Introduction
Decompressive craniectomy (DC) is a potentially life-saving procedure and it remains the treatment of choice for medically refractory intracranial hypertension, most commonly in the setting of severe traumatic brain injury with large vessel infarction, and less frequently in cases of intra-operative brain swelling, aneurysmal subarachnoid haemorrhage and encephalitis (1). People who have survived the first intracranial insult must undergo subsequent cranial reconstructive surgery.
Cranioplasty is considered to be a mandatory procedure, not only for cosmetic reasons, but also to protect the underlying brain to help ensure the potential for recovery of the injured brain (2). Reimplantation of the previously harvested autologous skull flap from DC is economical, and it provides an excellent anatomical fit (5). Complications of cranioplasty include infection, bone flap resorption, seizures and extra-axial haematoma (6). Post-cranioplasty infection adds considerable burden to patient recovery; it also has significant economic implications, including prolonged anti-microbial treatment, unscheduled returns to the operation theatre for removal of the infected bone flaps and an additional cranioplasty procedure using more costly synthetic material.
The two most common bone flap storage methods are cryopreservation, which involves storing the bone flaps in a bone freezer at a very low temperature, and storing the bone flaps in abdominal subcutaneous pockets. To date, studies comparing the outcomes of cranioplasty with cryopreserved and subcutaneously stored bone flaps have produced variable results; a significant deficiency in those studies is the lack of standardisation among the described techniques (7).
The current prospective study aims to investigate surgical outcomes, specifically surgical site infection (SSI), associated with these two bone flap preservation methods, frozen versus subcutaneous pockets, as well as other risk factors, such as the timing of the cranioplasty, the indication and types of DC (unilateral versus bifrontal), the status of the operating surgeon and the number of repeated surgeries before cranioplasty.
Methodology
This was a prospective study on patients subjected to autologous cranioplasty over a period of 18 months at two hospitals where one neurosurgical unit used cryopreserved bone flaps for cranioplasty and another unit used bone flaps stored in the abdominal subcutaneous pocket. The study subjects included all patients ranging in age from 18 to 70 who had undergone DC before and after June 2011 and who underwent autologous cranioplasty between June 2011 and November 2012 (18 months). Following the cranioplasty, the subjects were followed up for at least one year to evaluate for any SSI. Patients who had penetrating traumatic brain injury and those with documented fever before cranioplasty or signs of inflammation at the craniectomy and abdominal implant sites were excluded from the study. Two different bone flap storage methods (cryopreservation and subcutaneous pockets) and their handling methods were used based on the protocols available at the respective hospitals.
Cryopreservation Group
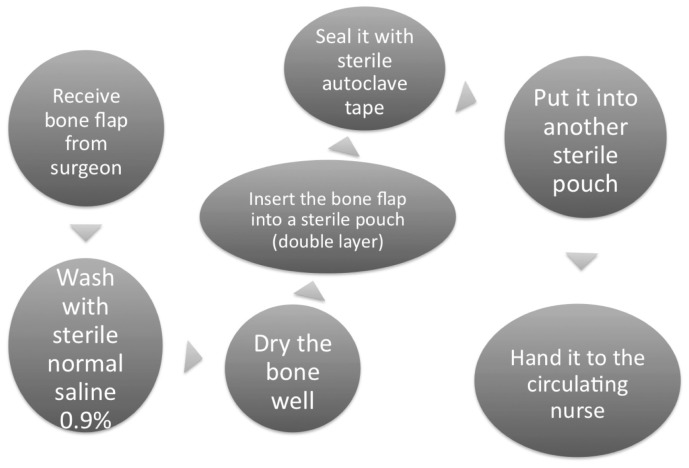
The steps and techniques used for packaging a bone flap for cryopreservation are shown in Figure 1. The autologous bone flap log was reviewed by appointed staff personnel every six months to verify the appropriateness of the method and the need for continued specimen storage. The temperature of the bone freezer was always kept between −20 °C and −40 °C. Dedicated neurosurgical staff members monitored the temperature of the bone bank to ensure optimum temperature consistency in order to maintain the viability of the bone flaps. On the day of surgery, the bone flap was taken from the bone freezer in the morning for thawing at room temperature one to two hours prior to cranioplasty. Once the cranioplasty operation had commenced, the bone flap packaging was removed and the bone flap was immersed in gentamicin solution until the surgeon fixed the bone flap onto the cranial defect for cranioplasty.
