Abstract
Background & Aims
Many cancers in the proximal colon develop via from sessile serrated adenomas (SSAs), which have flat, subtle features that are difficult to detect with conventional white-light colonoscopy. Many SSA cells have the V600E mutation in BRAF. We investigated whether this feature could be used with imaging methods to detect SSAs in patients.
Methods
We used phage display to identify a peptide that binds specifically to SSAs, using subtractive hybridization with HT29 colorectal cancer cells containing the V600E mutation in BRAF and Hs738.St/Int cells as a control. Binding of fluorescently labeled peptide to colorectal cancer cells was evaluated with confocal fluorescence microscopy. Rats received intra-colonic 0.0086 mg/kg, 0.026 mg/kg, or 0.86 mg/kg peptide or vehicle and morbidity, mortality, and injury were monitored twice daily to assess toxicity. In the clinical safety study, fluorescently labeled peptide was topically administered, using a spray catheter, to the proximal colon of 25 subjects undergoing routine outpatient colonoscopies (3 subjects were given 2.25 µmol/L and 22 patients were given 76.4 µmol/L). We performed blood cell count, chemistry, liver function, and urine analyses approximately 24 hrs after peptide administration. In the clinical imaging study, 38 subjects undergoing routine outpatient colonoscopies, at high risk for colorectal cancer, or with a suspected unresected proximal colonic polyp, were first evaluated by white-light endoscopy, to identify suspicious regions. The fluorescently labeled peptide (76.4 µmol/L) was administered topically to proximal colon, unbound peptide was washed away, and white-light, reflectance, and fluorescence videos were recorded digitally. Fluorescence intensities of SSAs were compared with those of normal colonic mucosa. Endoscopists resected identified lesions, which were analyzed histologically by gastrointestinal pathologists (reference standard). We also analyzed the ability of the peptide to identify SSAs vs adenomas, hyperplastic polyps, and normal colonic mucosa in specimens obtained from the tissue bank at the University of Michigan.
Results
We identified the peptide sequence KCCFPAQ, and measured an apparent dissociation constant of kd = 72 nM and an apparent association time constant of k = 0.174 min−1 (5.76 min). During fluorescence imaging of patients during endoscopy, regions of SSA had 2.43-fold higher mean fluorescence intensity than that for normal colonic mucosa. Fluorescence labeling distinguished SSAs from normal colonic mucosa with 89% sensitivity and 92% specificity. The peptide had no observed toxic effects in animals or patients. In the analysis of ex vivo specimens, peptide bound to SSAs had significantly higher mean fluorescence intensity than to hyperplastic polyps.
Conclusions
We have identified a fluorescently labeled peptide that has no observed toxic effects in animals or humans and can be used for wide-field imaging of lesions in the proximal colon. It distinguishes SSAs from normal colonic mucosa with 89% sensitivity and 92% specificity. This targeted imaging method might be used in early detection of pre-malignant serrated lesions during routine colonoscopies. ClinicalTrials.gov no: NCT02156557
Keywords: serrated, adenoma, colorectal cancer, peptide
Introduction
Colorectal cancer (CRC) is a leading cause of cancer-related mortality with ~1.4 million new cases diagnosed globally and ~690,000 annual deaths.1 Worldwide, these numbers are rising steadily as growth in obesity becomes epidemic and developing countries adopt a more Western diet.2 Colonoscopy is considered the gold standard for early detection of CRC where pre-malignant lesions are identified with white light illumination and resected. However, colonoscopy has been shown to have limited mortality benefit in the proximal compared with the distal colon.3–5 Previously, all CRCs were believed to arise from adenomas that progress through the traditional adenoma-carcinoma sequence.6 Recently, this pathway has been found to account for only ~60% of CRCs, and up to 35% is now attributed to the serrated pathway,7–9 while the remaining ~5% arises from Lynch Syndrome.10 Sessile serrated adenomas (SSA) are precursor lesions to the majority of CRCs with microsatellite instability (MSI),11 and are found predominantly in the proximal colon. They are molecularly characterized by a somatic point mutation in the V600E locus of BRAF that results in enhanced MEK and ERK signaling.12,13 Because SSAs are almost always are flat and/or have subtle mucosal features, these premalignant lesions are difficult to detect with conventional white light endoscopy (WLE), and are frequently missed.14 Thus, improved imaging methods for early detection of SSAs are needed to enhance the effectiveness of colonoscopy and better prevent serrated cancers.
White light endoscopy (WLE) is sensitive to gross morphological abnormalities, such as polyps. Combining endoscopy with targeted molecular probes is a promising approach to improve visualization of SSAs. Peptides are promising for use in clinical imaging because they can be developed with high specificity to cell surface targets with good binding affinity.15 Compared to antibodies, peptides are more attractive for diagnostic applications because they have faster binding kinetics, can be mass manufactured at low costs, and are less immunogenic, allowing for repeated use in high-risk patients that need frequent surveillance.16 Using topical administration, high contrast images can be achieved with minimal risk for toxicity, and avoids undesired probe biodistribution to non-targeted tissues, which is a major concern with systemic delivery. Moreover, peptides bind rapidly and predictably within only a few minutes.17–19 These properties are ideal for in vivo use in high volume diagnostic procedures, such as colonoscopy.
Imaging is a powerful tool that can rapidly visualize the entire mucosal surface of the proximal colon in real time. Wide-field endoscopes that are sensitive to fluorescence are being developed to detect specific imaging agents, such as peptides, in the digestive tract that generate high contrast images with low background.20,21 Using fluorophores as labels, targeting moieties that are specific to disease can be used as an adjunct to WLE to to guide detection and resection of pre-malignant lesions that may otherwise progress on to cancer. These instruments can be similar in appearance, dimensions, and handling to that of conventional colonoscopes. The endoscope, light source, video processor, and image digitizer are contained on a portable instrument cart that can be translated seamlessly into the clinic. The targeted fluorescence images collected can be segmented using simple image processing algorithms to provide a “red flag” region to guide physicians by identifying high risk areas for resection.22 Here, we aim to select and validate a peptide that binds specifically to pre-malignant lesions in the proximal colon that have sessile serrated pathology and are difficult to visualize with conventional white light colonoscopy.
Methods
Cells and culture
Human colorectal adenocarcinoma cells (HT29, DLD1, SW480, and SW620) and human normal intestinal cells Hs738.St/Int (CRL-7869) were obtained from the American Type Culture Collection (ATCC). HT29 cells were cultured in McCoy’s medium (Gibco) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen). These cells have the V600E BRAF point mutation found in SSA.23 DLD1, SW480, SW620, and Hs738.St/Int cells were cultured in DMEM (Gibco) containing 10% FBS, 1% penicillin/streptomycin, and 1% glutamax (Gibco). All cells were cultured at 37°C in 5% CO2 and passaged with 0.25% EDTA containing trypsin (Mediatech Inc).
