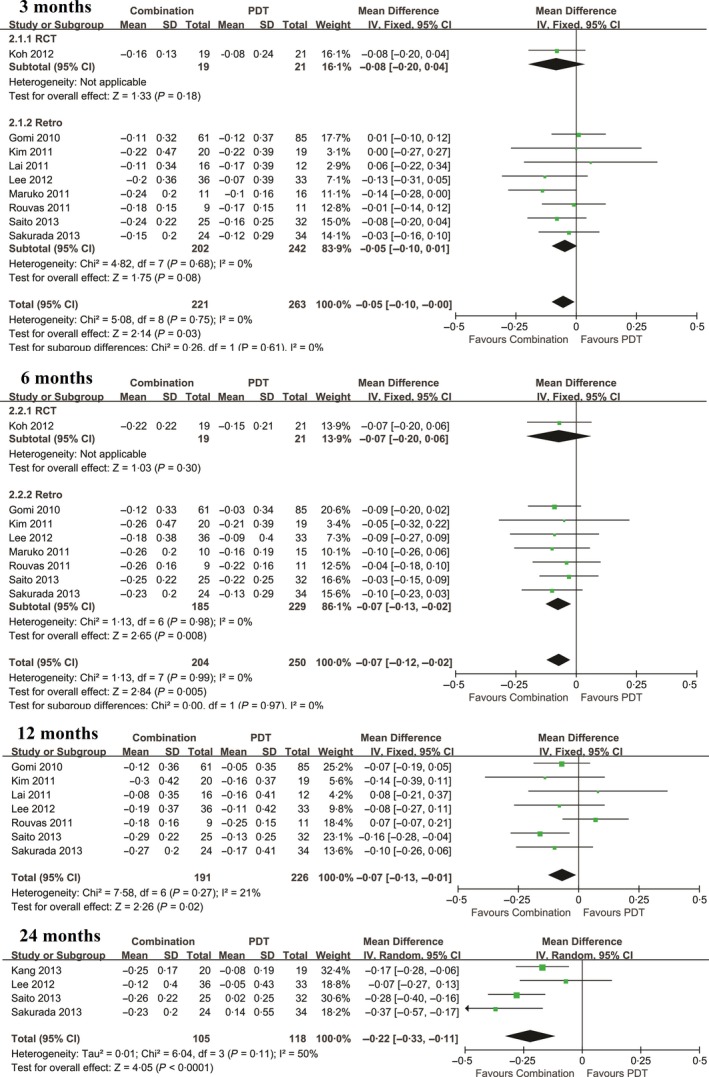
Mean change in BCVA for the comparison of combined therapy and PDT monotherapy at 3, 6, 12, and 24 mo. SD, standard deviation; IV, inverse variance; CI, confidence interval; BCVA; best corrected visual acuity; PDT: photodynamic therapy; PCV: polypoidal choroidal vasculopathy; anti‐VEGF: anti vascular endothelial growth factor
No significant heterogeneity was observed among studies with continuous data. Therefore, a random‐effects model was used to analyse changes in CRT occurring from baseline to 3 months and 6 months after each treatment. Combined treatment resulted in significantly greater reductions in CRT at 3‐months than PDT alone (P = .02), although no significant differences were observed at 6 months (Figure 4). No significant differences in polyp regression were observed between the two groups at either the 3‐ or ≥6‐month follow‐up (P = .32; and P = .75, respectively).
Figure 4.
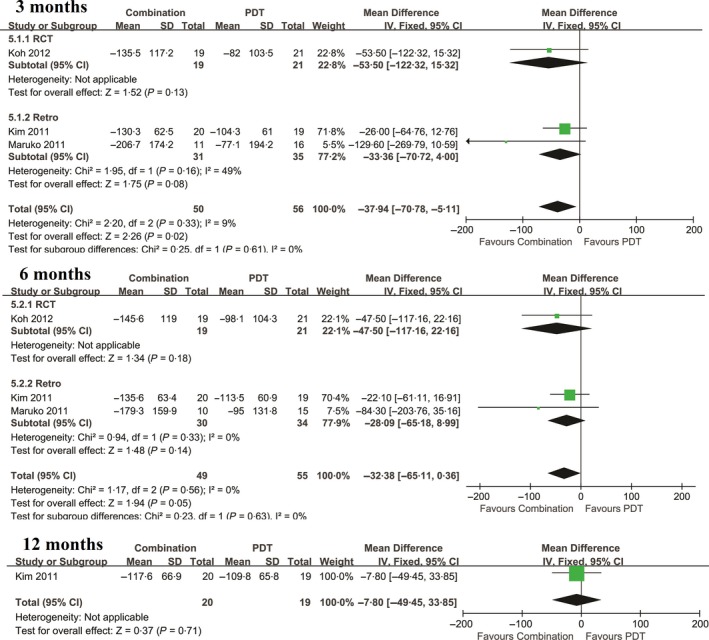
Mean change in CRT for the comparison of combined therapy and PDT at 3,6, and 12 mo. SD, standard deviation; IV, inverse variance; CI, confidence interval. CRT: central retinal thickness; PDT: photodynamic therapy
All results for the comparison of combined treatment and PDT monotherapy for PCV are shown in Table 3.
