Abstract
Purpose
Musculoskeletal events (MEs) resulting from breast cancer treatment can significantly interfere with the quality of life (QOL) of older adults. We evaluated the incidence of MEs in women 65 years and older who had surgery and adjuvant chemotherapy for breast cancer, and the impact of treatment on MEs and arm function.
Patients and Methods
Patient-reported data in Alliance/CALGB 49907 were collected using the EORTC QLQ-BR23 and physician-reported adverse events (AEs) to characterize self-reported MEs and incidence of lymphedema. EORTC QLQ-BR23 items related to musculoskeletal events were analyzed in this study and data collected at study entry (post-operative) and 12 and 24 months post entry.
Results
Lymphedema, arm function and ME data were available for 321 patients. One or more MEs were reported by 87% (median number=3) and 64% (median number=1) of patients post-operatively and at 24 months. At 24 months 2% had persistence of six MEs. Seventy-four percent experienced at least ≥3/6 types of MEs over the 24-month period. Detection of lymphedema at any time during the study was noted in 7.5% of the patients and appeared to be associated with type of chemotherapy given: CMF 16.4%, capecitabine 5.8% and AC 4%. Mastectomy and axillary node dissection were associated with the most MEs. LROM correlated with poorer arm function at all time periods.
Conclusion
Potentially debilitating MEs occur in three-fourths of elderly women undergoing standard therapy for breast cancer. Emphasis should be placed on prevention, identification and treatment of these MEs to improve QOL.
Keywords: Breast Cancer, Elderly, Musculoskeletal Events, Lymphedema
Introduction
Musculoskeletal symptoms in older patients treated for potentially curable breast cancer occur frequently, but we have little data on self-reported symptoms and their effects on function and quality of life. This analysis seeks to address these gaps. Common signs and symptoms include lymphedema[1, 2], self-reported arm or hand swelling[3], breast pain [4, 5], breast sensitivity[5, 6], pain in the arm/shoulder[3] and limitations in range of motion (LROM)[3, 6]. The incidences of these symptoms vary and depend on the type of surgery [1, 2, 7], receipt of radiation therapy [1, 2], whether chemotherapy was administered, the type of chemotherapy [7, 8, 9] and whether patients received aromatase inhibitors.
Lymphedema is of major concern with a meta-analysis of 72 studies showing a 17% risk of lymphedema after therapy [10]. At least 20% of women who receive therapy for breast cancer experience arm/shoulder pain and 66% experience breast/chest wall pain [5, 6]. A survey of patients of all ages at varying time intervals from original therapy revealed persistent operative site pain in 35% of 730 patients who underwent breast-conserving surgery (BCS) versus 30% who underwent mastectomy. Persistent arm swelling was self-reported in 14% who underwent BCS and 26% who underwent mastectomy, and LROM was reported in 32% having BCS versus 47% with mastectomy [7].
In older women, musculoskeletal problems during and after treatment might have more profound effects on physical function and quality of life (QOL) than in younger women and such complications could impact an individual’s ability to live independently. Cancer and Leukemia Group B (CALGB)/Alliance trial 49907 was a prospective randomized adjuvant trial of standard chemotherapy (doxorubicin and cyclophosphamide [AC] or cyclophosphamide, methotrexate, and fluorouracil [CMF]) versus capecitabine in women 65 and older with Stage I to III breast cancer [11]. This study provides a unique opportunity for the prospective analysis of LE and self-reported musculoskeletal events and their effect on function and quality of life in this older population of breast cancer patients.
Methods
Alliance study 171302 is a secondary analysis of CALGB/Alliance 49907, the details of which have been previously published [11]. Three hundred twenty-one of the CALGB 49907 patients were also enrolled in a QOL companion trial and as previously described were quite similar in demographic and other characteristics to the overall group; professionally-assessed lymphedema and patient self-assessed QOL data form the basis of this analysis [12]. For this study we matched the lymphedema adverse event (AE) data with the 321 patients who provided QOL data. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Lymphedema AE data were graded per Common Toxicity Criteria version 2 [13] during follow-up visits with the physician and the maximum physician-reported lymphedema grade experienced by each patient was recorded. Grade 0 Lymphedema indicates no lymphedema. Grade 1 indicates mild lymphedema and grade 2 indicates moderate lymphedema requiring compression. Six patient-reported musculoskeletal events (MEs) from the EORTC QLQ-BR23 were utilized in this study: 1) pain in the arm/shoulder, 2) arm/hand swelling, 3) difficulty in raising arm or moving it sideways (limited range of motion, LROM), 4) breast pain in the affected breast, 5) breast swelling in the affected breast, and 6) oversensitivity in the affected breast. Patients reported on severity of these symptoms with the following descriptors: 1) not at all, 2) a little, 3) quite a bit, or 4) very much. Patients completed the assessments at three time points: study baseline (within 84 days following surgery), 12 months from baseline, and 24 months from baseline. Patients with mastectomy and BCS completed the same questionnaires; we considered breast pain, breast sensitivity and breast swelling as referring to chest wall symptoms in patients who underwent mastectomy. One of the questions asked of participants at all 3 time points was their ability to lift a shopping bag or suitcase. We felt this question was one assessment of arm function.
We performed an analysis of physician-reported lymphedema and patient-reported data to determine the incidence of lymphedema and the six MEs experienced (i.e., pain in arms/shoulder, arm/hand swelling, LROM, and pain, swelling, and sensitivity in the affected breast). The relationship of the most extensive surgery (BCS or mastectomy), axillary surgery, receipt of radiation, number of examined nodes, and chemotherapy type (AC, CMF, or capecitabine) to lymphedema and the six patient-reported MEs was also assessed. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on November 19, 2015.Patients were dichotomized for each of the seven endpoints described above, (lymphedema AE and the six MEs) in two ways: (1) any event: at least one ≥ grade 1 lymphedema event (vs. no lymphedema events) or an answer of “a little” or worse (vs. “none”) for each of the six MEs and (2) severe: at least one ≥ grade 3 lymphedema event (vs. <grade 3 lymphedema events) or an answer of “quite a bit” or “very much” (vs. “none” or “a little”) for each of the six MEs.
Patients who enrolled into Alliance/CALGB 49907 and who answered at least one of the six MEs listed above at baseline were included in these analyses (N=321). Although not all of the 321 patients provided answers for all six MEs being assessed (as noted in the figures and tables), all available data were analyzed for associations, which led to different sample sizes when comparing variables at a given time point.
Chi‐squared tests [14] were used to determine if a given event happened at a higher or lower rate was associated with variables measured at baseline (most extensive surgery, axillary dissection, radiation, number of nodes examined, and chemotherapy type). These tests were performed at 3 time points: post-operative study entry and 12 and 24 months post study entry. Since any results will be viewed as hypothesis generating in general, all available data were used for each analysis and no adjustments were made for multiple comparisons.
Results
Of the 612 patients who participated in Alliance/CALGB 49907, both self-reported ME outcomes and physician-reported lymphedema (LE) data were available for 321 patients. These same patients also answered the question of whether or not they could lift a shopping bag or suitcase. The mean age was 72 years and 87% were Caucasian. Fifty-five percent underwent mastectomy and 45% BCS. Twenty percent had only a sentinel lymph node (SLN) biopsy and 80% had an axillary lymph node dissection (ALND). Fifty-four percent received radiation therapy (RT) and per protocol all received chemotherapy (48% capecitabine, 31% AC and 21% CMF) [Table 1].
Table 1.
Baseline Patient Characteristics
| Characteristic | Patients with Quality of Life information |
|---|---|
|
| |
| Age (years) | N=321 |
| Mean (SD) | 72.0 (4.8) |
| Age 65-69 | 129 (40.2%) |
| Age 70-79 | 179 (55.8%) |
| Age 80+ | 13 (4.1%) |
|
| |
| Gender | |
| Female | 321 (100%) |
|
| |
| Race | N=320 |
| White | 278 (86.9%) |
| Black | 35 (10.9%) |
| Other | 7 (2.2%) |
|
| |
| Most Extensive Surgery | N=319 |
| Breast Conservation Surgery | 143 (44.8%) |
| Mastectomy | 176 (55.2%) |
|
| |
| Axillary Dissection | N=321 |
| Yes | 256 (79.8%) |
| No | 65 (20.2%) |
|
| |
| Radiation | N=300 |
| Yes | 161 (53.7%) |
| No | 139 (46.3%) |
|
| |
| Number of Examined Nodes | N=314 |
| Mean (SD) | 11.8 (7.3) |
| Range | 0-32 |
|
| |
| Chemotherapy Type | N=321 |
| AC (Doxorubicin and Cyclophosphamide) | 99 (30.8%) |
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) | 67 (20.9%) |
| Capecitabine | 155 (48.3%) |
Eight-seven percent had at least one self-reported ME at study baseline (within 84 days post-operative) and the median number of MEs at baseline was 3. By 12 months, 72% had persistence of at least one ME symptom and 64% had at least one lingering ME symptom at 24 months [Table 2].