Selection and validation of KCC* phage
Peptide selection was performed with phage display technology (M13 Ph.D.-7, New England Biolabs) using a biopanning strategy based on a subtractive whole-cell approach, an unbiased method that maximizes binding and hence fluorescence signal for real time imaging.24 HT29 and Hs738.St/Int cells were grown to log-phase, detached with either cell dissociation buffer (Invitrogen) or trypsin with 0.25% EDTA, and immersed in blocking buffer consisting of PBS with 1% bovine serum albumin (BSA) for 30 min at 4°C with continuous agitation. 3 rounds of biopanning with a total of ~109 plaque-forming units (pfu) was performed at 4°C for 30 min with ~106 Hs738.St/Int cells (control) to remove non-specific binders. The supernatant containing the cleared phage pool was amplified to ~2×1011 pfu for positive selection against HT29 cells (~107) in PBS containing 0.2% Tween-20 (PBST) for 60 min at 4°C. The cells were washed 3X with cold PBST. The bound phages were then eluted with 1 mL of 0.2 M glycine (pH 2.2) containing 0.1% BSA for 8 min, and immediately neutralized with 150 µL of 1 M Tris buffer (pH 9.2). The resulting phages (~2×1011 pfu) were used to perform 4 additional rounds of biopanning against HT29 cells for further enrichment. After completion of biopanning, 60 plaques were randomly selected in each round and sequenced (ABI Automatic DNA Analyzer) with primer 5’-CCC TCATAG TTA GCG TAA CG-3’ (-96 gIII sequencing primer, New England Biolabs) that corresponds to the pIII gene sequence of M13 phage.
Selective binding of candidate phages to HT29 cells was validated by bound phage counts in triplicate. The candidate and non-insert wild-type (WT) phages (M13KE, New England Biolabs) were amplified, precipitated and tittered per manufacturer instructions. HT29 and Hs738.St/Int (~5×106) cells were grown to log-phase, detached, and blocked, as described above. ~2×1011 pfu of either candidate or WT phages were incubated with these cells for 15 min with continuous gentle agitation at room temperature (RT). After 3 rounds of washing with PBS/0.2% Tween-20, the bound phages were eluted and tittered, as described above.
Competitive inhibition of KCC* phage
Bound phage counts were measured with competition from unlabeled KCC* to evaluate specific binding by KCC* phage. Experiments were performed in triplicate. HT29 cells (~5×106) were detached with enzyme-free cell dissociation buffer and blocked with 1% BSA in PBS for 30 min at 4°C. The cells were washed and incubated with phage (~2×1011) for 15 min. The cells were then washed with PBS and immediately incubated with several concentrations (10, 25, 50, 75, 100, and 200 µM) of unlabeled KCC* for an additional 15 min. Cells were washed 3X and bound phages were recovered, as described above.
Characterization of KCC* peptide binding
We determined the apparent dissociation constant of KCC*-FITC to HT29 cells as a measurement of binding affinity.25 KCC*-FITC was serially diluted in PBS at increasing concentrations (0 to 0.6 µM) and incubated with HT29 cells (~105) at 4°C for 1 hour. The unbound peptides were rinsed away by washing 3X with cold PBS/0.2% Tween-20 solution. The cells were re-suspended in 200 µL of PBS and transferred to a 96 well plate. The fluorescence intensity was measured at 525 nm using excitation at λex = 485 nm in a multi-well plate reader (CytoFluor Series 4000, Applied Biosystems). The results were compared to the fluorescence intensities from the peptide alone (no cells). These intensities were used to calculate the equilibrium dissociation constant kd = 1/ka by performing a least squares fit of the data to the non-linear equation I[X] = (I0+Imaxka[X])/(I0+ka[X]). I0 and Imax are the initial and maximum fluorescence intensities, corresponding to no peptide and at saturation, respectively, and [X] represents the concentration of the bound peptide. Origin 6.1 data analysis software (Origin Lab Corp) was used to perform a non-linear least square fit of the data.
We measured the apparent association time constant for KCC*-FITC binding to HT29 cells to assess how rapidly the peptide binds.26 HT29 cells were grown to ~80% confluence in 10 cm dishes, and detached with PBS-based cell dissociation buffer (Invitrogen). Cells (~105) were incubated with 5 µM of KCC*-FITC at 4°C for various time intervals ranging from 0 to 30 min. The cells were centrifuged, washed with cold PBS, and fixed with 4% PFA. The mean fluorescence intensities were measured with flow cytometry (BD® LSRII, BD Biosciences). The median fluorescence intensity (y) was ratioed with that of HT29 cells without addition of peptide at different time points (t) using software (FlowJo, FlowJo, LLC). The rate constant k was calculated by fitting the data to a first order kinetics model, y(t) = Imax[1-exp(-kt)], where Imax = maximum value, using software (Prism 5.0, GraphPad Inc).
KCC* binding to human colorectal cancer cells in vitro
Binding of KCC*-FITC to cells in vitro was evaluated with confocal fluorescence microscopy (TCS SP5, Leica Microsystems). HT29, DLD1, SW480, SW620 and Hs738.St/Int. cells were grown on cover slips to ~80% confluence. PBS/0.5% BSA was added for 30 min to block non-specific binding. The cells were then incubated with 10 µM of either KCC*-FITC or PFA*-FITC (control) at 4°C for 30 min followed by counter staining with 1 µg/mL Hoechst dye (Sigma Aldrich). The cells were washed 3X with PBS and fixed with 4% paraformaldehyde (PFA) at RT for 10 min. The cells were washed again with PBS and mounted with ProLong Gold reagent (Invitrogen) prior to imaging.
GMP peptide synthesis
We synthesized KCC*-FITC under GMP conditions for the clinical study.27 The attributes of the imaging agent were documented on a certificate of analysis (COA). The specifications for the peptide test article, test methods, and results are shown, Table S1.
Animal pharmacology and toxicology study
We performed a rigorous pharmacology/toxicology study of GMP synthesized KCC*-FITC in rats to provide an initial assessment of safety. Intra-colonic administration with volume of 10 mL/kg was performed in 4 groups of 7 week-old rats, including vehicle (PBS) and at 0.0086 mg/kg, 0.026 mg/kg, and 0.86 mg/kg. Clinical signs (morbidity, mortality, injury) were monitored twice daily for all animals. 3 animals/gender/dose were evaluated by labs and necropsy on day 2 and 15. No acute peptide-related adverse effects in clinical signs, labs, or necropsy were found in any of the animals, Table S2.