Table 3.
Comparison of combined treatment and PDT monotherapy for PCV
| Outcome of interest | Studies (n) | WMD/OR (95% CI) | P | Study heterogeneity | ||
|---|---|---|---|---|---|---|
| χ2 | P | I 2 (%) | ||||
| Comparison of mean change in BCVA from baseline at follow‐up points (logMAR) | ||||||
| 3 mo | 9 | −0.05 (−0.10, 0.00) | .03 | 5.08 | .75 | 0 |
| 6 mo | 8 | −0.07 (−0.12, −0.02) | .005 | 1.13 | .99 | 0 |
| 12 mo | 7 | −0.07 (−0.13, −0.01) | .02 | 7.58 | .27 | 21 |
| 24 mo | 4 | −0.22 (−0.33, −0.11) | <.0001 | 6.04 | .11 | 50 |
| Comparison of mean change in CRT from baseline at follow‐up points | ||||||
| 3 mo | 3 | −37.94 (−70.78, −5.11) | .02 | 2.20 | .33 | 9 |
| 6 mo | 3 | −28.09 (−65.18, 0.36) | .05 | 1.17 | .56 | 0 |
| 12 mo | 1 | −7.80 (−49.45, 33.85) | ‐ | ‐ | ‐ | ‐ |
| Comparison of the proportions of patients with polyp regression | ||||||
| 3 mo | 4 | 1.36 (0.74, 2.52) | .32 | 1.37 | .71 | 0 |
| ≥6 mo | 4 | 1.16 (0.47, 2.87) | .75 | 1.31 | .73 | 0 |
PDT, photodynamic therapy; NA, not available; logMAR, logarithm of the minimal angle of resolution; CRT, central retinal thickness; PCV, polypoidal choroidal vasculopathy; WMD, weighted mean difference; anti‐VEGF: anti‐vascular endothelial growth factor; combined treatment: treatment with PDT and anti‐VEGF agents; OR, odds ratio; CI, confidence interval; χ 2, chi‐square statistic; P, P‐value; I 2, I‐square heterogeneity statistic; Bold italic values indicate statistically significant results.
3.5. Comparison of combined treatment and intravitreal anti‐VEGF monotherapy
The mean change in BCVA from baseline for each study was analysed at 3, 6, 12, and 24 months. Combined treatment resulted in significantly greater improvements in BCVA when compared with anti‐VEGF treatment at 6 and 24 months (P = .001; P < .00001, respectively), with no heterogeneity (P = .60, I 2 = 0%; P = .43, I 2 = 0%, respectively). However, the pooled results revealed that there was no significant difference in BCVA change between combined treatment and anti‐VEGF treatment at 3 and 12 months. Furthermore, no significant differences in CRT reduction were observed between combined treatment and anti‐VEGF treatment at 6 and 12 months.
Data regarding the regression of polyps at 3 and ≥6 months were available for three and five studies, respectively (Figure 5). The pooled results revealed that there were significant statistical differences between the two groups at 3 and ≥6 months (P < .00001; P < .0001, respectively).
Figure 5.

Proportion of patients with polyp regression following combination therapy and Anti‐VEGF monotherapy at 3, and ≥6 mo. CI, confidence interval; PDT: photodynamic therapy; anti‐VEGF: anti vascular endothelial growth factor
All results for the comparison of combined treatment and anti‐VEGF monotherapy for PCV are shown in Table 4.
Table 4.