Table 2.
Patient-reported musculoskeletal events (MEs) over time
| Number of patient-reported MEs experienced |
Post-operative (N=321) |
12 months (N=259) |
24 months (N=240) |
|---|---|---|---|
|
| |||
| Mean (Median) | 3.0 (3) | 1.7 (2) | 1.6 (1) |
|
| |||
| None | 43 (13.4%) | 72 (27.8%) | 86 (35.8%) |
| 1 | 31 (9.7%) | 38 (14.7%) | 40 (16.7%) |
| 2 | 50 (15.6%) | 58 (22.4%) | 46 (19.2%) |
| 3 | 68 (21.2%) | 34 (13.1%) | 39 (16.3%) |
| 4 | 52 (16.2%) | 30 (11.6%) | 13 (5.4%) |
| 5 | 44 (13.7%) | 20 (7.7%) | 12 (5.0%) |
| 6 | 33 (10.3%) | 7 (2.7%) | 4 (1.7%) |
Over this same 24-month period, 7.5% of these 321 women developed grade 1 or grade 2 LE [Table 3]. LE incidence was associated with type of chemotherapy received: CMF (13.4% grade 1 and 3.0% grade 2), capecitabine (4.5% grade 1 and 1.3% grade 2) and AC (4.0% grade 1 and 0% grade 2) [p=0.007]. There was no association between mastectomy or BCS and LE incidence (p=0.453) nor any association with receipt of RT or not (p=0.308) and lymphedema incidence. Performance of an ALND or not and removal of ≥ 8 ALNs was however associated with the development of LE (p=0.042 and p=0.023, respectively). If no ALND was performed, only 1.5% developed grade 1 LE but if an ALND was done, 7.4% developed grade1 LE and 1.6% grade 2 (p=0.042). If 8 or more ALN were removed, 8.5% developed grade 1 LE and 1.9% grade 2 [Table 3].
Table 3.
Maximum grade of physician-reported lymphedema during the 24-month reporting period.
| Lymphedema Severity | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| None | Grade 1 | Grade 2 | Grade 3 | p-value (any) | p-value (grade 3+) | |
|
| ||||||
| For all patients (N=321) | 297 (92.5%) | 20 (6.2%) | 4 (1.3%) | 0 (0%) | ||
|
| ||||||
| Vs treatment arm (N=321) | ||||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=67) | 56 (88.6%) | 9 (13.4%) | 2 (3.0%) | 0 (0%) | 0.007 | NA |
| AC (Doxorubicin and Cyclophosphamide) (N=99) | 95 (96.0%) | 4 (4.0%) | 0 (0%) | 0 (0%) | ||
| Capecitabine (N=155) | 146 (94.2%) | 7 (4.5%) | 2 (1.3%) | 0 (0%) | ||
|
| ||||||
| Vs Most Extensive Surgery (N=319) | ||||||
| Breast Conserving Surgery (N=143) | 134 (93.7%) | 8 (5.6%) | 1 (0.7%) | 0 (0%) | 0.453 | NA |
| Full Mastectomy (N=176) | 161 (91.5%) | 12 (6.8%) | 3 (1.7%) | 0 (0%) | ||
|
| ||||||
| Vs Axillary Dissection (N=321) | ||||||
| No (N=65) | 64 (98.5%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 0.042 | NA |
| Yes (N=256) | 233 (91.0%) | 19 (7.4%) | 4 (1.6%) | 0 (0%) | ||
|
| ||||||
| Vs Radiation Therapy (N=300) | ||||||
| No (N=139) | 126 (90.7%) | 11 (7.9%) | 2 (1.4%) | 0 (0%) | 0.308 | NA |
| Yes (N=161) | 151 (93.8%) | 9 (5.6%) | 1 (0.6%) | 0 (0%) | ||
|
| ||||||
| Vs Number of Nodes Examined (N=314) | ||||||
| 0-3 (N=49) | 47 (95.9%) | 2 (4.1%) | 0 (0%) | 0 (0%) | 0.023 | NA |
| 4-7 (N=58) | 53 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| 8+ (N=212) | 190 (89.6%) | 18 (8.5%) | 4 (1.9%) | 0 (0%) | ||
Patient-reported arm/hand swelling at any time point post-surgery was significantly related to health care professional reported LE with p values from <0.001 to 0.003. Despite that association, only about 20% of women who reported arm/hand swelling over the 24-month period of the study developed sufficient LE to be noted by a healthcare professional. Conversely, 80% of women reporting arm/hand swelling were not noted to have enough swelling by healthcare professionals to diagnose LE [Table 4]. There was little change in arm/hand swelling over time from post-operative baseline, 12 months, and 24 months: 22%, 21%, and 20%, respectively [Figure 1]. Arm and hand swelling did not affect arm function (ability to lift a shopping bag or suitcase) at any of the measured periods (p=0.260, p=0.136, and p=0.060) [Table 5]. Presence of lymphedema did not affect arm function either (p=0.716) [Data not shown].
Table 4.
Lymphedema reported as an adverse event (AE) vs patient-reported swelling of arm and hand.
| Time Frame (Patient Reported) | Lymphedema (AE)
|
p-value | |
|---|---|---|---|
| Yes | No | ||
|
| |||
| Arm/Hand Swelling at Post-operative (N=321) | <0.001 | ||
| Yes | 13 (18.3%) | 58 (81.7%) | |
| No | 11 (4.4%) | 239 (95.6%) | |
|
| |||
| Arm/Hand Swelling at 12 mo Post Op (N=259) | 0.003 | ||
| Yes | 10 (18.5%) | 44 (81.5%) | |
| No | 12 (5.9%) | 193 (94.1%) | |
|
| |||
| Arm/Hand Swelling at 24 mo Post Op (N=240) | <0.001 | ||
| Yes | 10 (21.3%) | 37 (78.7%) | |
| No | 6 (3.1%) | 187 (96.9%) | |
Figure 1.
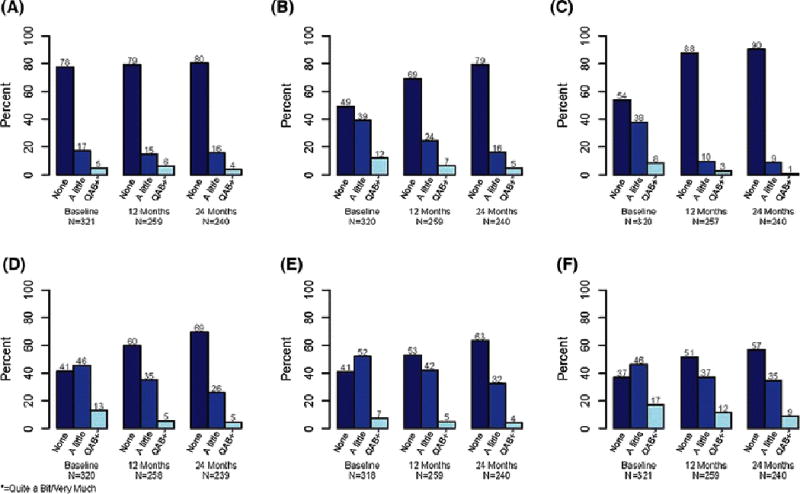
Severity of EORTC QLQ-BR23 musculoskeletal events at baseline, 12 months, and 24 months in: arm/hand swelling (A), limited range of motion (B), breast swelling (C), breast sensitivity (D), breast pain, (E), and arm/shoulder pain (F).
Table 5.