Figure 1.
Steps to prepare bone flap for cryopreservation
Subcutaneous Pocket Group
Following DC, the bone flap was cleaned by stripping off the muscle fascia and pericranium from its surface, and the sharp edges were trimmed or rongeured to ensure smooth surfaces. The bone flap was then stored in the abdominal subcutaneous pocket between the subcutaneous fat and muscular fascia. On the day of cranioplasty, the bone flap was retrieved from the abdomen, and then immersed in gentamicin solution until it was placed on the craniectomy site.
For both study groups, sterile dressing was applied to the primarily closed incisional wound as protection for at least 48 hours. A subgaleal drain was left in-situ for less than 72 hours.
Data Collection and Statistical Analysis
The follow-ups occurred at different intervals over a period of 18 months after the date of the cranioplasty. Data were strictly collected by a single investigator to minimise the risk of error in the evaluation of specific data needed for the study. Data collection included details of the bone flap storage methods, indications of DC, types of DC, pre-existing medical illness, status of the surgeon, timing of the cranioplasty and any cranial surgery between the DC and cranioplasty. The patients were assessed for SSI using Centre for Disease Control Criteria (8).
The study was submitted to the National Medical Research Register for ethical approval (NMRR-07-484-757). Data entry and analysis were done using Statistical Package for Social Sciences (SPSS) version 20.0. Descriptive statistics are shown where appropriate. Univariate and multivariate regressions were conducted. Multiple logistic regression was used to determine which variables were significantly associated with the outcomes. Variables that were significant at a 5% significance level were retained in the final model. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for these models.
Results
A total of 101 patients underwent autologous cranioplasty between June 2011 and November 2012 from both of the hospitals enrolled in the study based on the inclusion and exclusion criteria. The youngest patient was 18 and the oldest patient was 66. The mean age was 27 (SD 14.70). More than half of the patients (N = 52, 51.4%) were younger than 30. Four elderly (> 60 years) patients were included in the study. Eighty-three of the patients (82.2%) were male and 18 (17.8%) were female.
Among the 101 cases, cryopreserved bone flaps were used in 55 cases (54.5%); the remaining 46 bone flaps (45.5%) were stored in the subcutaneous pockets. Three cases of infection (5.45%) were found in the cryopreserved group and three cases of infection (6.52%) were also found in the subcutaneous pocket group. Eighteen (17.8%) patients had underlying medical illness prior to cranioplasty. The remaining 83 (82.2%) were healthy individuals. The documented medical illnesses included hypertension, diabetes mellitus, bronchial asthma, hypercholestrolaemia and coronary heart disease.
The most common indication of DC was traumatic brain injury, which was found in 86 cases (85.1%); 15 cases (14.9%) were found to have non-traumatic brain swelling, such as spontaneous intracerebral haemorrhage, malignant middle cerebral artery infarction and tumour surgery. Among the traumatic brain injury cases, 50 patients (58.1%) had their bone flap stored in the bone freezer and 36 (41.9%) had their bone flaps stored in subcutaneous pockets. Within the traumatic brain injury cases, three cases of infection (6%) were seen in the cryopreserved group and three cases of infection (5.5%) were seen the subcutaneous pocket group.
Seventy-nine bone flaps (78.2%) were unilateral (either right or left) and 22 (21.8%) were bifrontal. Of the infected cases, half (three cases) were bifrontal bone flaps and half (three cases) were unilateral bone flaps. Cranioplasty was performed by specialists in 58 cases (57.5%) and by non-specialists in 43 cases (42.5%). All six of the patients with cases of infection were operated on by non-specialists.
