Abstract
Endothelial progenitor cells (EPCs) repair damaged vascular endothelium, and low circulating EPC levels have been associated with cardiovascular disease (CVD). CD34+/KDR+ EPCs are commonly reported in the literature and CD34+/CD133+/KDR+ EPCs are rare in circulation but highly specific for endothelial lineage. HIV-infected (HIV+) adults have chronic inflammation and increased CVD risk, but the relationship between CVD, vascular inflammation, and EPCs in HIV remains unclear. In a pilot study, EPCs were measured in 57 HIV+ men [≥50 years old, HIV-1 RNA <50 copies/ml on antiretroviral therapy (ART)] by real-time flow cytometry using cellular immaturity (CD34 and/or CD133) and endothelial commitment (KDR) markers. Fasting inflammatory biomarker levels were measured by ELISA. Median age was 57 years; CD4+ T lymphocyte count was 570 cells/mm3. Prevalent CVD risk factors included 16% diabetes, 28% hypertension, 53% dyslipidemia, and 33% smoking. Median (interquartile range) EPC values were CD34+/KDR+ 0.1 (0.0–0.9) cells/105 peripheral blood mononuclear cells (PBMCs) and CD34+/CD133+/KDR+ 0.1 (0.0–0.9) cells/105 PBMCs. We observed a high prevalence of undetectable CD34+/KDR+ (40%) and CD34+/CD133+/KDR+ EPCs (44%). Men with undetectable EPCs were more likely to have ≥2 CVD risk factors, lower interleukin-6 (IL-6), and higher sCD163 levels. In these older HIV+ men on suppressive ART, CD34+/KDR+ and CD34+/CD133+/KDR+ EPC levels were low and often undetectable. Undetectable EPC levels were associated with greater CVD risk factor burden, lower IL-6 (consistent with decreased EPC production stimulus), and higher sCD163 (consistent with monocyte activation and prior CVD associations) levels, suggesting a potential relationship between EPCs and atherosclerotic burden in this population.
Keywords: : endothelial progenitor cells, HIV, inflammation
Introduction
Although effective antiretroviral therapy (ART) prevents AIDS-defining illnesses and prolongs life, morbidity and mortality rates in HIV-infected (HIV+) individuals due to non-AIDS events remain high.1,2 HIV+ individuals have earlier and accelerated development of subclinical atherosclerosis,3 leading to a higher risk of cardiovascular disease (CVD) than HIV-uninfected (HIV−) individuals.2 The exact physiologic mechanisms contributing to heightened CVD risk in HIV infection are not fully understood.
Disturbances in vascular reparative capacity, as measured by the number of circulating endothelial progenitor cells (EPCs), have been suggested as a potential driver of endothelial dysfunction in HIV infection.4–6 EPCs are bone marrow-derived cells characterized by having cellular markers of both hematopoietic immaturity7 and endothelial commitment.8 Physiologically, EPCs are released from the bone marrow into the circulation after vascular injury and contribute to endothelial repair. In HIV− persons, the number and function of circulating EPCs are altered by traditional CVD risk factors, and number of EPCs are inversely correlated with severity of atherosclerosis.9
In HIV+ individuals, alterations in the number and/or functionality of EPCs compared with HIV− individuals have been reported.4–6 However, the association between EPC levels and subclinical atherosclerosis remains unclear, particularly among HIV+ individuals with suppressed viremia.4,10 In addition, data are limited to a small number of studies using varied EPC isolation techniques, and results are conflicting.10–12
In this pilot study, we first aimed to describe the cross-sectional distribution of number of EPCs and immunophenotype in older HIV+ men on suppressive ART, a group at increased CVD risk.13 Considering the influence of traditional CVD risk factors, including sex and age, on EPC levels in the general population,14 we chose to focus our study on a homogeneous HIV+ group of men with age ≥50 years. We then analyzed the relationship between EPC subset frequency and CVD risk factor burden, and explored relationships between EPC subset frequency and levels of circulating biomarkers of inflammation and vascular disease.