Clinical safety study
We performed a “first-in-human” safety study of KCC*-FITC in n = 25 patients. The FDA approved an Investigational New Drug (IND, #116,907, sponsor-investigator DKT) application that included the chemistry, manufacturing, and control (CMC) document for GMP peptide synthesis, results of the animal pharmacology/toxicology study, and clinical study protocol. The safety study was approved by the University of Michigan IRB, and was registered on-line (ClinicalTrials.gov NCT01722058). Patients previously scheduled for routine outpatient colonoscopy were enrolled. Dose escalation was performed with n = 3 subjects receiving a lower dose of 25.5 µM (0.4 mg in 10 mL of 0.9% NaCl). The remaining n = 22 patients receive a higher dose of 76.4 µM. No images were collected in this study. Labs, including complete blood count (CBC), chemistries, liver function tests (LFTs) and urninalysis (UA), were collect prior to and ~24 hours after peptide administration. Vital signs were monitored continuously during the procedure. KCC*-FITC was topically administered in the proximal colon using a standard spray catheter (PW-5V-1, Olympus). Biopsies were taken as clinically indicated and evaluated by routine histology.
Clinical imaging study
We then performed a clinical imaging study (ClinicalTrials.gov NCT02156557) with topically administered KCC*-FITC by submitting an amendment of IND, #116,907 to the FDA. All authors have access to the study data and have reviewed and approved the final manuscript. A total of n = 45 prospective patients referred to the University of Michigan for routine colonoscopy were consented, and n = 38 subjects were evaluated. Inclusion criteria included 1) scheduled for colonoscopy, 2) high risk for CRC, 3) suspected unresected proximal colonic polyp; 4) medically cleared for colonoscopy; and 5) age over 18. Exclusion criteria included known allergy or negative reaction to FITC/fluorescein. We used a multi-modal video colonoscope (CF-Y0012, Olympus Medical Systems Corp, Tokyo, Japan) that has a standard definition (SD) CCD detector to collect sequential fluorescence and reflectance images. A separate SD detector collects white light images. This prototype instrument provides excitation to match the peak absorption of FITC, and detects fluorescence emission, Fig. 1B.22
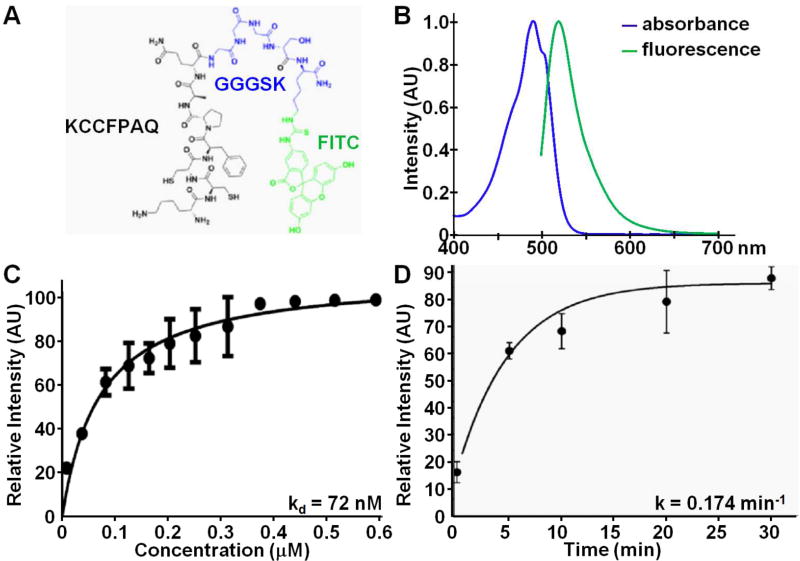
Fig. 1. Fluorescently-labeled peptide.
A) Chemical structure of KCCFPAQ peptide (black) attached via GGGSK linker (blue) to FITC fluorophore (green), hereafter KCC*-FITC. B) Fluorescence spectra of KCC*-FITC show peak absorbance and fluorescence emission at 490 and 519 nm, respectively. We measured C) an apparent dissociation constant of kd = 72 nM, R2 = 0.925, that reflects binding affinity, and D) an apparent association time constant of k = 0.174 min−1, R2 = 0.989, for binding of KCC*-FITC to HT29 cells, resulting in a time scale of 5.76 min. Results are an average of 5 independent experiments for kd and two independent experiments for k.
The colonoscopy procedure was performed by experienced therapeutic endoscopists (AP, RSK, EJW, GHE, EMS). White light was used first to identify regions of mucosa in the proximal colon that appear suspicious for harboring disease. Vigorous rinsing was performed to remove any overlying mucous, debris, or bubbles. Images were collected prior to peptide administration to assess background. The dimensions and macroscopic description of all lesions found were recorded, Table S4. KCC*-FITC was topically administered at the higher concentration of 76.4 µmol/L (1.2 mg in 10 ml of 0.9% NaCl) found in the safety study via a spray catheter passed through the instrument channel. After six minutes of incubation, the unbound peptides were washed away and the residual liquid was suctioned. White light, reflectance and fluorescence videos were recorded using an image digitizer (IMH-10, Olympus Medical Systems Corp) in high definition (1920×1080) with 8-bit intensity (0–255 gray levels) in MP4 format. The imaging mode was changed using a push button on the instrument handle. The average time for peptide administration and imaging was <10 min. The endoscopist then resected the lesion as clinically indicated, and expert gastrointestinal pathologists (HDA, SO) classified the histology per standard criteria.9
In vivo imaging performance
We defined a “red flag” region that can be displayed as an overlay on the white light image to help guide physician biopsy. We first subtracted 12 gray levels from each image that corresponded to detector read-out with no illumination (dark frame). The co-registered fluorescence and reflectance images (540×540 pixels) were used in a ratio to correct for differences in distance and geometry between the tissue and detector over the image field-of-view to quantify image intensities.28 We segmented the ratio image using the Otsu algorithm by seeking a threshold that minimizes the intra-class variance between pixel intensities from disease and normal mucosa.29 We classified each image pixel as either higher or lower in intensity than the candidate threshold to create a binary image (high = 1, low = 0). We defined a contour around regions of high intensity pixels for the target region. The neighboring contour with width of 30 pixels was assigned as background. We then calculated the target-to-background (T/B) ratio for each lesion.