Comparison of combined treatment and intravitreal Anti‐VEGF monotherapy for PCV
| Outcome of interest | Studies (n) | WMD/OR (95% CI) | P | Study heterogeneity | ||
|---|---|---|---|---|---|---|
| χ2 | P | I 2 (%) | ||||
| Comparison of mean change in BCVA from baseline at follow‐up points (logMAR) | ||||||
| 3 mo | 4 | −0.06 (−0.17, 0.06) | .34 | 7.60 | .06 | 61 |
| 6 mo | 5 | −0.12 (−0.19, −0.05) | .001 | 2.77 | .60 | 0 |
| 12 mo | 7 | −0.06 (−0.17, 0.06) | .34 | 12.72 | .05 | 53 |
| 24 mo | 2 | −0.29 (−0.39, −0.19) | <.00001 | 0.63 | .43 | 0 |
| Comparison of mean change in CRT from baseline at follow‐up points | ||||||
| 6 mo | 2 | −28.50 (−123.29, 66.28) | .56 | 4.34 | .04 | 77 |
| 12 mo | 3 | 5.84 (−79.86, 91.55) | .89 | 4.72 | .09 | 58 |
| Comparison of the proportions of patients with polyp regression | ||||||
| 3 mo | 3 | 7.75 (3.31, 18.15) | <.00001 | 3.07 | .22 | 35 |
| ≥6 mo | 5 | 4.07 (2.17, 7.64) | <.0001 | 6.16 | .19 | 35 |
PDT, photodynamic therapy; NA, not available; logMAR, logarithm of the minimal angle of resolution; CRT, central retinal thickness; PCV, polypoidal choroidal vasculopathy; WMD, weighted mean difference; anti‐VEGF: anti‐vascular endothelial growth factor; combined treatment: treatment with PDT and anti‐VEGF agents; OR, odds ratio; CI, confidence interval; χ 2, chi‐square statistic; P, P‐value; I 2, I‐square heterogeneity statistic; Bold italic values indicate statistically significant results.
3.6. Adverse events
As it has been widely reported that PDT may result in subretinal haemorrhage, we also evaluated whether intravitreal anti‐VEGF agents reduced the incidence of haemorrhage in the included studies. When compared to PDT monotherapy, combined treatment was associated with a significantly lower incidence of subretinal haemorrhage (1 optic disc diameter) during the follow‐up period, with no heterogeneity (P = .17, I 2 = 38%). The pooled results are shown in Figure 6 (P = .02). However, no significant differences in the incidence of subretinal haemorrhage were observed between combined treatment and anti‐VEGF monotherapy (P = .85; Figure 7).
Figure 6.
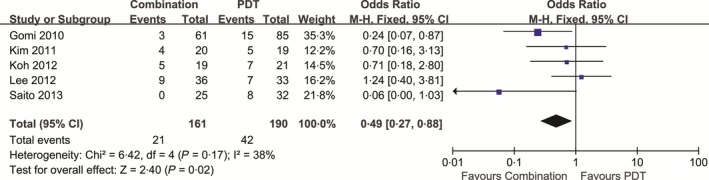
Proportion of patients with subretinal haemorrhage following combination and PDT therapy. CI, confidence interval. PDT: photodynamic therapy
Figure 7.
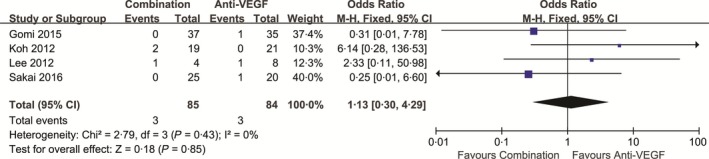
Proportion of patients with subretinal haemorrhage following combination and anti‐VEGF monotherapy. CI, confidence interval. PDT: photodynamic therapy
4. DISCUSSION
Whether PCV is a subtype of neovascular age‐related macular degeneration (nAMD) remains controversial38 As some researchers have suggested that the two conditions are associated with different demographic features, clinical characteristics, and natural history, etc.39, 40 However, both PCV and nAMD are regarded as abnormal vasculopathies arising from the choroidal vasculature, potentially resulting in recurrent serous exudation and haemorrhages.38 Furthermore, increased levels of VEGF are observed in the affected eyes or patients with PCV and nAMD. Currently, anti‐VEGF therapy is the standard treatment for nAMD. Thus, anti‐VEGF may also represent a valuable treatment option for PCV. Intravitreal anti‐VEGF injection results in rapid resolution of retinal oedema and subretinal fluid, restoring macular morphology and visual function41 In addition, PDT has proven more effective for the treatment of PCV than thermal laser photocoagulation, as it is associated with minimal retinal damage.