The shortest interval from DC to cranioplasty was 25 days. The longest interval was 538 days. The mean interval was 168 days. The timing of the cranioplasty was further subdivided into early (< 90 days from DC to cranioplasty) and late (> 90 days from DC to cranioplasty). Only 12 patients (11.9%) had early cranioplasty; 89 patients (88.1%) underwent late cranioplasty. The majority of the cryopreserved (96.4%) and subcutaneous pocket (78.3%) bone flap cases had late cranioplasty. Among the 12 early cranioplasty cases, two (16.7%) were found to have post-cranioplasty SSI. Of the 89 cases in the late cranioplasty group, only four cases of infection (4.5%) were found.
Seventy (69.3%) of the patients who had DC did not have any further cranial operations, and 31 patients (30.7%) had one or more subsequent cranial operations prior to cranioplasty. The subsequent cranial operations were recraniotomy and evacuation of extra-axial collection, recraniotomy and evacuation of intracerebral haematoma, wound debridement and desloughing of the craniotomy wound.
Fifty-eight cranioplasty operations (57.5%) were performed by specialists with neurosurgical qualifications and 43 (42.5%) were performed by non-specialist neurosurgical medical officers who were trained in cranioplasty. All six of the patients with infections were operated on by non-specialist surgeons. No SSI cases were reported in the group in which the cranioplasty was performed by a qualified neurosurgical specialist.
In these two neurosurgical units, the overall incidence of post-cranioplasty SSI from 1 June 2011 to 30 Nov 2012 was found to be six cases (5.9%). The simple logistic regression analysis results showed that the type of bone flap storage method (P = 0.572), type of bone flap (P = 0.116), indication of DC (P = 0.629), pre-existing medical illness (P = 0.588) and the timing of the cranioplasty (early versus late) (P = 0.148) have no significant association with post-cranioplasty SSI. The significant factors associated with the development SSI were repeat cranial operations (P = 0.014) and the status of the operating surgeon (P = 0.032) (Table 1).
Table 1.
Different variables and their p-values in association with post-cranioplasty surgical site infection
| Variables | Surgical Site Infection n (%) |
Odds Ratio (95% Confidence Interval) | P-value | |
|---|---|---|---|---|
|
| ||||
| Yes | No | |||
| Pre-existing Illness | ||||
| Yes | 0 (0) | 18 (19) | 0.93 (0.88–0.99) | 0.588 |
| No | 6 (100) | 77 (81) | ||
| Indication for DC | ||||
| TBI | 5 (83.3) | 81 (85.3) | 0.82 (0.09–7.96) | 0.629 |
| Non-TBI | 1 (16.7) | 14 (14.7) | ||
| Types of DC | ||||
| Unilateral | 3 (50) | 76 (80) | 4.00 (0.75–21.41) | 0.116 |
| Bifrontal | 3 (50) | 19 (20) | ||
| Method of bone storage | ||||
| Cryopreservation | 3 (50) | 52 (54.7) | 1.2 (0.23–6.30) | 0.572 |
| Subcutaneous pocket | 3 (50) | 43 (45.3) | ||
| Repeat operation | ||||
| Yes | 5 (83.3) | 26 (27.4) | 13 (1.48–119.03) | 0.010 |
| No | 1 (16.7) | 69 (72.6) | ||
| Timing of cranioplasty | ||||
| Early | 2 (33.3) | 10 (10.5) | 0.24 (0.04–1.45) | 0.148 |
| Late | 4 (66.7) | 85 (89.5) | ||
| Operating Personnel | ||||
| Specialist | 0 (0) | 43 (45.3) | 1.1 (1.02–1.22) | 0.032 |
| Non-specialist | 6 (100) | 52 (54.7) | ||
A multiple logistic regression analysis was conducted to predict the potential for post-cranioplasty SSI using the status of the operating surgeons and the number of repeat cranial operations as predictors. The Wald criterion demonstrated that only repeat cranial operations had a significant impact on the potential for SSI (P = 0.0010. The Exp(B) value indicates that if patients undergo more than two repeat cranial operations before undergoing cranioplasty they are almost 11 times more likely to get SSI (Table 2).