KCC* binding to human colon specimens ex vivo
Formalin-fixed, paraffin-embedded (FFPE) human specimens of sessile serrated adenomas, adenomas, hyperplastic polyps, and normal colonic mucosa were obtained from the archived tissue bank in the Department of Pathology at the University of Michigan. 5 µm thick sections were cut, and mounted onto glass slides (Superfrost Plus, Fischer Scientific). The tissues were deparaffinized, and antigen retrieval was performed, following the standard protocol. The sections were blocked with protein serum for 15 min at RT followed by rinsing with PBS. The sections were then stained with 5 µM of KCC*-FITC for 10 min at RT. The sections were then washed for 3 min 3X with PBST and mounted with Prolong Gold reagent containing DAPI (Invitrogen) using #1 cover glass (1.5 µm thickness). The images were collected with a confocal microscope (Leica TCS SP5, Leica Microsystems) using the same exposure time for all specimens. We measured the mean fluorescence intensities from each image by placing 3 boxes with dimensions of 20×20 µm2 completely within colonic epithelium using custom Matlab software (Mathworks, Inc). Regions of saturated intensities were avoided. The mean intensities were fit with a two-sample t-test. Adjacent sections were processed for routine histology (H&E) and evaluated with standard criteria by expert gastrointestinal pathologists (HDA, SO).9
Results
Selection and validation of KCC* phage
We identified the peptide sequence KCCFPAQ, hereafter KCC*, using phage display technology by biopanning against human HT29 colorectal cancer cells that have the V600E BRAF point mutation found in SSA. We used a subtractive whole cell approach to identify peptides that bind specifically to the cell surface and are accessible to imaging with topical administration. We used normal human intestinal cells (Hs738.St/Int) as control. We performed 3 rounds of negative selection against Hs738.St/Int cells to remove non-specific binders and then 5 rounds of positive selection with HT29 cells. The pool of candidate phage binders was enriched by 78-fold (10×102/µL in first round versus 78×103/µL in the 5th round). Sequencing of 60 clones from the final round yielded the following peptide sequences in multiplicity (phage number): KCCFPAQ (13), WPTPPYA (1), MHAPPFY (1), SILPYPY (4), YRAPWPP (3), and QPWPTSI (3), Fig. S1A. Wild-type (WT) phage has no peptide insert, and is used as negative control. Multiple sequence alignment analysis using RELIC software did not reveal significant homology among these repeated sequences.30 Only some short motifs such as PP/FP/WP were found. The candidate sequences were further tested by counting the number of bound phages to HT29 and Hs738.St/Int cells. KCC* phage showed significantly greater binding to HT29 than to Hs738.St/Int. cells, and was significantly higher than that compared with the other phages, including WT. Based on these results, we synthesized the KCC* peptide for further validation.
Synthetic KCC*-FITC peptide
We labeled KCC* (black) via a GGGSK linker (blue) with a FITC (green) fluorophore on the C-terminus to prevent steric hindrance, Fig. 1A. FITC was chosen because fluorescein is safe and FDA approved for human use. KCC*-FITC showed peak absorbance at 490 nm, and maximum fluorescence emission at 519 nm, Fig. 1B. We purified the FITC-labeled peptides to >98.7% on HPLC, and measured a mass-to-charge (m/z) ratio of 1570.59 on mass spectrometry that was consistent with the expected value of 1570.78, Fig. S2.
Competitive inhibition of KCC* phage
We used unlabeled KCC* peptide in concentrations ranging from 0 – 200 µM to compete with KCC* phage for binding to HT29 cells, and found a dose-dependent reduction in bound phage counts, Fig. S1B. Peak counts were reduced by >98% at concentrations of 75 µM and above. These results suggest that binding of KCC* phage to the surface of HT29 cells is mediated by the specific insert peptide sequence rather than by other phage coat proteins.
Characterization of KCC* peptide binding
We measured an apparent dissociation constant of kd = 72 nM for binding of KCC*-FITC to HT29 cells, Fig. 1C, providing a measure of binding affinity. Also, we measured an apparent association time constant k = 0.174 min−1 for binding KCC*-FITC to HT29 cells, Fig. 1D, providing a time scale of 5.76 min.
KCC* binding to human colorectal cancer cells in vitro
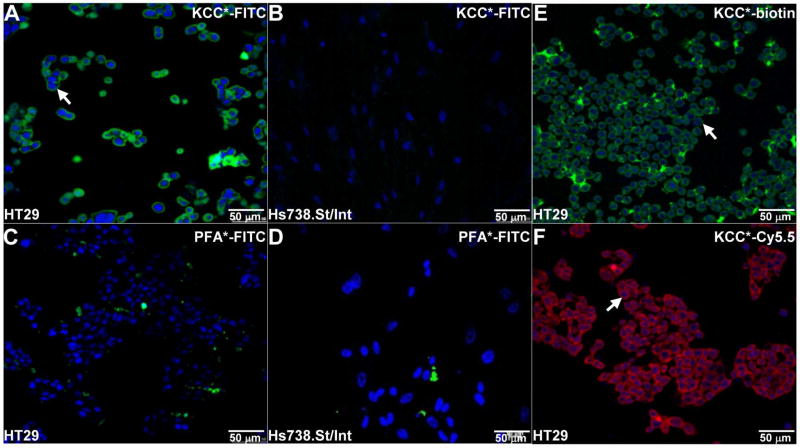
We validated binding of KCC*-FITC to the surface (arrows) of HT29 cells on confocal fluorescence microscopy, Fig. 2A. No binding was observed to Hs738.St/Int cells Fig. 2B. The peptide sequence PFAAPLP (PFA*) was identified from the pool of non-binders to HT29, and was used as control. PFA*-FITC (control) did not bind to any of the cells, Fig. 2C,D. We found that KCC* labeled with either biotin or Cy5.5 also bound comprehensively to the surface (arrows) of HT29 cells, Fig. 2E,F.
Fig. 2. KCC* peptide binding to human colon cells in vitro.
On confocal fluorescence microscopy, A) intense staining was observed for binding of KCC*-FITC to the surface (arrows) of HT29 cells with a V600E point mutation in BRAF, but not to B) Hs738St/Int (control) cells. C,D) No binding was observed for the PFA*-FITC control peptide to either cell. Labeling the KCC* peptide with either E) biotin or F) Cy5.5 also resulted in strong cell surface binding. Experiments were performed in triplicate.
Clinical safety study
We first performed a rigorous pharmacology/toxicology study of GMP synthesized KCC*-FITC in rats, and found no acute peptide-related adverse effects in clinical signs, labs, or necropsy in any of the animals. We then performed a “first-in-human” safety study in n = 25 human subjects using KCC*-FITC synthesized under GMP conditions. The study was approved by the FDA (IND #116,907) and University of Michigan IRB, and was registered on-line (ClinicalTrials.gov NCT01722058). We topically administered KCC*-FITC to the proximal colon in patients undergoing routine colonoscopy. We monitored clinical signs during the procedure, and labs, including chemistry, hematology, and urinalysis, before and ~24 hours after administration, Table S3. There were no serious adverse events (SAEs). Adverse events (AEs) included n = 1 patient who developed transient transaminitis (↑AST/ALT) and n = 3 patients who developed petechiae at the site of peptide administration.