Arm function versus musculoskeletal events over time*
| Rates with symptom | Post-operative | 12 months | 24 months |
|---|---|---|---|
|
| |||
| Vs LROM* | p<0.001 | p=0.002 | p<0.001 |
| Poor Arm Function | 111/186 (60%) | 57/143 (39%) | 38/133 (29%) |
| Normal Arm Function | 51/130 (39%) | 24/113 (21%) | 11/105 (10%) |
|
| |||
| Vs Breast Sensitivity | p=0.004 | p=0.038 | p=0.164 |
| Poor Arm Function | 122/186 (66%) | 69/142 (46%) | 45/133 (34%) |
| Normal Arm Function | 64/130 (49%) | 38/113 (34%) | 27/105 (26%) |
|
| |||
| Vs Breast Swelling | p=0.352 | p=0.018 | p=0.343 |
| Poor Arm Function | 90/186 (48%) | 24/141 (17%) | 15/133 (11%) |
| Normal Arm Function | 56/130 (43%) | 8/113 (7%) | 8/105 (8%) |
|
| |||
| Vs Arm/Shoulder Pain | p=0.078 | p=0.005 | p=0.001 |
| Poor Arm Function | 126/187 (67%) | 81/143 (57%) | 70/133 (53%) |
| Normal Arm Function | 75/130 (58%) | 44/113 (39%) | 33/105 (31%) |
|
| |||
| Vs Breast Pain | p<0.001 | p=0.266 | p=0.065 |
| Poor Arm Function | 126/186 (68%) | 72/143 (50%) | 56/133 (42%) |
| Normal Arm Function | 60/128 (47%) | 49/113 (44%) | 32/105 (30%) |
|
| |||
| Vs Arm/Hand Swelling | P=0.260 | p=0.136 | p=0.060 |
| Poor Arm Function | 46/187 (25%) | 35/143 (24%) | 32/133 (24%) |
| Normal Arm Function | 25/130 (19%) | 19/113 (17%) | 15/105 (14%) |
For example, at the post-operative time point, patients with poor arm function at baseline had limited range of motion (LROM) 60% of the time compared to 39% for those with normal arm function
Limited range of motion as defined by difficulty in raising arm or moving it sideways did interfere with arm function at all measured time intervals: post-operative baseline p<0.001; 12 months p=0.002; and 24 months p<0.001. Limited range of motion fell from 51% at baseline to 31% at 12 months and 21% by 24 months [Figure 1]. Breast sensitivity interfered with arm function in the post-operative (p=0.004) and 12-month (p=0.038) periods but resolved by 24 months (p=0.164). Breast sensitivity was present in 59% of women post-operatively and declined to 40% at 12 months but persisted in 31% at 24 months [Figure 1]. Breast swelling also interfered with arm function at 12 months (p=0.018) but surprisingly did not interfere in the post-operative or 24-month period: p=0.352 and p=0.343. Breast swelling was present in 46% of participants in the post-operative period and dropped to 12% by 12 months and 10% by 24 months [Figure 1]. Arm and shoulder pain significantly interfered with arm function at all three measured intervals: p=0.078 post-operatively, p=0.005 at 12 months, and p=0.001 at 24 months. Arm and shoulder pain was seen in 63% at baseline, 49% at 12 months and 43% at 24 months [Figure 1]. Women with this post-operative arm/shoulder pain also experienced significant breast pain with an incidence of 57% at 12 months and 44% at 24 months (p<0.001 and p=0.002) respectively (Appendix Table 7). Breast pain only interfered with arm function in the post-operative period (p<0.001). Similarly breast pain was also present at 59% at baseline, 47% at 12 months and 37% at 24 months.
Those that underwent an axillary dissection or had ≥ 8 axillary lymph nodes removed had an increased risk of arm/shoulder pain in the post-operative period with a 43% incidence (p<0.001) and 71% incidence (p<0.001) respectively. This association resolved by 12 months (Appendix Table 6).
Discussion
This study in older women with early stage breast cancer with prospectively collected data on professionally assessed lymphedema and self-reported musculoskeletal events is one of the few to address these complications in this age group, to evaluate these symptoms over time, and to correlate these symptom changes with function. The persistence of self-reported arm/hand swelling, limited range of motion and arm/shoulder pain at 24 months in a quarter or more of these older women is substantial and threatens their ability to live independently. LE is a major complication of surgery and radiation and our physician-reported LE incidence of 7.5% at any time over 24 months compared poorly to the incidence of patient-reported post-operative arm/hand swelling of 20% at 24 months. This discrepancy confirms current literature that shows that the incidence of treatment side-effects is higher when patient-reported compared to provider-reported, and may explain the large variability in the incidence of lymphedema that has been previously reported (0-56%) [15]. A recent review showed that patient-reported outcomes were indeed more accurate than provider adverse event reporting [16]. Part of the difference may be explained by our study’s reliance on provider and not volumetric LE assessment, further underestimating true incidence.
Limited range of motion and arm/shoulder pain, as expected, interfered with arm function at all measured time intervals, while neither arm/hand swelling nor LE did. Our 63% incidence of arm/shoulder pain at baseline in older women is similar to another study that showed an incidence of 60% in breast cancer survivors of all ages [17]. This latter study revealed a 67% incidence of decreased upper body strength while we found 60% of our patients had LROM and poor arm function (as defined by ability to lift a suitcase or shopping bag). Surprisingly, limited range of motion was not associated with lymphedema in our study [data not shown] in contradistinction to others [18]. For the elderly this may convert those living independently into those requiring assistance.
ALND and removal of ≥8 ALN as compared to breast-conserving surgery or mastectomy were associated with greater arm/hand swelling (see Appendix). Our findings regarding ALND versus not and removal of ≥8 ALN and their association with lymphedema, self-reported arm/hand swelling, limited range of motion, breast pain/sensitivity, and pain in arm/shoulder are similar to that seen in American College of Surgeons Oncology Group (Alliance) Z0011 [19] (ALND versus no ALND in women with invasive breast cancer and sentinel lymph node metastasis) and National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 [20, 21] (SLN versus ALND in clinically node negative breast cancer). For older women, our data and that of others suggest that the surgical approach of choice, if possible, is a lumpectomy and sentinel lymph node (SLN) biopsy [22].
Breast/chest wall swelling was commonly reported post-operatively but almost completely resolved by 24 months. It was more commonly reported in patients receiving radiation treatment, as was breast swelling and breast sensitivity, but this association was lost by 24 months. These findings may represent lymphedema of the breast and their resolution with time should be reassuring to clinicians and older women with these symptoms. The incidence of breast or chest wall pain in our elderly patients post-operatively, at 12 months, and at 24 months of 59%, 47% and 36%, respectively, was somewhat dissimilar to an analysis in breast cancer patients of all ages of 68% at 6 months6 and 66% at 12 months [23], and to another analysis that revealed persistent pain in 33% at 12 months [5]. Our data and that of others confirm that pain diminishes significantly over time. We also found that post-operative pain in the arm/shoulder was highly associated with continuing breast/chest wall pain and was increased with more extensive axillary surgery (data not shown). Limiting axillary surgery to sentinel lymph node biopsy or omitting it unless it will change treatment planning or outcomes, should help minimize these symptoms.
This study has several limitations. All patients were in good health and capable of participating in a clinical trial; therefore, these patients may differ from other older early stage breast cancer patients in the general population and our findings might underestimate the true frequency of musculoskeletal symptoms. Moreover, only one-half of the study population participated in the QOL portion of this study and not all ME data were available for each time point. We did not have baseline data on musculoskeletal events prior to surgery, nor what interventions or medications were implemented for these symptoms either before or after surgery and whether they affected the improvement in symptoms that were noted over time. Conversely, this is one of the few studies that prospectively captured these LE and ME complications in elderly patients on a clinical trial.
Overall, our data are reassuring and suggest that the frequency of major musculoskeletal symptoms in an older patient population participating in a clinical trial is substantial but dramatically decreases with time. However, for many patients these symptoms are quite bothersome and affect quality of life. Oncologists should query older patients concerning musculoskeletal symptoms and refer patients for interventions when appropriate. In addition, these data should be shared with patients and families to optimize treatment planning.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA032291, U10CA047559, U10CA047577, U10CA077597, U10CA077651, U10CA180791, U10CA180838, U10CA180857, and U10CA180867. Supported, in part, by grants from the National Cancer Institute (U10CA031946 and U10CA033601), the National Institute on Aging (U10CA85850), the Breast Cancer Research Foundation, the Coalition of Cancer Cooperative Groups, and Roche Biomedical Laboratories. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
Table 1.