The scientific debate regarding the comparative effectiveness of these treatment strategies for PCV has remained unsettled for many years. Hence, this meta‐analysis aimed to comprehensively compare the three strategies in terms of BCVA, CRT, proportion of patients experiencing polyp regression, etc. BCVA, an exceedingly important outcome, can be regarded as a primary measure of treatment efficacy, although CRT and polyp regression are important for evaluating treatment efficacy, as these reflect anatomical outcomes.
Our findings revealed that anti‐VEGF treatment was more effective in reducing CRT than PDT at 3 months but not at 6 months, while PDT was more effective than anti‐VEGF in achieving regression of polyps at 3 months and 6 or more months. However, these two monotherapy procedures appear to be equivalent in terms of BCVA change from baseline at all follow‐up time points.
Although PDT monotherapy aims to produce polyp regression, no significant reductions in CRT were observed. In contrast, anti‐VEGF monotherapy significantly reduced CRT at 3 months, although it was significantly less effective in regressing and suppressing the recurrence of polyps than PDT. Generally, these findings suggest that neither PDT monotherapy nor anti‐VEGF monotherapy is the best option for the treatment of PCV, although each possesses distinct advantages over the other.
Considering the shortcomings of the two monotherapies, combination therapy may allow for more comprehensive treatment with regard to RCT reduction, polyp regression, and BCVA improvement than PDT monotherapy or anti‐VEGF monotherapy. Specifically, the direct effect of PDT on polypoidal lesions, as confirmed via ICGA, and the subsequent resolution of exudative fluid can likely lead to a favourable visual outcome.42 Some studies have reported that PDT was superior to anti‐VEGF therapy in terms of polyp regression.25, 27, 32 As for intravitreal anti‐VEGF injections, the rapid resolution of exudative fluid and retinal oedema can play an important role in ensuring favourable BCVA outcomes.10
In this meta‐analysis, combined therapy utilising PDT and anti‐VEGF was superior to PDT in improving BCVA at all follow‐up points, suggesting that anti‐VEGF may overcome the deficiencies of PDT monotherapy with regard to this aspect. Furthermore, BCVA improvements seemed to decrease with time in the PDT monotherapy group, suggesting that additional PDT may be necessary to sustain these improvements. Sato et al43 also suggested that several additional PDT sessions would be necessary to treat patients with PCV recurrences over longer follow‐up periods.
We further observed that combined treatment resulted in significantly greater improvements in BCVA when compared to anti‐VEGF monotherapy at the 6‐ and 24‐month follow‐up points, suggesting that combination therapy is more effective than anti‐VEGF monotherapy in improving BCVA.
At 3 months, combined therapy was associated with significantly greater decreases in CRT than PDT, indicating that anti‐VEGF treatment also plays a role in reducing exudative lesions. However, no significant differences in CRT reduction were observed between the groups at 6 months, suggesting that the efficacy of anti‐VEGF in reducing CRT is only maintained over a short period of time. Furthermore, no significant difference in CRT reduction was observed between combined treatment and intravitreal anti‐VEGF treatment, suggesting that PDT has little influence on CRT. Taken together, our findings indicate that PDT plays a greater role in polyp regression than anti‐VEGF, consistent with the results of two previous meta‐analyses.11, 12
Many studies have reported that PDT may lead to an increased risk of intraretinal or subretinal haemorrhage, thereby resulting in ischaemic damage to normal choroidal tissue.16, 17, 22, 23, 24, 25 Among them, subretinal haemorrhage, the most common and most reported adverse event, may also limit visual improvement following PDT.44 However, our findings indicate that these disadvantages can be reduced via combined therapy with PDT and anti‐VEGF, in accordance with the conclusions of Cheung et al45 As the risk of subretinal haemorrhage decreases with anti‐VEGF, combined treatment may be both safer and more effective than PDT monotherapy.