Clinical imaging study
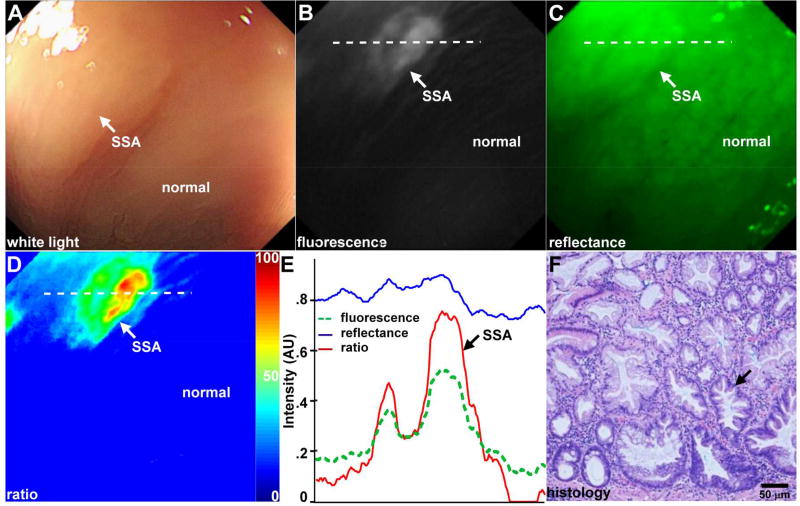
We performed an imaging study of KCC*-FITC in the proximal colon of n = 38 patients (n = 44 lesions) with an amendment to IND #116,907 (ClinicalTrials.gov NCT02156557), Table 1. We observed minimal signal before the peptide was administered from autofluorescence background using the molecular colonoscope. We imaged a total of n = 19 SSAs, including with flat (n = 4), sessile (n = 14), and pedunculated (n = 1) morphology. We observed minimal background from autofluorescence images collected prior to peptide administration, Fig. S3. A set of white light, fluorescence, and reflectance images collected in vivo from a flat lesion is shown, Fig. 3A–C. On white light, a subtle area (arrow) is seen that was found later to be SSA on pathology (Visualization1), Fig. 3A. This same region was easily identified by increased fluorescence intensity (Visualization2), Fig. 3B. We combined co-registered fluorescence and reflectance (Visualization3), Fig. 3C, in a ratio to correct for differences in distance and geometry from the detector to the mucosal surface over the field-of-view. The quantified image intensities are shown in pseudocolor, Fig. 3D. The intensities along the dashed line passing through the lesion in Fig. 3B–D are shown in Fig. 3E. A peak on fluorescence is seen at the location of the SSA. On pathology, the basally located crypts (arrow) are dilated and expanded laterally with numerous goblet cells to suggest abnormal maturation and are consistent with SSA, Fig. 3F. We show representative images from fluorescence videos collected in the proximal colon from hyperplastic polyp (HP, Visualization4), adenoma (Visualization5), and adenocarcinoma (ACA, Visualization6), Fig. 4A–C. Images from corresponding white light videos (Visualization7–9) are shown, Fig. 4D–F. At a frame rate of 20 Hz, we observed minimal motion artifacts between consecutive image frames.
Table 1.
Patient demographics
| Patient characteristics | ||
| Number of subjects | 38 (22 male, 16 female) | |
| Mean age in years | 61.9±10.8 (49–87) | |
| BMI | 30.2±5.3 | |
|
| ||
| Location of lesions | Number (%) | |
| total | 44 (100%) | |
| rectum | 1 ( 3%) | |
| sigmoid | 4 (11%) | |
| descending | 4 (11%) | |
| transverse | 3 (8%) | |
| hepatic flexure | 6 (16%) | |
| ascending | 12 (32%) | |
| cecum | 8 (21%) | |
|
| ||
| Endoscopic description of lesions | Number (%) | |
| flat | 11 (25%) | |
| sessile | 30 (68%) | |
| pedunculated | 3 (7%) | |
|
| ||
| Pathology | Number (%) | Size (mm) |
| HP | 1 ( 2%) | 3 |
| SSA | 19 (43%) | 15.7±9.3 |
| Adenoma | 23 (52%) | 25.9±15.5 |
| ACA | 1 ( 2%) | 40 |
| NML | 24 (55%) | – |
Key: BMI, body mass index; NML, normal; HP, hyperplastic; SSA, sessile serrated adenoma; TA, tubular adenoma; ACA, adenocarcinoma
Fig. 3. Targeted in vivo images of flat SSA in proximal colon.
A) Endoscopic image with white light illumination (Visualization1) shows limited visualization of SSA (arrow) with flat morphology. B) Fluorescence image (Visualization2) shows increased intensity and high contrast from lesion (arrow) while normal colonic mucosa shows minimal background. C) Reflectance (Visualization3) and co-registered fluorescence images are combined in a ratio to correct for differences in distance over the image field-of-view. D) Ratio image shown in pseudo-color enhances signal from SSA (arrow). E) Intensities from fluorescence, reflectance, and ratio images along horizontal dashed line in B–D) show a peak at location of SSA (arrow). F) Corresponding histology shows pathological features of SSA (arrow).
Fig. 4. Endoscopic images from proximal colon.
Representative fluorescence images of A) hyperplastic polyp (HP, Visualization4), B) adenoma (Visualization5), and C) adenocarcinoma (ACA, Visualization6) are shown along with corresponding D–F) white light images (Visualization7–9).