Arm/Hand Swelling
| None | A little | Quite a bit | Very Much | p-value (little+) | p-value (quite a bit+) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| POST OP | Overall ARM/HAND SWELLING (N=321) | 250 (77.9%) | 56 (17.4%) | 11 (3.4%) | 4 (1.2%) | ||
|
| |||||||
| Vs Arm (N=321) | 0.572 | 0.720 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=67) | 49 (73.1%) | 15 (22.4%) | 2 (3.0%) | 1 (1.5%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=99) | 78 (78.8%) | 15 (15.2%) | 4 (4.0%) | 2 (2.0%) | |||
| Capecitabine (N=155) | 123 (79.4%) | 26 (16.8%) | 5 (3.2%) | 1 (0.6%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=319) | 0.621 | 0.359 | |||||
| Breast Conserving Surgery (N=143) | 113 (79.0%) | 25 (17.5%) | 4 (2.8%) | 1 (0.7%) | |||
| Full Mastectomy (N=176) | 135 (76.7%) | 31 (17.6%) | 7 (4.0%) | 3 (1.7%) | |||
|
| |||||||
| Vs Axillary Dissection (N=321) | <0.001 | 0.046 | |||||
| No (N=65) | 62 (95.4%) | 3 (4.6%) | 0 (0%) | 0 (0%) | |||
| Yes (N=256) | 188 (73.4%) | 53 (20.7%) | 11 (4.3%) | 4 (1.6%) | |||
|
| |||||||
| Vs RT (N=300) | 0.570 | 0.789 | |||||
| No (N=139) | 110 (79.1%) | 23 (16.6%) | 4 (2.9%) | 2 (1.4%) | |||
| Yes (N=161) | 123 (76.4%) | 30 (18.6%) | 6 (3.7%) | 2 (1.2%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=314) | <0.001 | 0.083 | |||||
| 0-3 (N=49) | 45 (91.8%) | 4 (8.2%) | 0 (0%) | 0 (0%) | |||
| 4-7 (N=53) | 49 (92.5%) | 3 (5.7%) | 1 (1.9%) | 0 (0%) | |||
| 8+ (N=212) | 149 (70.3%) | 49 (23.1%) | 10 (4.7%) | 4 (1.9%) | |||
|
| |||||||
| AT 12 MONTHS | Overall ARM/HAND SWELLING (N=259) | 205 (79.2%) | 38 (14.7%) | 12 (4.6%) | 4 (1.5%) | ||
|
| |||||||
| Vs Arm (N=259) | 0.957 | 0.226 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=54) | 43 (79.6%) | 9 (16.7%) | 2 (3.7%) | 0 (0%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=82) | 64 (78.1%) | 10 (12.2%) | 6 (7.3%) | 2 (2.4%) | |||
| Capecitabine (N=123) | 98 (79.7%) | 19 (15.4%) | 4 (3.3%) | 2 (1.6%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=257) | 0.968 | 0.929 | |||||
| Breast Conserving Surgery (N=117) | 93 (79.5%) | 17 (14.5%) | 5 (4.3%) | 2 (1.7%) | |||
| Full Mastectomy (N=140) | 111 (79.3%) | 20 (14.3%) | 7 (5.0%) | 2 (1.4%) | |||
|
| |||||||
| Vs Axillary Dissection (N=259) | 0.320 | 0.628 | |||||
| No (N=56) | 47 (83.9%) | 5 (8.9%) | 3 (5.4%) | 1 (1.8%) | |||
| Yes (N=203) | 158 (77.8%) | 33 (16.3%) | 9 (4.4%) | 3 (1.5%) | |||
|
| |||||||
| Vs RT (N=245) | 0.368 | 0.415 | |||||
| No (N=109) | 90 (82.6%) | 14 (12.8%) | 5 (4.6%) | 0 (0%) | |||
| Yes (N=136) | 106 (77.9%) | 21 (15.4%) | 6 (4.4%) | 3 (2.2%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=254) | 0.479 | 0.582 | |||||
| 0-3 (N=44) | 37 (84.1%) | 4 (9.1%) | 3 (6.8%) | 0 (0%) | |||
| 4-7 (N=39) | 32 (82.1%) | 3 (7.7%) | 1 (2.6%) | 3 (7.7%) | |||
| 8+ (N=171) | 131 (76.6%) | 31 (18.1%) | 8 (4.7%) | 1 (0.6%) | |||
|
| |||||||
| AT 24 MONTHS | Overall ARM/HAND SWELLING (N=240) | 193 (80.4%) | 38 (15.8%) | 8 (3.3%) | 1 (0.4%) | ||
|
| |||||||
| Vs Arm (N=240) | 0.116 | 0.211 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=49) | 36 (73.5%) | 9 (18.4%) | 3 (6.1%) | 1 (2.0%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=83) | 64 (77.1%) | 17 (20.5%) | 2 (2.4%) | 0 (0%) | |||
| Capecitabine (N=108) | 93 (86.1%) | 12 (11.1%) | 3 (2.8%) | 0 (0%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=238) | 0.202 | 0.039 | |||||
| Breast Conserving Surgery (N=108) | 91 (84.3%) | 16 (14.8%) | 1 (0.9%) | 0 (0%) | |||
| Full Mastectomy (N=130) | 101 (77.7%) | 21 (16.2%) | 7 (5.3%) | 1 (0.8%) | |||
|
| |||||||
| Vs Axillary Dissection (N=240) | 0.209 | 0.489 | |||||
| No (N=52) | 45 (86.5%) | 6 (11.5%) | 1 (1.9%) | 0 (0%) | |||
| Yes (N=188) | 148 (77.7%) | 32 (17.0%) | 7 (3.7%) | 1 (0.5%) | |||
|
| |||||||
| Vs RT (N=226) | 0.522 | 0.833 | |||||
| No (N=100) | 82 (82.0%) | 14 (14.0%) | 4 (4.0%) | 0 (0%) | |||
| Yes (N=126) | 99 (78.6%) | 23 (18.3%) | 3 (2.4%) | 1 (0.8%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=236) | 0.079 | 0.406 | |||||
| 0-3 (N=41) | 38 (92.7%) | 2 (4.9%) | 1 (2.4%) | 0 (0%) | |||
| 4-7 (N=39) | 31 (79.5%) | 5 (12.8%) | 2 (5.1%) | 1 (2.6%) | |||
| 8+ (N=156) | 120 (76.9%) | 31 (19.9%) | 5 (3.2%) | 0 (0%) | |||
Table 2.