Table 2.
Variables associated with autologous cranioplasty post-operative SSI in the last stage of multivariate analysis using multiple logistic regression model
| Risk Factors | Adjusted OR (95% CI) | B | Wald Statistic | P-value |
|---|---|---|---|---|
| Operating personnel | ||||
| Specialist | 1 | 2.81 | 6.05 | 0.997 |
| Non-specialist | 1 (0.77–15.85) | |||
| Repeat operation | ||||
| Yes | 10.46 (3.38–32.32) | 2.35 | 16.59 | 0.001 |
| No | 1 | |||
Discussion
Bone flap infection following any cranial surgery is a serious and burdensome complication. This complication ranges from simple superficial wound infections to deeper infections, such as bone flap osteomyelitis, meningitis or brain abscesses (9). Therefore, it is important to identify the predictive parameters for cranioplasty SSI in order to improve patient outcomes.
This prospective study was conducted to determine if there are any differences in SSI following autologous cranioplasty based on the mode of bone flap storage. The study found that the incidence of SSI in cranioplasty using the autologous bone flap method at the two studied centres was 5.9% in 101 patients within a period of 18 months. This finding is consistent with previously reported rates of infection, ranging between 7% and 22% (10–11). The rate of post-cranioplasty SSI was 5.5% and 6.5% for the cryopreserved bone flaps and the bone flaps stored in the abdominal subcutaneous pocket, respectively. However, the difference between the two modes of bone flap storage was not statistically significant. A literature review of multiple retrospective studies revealed that the rate of post-cranioplasty SSI with cryopreserved bone flaps ranges between 0% and 25.9%; for subcutaneous preserved bone flaps, the rate ranges between 2.3% and 5.1% (12). In a systematic review of non-prospective studies, Yadla et al. (13) reported no significant difference in infection rates between subcutaneous or extracorporeal preservation of autologous bone flaps.
A retrospective study by Inamasu et al. (14) over 9 years with 70 patients at one centre found that the SSI rate was 16.1% and 5.1% in the cryopreservation and subcutaneous pocket groups, respectively, with no significant difference in the incidence of infection between the two bone flap storage methods. However, a subgroup analysis of traumatic brain injury patients showed a significant rate of infection with cryopreserved bone flaps. In the present prospective study, the association between the methods of storage and post-cranioplasty SSI among the 86 traumatic brain injury cases was not statistically significant.
Even though the outcome (infection) for both groups did not differ significantly, the cryopreservation method has many advantages over the method that buries a bone flap in abdominal subcutaneous tissues. Storing bone flaps in the bone freezer seems to be the simplest and safest strategy because the surgeon does not have to spend extra time creating subcutaneous pockets and the patient does not experience additional surgical scarring. However, if the abdominal storage site becomes infected additional surgery for wound debridement of the abdomen and removal of the stored bone flap is required. In this context, the patient and hospital bear greater burdens including extra theatre time, additional (patient) suffering, the need for more antibiotics (increased costs), prolonged hospital stay and the cost of an alloplastic implant for future cranioplasty.
The abdominal subcutaneous pocket method may be more beneficial than cryopreservation because it is affordable and it does not require the maintenance of special expensive freezers. However, serious consequences can occur with this method, and these pose a higher danger and more harm to patients than cryopreserved bone flaps. The harmful consequences include the risk of infection as well as dissatisfaction with a bone flap stored in the abdomen. In the present study, among the 293 DCs in which the bone flaps were stored in the abdomen, 26 patients (8.9%) reported abdominal pain and discomfort. The phenomenon of the bone flap implantation site giving rise to pain and discomfort is more commonly seen in thin-built patients. Some patients also complained of the bone flap “bulging out”, and some reported having pain only when they sat in an upright position when the bone flap edges cause friction with the iliac bone. This phenomenon is also reported by Morina et al. (15).