In vivo imaging performance
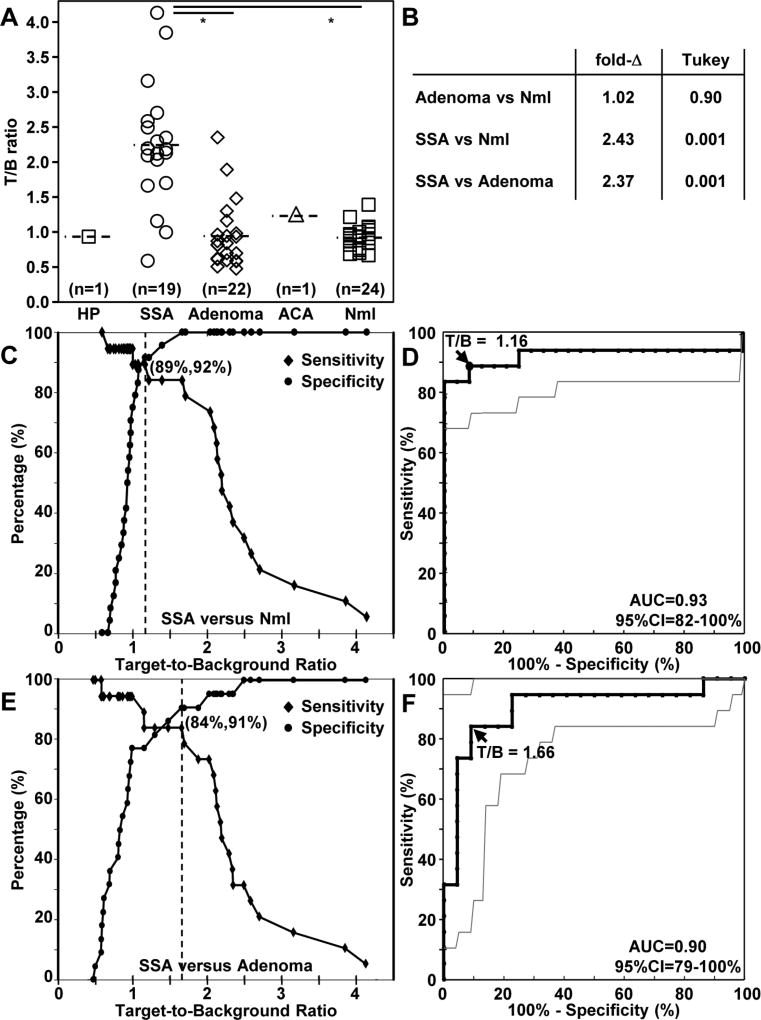
We measured the mean T/B ratio from the “red flag” regions for each lesion, and classified according to pathology, Fig. 5A. We were able to identify a region of adjacent normal for some (n = 24) of the lesions imaged. We found the mean fold-change for SSA to be significantly greater than that for normal and adenoma based on Tukey’s test, Fig. 5B. At a T/B ratio of 1.16, we found 89% sensitivity and 92% specificity for identifying SSA from normal colonic mucosa, Fig. 5C. The receiver-operator-characteristic (ROC) curve comparing T/B ratios for SSA with that for normal colonic mucosa has an area-under-curve (AUC) of 0.93 with a 95% confidence interval of 82–100%, Fig. 5D. The 95% confidence intervals for sensitivity, specificity, and positive and negative likelihood ratios are shown in Fig. S4. At a T/B ratio of 1.66, we found 84% sensitivity and 91% specificity for identifying SSA from adenoma, Fig. 5E. The ROC curve comparing T/B ratios for SSA with that for adenoma has an AUC of 0.90 with a 95% confidence interval of 79–100%, Fig. 5F. The 95% confidence intervals for sensitivity, specificity, and positive and negative likelihood ratios are shown in Fig. S5.
Fig. 5. In vivo imaging performance.
A) The mean T/B ratios from individual patients are calculated from the “red flag” contours drawn from a ratio of the fluorescence and reflectance images and are classified by histology: hyperplastic (HP), sessile serrated adenoma (SSA), adenoma, adenocarcinoma (ACA), and normal colonic mucosa (Nml). The mean (±std) values were found to be 0.94 for HP (n = 1), 2.24±0.87 for SSA (n = 19), 0.94±0.47 for adenoma (n = 23), 1.26 for ACA (n = 1), and 0.92±0.17 for Nml (n = 24). Fluorescence intensities were measured from adjacent normal colonic mucosa in only n = 24 cases because of the large size of some of the lesions. B) Based on the estimated fold-change, we found the mean fluorescence intensities for SSA to be significantly greater than that for normal and adenoma using Tukey’s single step multiple comparison test, C) For distinguishing SSA from Nml, 89% sensitivity and 92% specificity was achieved at a T/B ratio of 1.16 (dashed line). D) The corresponding receiver operator characteristic (ROC) curve has an AUC of 0.93 with 95% confidence interval: 82–100%. E) For distinguishing SSA from adenoma, 84% sensitivity and 91% specificity was achieved at a T/B ratio of 1.66 (dashed line). F) The corresponding ROC curve has an AUC of 0.90 with 95% confidence interval: 79–100%.
KCC* binding to human colon specimens ex vivo
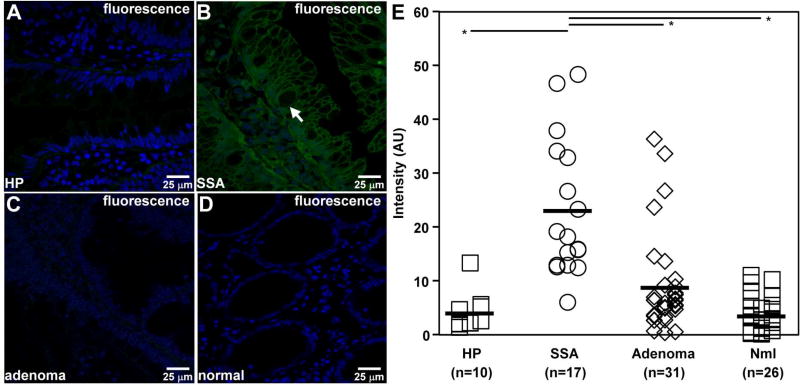
We performed ex vivo confocal imaging to validate specific binding of KCC*-FITC to SSA on a microscopic scale and to better compare peptide staining with hyperplastic polyps (HP). We used human specimens obtained from various anatomic segments of colon. We observed much stronger binding to the surface (arrow) of SSA colonocytes by comparison to that for HPs, adenomas, and normal colonic mucosa, Fig. 6A–D. We found the mean fluorescence intensity for SSA to be significantly greater than that for the other histological classifications, Fig. 6E.
Fig. 6. Ex vivo peptide binding to human colon specimens.
A–D) On confocal microscopy, KCC*-FITC (green) binds brightly to surface (arrow) of colonocytes from sessile serrated adenoma (SSA), while minimal signal is seen for hyperplasia (HP), adenoma, and normal colonic mucosa. E) The mean (±std) fluorescence intensities for HP (n = 10), SSA (n = 17), adenoma (n = 31), and normal (n = 26) were found to be 4.04±1.12, 22.93±3.07, 8.81±1.63, and, 3.56±0.64 respectively. The mean fluorescence intensity for SSA was found to be significantly higher than that for HP, adenoma, and normal, *P=<0.01, by unpaired t-test.
Discussion
Here, we demonstrate a systematic approach to identify, characterize, and validate a peptide that is specific for sessile serrated pathology. These pre-malignant lesions are responsible for between ~15–35% of all CRCs,7–9 and likely contribute to reduced protection of colonoscopy for CRC in the proximal colon.3–5 We identified the sequence KCCFPAQ using HT29 cells that have the V600E point mutation in BRAF that is common to sporadic SSAs.11–13 This mutation is not found in conventional adenomas, which were distinguished from SSAs on imaging in vivo, Fig. 5. We collected fluorescence images with high resolution and contrast using a prototype wide-field colonoscope,22 and identified SSAs with high sensitivity and specificity, including n = 19 lesions that were poorly visualized with white light alone. The presence of a mucous cap or rim of debris is characteristic of SSAs,31 but did not interfere with imaging because of vigorous rinsing. We quantified the ratio of co-registered fluorescence and reflectance images using a simple segmentation algorithm to objectively define a “red flag” region, Fig. 3. We have demonstrated an integrated imaging methodology that may be useful as an adjunct to routine colonoscopy to detect and localize SSAs for improved early detection of CRC in the proximal colon.