Limited Range of Motion
| None | A little | Quite a bit | Very Much | p-value (little+) | p-value (quite a bit+) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| POST OP | Overall LROM (N=320) | 157 (49.1%) | 125 (39.1%) | 31 (9.7%) | 7 (2.2%) | ||
|
| |||||||
| Vs Arm (N=320) | 0.929 | 0.698 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=67) | 33 (49.3%) | 28 (41.8%) | 5 (7.5%) | 1 (1.5%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=99) | 50 (50.5%) | 36 (36.4%) | 11 (11.1%) | 2 (2.0%) | |||
| Capecitabine (N=154) | 74 (48.1%) | 61 (39.6%) | 15 (9.7%) | 4 (2.6%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=318) | 0.034 | 0.480 | |||||
| Breast Conserving Surgery (N=143) | 80 (55.9%) | 48 (33.6%) | 14 (9.8%) | 1 (0.7%) | |||
| Full Mastectomy (N=175) | 77 (44.0%) | 75 (42.9%) | 17 (9.7%) | 6 (3.4%) | |||
|
| |||||||
| Vs Axillary Dissection (N=320) | <0.001 | 0.466 | |||||
| No (N=65) | 48 (73.8%) | 11 (16.9%) | 6 (9.2%) | 0 (0%) | |||
| Yes (N=255) | 109 (42.7%) | 114 (44.7%) | 25 (9.8%) | 7 (2.7%) | |||
|
| |||||||
| Vs RT (N=299) | 0.668 | 0.938 | |||||
| No (N=138) | 66 (47.8%) | 56 (40.6%) | 14 (10.1%) | 2 (1.4%) | |||
| Yes (N=161) | 81 (50.3%) | 61 (37.9%) | 16 (9.9%) | 3 (1.9%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=313) | <0.001 | 0.871 | |||||
| 0-3 (N=49) | 35 (71.4%) | 9 (18.4%) | 5 (10.2%) | 0 (0%) | |||
| 4-7 (N=53) | 32 (60.4%) | 15 (28.3%) | 6 (11.3%) | 0 (0%) | |||
| 8+ (N=211) | 85 (40.3%) | 99 (46.9%) | 20 (9.5%) | 7 (3.3%) | |||
|
| |||||||
| AT 12 MONTHS | Overall LROM (N=259) | 179 (69.1%) | 63 (24.3%) | 14 (5.4%) | 3 (1.2%) | ||
|
| |||||||
| Vs Arm (N=259) | 0.779 | 0.666 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=54) | 36 (66.7%) | 15 (27.8%) | 2 (3.7%) | 1 (1.9%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=82) | 59 (72.0%) | 19 (23.2%) | 4 (4.9%) | 0 (0%) | |||
| Capecitabine (N=123) | 84 (68.3%) | 29 (23.6%) | 8 (6.5%) | 2 (1.6%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=257) | 0.339 | 0.417 | |||||
| Breast Conserving Surgery (N=117) | 85 (72.6%) | 26 (22.2%) | 5 (4.3%) | 1 (0.9%) | |||
| Full Mastectomy (N=140) | 94 (67.1%) | 35 (25.0%) | 9 (6.4%) | 2 (1.4%) | |||
|
| |||||||
| Vs Axillary Dissection (N=259) | 0.160 | 0.730 | |||||
| No (N=56) | 43 (76.8%) | 9 (16.1%) | 4 (7.1%) | 0 (0%) | |||
| Yes (N=203) | 136 (67.0%) | 54 (26.6%) | 10 (4.9%) | 3 (1.4%) | |||
|
| |||||||
| Vs RT (N=245) | 0.796 | 0.467 | |||||
| No (N=109) | 77 (70.6%) | 26 (23.9%) | 5 (4.6%) | 1 (0.9%) | |||
| Yes (N=136) | 94 (69.1%) | 32 (23.5%) | 8 (5.9%) | 2 (1.5%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=254) | 0.536 | 0.904 | |||||
| 0-3 (N=44) | 33 (75.0%) | 9 (20.5%) | 2 (4.5%) | 0 (0%) | |||
| 4-7 (N=39) | 25 (64.1%) | 11 (28.2%) | 3 (7.7%) | 0 (0%) | |||
| 8+ (N=171) | 116 (67.8%) | 43 (25.1%) | 9 (5.3%) | 3 (1.8%) | |||
|
| |||||||
| AT 24 MONTHS | Overall LROM (N=240) | 190 (79.1%) | 39 (16.3%) | 8 (3.3%) | 3 (1.3%) | ||
|
| |||||||
| Vs Arm (N=240) | 0.096 | 0.120 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=49) | 35 (71.4%) | 10 (20.4%) | 3 (6.1%) | 1 (2.0%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=83) | 63 (75.9%) | 15 (18.1%) | 4 (4.8%) | 1 (1.2%) | |||
| Capecitabine (N=108) | 92 (85.2%) | 14 (13.0%) | 1 (0.9%) | 1 (0.9%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=238) | 0.028 | 0.234 | |||||
| Breast Conserving Surgery (N=108) | 93 (86.1%) | 12 (11.1%) | 3 (2.8%) | 0 (0%) | |||
| Full Mastectomy (N=130) | 97 (74.6%) | 25 (19.2%) | 5 (3.8%) | 3 (2.3%) | |||
|
| |||||||
| Vs Axillary Dissection (N=240) | 0.748 | 0.862 | |||||
| No (N=52) | 42 (80.8%) | 8 (15.4%) | 2 (3.8%) | 0 (0%) | |||
| Yes (N=188) | 148 (78.7%) | 31 (16.5%) | 6 (3.2%) | 3 (1.6%) | |||
|
| |||||||
| Vs RT (N=226) | 0.691 | 0.292 | |||||
| No (N=100) | 78 (78.0%) | 19 (19.0%) | 3 (3.0%) | 0 (0%) | |||
| Yes (N=126) | 101 (80.2%) | 18 (14.3%) | 5 (4.0%) | 2 (1.6%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=236) | 0.465 | 0.832 | |||||
| 0-3 (N=41) | 34 (82.9%) | 6 (14.6%) | 1 (2.4%) | 0 (0%) | |||
| 4-7 (N=39) | 33 (84.6%) | 4 (10.3%) | 0 (0%) | 2 (5.1%) | |||
| 8+ (N=156) | 120 (76.9%) | 28 (18.0%) | 7 (4.5%) | 1 (0.6%) | |||
Table 3.
Breast Swelling
| None | A little | Quite a bit | Very Much | p-value (little+) | p-value (quite a bit+) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| POST OP | Overall Breast Swelling (N=320) | 172 (53.8%) | 121 (37.8%) | 19 (5.9%) | 8 (2.5%) | ||
|
| |||||||
| Vs Arm (N=320) | 0.324 | 0.476 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=66) | 33 (50.0%) | 29 (43.9%) | 2 (3.0%) | 2 (3.0%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=99) | 49 (49.5%) | 43 (43.4%) | 4 (4.0%) | 3 (3.0%) | |||
| Capecitabine (N=155) | 90 (58.1%) | 49 (31.6%) | 13 (8.4%) | 3 (1.9%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=318) | 0.981 | 0.395 | |||||
| Breast Conserving Surgery (N=143) | 77 (53.8%) | 56 (39.2%) | 6 (4.2%) | 4 (2.8%) | |||
| Full Mastectomy (N=175) | 94 (53.7%) | 64 (36.6%) | 13 (7.4%) | 4 (2.3%) | |||
|
| |||||||
| Vs Axillary Dissection (N=320) | 0.091 | 0.463 | |||||
| No (N=65) | 41 (63.1%) | 20 (30.8%) | 2 (3.1%) | 2 (3.1%) | |||
| Yes (N=255) | 131 (51.4%) | 101 (39.6%) | 17 (6.7%) | 6 (2.4%) | |||
|
| |||||||
| Vs RT (N=300) | 0.029 | 0.507 | |||||
| No (N=139) | 84 (60.4%) | 45 (32.4%) | 9 (6.5%) | 1 (0.7%) | |||
| Yes (N=161) | 77 (47.8%) | 69 (42.9%) | 9 (5.6%) | 6 (3.7%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=313) | 0.065 | 0.562 | |||||
| 0-3 (N=49) | 33 (67.3%) | 13 (26.5%) | 1 (2.0%) | 2 (4.1%) | |||
| 4-7 (N=53) | 31 (58.5%) | 19 (35.8%) | 1 (1.9%) | 2 (3.8%) | |||
| 8+ (N=211) | 105 (49.8%) | 86 (40.8%) | 16 (7.6%) | 4 (1.9%) | |||
|
| |||||||
| AT 12 MONTHS | Overall Breast Swelling (N=257) | 225 (87.5%) | 25 (9.7%) | 5 (1.9%) | 2 (0.8%) | ||
|
| |||||||
| Vs Arm (N=257) | 0.764 | 0.452 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=54) | 48 (88.9%) | 5 (9.3%) | 0 (0%) | 1 (1.9%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=82) | 73 (89.0%) | 8 (9.8%) | 1 (1.2%) | 0 (0%) | |||
| Capecitabine (N=121) | 104 (86.0%) | 12 (9.9%) | 4 (3.3%) | 1 (0.8%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=255) | 0.329 | 0.028 | |||||
| Breast Conserving Surgery (N=115) | 98 (85.21%) | 11 (9.6%) | 5 (4.3%) | 1 (0.9%) | |||
| Full Mastectomy (N=140) | 125 (89.3%) | 14 (10.0%) | 0 (0%) | 1 (0.7%) | |||
|
| |||||||
| Vs Axillary Dissection (N=257) | 0.076 | 0.691 | |||||
| No (N=55) | 52 (94.5%) | 2 (3.6%) | 1 (1.8%) | 0 (0%) | |||
| Yes (N=202) | 173 (85.6%) | 23 (11.4%) | 4 (2.0%) | 2 (1.0%) | |||
|
| |||||||
| Vs RT (N=243) | 0.011 | 0.013 | |||||
| No (N=109) | 102 (93.6%) | 7 (6.4%) | 0 (0%) | 0 (0%) | |||
| Yes (N=134) | 111 (82.8%) | 16 (11.9%) | 5 (3.7%) | 2 (1.5%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=252) | 0.097 | 0.172 | |||||
| 0-3 (N=43) | 41 (95.3%) | 1 (2.3%) | 1 (2.3%) | 0 (0%) | |||
| 4-7 (N=39) | 31 (79.5%) | 5 (12.8%) | 1 (2.6%) | 2 (5.1%) | |||
| 8+ (N=170) | 148 (87.1%) | 19 (11.2%) | 3 (1.8%) | 0 (0%) | |||
|
| |||||||
| AT 24 MONTHS | Overall Breast Swelling (N=240) | 217 (90.4%) | 21 (8.8%) | 1 (0.4%) | 1 (0.4%) | ||
|
| |||||||
| Vs Arm (N=240) | 0.742 | 0.363 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=49) | 43 (87.8%) | 5 (10.2%) | 0 (0%) | 1 (2.0%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=83) | 75 (90.4%) | 7 (8.4%) | 1 (1.2%) | 0 (0%) | |||
| Capecitabine (N=108) | 99 (91.7%) | 9 (8.3%) | 0 (0%) | 0 (0%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=238) | 0.804 | 0.201 | |||||
| Breast Conserving Surgery (N=108) | 97 (89.8%) | 11 (10.2%) | 0 (0%) | 0 (0%) | |||
| Full Mastectomy (N=130) | 118 (90.8%) | 10 (7.7%) | 1 (0.8%) | 1 (0.8%) | |||
|
| |||||||
| Vs Axillary Dissection (N=240) | 0.112 | 0.475 | |||||
| No (N=52) | 50 (96.2%) | 2 (3.8%) | 0 (0%) | 0 (0%) | |||
| Yes (N=188) | 167 (88.8%) | 19 (10.1%) | 1 (0.5%) | 1 (0.5%) | |||
|
| |||||||
| Vs RT (N=226) | 0.551 | 0.919 | |||||
| No (N=100) | 92 (92.0%) | 7 (7.0%) | 1 (1.0%) | 0 (0%) | |||
| Yes (N=126) | 113 (89.7%) | 12 (9.5%) | 0 (0%) | 1 (0.8%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=236) | 0.509 | 0.425 | |||||
| 0-3 (N=41) | 39 (95.1%) | 2 (4.9%) | 0 (0%) | 0 (0%) | |||
| 4-7 (N=39) | 35 (89.7%) | 3 (7.7%) | 0 (0%) | 1 (2.6%) | |||
| 8+ (N=156) | 139 (89.1%) | 16 (10.3%) | 1 (0.6%) | 0 (0%) | |||
Table 4.