Recently, the administration of intravitreal corticosteroids has been utilised as an auxiliary treatment strategy in many clinical trials. Intraocular inflammatory cytokines are likely to be elevated in PCV eyes, especially in the presence of subretinal haemorrhage or macular oedema.46 Ho et al47 reported that the majority of patients responded well to corticosteroid treatment, with significant responses observed as early as 1 week after initiation of triple therapy including photodynamic therapy, intravitreal aflibercept, and intravitreal dexamethasone. Liang et al48 also evaluated the efficacy and adverse effects of treatment with low‐dose intravitreal triamcinolone acetonide (IVTA).
PCV results in alterations of the choroidal structure, which can be detected by enhanced depth imaging optical coherence tomography (EDI‐OCT).49 Thus, such alterations may be useful in evaluating the efficacy of particular treatment strategies. Daizumoto et al50 reported that intravitreal aflibercept was effective in the treatment of PCV via significant correlations of the pachychoroid index, a kind of index reflecting choroidal structures.
Recently, several studies reported favourable visual and anatomical outcomes following aflibercept monotherapy with fixed or treat‐and‐extend (TAE) retreatment regimens in patients with PCV. Hosokawa et al51 reported that intravitreal aflibercept injections delivered as part of a TAE regimen are effective in improving BCVA and CRT in eyes with PCV. In another retrospective interventional case series of 58 eyes, TAE intravitreal aflibercept was associated with improvements in BCVA and regression of polypoidal lesions.52 In one study involving Japanese patients with treatment‐naive PCV, treatment with intravitreal aflibercept every 2 months following three initial monthly doses resulted in significant increases in BCVA and a high rate of polyp resolution at 12 months.53 Lee et al54 reported that fixed‐dose treatment with aflibercept was associated with favourable outcomes in patients with PCV patients at the 12‐month follow‐up. The results of these studies51, 52, 53, 54 have led many physicians to prefer the use of this modality, rather than PDT. As only one study involving aflibercept was included was included in the present meta‐analysis, we were unable to sufficiently compare outcomes between aflibercept monotherapy and combination therapy. Thus, future studies should aim to perform this comparison.
Our study possesses several strengths in comparison with previous systematic reviews of PCV treatment. First, our meta‐analysis included the most recent work, analysing studies published as late as December 31, 2016. Second, we performed a comprehensive comparison of PDT monotherapy, anti‐VEGF monotherapy, and combined treatment using strict inclusion and exclusion criteria. Third, we included studies of all anti‐VEGF agents (ranibizumab, bevacizumab, and aflibercept) approved by the Food and Drug Administration (FDA), rather than those evaluating a single anti‐VEGF.
However, this work may have some limitations. First, inadequate random sequence generation and blinding may have resulted in selection bias, and patients with PCV whose condition worsened may have switched treatment. Nonetheless, selection bias was less likely to occur, as the major study characteristics of the eyes in the three groups were comparable at baseline. Second, some studies did not report all detailed outcome indicators, which caused some comparison only included two or three studies. For example, when comparing the mean change in CRT from baseline between combined treatment and PDT monotherapy at 6 months, the pooled results were insufficient for determining a statistically significant difference (P = .05). Therefore, more data may have resulted in a statistically significant difference. Third, publication bias cannot be fully excluded because we could not gain access to unopen results and unpublished studies. Nevertheless, the findings of the present meta‐analysis suggest that combined treatment with PDT and intravitreal anti‐VEGF injection therapy results in better long‐term improvements in VA, reductions in CRT, and more substantial regression of polyps. These findings indicate that combined therapy may be more effective than monotherapy in the treatment of PCV. Despite these encouraging findings, the inherent limitations of the included studies should be considered. Hence, more detailed data at different follow‐up time points and further large‐volume RCTs are required to improve the accuracy and robustness of our findings for clinical application.
CONFLICTS OF INTEREST
All authors declare that they have no conflicts of interest.
AUTHORS' CONTRIBUTIONS
All authors conceived of and designed the experimental protocol. Qian TW & Li XX collected the data. Qian TW & Zhao MY analysed the data. Qian TW and Li XX wrote the first draft of the manuscript. Qian TW & Xu X reviewed and revised the manuscript and produced the final version. All authors read and approved the final manuscript.
Qian TW & Li XX contributed equally to this work and should be considered co‐first authors.
Qian T, Li X, Zhao M, Xu X. Polypoidal choroidal vasculopathy treatment options: A meta‐analysis. Eur J Clin Invest. 2018;48:e12840 https://doi.org/10.1111/eci.12840
Funding information
Supported by the National Natural Science Foundation of China (No. 81570851).