Methods
Participants
Participants were enrolled into this cross-sectional study at the University of California, Los Angeles (UCLA) Clinical AIDS Research and Education Center (May–November 2014). Inclusion criteria included HIV+, male sex, ≥50 years old, and HIV-1 RNA <200 copies/ml on stable ART for ≥24 weeks. Exclusion criteria included active, untreated opportunistic, or AIDS-defining illness; active autoimmune disease; acute infection or vaccination in the 21 days before entry; change in medications with anti-inflammatory properties in the 12 weeks before entry; regular use of prednisone at doses ≥5 mg/day; and any ischemic event in the 24 weeks before entry. All study documents and procedures were approved by the institutional review board at UCLA, and all subjects provided written informed consent before initiation of study procedures.
EPC characterization and isolation
Number of EPCs and immunophenotype were measured using real-time flow cytometry on whole blood samples at room temperature.8,15,16 Blood was collected in sodium heparin tubes in the fasting state (nothing to eat or drink for >8 h before the blood draw except water or medications). Flow cytometry was performed on freshly isolated peripheral blood mononuclear cells (PBMCs) using the following fluorochrome-conjugated antibodies: anti-CD45 FITC (clone HI30), anti-CD3 PerCP-Cy5.5 (clone UCHT1), anti-CD33 PerCP-Cy5.5 (clone P67.6), anti-CD19 (clone HIB19), and anti-CD34 PE-Cy7 (clone 8G12), all from BD Biosciences (San Jose, CA); anti CD235ab (glycophorin) PerCP-Cy5.5 (clone HIR2) from Biolegend (San Diego, CA); anti-CD133/2 PE (clone 293C3) from Beckman-Coulter (Brea, CA); and anti-VEGF R2 (KDR/Flk-1) APC (clone 89106) from R&D (Minneapolis, MN). Using CD34 and CD133 as markers of EPC early maturity and KDR as a marker of endothelial lineage commitment,17 EPCs were defined as viable CD45−(or dim)/CD3−/CD33−/CD19−/glycophorin− cells with one of the following four immunophenotypes: CD133+/KDR+, CD34+/KDR+, CD34+/CD133+, or CD34+/KDR+/CD133+(expressed per 105 PBMCs).
An LSR-II flow cytometer (BD) was used to analyze ≥500,000 cells per sample. Cell Preparation Tube beads (BD Biosciences, San Jose, CA) were used for instrument setup before each analysis, and Rainbow beads (Spherotech, Lake Forest, IL) to standardize instrument settings between sampling runs. Fluorescence Minus One controls were prepared for each run to ensure between-run gate consistency. All samples had >75% viability; only live cells (defined by negative 7-aminoactinomycin D staining) were included. For each sample, a minimum of 500,000 events was acquired. Data were analyzed in FlowJo software, version 9.3.3 (Treestar, Ashland, OR). Undetectable level of EPCs was defined as no signal detected through real-time flow cytometry.
Inflammatory biomarker testing
Blood was collected in the fasting state in EDTA and serum separator tubes for isolation of plasma and serum, respectively, and stored at −70°C until biomarker measurement could occur. Concentrations of plasma interleukin-6 (IL-6, sensitivity 0.7 pg/ml), soluble tumor necrosis factor-α receptor 1 (sTNFR-I, sensitivity 1.2 pg/ml), soluble vascular cell adhesion molecule-1 (sVCAM-1, sensitivity 1.26 ng/ml), soluble CD14 (sCD14, sensitivity 125 pg/ml), and serum-soluble CD163 (sCD163, sensitivity 0.613 ng/ml) were measured by R&D Systems (Minneapolis, MN) ELISAs; plasma asymmetric dimethylarginine (ADMA, sensitivity 0.05 μmol/ml) was measured through DLD Diagnostika GmbH (Hamburg, Germany) ELISA. All biomarker measurements occurred at the University of Vermont Laboratory for Clinical Biochemistry Research.
Clinical parameters
Medical records were reviewed for clinical and demographic data, medical and medication history. Assessment of CVD risk factor burden [age ≥50 years, dyslipidemia, diabetes, hypertension, current smoking or ≤2 years since smoking cessation, body mass index (BMI) ≥25 kg/m2, and history of CVD] was performed. Current ART and cumulative time on nucleoside reverse transcriptase inhibitor (NRTI), protease inhibitor (PI), non-NRTI (NNRTI), and integrase strand transfer inhibitor (INSTI) therapy were recorded.
Outcomes
The primary endpoints were the differences in EPC subset frequency by CVD risk factor profile and the associations between EPC subset frequency and levels of circulating biomarkers of inflammation and vascular disease.