Breast Sensitivity
| None | A little | Quite a bit | Very Much | p-value (little+) | p-value (quite a bit+) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| POST OP | Overall Breast Sensitivity (N=320) | 132 (41.3%) | 146 (45.6%) | 33 (10.3%) | 9 (2.8%) | ||
|
| |||||||
| Vs Arm (N=320) | 0.126 | 0.447 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=66) | 20 (30.3%) | 38 (57.6%) | 6 (9.1%) | 2 (3.0%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=99) | 43 (43.4%) | 46 (46.5%) | 9 (9.1%) | 1 (1.0%) | |||
| Capecitabine (N=155) | 69 (44.5%) | 62 (40.0%) | 18 (11.6%) | 6 (3.9%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=318) | 0.176 | 0.530 | |||||
| Breast Conserving Surgery (N=143) | 53 (37.1%) | 73 (51.0%) | 14 (9.8%) | 3 (2.1%) | |||
| Full Mastectomy (N=175) | 78 (44.6%) | 72 (41.1%) | 19 (10.9%) | 6 (3.4%) | |||
|
| |||||||
| Vs Axillary Dissection (N=320) | 0.081 | 0.062 | |||||
| No (N=65) | 33 (50.8%) | 28 (43.1%) | 3 (4.6%) | 1 (1.5%) | |||
| Yes (N=255) | 99 (38.8%) | 118 (46.3%) | 30 (11.8%) | 8 (3.1%) | |||
|
| |||||||
| Vs RT (N=300) | 0.019 | 0.051 | |||||
| No (N=139) | 67 (48.2%) | 60 (43.2%) | 9 (6.5%) | 3 (2.2%) | |||
| Yes (N=161) | 56 (34.8%) | 79 (49.1%) | 22 (13.7%) | 4 (2.5%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=313) | 0.137 | 0.424 | |||||
| 0-3 (N=49) | 23 (46.9%) | 21 (42.9%) | 4 (8.2%) | 1 (2.0%) | |||
| 4-7 (N=53) | 27 (50.9%) | 21 (39.6%) | 3 (5.7%) | 2 (3.8%) | |||
| 8+ (N=211) | 79 (37.4%) | 100 (47.4%) | 26 (12.3%) | 6 (2.8%) | |||
|
| |||||||
| AT 12 MONTHS | Overall Breast Sensitivity (N=258) | 154 (59.7%) | 90 (34.9%) | 11 (4.3%) | 3 (1.2%) | ||
|
| |||||||
| Vs Arm (N=258) | 0.014 | 0.343 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=54) | 24 (44.4%) | 26 (48.2%) | 2 (3.7%) | 2 (3.7%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=82) | 57 (69.5%) | 23 (28.1%) | 2 (2.4%) | 0 (0%) | |||
| Capecitabine (N=122) | 73 (59.8%) | 41 (33.6%) | 7 (5.7%) | 1 (0.8%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=256) | <0.001 | 0.440 | |||||
| Breast Conserving Surgery (N=117) | 57 (48.7%) | 55 (47.0%) | 4 (3.4%) | 1 (0.9%) | |||
| Full Mastectomy (N=139) | 96 (69.1%) | 34 (24.5%) | 7 (5.0%) | 2 (1.4%) | |||
|
| |||||||
| Vs Axillary Dissection (N=258) | 0.043 | 0.043 | |||||
| No (N=56) | 40 (71.4%) | 16 (28.6%) | 0 (0%) | 0 (0%) | |||
| Yes (N=202) | 114 (56.4%) | 74 (36.6%) | 11 (5.4%) | 3 (1.5%) | |||
|
| |||||||
| Vs RT (N=244) | <0.001 | 0.643 | |||||
| No (N=109) | 84 (77.1%) | 20 (18.3%) | 5 (4.6%) | 0 (0%) | |||
| Yes (N=135) | 61 (45.2%) | 66 (48.9%) | 5 (3.7%) | 3 (2.2%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=253) | 0.725 | 0.201 | |||||
| 0-3 (N=44) | 27 (61.4%) | 17 (38.6%) | 0 (0%) | 0 (0%) | |||
| 4-7 (N=39) | 25 (64.1%) | 11 (28.2%) | 1 (2.6%) | 2 (5.1%) | |||
| 8+ (N=170) | 98 (57.6%) | 61 (35.9%) | 10 (5.9%) | 1 (0.6%) | |||
|
| |||||||
| AT 24 MONTHS | Overall Breast Sensitivity (N=239) | 166 (69.5%) | 62 (25.9%) | 8 (3.3%) | 3 (1.3%) | ||
|
| |||||||
| Vs Arm (N=239) | 0.140 | 0.724 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=49) | 32 (65.3%) | 15 (30.6%) | 1 (2.0%) | 1 (2.0%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=82) | 52 (63.4%) | 25 (30.5%) | 3 (3.7%) | 2 (2.4%) | |||
| Capecitabine (N=108) | 82 (75.9%) | 22 (20.4%) | 4 (3.7%) | 0 (0%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=237) | 0.320 | 0.103 | |||||
| Breast Conserving Surgery (N=107) | 78 (72.9%) | 27 (25.2%) | 2 (1.9%) | 0 (0%) | |||
| Full Mastectomy (N=130) | 87 (66.9%) | 35 (26.9%) | 5 (3.8%) | 3 (2.3%) | |||
|
| |||||||
| Vs Axillary Dissection (N=239) | 0.003 | 0.077 | |||||
| No (N=51) | 44 (86.3%) | 7 (13.7%) | 0 (0%) | 0 (0%) | |||
| Yes (N=188) | 122 (64.9%) | 55 (29.3%) | 8 (4.3%) | 3 (1.6%) | |||
|
| |||||||
| Vs RT (N=225) | 0.347 | 0.494 | |||||
| No (N=100) | 73 (73.0%) | 24 (24.0%) | 3 (3.0%) | 0 (0%) | |||
| Yes (N=125) | 84 (67.2%) | 365(28.0%) | 3 (2.4%) | 3 (2.4%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=235) | 0.293 | 0.756 | |||||
| 0-3 (N=41) | 29 (70.7%) | 11 (26.8%) | 1 (2.4%) | 0 (0%) | |||
| 4-7 (N=38) | 30 (79.0%) | 6 (15.8%) | 1 (2.6%) | 1 (2.6%) | |||
| 8+ (N=156) | 103 (66.0%) | 45 (28.9%) | 6 (3.8%) | 2 (1.3%) | |||
Table 5.