Lesions that arise from the serrated pathway were originally believed to be benign when first identified. Over the past decade, the role of SSAs in CRC development has become better recognized as having clinical importance.9 SSAs have a CpG island methylator phenotype (CIMP) that may stimulate proliferation of hyperplastic polyps,11–13 and are more likely to result in interval cancers where CRC is diagnosed after colonoscopy.32 SSAs are frequently missed because they appear either flat or minimally raised and have indistinct margins. Targeted imaging is an emerging methodology that is promising for detection of pre-malignant lesions based on unique protein expression patterns.33 Recently, a peptide specific for c-Met labeled with Cy5 was shown to detect traditional colonic adenomas in n = 15 human subjects.20 In that study, a fiber-optic colonoscope was used that has much lower resolution that the video colonoscope used here, and may explain the poor visualization of flat adenomas with white light illumination. In another study, a peptide specific for Caspr-1 labeled with fluorescein was administered topically in n = 18 patients.34 A confocal laser endomicroscope was used to validate specific peptide binding to the surface of dysplastic colonocytes in vivo over a microscopic FOV.
Other advanced imaging techniques, including high-definition white light endoscopy, chromoendoscopy (HD-WLE), narrowband imaging (NBI), magnification endoscopy (ME), and autofluorescence imaging (AFI), have been used to detect SSAs in the clinic. These methods detect abnormalities on the mucosal surface based on non-specific contrast mechanisms. They are limited in effectiveness because the morphologically distinct features of SSAs are found primarily in the crypt base. HD-WLE has been used to evaluate endoscopic features, including indistinctive borders, cloud-like surface, pit pattern, dark spots, vascular pattern intensity, and irregular shape, and was found to have 75% sensitivity, 79% specificity, and 77% accuracy for detection of SSA.35 Chromoendoscopy uses topical administration of intra-vital dyes to enhance contrast from mucosal surface patterns. Clinical studies aimed to discriminate SSAs from HPs was shown to have shown low sensitivity.36 Narrow-band imaging (NBI) uses restricted bands of visible light to enhance contrast from pit patterns and vasculature features, and is often performed with magnification. Subjective descriptions of the mucosa, including cloud-like surface, expanded crypt openings, thick branched vessels, have been used to distinguish SSAs from HPs.37 Using 100-fold magnification, NBI has been able to visualize expanded crypt openings and thick branched vessels with improved performance, resulting in 84.3% sensitivity, 81.1% specificity, and 82.4% accuracy.38 Autofluorescence imaging (AFI) uses differences in the fluorescence emission spectra from endogenous fluorophores. AFI has been used in combination with HD-WLE (high definition) and NBI in a tri-modal system to distinguish SSAs, but the diagnostic accuracy in a prospective study was poor.39
Peptides represent a new class of targeting agent that is highly compatible with endoscopic imaging because of their small size and rapid binding kinetics. By comparison, antibodies are much larger in size and require several hours to bind. Also, they are more expensive to mass manufacture and prone to immunogenicity. Previously, the peptide VRPMPLQ was identified using a tissue-based biopanning strategy that was limited by disease heterogeneity and the assumption that all patients over express similar molecular targets.34 Using this selection strategy, rigorous validation of specific binding to dysplasia was difficult because adenomas are a mixture of cell types including epithelial, stromal, and inflammatory. With cell based biopanning, the surface targets are preserved in their native context and cell membrane topography is defined by the arrangement and expression levels of membrane bound proteins. Also, specific peptides can be isolated without complete knowledge of the cellular landscape, and allows for detection of cell surface macromolecules that may not otherwise be exposed.40 However, the cell surface target is not completely known using this selection strategy. Alternate strategies have been used to identify peptides for known cell surface targets, including EGFR, ErbB2, claudin-1, and c-MET.17–20 In vivo images have also been collected in the pre-clinical and clinical settings using these peptides.
While this method has shown promise, use of fluorescently-labeled peptides requires additional time and cost, suggesting that future clinical use of this approach will most likely be appropriate for high risk rather than routine colonoscopy. In our in vivo study, we focused our imaging in the proximal colon, thus detected only one hyperplastic polyp. Greater fluorescence intensity from specific peptide binding to SSAs was validated ex vivo, where we studied n = 10 hyperplastic polyps. Our results need to be validated in prospective, multi-center studies that include additional hyperplastic polyps and adenocaricinomas. We are currently investigating the cell surface protein target, and its role in the pathogenesis of disease. Image resolution can be improved with use of a high definition (HD) rather than standard detector. Future imaging performance can be improved with use of near-infrared (NIR) fluorophores that have brighter intensity and less photobleaching compared with FITC. NIR fluorescence will result in reduced hemoglobin absorption, less tissue background, and greater image contrast, all significant advantages for in vivo imaging.
Topical administration of contrast agents in the proximal colon during colonoscopy is practical. This approach is used in chromoendoscopy,41 which is now being recommended for CRC screening in patients with inflammatory bowel disease (IBD). The peptides can also be administered with the prep or as an enema. Peptides that are specific for either the traditional adenoma-carcinoma or the serrated pathways may be combined by using endoscopes that can perform multiplexed imaging to maximize patient protection against all pre-malignant lesions.42 Improved methods of imaging are critical because other methods for screening, such as fecal immunochemical and occult blood tests, have been shown to be less sensitive to SSA than to conventional adenomas.43 Our imaging results demonstrate a promising new methodology for the early detection of SSAs that may help physicians guide biopsy, and perform resection of precancerous lesions that are difficult to visualize and may otherwise progress to malignancy. The practice of colonoscopy may be improved by optimizing detection, removal, and surveillance of serrated precursors and better protect against proximal CRCs.
Supplementary Material
Acknowledgments
Funding was provided in part by National Institutes of Health R01 CA193377 (TDW) and by gift funds from the Rose and Lawrence C. Page, Sr. Family Charitable Foundation (DKT) and Mary L. Petrovich (TDW). We thank E. Brady and N. Khan for clinical support, and B. Reisdorph and K. Weatherwax for regulatory support.
Abbreviations
- CRC
Colorectal Cancer
- EMR
Endoscopic Mucosal Resection
- IRB
Institutional Review Board
- IND
Investigational New Drug
- FDA
Food and Drug Administration
- FITC
Fluorescein Isothiocyanate
- SSA
Sessile Serrated Adenoma
- HP
Hyperplastic Polyp
- ACA
Adenocarcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: BPJ, DKT, and TDW conceived and designed the experiments. JHL, NG, JC, AP, EJW, RSK, GHE, AP, TK, and RMS performed the experiments. ZD, HDA, RK, DKT and TDW analyzed the data. ZD, TK, and RMS contributed materials/analysis tools. ZD, DKT, and TDW wrote the manuscript.