Repeated cranial operations and evacuation of extra-axial collections have contributed to an increased incidence of SSI. In one study, the rate of bone flap infection increased from 4.3% to 33% following more than three cranial operations (16). Studies by Cheng et al. (17) and Matsuno et al. (18) reported that multiple cranial operations prior to cranioplasty increase the risk of infection. Repetitive disruption to wound healing from multiple cranial operations may be related to post-cranioplasty SSI. The current study found that patients who underwent repeat cranial surgery more than two times after DC and before cranioplasty had an almost 11 times higher risk of developing post-cranioplasty SSI. Attempts to minimise the total number of cranial procedures prior to cranioplasty can prevent SSI
Conclusion
The results of this prospective study provide valuable information for predicting outcomes following cranioplasty with autologous bone flaps stored with cryopreservation or in subcutaneous pockets. In conclusion, there is no difference in the incidence of post-cranioplasty SSI using autologous bone flaps stored either by cryopreservation or in subcutaneous pockets. However, undergoing multiple cranial operations following craniectomy is a significant risk factor for post-cranioplasty SSI. Attempts to reduce cerebrospinal fluid leaks, subgaleal haematoma and post-craniectomy haematoma are necessary in order to reduce repeated cranial operations before cranioplasty.
Acknowledgements
The results of this study was presented by Dr Cheah Pooi Pooi and won the best paper presentation at 21st Annual Meeting of the Japan Society of Neurosurgical Emergency in Tokyo, January 29–30, 2016.
Footnotes
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interests (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript
Ethical Approval
All the procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from each of the participants included in the study.
Authors’ Contributions
Conception and design: CPP, CCK
Analysis and interpretation of the data: CPP, BI
Drafting of the article: CPP, BI
Critical revision of the article for important intellectual content: BI
Final approval of the article: BI
Provision of study materials or patients: CPP, AKR, CCK
Administrative, technical, or logistic support: AKR, CCK
Collection and assembly of data: CPP, AKR, CCK
References
- 1.Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 2009;26 doi: 10.3171/2009.3.FOCUS0962. http://dx.doi.org/10.3171/2009.3.FOCUS0962. [DOI] [PubMed] [Google Scholar]
- 2.Honeybul S, Janzen C, Kruger K, Ho KM. The impact of cranioplasty on neurological function. Br J Neurosurg. 2013;27:636–641. doi: 10.3109/02688697.2013.817532. http://dx.doi.org/10.3109/02688697.2013.817532. [DOI] [PubMed] [Google Scholar]
- 3.Jelcic N, De Pellegrin S, Cecchin D, Della Puppa A, Cagnin A. Cognitive improvement after cranioplasty: a possible volume transmission-related effect. Acta Neurochir (Wien) 2013;155:1597–1599. doi: 10.1007/s00701-012-1519-6. http://dx.doi.org/10.1007/s00701-012-1519-6. [DOI] [PubMed] [Google Scholar]
- 4.Di Stefano C, Sturiale C, Trentini P, Bonora R, Rossi D, Cervigni G, et al. Unexpected neuropsychological improvement after cranioplasty: a case series study. Br J Neurosurg. 2012;26:827–831. doi: 10.3109/02688697.2012.692838. http://dx.doi.org/10.3109/02688697.2012.692838. [DOI] [PubMed] [Google Scholar]