Breast Pain
| None | A little | Quite a bit | Very Much | p-value (little+) | p-value (quite a bit+) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| POST OP | Overall Breast Pain (N=318) | 130 (40.9%) | 165 (51.9%) | 21 (6.6%) | 2 (0.6%) | ||
|
| |||||||
| Vs Arm (N=318) | 0.364 | 0.863 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=65) | 22 (33.8%) | 38 (58.5%) | 4 (6.2%) | 1 (1.5%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=99) | 40 (40.4%) | 53 (53.5%) | 5 (5.1%) | 1 (1.0%) | |||
| Capecitabine (N=154) | 68 (44.2%) | 74 (48.1%) | 12 (7.8%) | 0 (0%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=316) | 0.031 | 0.540 | |||||
| Breast Conserving Surgery (N=143) | 49 (34.3%) | 85 (59.4%) | 9 (6.3%) | 0 (0%) | |||
| Full Mastectomy (N=173) | 80 (46.2%) | 79 (45.7%) | 12 (6.9%) | 2 (1.2%) | |||
|
| |||||||
| Vs Axillary Dissection (N=318) | 0.210 | 0.147 | |||||
| No (N=65) | 31 (47.7%) | 32 (49.2%) | 2 (3.1%) | 0 (0%) | |||
| Yes (N=253) | 99 (39.1%) | 133 (52.6%) | 19 (7.5%) | 2 (0.8%) | |||
|
| |||||||
| Vs RT (N=298) | 0.007 | 0.308 | |||||
| No (N=137) | 68 (49.6%) | 62 (45.3%) | 6 (4.4%) | 1 (0.7%) | |||
| Yes (N=161) | 55 (34.2%) | 93 (57.8%) | 12 (7.5%) | 1 (0.6%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=311) | 0.846 | 0.038 | |||||
| 0-3 (N=49) | 21 (42.9%) | 27 (55.1%) | 1 (2.0%) | 0 (0%) | |||
| 4-7 (N=53) | 23 (43.4%) | 29 (54.7%) | 0 (0%) | 1 (1.9%) | |||
| 8+ (N=209) | 83 (39.7%) | 105 (50.2%) | 20 (9.6%) | 1 (0.5%) | |||
|
| |||||||
| AT 12 MONTHS | Overall Breast Pain (N=259) | 137 (52.9%) | 109 (42.1%) | 8 (3.1%) | 5 (1.9%) | ||
|
| |||||||
| Vs Arm (N=259) | 0.061 | 0.157 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=54) | 21 (38.9%) | 29 (53.7%) | 2 (3.7%) | 2 (3.7%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=82) | 48 (58.5%) | 33 (40.2%) | 0 (0%) | 1 (1.2%) | |||
| Capecitabine (N=123) | 68 (55.3%) | 47 (38.2%) | 6 (4.9%) | 2 (1.6%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=257) | 0.006 | 0.234 | |||||
| Breast Conserving Surgery (N=117) | 51 (43.6%) | 58 (49.6%) | 6 (5.1%) | 2 (1.7%) | |||
| Full Mastectomy (N=140) | 85 (60.7%) | 50 (35.7%) | 2 (1.4%) | 3 (2.1%) | |||
|
| |||||||
| Vs Axillary Dissection (N=259) | 0.909 | 0.896 | |||||
| No (N=56) | 30 (53.6%) | 23 (41.1%) | 1 (1.8%) | 2 (3.6%) | |||
| Yes (N=203) | 107 (52.7%) | 86 (42.4%) | 7 (3.4%) | 3 (1.5%) | |||
|
| |||||||
| Vs RT (N=245) | <0.001 | 0.006 | |||||
| No (N=109) | 74 (67.9%) | 34 (31.8%) | 1 (0.9%) | 0 (0%) | |||
| Yes (N=136) | 55 (40.4%) | 69 (50.7%) | 7 (5.1%) | 5 (3.7%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=254) | 0.708 | 0.284 | |||||
| 0-3 (N=44) | 22 (50.0%) | 20 (45.5%) | 1 (2.3%) | 1 (2.2%) | |||
| 4-7 (N=39) | 23 (59.0%) | 12 (30.8%) | 1 (2.6%) | 3 (7.7%) | |||
| 8+ (N=171) | 91 (53.2%) | 73 (42.7%) | 6 (3.5%) | 1 (0.6%) | |||
|
| |||||||
| AT 24 MONTHS | Overall Breast Pain (N=240) | 152 (63.3%) | 78 (32.5%) | 8 (3.3%) | 2 (0.8%) | ||
|
| |||||||
| Vs Arm (N=240) | 0.214 | 0.744 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=49) | 26 (53.1%) | 20 (40.8%) | 2 (4.1%) | 1 (2.0%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=83) | 53 (63.9%) | 27 (32.5%) | 2 (2.4%) | 1 (1.2%) | |||
| Capecitabine (N=108) | 73 (67.6%) | 31 (28.7%) | 4 (3.7%) | 0 (0%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=238) | 0.281 | 0.100 | |||||
| Breast Conserving Surgery (N=108) | 65 (60.2%) | 41 (38.0%) | 2 (1.9%) | 0 (0%) | |||
| Full Mastectomy (N=130) | 87 (66.9%) | 35 (26.9%) | 6 (4.6%) | 2 (1.5%) | |||
|
| |||||||
| Vs Axillary Dissection (N=240) | 0.049 | 0.089 | |||||
| No (N=52) | 39 (75.0%) | 13 (25.0%) | 0 (0%) | 0 (0%) | |||
| Yes (N=188) | 113 (60.1%) | 65 (34.6%) | 8 (4.3%) | 2 (1.1%) | |||
|
| |||||||
| Vs RT (N=226) | 0.032 | 0.990 | |||||
| No (N=100) | 71 (71.0%) | 25 (25.0%) | 4 (4.0%) | 0 (0%) | |||
| Yes (N=126) | 72 (57.1%) | 49 (38.9%) | 3 (2.4%) | 2 (1.6%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=236) | 0.265 | 0.334 | |||||
| 0-3 (N=41) | 24 (58.5%) | 17 (41.5%) | 0 (0%) | 0 (0%) | |||
| 4-7 (N=39) | 29 (74.4%) | 8 (20.5%) | 1 (2.6%) | 1 (2.6%) | |||
| 8+ (N=156) | 96 (61.5%) | 52 (33.3%) | 7 (4.5%) | 1 (0.6%) | |||
Table 6.