Qian and Li contributed equally to the work of this manuscript.
REFERENCES
- 1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV)[J]. Retina. 1990;10:1‐8. [PubMed] [Google Scholar]
- 2. Sakurada Y, Yoneyama S, Imasawa M, Iijima H. Systemic risk factors associated with polypoidal choroidal vasculopathy and neovascular age‐related macular degeneration[J]. Retina. 2013;33:841‐845. [DOI] [PubMed] [Google Scholar]
- 3. Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy[J]. Retina. 1995;15:100‐110. [DOI] [PubMed] [Google Scholar]
- 4. Nowak‐Sliwinska P, Van Den Bergh H, Sickenberg M, Koh AH. Photodynamic therapy for polypoidal choroidal vasculopathy[J]. Prog Retin Eye Res. 2013;37:182‐199. [DOI] [PubMed] [Google Scholar]
- 5. Wong CW, Cheung CMG, Mathur R, et al. Three‐year results OF polypoidal choroidal vasculopathy treated with photodynamic therapy: retrospective study and systematic review[J]. Retina. 2015;35:1577‐1593. [DOI] [PubMed] [Google Scholar]
- 6. Kim JH, Lee DW, Choi SC, et al. Intravitreal anti‐vascular endothelial growth factor for newly diagnosed symptomatic polypoidal choroidal vasculopathy with extrafoveal polyps[J]. Korean J Ophthalmol. 2015;29:404‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho HJ, Kim JW, Lee DW, Cho SW, Kim CG. Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy[J]. Eye. 2012;26:426‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akaza E, Mori R, Yuzawa M. Long‐term results of photodynamic therapy of polypoidal choroidal vasculopathy[J]. Retina. 2008;28:717‐722. [DOI] [PubMed] [Google Scholar]
- 9. Tong JP, Chan WM, Liu DTL, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium–derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization[J]. Am J Ophthalmol. 2006;141:456‐462. [DOI] [PubMed] [Google Scholar]
- 10. Yong M, Zhou M, Deng G. Photodynamic therapy versus anti‐vascular endothelial growth factor agents for polypoidal choroidal vasculopathy: a meta‐analysis[J]. BMC Ophthalmo. 2015;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang K, Si JK, Guo DD, et al. Ranibizumab alone or in combination with photodynamic therapy vs photodynamic therapy for polypoidal choroidal vasculopathy: a systematic review and Meta‐analysis[J]. Int J ophthalmol. 1056;2015:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang W, He M, Zhang X. Combined intravitreal anti‐VEGF and photodynamic therapy versus photodynamic monotherapy for polypoidal choroidal vasculopathy: a systematic review and meta‐analysis of comparative studies[J]. PLoS ONE. 2014;9:e110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research[J]. Eur J Clin Invest. 2010;40:35‐53. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Green S (2011) Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March, 2011]: The Cochrane Collaboration. http://handbook.cochrane.org/
- 15. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 16. Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy[J]. Retina. 2012;32:1453‐1464. [DOI] [PubMed] [Google Scholar]
- 17. Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12‐month LAPTOP study results[J]. Am J Ophthalmol. 2013;156:644‐651. e1 [DOI] [PubMed] [Google Scholar]
- 18. Lee MY, Lee WK, Baek J, Kwon OW, Lee JH. Photodynamic therapy versus combination therapy in polypoidal choroidal vasculopathy: changes of aqueous vascular endothelial growth factor[J]. Am J Ophthalmol. 2013;156:343‐348. [DOI] [PubMed] [Google Scholar]
- 19. Lai TYY, Chan WM, Liu DTL, Luk FO, Lam DS. Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy[J]. Br J Ophthalmol. 2008;92:661‐666. [DOI] [PubMed] [Google Scholar]