- 5.Brommeland Tor, Rydning Pål Nicolay, Pripp Are Hugo, Helseth Eirik. Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med. 2015;23:75. doi: 10.1186/s13049-015-0155-6. http://dx.doi.org/10.1186/s13049-015-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobani ZA, Shamim MS, Zafar SN, Qadeer M, Bilal N, Murtaza SG, et al. Cranioplasty after decompressive craniectomy: an institutional audit and analysis of factors related to complications. Surg Neurol Int. 2011;2:123. doi: 10.4103/2152-7806.85055. http://dx.doi.org/10.4103/2152-7806.85055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sultan SM, Davidson EH, Butala P, Schachar JS, Witek L, Szpalski C, et al. Interval cranioplasty: comparison of current standards. Plast Reconstr Surg. 2011 May;127(5):1855–1864. doi: 10.1097/PRS.0b013e31820e89a5. http://dx.doi.org/10.1097/PRS.0b013e31820e89a5. [DOI] [PubMed] [Google Scholar]
- 8.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. http://dx.doi.org/10.1017/S0195941700015241. [PubMed] [Google Scholar]
- 9.Cassir N, De La Rosa S, Melot A, Touta A, Troude L, Loundou A, et al. Risk factors for surgical site infections after neurosurgery: A focus on the postoperative period. Am J Infect Control. 2015 Dec 1;43(12):1288–1291. doi: 10.1016/j.ajic.2015.07.005. http://dx.doi.org/10.1016/j.ajic.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Coulter IC, Pesic-Smith JD, Cato-Addison WB, Khan SA, Thompson D, Jenkins AJ, et al. Routine but risky: a multi-centre analysis of the outcomes of cranioplasty in the Northeast of England. Acta Neurochir (Wien) 2014;156:1361–1368. doi: 10.1007/s00701-014-2081-1. http://dx.doi.org/10.1007/s00701-014-2081-1. [DOI] [PubMed] [Google Scholar]
- 11.Lee L, Ker J, Quah BL, Chou N, Choy D, Yeo TT. A retrospective analysis and review of an institution’s experience with the complications of cranioplasty. Br J Neurosurg. 2013;27:629–635. doi: 10.3109/02688697.2013.815313. http://dx.doi.org/10.3109/02688697.2013.815313. [DOI] [PubMed] [Google Scholar]
- 12.Hng D, Bhaskar I, Khan M, Budgeon C, Damodaran O, Knuckey N, et al. Delayed cranioplasty: outcomes using frozen autologous bone flaps. Craniomaxillofacial Trauma & Reconstruction. 2015;8(3):190–197. doi: 10.1055/s-0034-1395383. http://dx.doi.org/10.1055/s-0034-1395383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: a systematic review. Neurosurgery. 2011 Apr;68(4):1124–1129. doi: 10.1227/NEU.0b013e31820a5470. http://dx.doi.org/10.1227/NEU.0b013e31820a5470. [DOI] [PubMed] [Google Scholar]
- 14.Inamasu J, Kuramae T, Nakatsukasa M. Does difference in the storage method of bone flaps after decompressive craniectomy affect the incidence of surgical site infection after cranioplasty? Comparison between subcutaneous pocket and cryopreservation. J Trauma Acute Care Surg. 2010;68(1):183–187. doi: 10.1097/TA.0b013e3181c45384. http://dx.doi.org/10.1097/TA.0b013e3181c45384. [DOI] [PubMed] [Google Scholar]
- 15.Morina A, Kelmendi F, Morina Q, et al. Cranioplasty with subcutaneously preserved autologous bone grafts in abdominal wall—Experience with 75 cases in a post-war country Kosova. Surgical Neurology International. 2011;2:72. doi: 10.4103/2152-7806.81735. http://dx.doi.org/10.4103/2152-7806.81735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CH, Chung YS, Lee SH, Yang HJ, Son YJ. Analysis of the factors influencing bone graft infection after cranioplasty. J Trauma Acute Care Surg. 2012;73(1):255–260. doi: 10.1097/TA.0b013e318256a150. http://dx.doi.org/10.1097/TA.0b013e318256a150. [DOI] [PubMed] [Google Scholar]
- 17.Cheng YK, Weng HH, Yang JT, Lee MH, Wang TC, Chang CN. Factors affecting graft infection after cranioplasty. J Clin Neurosci. 2008 Oct;15(10):1115–1119. doi: 10.1016/j.jocn.2007.09.022. http://dx.doi.org/10.1016/j.jocn.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir [Internet] 2006;148(5):535–540. doi: 10.1007/s00701-006-0740-6. http://dx.doi.org/10.1007/s00701-006-0740-6. [DOI] [PubMed] [Google Scholar]