Arm/Shoulder Pain
| None | A little | Quite a bit | Very Much | p-value (little+) | p-value (quite a bit+) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| POST OP | Overall Arm/Shoulder Pain (N=321) | 118 (36.8%) | 148 (46.1%) | 45 (14.0%) | 10 (3.1%) | ||
|
| |||||||
| Vs Arm (N=321) | 0.218 | 0.656 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=67) | 19 (28.4%) | 34 (50.8%) | 14 (20.9%) | 0 (0%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=99) | 36 (36.4%) | 47 (47.5%) | 14 (14.1%) | 2 (2.0%) | |||
| Capecitabine (N=155) | 63 (40.6%) | 67 (43.2%) | 17 (11.0%) | 8 (5.2%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=319) | 0.834 | 0.276 | |||||
| Breast Conserving Surgery (N=143) | 52 (36.4%) | 70 (49.0%) | 17 (11.9%) | 4 (2.8%) | |||
| Full Mastectomy (N=176) | 66 (37.5%) | 76 (43.2%) | 28 (15.9%) | 6 (3.4%) | |||
|
| |||||||
| Vs Axillary Dissection (N=321) | <0.001 | 0.009 | |||||
| No (N=65) | 37 (56.9%) | 24 (36.9%) | 4 (6.2%) | 0 (0%) | |||
| Yes (N=256) | 81 (31.6%) | 124 (48.4%) | 41 (16.0%) | 10 (3.9%) | |||
|
| |||||||
| Vs RT (N=300) | 0.112 | 0.397 | |||||
| No (N=139) | 59 (42.4%) | 60 (43.2%) | 15 (10.8%) | 5 (3.6%) | |||
| Yes (N=161) | 54 (33.5%) | 78 (48.4%) | 25 (15.5%) | 4 (2.5%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=314) | <0.001 | 0.087 | |||||
| 0-3 (N=49) | 25 (51.0%) | 18 (36.7%) | 6 (12.2%) | 0 (0%) | |||
| 4-7 (N=53) | 28 (52.8%) | 20 (37.7%) | 5 (9.4%) | 0 (0%) | |||
| 8+ (N=212) | 62 (29.2%) | 106 (50.0%) | 34 (16.0%) | 10 (4.7%) | |||
|
| |||||||
| AT 12 MONTHS | Overall Arm/Shoulder Pain (N=259) | 133 (51.4%) | 96 (37.1%) | 22 (8.5%) | 8 (3.1%) | ||
|
| |||||||
| Vs Arm (N=259) | 0.514 | 0.274 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=54) | 24 (44.4%) | 24 (44.4%) | 5 (9.3%) | 1 (1.9%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=82) | 43 (52.4%) | 33 (40.2%) | 5 (6.1%) | 1 (1.2%) | |||
| Capecitabine (N=123) | 66 (53.7%) | 39 (31.7%) | 12 (9.8%) | 6 (4.9%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=257) | 0.523 | 0.798 | |||||
| Breast Conserving Surgery (N=117) | 58 (49.6%) | 46 (39.3%) | 9 (7.7%) | 4 (3.4%) | |||
| Full Mastectomy (N=140) | 75 (53.6%) | 48 (34.3%) | 13 (9.3%) | 4 (2.9%) | |||
|
| |||||||
| Vs Axillary Dissection (N=259) | 0.327 | 0.475 | |||||
| No (N=56) | 32 (57.1%) | 16 (28.6%) | 6 (10.7%) | 2 (3.6%) | |||
| Yes (N=203) | 101 (49.8%) | 80 (39.4%) | 16 (7.9%) | 6 (3.0%) | |||
|
| |||||||
| Vs RT (N=245) | 0.074 | 0.556 | |||||
| No (N=109) | 63 (57.8%) | 35 (32.1%) | 9 (8.3%) | 2 (1.8%) | |||
| Yes (N=136) | 63 (46.3%) | 56 (41.2%) | 11 (8.1%) | 6 (4.4%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=254) | 0.741 | 0.878 | |||||
| 0-3 (N=44) | 25 (56.8%) | 13 (29.5%) | 4 (9.1%) | 2 (4.5%) | |||
| 4-7 (N=39) | 20 (51.3%) | 14 (35.9%) | 5 (12.8%) | 0 (0%) | |||
| 8+ (N=171) | 86 (50.3%) | 66 (38.6%) | 13 (7.6%) | 6 (3.5%) | |||
|
| |||||||
| AT 24 MONTHS | Overall Arm/Shoulder Pain (N=240) | 136 (56.7%) | 83 (34.6%) | 16 (6.7%) | 5 (2.1%) | ||
|
| |||||||
| Vs Arm (N=240) | 0.069 | 0.104 | |||||
| CMF (Cyclophosphamide, Methotrexate and Fluorouracil) (N=49) | 24 (50.0%) | 20 (40.8%) | 3 (6.1%) | 2 (4.1%) | |||
| AC (Doxorubicin and Cyclophosphamide) (N=83) | 42 (50.6%) | 30 (36.1%) | 9 (10.8%) | 2 (2.4%) | |||
| Capecitabine (N=108) | 70 (64.8%) | 33 (30.6%) | 4 (3.7%) | 1 (0.9%) | |||
|
| |||||||
| Vs Most Extensive Surgery (N=238) | 0.851 | 0.149 | |||||
| Breast Conserving Surgery (N=108) | 61 (56.5%) | 41 (38.0%) | 5 (4.6%) | 1 (0.9%) | |||
| Full Mastectomy (N=130) | 75 (57.7%) | 41 (31.5%) | 10 (7.7%) | 4 (3.1%) | |||
|
| |||||||
| Vs Axillary Dissection (N=240) | 0.883 | 0.049 | |||||
| No (N=52) | 29 (55.8%) | 22 (42.3%) | 1 (1.9%) | 0 (0%) | |||
| Yes (N=188) | 107 (56.9%) | 61 (32.4%) | 15 (8.0%) | 5 (2.7%) | |||
|
| |||||||
| Vs RT (N=226) | 0.346 | 0.453 | |||||
| No (N=100) | 61 (61.0%) | 30 (30.0%) | 9 (9.0%) | 0 (0%) | |||
| Yes (N=126) | 69 (54.8%) | 49 (38.9%) | 4 (3.2%) | 4 (3.2%) | |||
|
| |||||||
| Vs Number of Nodes Examined (N=236) | 0.788 | 0.229 | |||||
| 0-3 (N=41) | 25 (61.0%) | 15 (36.6%) | 1 (2.4%) | 0 (0%) | |||
| 4-7 (N=39) | 21 (53.9%) | 15 (38.5%) | 2 (5.1%) | 1 (2.6%) | |||
| 8+ (N=156) | 87 (55.8%) | 52 (33.3%) | 13 (8.3%) | 4 (2.6%) | |||
Table 7.
Post Op Arm/Shoulder Pain versus Breast Pain
| Breast Pain – 12 months post op | p-value | |
|---|---|---|
|
| ||
| Arm/Shoulder Pain at Post Op (N=259) | <0.001 | |
| Yes (N=161) | 91 (56.5%) | |
| No (N=98) | 31 (31.6%) | |
|
| ||
| Breast Pain – 24 months post op | ||
|
| ||
| Arm/Shoulder Pain at Post Op (N=240) | 0.002 | |
| Yes (N=149) | 66 (44.3%) | |
| No (N=91) | 22 (24.2%) | |
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Shah C, Arthur D, Riutta J, et al. Breast-cancer related lymphedema: a review of procedure-specific incidence rates, clinical assessment AIDS, treatment paradigms, and risk reduction. Breast J. 2012;18:357–61. doi: 10.1111/j.1524-4741.2012.01252.x. [DOI] [PubMed] [Google Scholar]
- 2.Ozcinar B, Guler SA, Kocaman N, et al. Breast cancer related lymphedema in patients with different loco-regional treatments. Breast. 2012;21:361–5. doi: 10.1016/j.breast.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Kwan W, Jackson J, Weir LM, et al. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol. 2002;20:4242–8. doi: 10.1200/JCO.2002.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Tasmuth T, von Smitten K, Kalso E. Pain and other symptoms during the first year after radical and conservative surgery for breast cancer. Br J Cancer. 1996;74:2024–31. doi: 10.1038/bjc.1996.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miaskowski C, Cooper B, Paul SM, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13:1172–87. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karki A, Simonen R, Malkia E, et al. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37:180–8. doi: 10.1080/16501970410024181. [DOI] [PubMed] [Google Scholar]
- 7.Feiten S, Dunnebacke J, Heymanns J, et al. Breast cancer morbidity: questionnaire survey of patients on the long term effects of disease and adjuvant therapy. Dtsch Arztebl Int. 2014;111:537–44. doi: 10.3238/arztebl.2014.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norman SA, Localio AR, Kallan MJ, et al. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev. 2010;19:2734–46. doi: 10.1158/1055-9965.EPI-09-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–83. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 10.DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 11.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–65. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornblith AB, Lan L, Archer L, et al. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J Clin Oncol. 2011;29:1022–8. doi: 10.1200/JCO.2010.29.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Therapy Evaluation Program, Common Toxicity Criteria (CTC) Version 2.0. 1999 [Google Scholar]
- 14.Mantel N. Chi-Square Tests with One Degree of Freedom; Extensions of the Mantel-Haenszel Procedure. Journal of the American Statistical Association. 1963;58:690–700. [Google Scholar]
- 15.Sakorafas GH, Peros G, Cataliotti L, et al. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. 2006;15:153–65. doi: 10.1016/j.suronc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480–501. doi: 10.1200/JCO.2013.53.5948. [DOI] [PubMed] [Google Scholar]
- 17.Pedro-Guzman J. Managing Upper Extremity Dysfunction in Breast Cancer Survivors. 2016 [Google Scholar]
- 18.Smoot B, Wong J, Cooper B, et al. Upper extremity impairments in women with or without lymphedema following breast cancer treatment. J Cancer Surviv. 2010;4:167–78. doi: 10.1007/s11764-010-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–63. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 20.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102:111–8. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Land SR, Kopec JA, Julian TB, et al. Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B-32. J Clin Oncol. 2010;28:3929–36. doi: 10.1200/JCO.2010.28.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnant M, Thomssen C, Harbeck N. St. Gallen/Vienna 2015: A Brief Summary of the Consensus Discussion. Breast Care (Basel) 2015;10:124–30. doi: 10.1159/000430488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meretoja TJ, Leidenius MH, Tasmuth T, et al. Pain at 12 months after surgery for breast cancer. Jama. 2014;311:90–2. doi: 10.1001/jama.2013.278795. [DOI] [PubMed] [Google Scholar]