- 20. Lee SY, Kim JG, Joe SG, Chung H, Yoon YH. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy[J]. Korean J Ophthalmol. 2008;22:92‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim JY, Lee SY, Kim JG, Lee JY, Chung H, Yoon YH. Intravitreal bevacizumab alone versus in combination with photodynamic therapy for the treatment of neovascular maculopathy in patients aged 50 years or older: 1‐year results of a prospective clinical study[J]. Acta Ophthalmol. 2012;90:61‐67. [DOI] [PubMed] [Google Scholar]
- 22. Gomi F, Sawa M, Wakabayashi T, Sesamoto Y, Suzuki M, Tsujikawa M. Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy[J]. Am J Ophthalmol. 2010;150:48‐54. e1 [DOI] [PubMed] [Google Scholar]
- 23. Mitamura Y, Kitahashi M, Kubota‐Taniai M, Yamamoto S. Comparison of intravitreal bevacizumab to photodynamic therapy for polypoidal choroidal vasculopathy: short‐term results[J]. Indian J Ophthalmol. 2010;58:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SJ, Yu HG. Efficacy of combined photodynamic therapy and intravitreal bevacizumab injection versus photodynamic therapy alone in polypoidal choroidal vasculopathy[J]. Retina. 2011;31:1827‐1834. [DOI] [PubMed] [Google Scholar]
- 25. Lai TYY, Lee GKY, Luk FOJ, Lam DS. Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy[J]. Retina. 2011;31:1581‐1588. [DOI] [PubMed] [Google Scholar]
- 26. Maruko I, Iida T, Sugano Y, Sekiryu T. Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy[J]. Am J Ophthalmol. 2011;151:594‐603. e1 [DOI] [PubMed] [Google Scholar]
- 27. Rouvas AA, Papakostas TD, Ntouraki A, Douvali M, Vergados I, Ladas ID. Photodynamic therapy, ranibizumab, and ranibizumab with photodynamic therapy for the treatment of polypoidal choroidal vasculopathy[J]. Retina. 2011;31:464‐474. [DOI] [PubMed] [Google Scholar]
- 28. Saito M, Iida T, Kano M. Intravitreal ranibizumab for polypoidal choroidal vasculopathy with recurrent or residual exudation[J]. Retina. 2011;31:1589‐1597. [DOI] [PubMed] [Google Scholar]
- 29. Song MH, Ryu HW, Roh YJ. One‐year results of intravitreal ranibizumab with or without photodynamic therapy for polypoidal choroidal vasculopathy[J]. Ophthalmol. 2011;226:119‐126. [DOI] [PubMed] [Google Scholar]
- 30. Lee YA, Yang CH, Yang CM, et al. Photodynamic therapy with or without intravitreal bevacizumab for polypoidal choroidal vasculopathy: two years of follow‐up[J]. Am J Ophthalmol. 2012;154:872‐880. e2 [DOI] [PubMed] [Google Scholar]
- 31. Inoue M, Arakawa A, Yamane S, Kadonosono K. Long‐term outcome of intravitreal ranibizumab treatment, compared with photodynamic therapy, in patients with polypoidal choroidal vasculopathy[J]. Eye. 2013;27:1013‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang HM, Koh HJ. Two‐year outcome after combination therapy for polypoidal choroidal vasculopathy: comparison with photodynamic monotherapy and anti‐vascular endothelial growth factor monotherapy[J]. Ophthalmol. 2013;231:86‐93. [DOI] [PubMed] [Google Scholar]
- 33. Saito M, Iida T, Kano M, Itagaki K. Two‐year results of combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy[J]. Graefes Arch Clin Exp Ophthalmol. 2013;251:2099‐2110. [DOI] [PubMed] [Google Scholar]
- 34. Sakurada Y, Iijima H. Two‐year results of photodynamic therapy with or without intravitreal ranibizumab for polypoidal choroidal vasculopathy[J]. J Ocul Pharmacol Ther. 2013;29:832‐836. [DOI] [PubMed] [Google Scholar]
- 35. Gomi F, Oshima Y, Mori R, et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: the Fujisan Study[J]. Retina. 2015;35:1569‐1576. [DOI] [PubMed] [Google Scholar]
- 36. Kikushima W, Sakurada Y, Sugiyama A, Tanabe N, Kume A, Iijima H. Comparison of initial treatment between 3‐monthly intravitreal aflibercept monotherapy and combined photodynamic therapy with single intravitreal aflibercept for polypoidal choroidal vasculopathy[J]. Graefes Arch Clin Exp Ophthalmol. 2016;255:1‐6. [DOI] [PubMed] [Google Scholar]
- 37. Sakai T, Okano K, Kohno H, Tsuneoka H. Three‐year visual outcomes of intravitreal ranibizumab with or without photodynamic therapy for polypoidal choroidal vasculopathy[J]. Acta Ophthalmol. 2016;94:e765‐e771. [DOI] [PubMed] [Google Scholar]