Conflict of Interest: BPJ and TDW are inventors on a patent filed by the University of Michigan on the peptide presented in this study. The other authors have nothing to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Goh LY, Goh KL. Obesity: an epidemiological perspective from Asia and its relationship to gastrointestinal and liver cancers. J Gastroenterol Hepatol. 2013;28S4:54–8. doi: 10.1111/jgh.12293. [DOI] [PubMed] [Google Scholar]
- 3.Singh H, Nugent Z, Demers AA, et al. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–37. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Hoffmeister M, Arndt V, et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 5.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Huang CS, Farraye FA, Yang S, et al. The clinical significance of serrated polyps. Am J Gastroenterol. 2011;106:229–40. doi: 10.1038/ajg.2010.429. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–29. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 11.Rad R, Cadiñanos J, Rad L, et al. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell. 2013;24:15–29. doi: 10.1016/j.ccr.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rustgi AK. BRAF: a driver of the serrated pathway in colon cancer. Cancer Cell. 2013;24:1–2. doi: 10.1016/j.ccr.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–7. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Payne SR, Church TR, Wandell M, et al. Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol. 2014;12:1119–26. doi: 10.1016/j.cgh.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Xie J, Chen X. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem Rev. 2010;110:3087–111. doi: 10.1021/cr900361p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118–29. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Joshi BP, Duan X, et al. EGFR overexpressed in colonic neoplasia can be detected on wide-field endoscopic imaging. Clinical Translational Gastroenterology. 2015;6:e102. doi: 10.1038/ctg.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi BP, Zhou J, Pant A, et al. Design and Synthesis of Near-Infrared Peptide for In Vivo Molecular Imaging of HER2. Bioconjugate Chemistry. 2016;27:481–94. doi: 10.1021/acs.bioconjchem.5b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinsky EF, Joshi BP, Zhou J, et al. Claudin-1 peptide can detect colonic neoplasia in vivo on endoscopic imaging. Cellular and Molecular Gastroenterology and Hepatology. 2016;2:222–37. doi: 10.1016/j.jcmgh.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burggraaf J, Kamerling IM, Gordon PB, et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat Med. 2015;21:955–61. doi: 10.1038/nm.3641. [DOI] [PubMed] [Google Scholar]
- 21.Joshi BP, Duan X, Kwon RS, et al. Multimodal endoscope can quantify wide-field fluorescence detection of Barrett's neoplasia. Endoscopy. 2016;48:A1–A13. doi: 10.1055/s-0034-1392803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi BP, Pant A, Duan X, et al. Multimodal Video Colonoscope for Targeted Wide-Field Detection of Nonpolypoid Colorectal Neoplasia. Gastroenterology. 2016;150:1084–6. doi: 10.1053/j.gastro.2016.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed D, Eide PW, Eilertsen IA, et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;16(2):e71. doi: 10.1038/oncsis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Anastassiades CP, Joshi B, et al. Affinity peptide for targeted detection of dysplasia in Barrett's esophagus. Gastroenterology. 2010;139:1472–80. doi: 10.1053/j.gastro.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas R, Chen J, Roudier MM, et al. In vitro binding evaluation of 177Lu-AMBA, a novel 177Lu-labeled GRP-R agonist for systemic radiotherapy in human tissues. Clin Exp Metastasis. 2009;26:105–19. doi: 10.1007/s10585-008-9220-0. [DOI] [PubMed] [Google Scholar]
- 26.Joshi BP, Liu Z, Elahi SF, et al. Near-infrared-labeled peptide multimer functions as phagemimic for high affinity, specific targeting of colonic adenomas in vivo. Gastrointestinal Endoscopy. 2012;76:1197-206.e1–5. doi: 10.1016/j.gie.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields GB, Noble RL. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang TD, Janes GS, Wang Y, et al. Mathematical model of fluorescence endoscopic image formation. Appl Opt. 1998;37:8103–11. doi: 10.1364/ao.37.008103. [DOI] [PubMed] [Google Scholar]
- 29.Otsu N. A threshold selection method from gray-level histograms. Automatica. 1975;11:285–296. [Google Scholar]
- 30.Mandava S, Makowski L, Devarapalli S, et al. RELIC - a bioinformatics server for combinatorial peptide analysis and identification of protein-ligand interaction sites. Proteomics. 2004;4:1439–1460. doi: 10.1002/pmic.200300680. [DOI] [PubMed] [Google Scholar]
- 31.Tadepalli US, Feihel D, Miller KM, et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video) Gastrointest Endosc. 2011;74:1360–8. doi: 10.1016/j.gie.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Stoffel EM, Erichsen R, Frøslev T, et al. Clinical and Molecular Characteristics of Post- Colonoscopy Colorectal Cancer: A Population-based Study. Gastroenterology. 2016;151:870–78. doi: 10.1053/j.gastro.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology. 2010;138:828–33. doi: 10.1053/j.gastro.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsiung PL, Hardy J, Friedland S, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008;14:454–8. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazewinkel Y, López-Cerón M, East JE, et al. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916–24. doi: 10.1016/j.gie.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Murakami T, Sakamoto N, Yao T. Sessile serrated adenoma/polyp with cytological dysplasia diagnosed accurately by magnifying chromoendoscopy. Dig Endosc. 2016;28:98. doi: 10.1111/den.12540. [DOI] [PubMed] [Google Scholar]
- 37.Yamada M, Sakamoto T, Otake Y, et al. Investigating endoscopic features of sessile serrated adenomas/polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc. 2015;82:108–17. doi: 10.1016/j.gie.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Yamashina T, Takeuchi Y, Uedo N, et al. Diagnostic features of sessile serrated adenoma/polyps on magnifying narrow band imaging: a prospective study of diagnostic accuracy. J Gastroenterol Hepatol. 2015;30:117–23. doi: 10.1111/jgh.12688. [DOI] [PubMed] [Google Scholar]
- 39.Boparai KS, van den Broek FJ, van Eeden S, et al. Hyperplastic polyposis syndrome: a pilot study for the differentiation of polyps by using high-resolution endoscopy, autofluorescence imaging, and narrow-band imaging. Gastrointest Endosc. 2009;70:947–55. doi: 10.1016/j.gie.2009.03.1172. [DOI] [PubMed] [Google Scholar]
- 40.Elayadi AN, Samli KN, Prudkin L, et al. A peptide selected by biopanning identifies the integrin αvβ6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007;67:5889–95. doi: 10.1158/0008-5472.CAN-07-0245. [DOI] [PubMed] [Google Scholar]
- 41.Laine L, Kaltenbach T, Barkun A, et al. SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489–501. doi: 10.1016/j.gie.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Joshi BP, Miller SJ, Lee CM, et al. Multispectral endoscopic imaging of colorectal dysplasia in vivo. Gastroenterology. 2012;143:1435–7. doi: 10.1053/j.gastro.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang LC, Shun CT, Hsu WF, et al. Fecal Immunochemical Test Detects Sessile Serrated Adenomas and Polyps With a Low Level of Sensitivity. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.07.029. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.