- 38. Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age‐related macular degeneration: same or different disease?[J]. Prog Retin Eye Res. 2010;29:19‐29. [DOI] [PubMed] [Google Scholar]
- 39. Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics[J]. Arch Ophthalmol. 2003;121:1392‐1396. [DOI] [PubMed] [Google Scholar]
- 40. Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation[J]. Br J Ophthalmol. 2002;86:321‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Golbaz I, Ahlers C, Stock G, et al. Quantification of the therapeutic response of intraretinal, subretinal, and subpigment epithelial compartments in exudative AMD during anti‐VEGF therapy[J]. Invest Ophthalmol Vis Sci. 2011;52:1599‐1605. [DOI] [PubMed] [Google Scholar]
- 42. Chan WM, Lam DSC, Lai TYY, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one‐year results of a prospective case series[J]. Ophthalmology. 2004;111:1576‐1584. [DOI] [PubMed] [Google Scholar]
- 43. Sato T, Kishi S, Matsumoto H, Mukai R. Comparisons of outcomes with different intervals between adjunctive ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy[J]. Am J Ophthalmol. 2013;156:95‐105. e1 [DOI] [PubMed] [Google Scholar]
- 44. Honda S, Imai H, Yamashiro K, et al. Comparative assessment of photodynamic therapy for typical age‐related macular degeneration and polypoidal choroidal vasculopathy: a multicenter study in Hyogo prefecture, Japan[J]. Ophthalmol. 2009;223:333‐338. [DOI] [PubMed] [Google Scholar]
- 45. Cheung CMG, Yeo I, Li X, et al. Argon laser with and without anti‐vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy[J]. Am J Ophthalmol. 2013;155:295‐304. e1 [DOI] [PubMed] [Google Scholar]
- 46. Wang K, Wang Y, Gao L, Li X, Li M, Guo J. Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin‐induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule‐1 expression[J]. Biol Pharm Bull. 2008;31:1541‐1546. [DOI] [PubMed] [Google Scholar]
- 47. Ho M, Woo DCF, Chan VCK, Young AL, Brelen ME. Treatment of polypoidal choroidal vasculopathy by photodynamic therapy, aflibercept and dexamethasone triple therapy[J]. Sci Rep. 2016;6:36870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liang IC, Lin YR, Chien HW, Liu KR. Vision preservation in eyes of polypoidal choroidal vasculopathy with low‐dose intravitreal triamcinolone acetonide[J]. J Ocul Pharmacol Ther. 2017;33:42‐49. [DOI] [PubMed] [Google Scholar]
- 49. Sonoda S, Sakamoto T, Yamashita T, et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images[J]. Am J Ophthalmol. 2015;159:1123‐1131. e1 [DOI] [PubMed] [Google Scholar]
- 50. Daizumoto E, Mitamura Y, Sano H, et al. Changes of choroidal structure after intravitreal aflibercept therapy for polypoidal choroidal vasculopathy[J]. Br J Ophthalmol. 2017;101:56‐61. [DOI] [PubMed] [Google Scholar]
- 51. Hosokawa M, Morizane Y, Hirano M, et al. One‐year outcomes of a treat‐and‐extend regimen of intravitreal aflibercept for polypoidal choroidal vasculopathy[J]. Jpn J Ophthalmol. 2017;61:150‐158. [DOI] [PubMed] [Google Scholar]
- 52. Morimoto M, Matsumoto H, Mimura K, Akiyama H. Two‐year results of a treat‐and‐extend regimen with aflibercept for polypoidal choroidal vasculopathy[J]. Graefes Arch Clin Exp Ophthalmol. 2017;2017:1‐7. [DOI] [PubMed] [Google Scholar]
- 53. Arakawa A, Inoue M, Sato S, Yamane S. Efficacy of intravitreal aflibercept injections for Japanese patients with polypoidal choroidal vasculopathy[J]. Clin Ophthalmol (Auckland, NZ), 2017;11:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee JE, Shin JP, Kim HW, et al. Efficacy of fixed‐dosing aflibercept for treating polypoidal choroidal vasculopathy: 1‐year results of the VAULT study[J]. Graefes Arch Clin Exp Ophthalmol. 2017;255:493‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]