Statistical analyses
Continuous variables are presented as medians and interquartile ranges (IQRs), and nominal data are described as absolute values or percentages. Statistical analyses were performed using the Mann–Whitney U-test, and multiple groups were compared using one-way analysis of variance. Correlations were assessed using simple regression and Kendall's tau test. Significance was defined as two-sided α ≤ 0.05. The sample size for the EPC measurement was based upon EPC frequencies and standard deviations in a prior cohort of older HIV+ adults.18
Results
Participant characteristics
Clinical and demographic characteristics are detailed in Table 1. Among the 57 men enrolled in this study (89% white, median age 57 years), 91% had ≥2 CVD risk factors. Current ART use included 95% NRTI, 26% PI, 33% NNRTI, and 26% INSTI; 96% of men had been on their current ART regimen for ≥1 year before enrollment. Median CD4+ T lymphocyte count was 570 cells/mm3. Chronic hepatitis B virus (HBV, defined as the presence of positive HBV surface antigen) and chronic hepatitis C virus (HCV, defined as detectable HCV RNA) were reported in one and five subjects, respectively.
Table 1.
Clinical and Demographical Characteristics
| N = 57 | |
|---|---|
| Age (years) | 57 (53–61) |
| Race, n (%) | |
| White | 51 (89) |
| African American | 4 (7) |
| Asian | 2 (4) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 11 (19) |
| Non-Hispanic or non-Latino | 46 (81) |
| BMI (kg/m2) | 26 (23–30) |
| Current smoking, n (%) | 19 (33) |
| Diabetes, n (%) | 9 (16) |
| Hypertension, n (%) | 16 (28) |
| Dyslipidemia, n (%) | 30 (53) |
| History of CVD event, n (%) | 4 (7) |
| Framingham risk score (%)a | 13 (9–20) |
| Blood pressure (mmHg) | |
| SBP | 122 (116–135) |
| DBP | 78 (72–83) |
| Lipid profile (mg/dL) | |
| Total cholesterol | 170 (148–199) |
| HDL cholesterol | 46 (41–54) |
| LDL cholesterol | 102 (80–118) |
| Triglycerides | 116 (72–170) |
| Fasting glucose (mg/dL) | 97 (89–105) |
| CD4+ T lymphocyte count (cells/ml) | 570 (404–765) |
| CD4+ T lymphocyte percentage (%) | 33 (26–40) |
| CD4+:CD8+ T lymphocyte ratio | 0.8 (0.5–1.4) |
| Time since HIV diagnosis (years) | 22 (12–26) |
| Time on ART (years) | 12 (7–18) |
| AIDS diagnosis, n (%) | 21 (37) |
| ART class, n (%) | |
| NRTI | 54 (95) |
| ABC | 12 (22) |
| TDF | 32 (59) |
| PI | 15 (26) |
| NNRTI | 19 (33) |
| INSTI | 15 (26) |
| Concomitant medication | |
| Statin, n (%) | 27 (47) |
| Angiotensin receptor blocker, n (%) | 9 (16) |
| Aspirin, n (%) | 24 (42) |
Data are expressed as median (IQR) unless otherwise indicated.
10-year risk of a cardiovascular event, https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php#
ABC, abacavir; ART, antiretroviral therapy; CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL, high-density lipoprotein; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; LDL, low-density lipoprotein; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SBP, systolic blood pressure; TDF, tenofovir.
Number of EPCs and immunophenotype
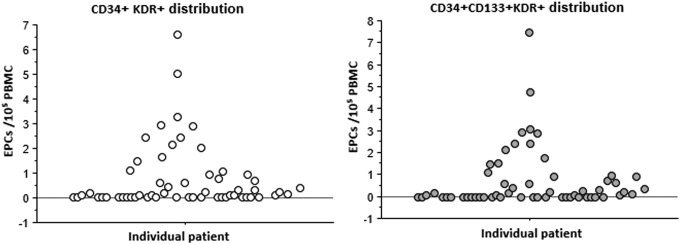
Median (IQR) EPC values were as follows: CD34+/CD133+: 23.7 (16.3–30.1) cells/105 PBMCs; CD34+/KDR+: 0.1 (0.0–0.9) cells/105 PBMCs; CD133+/KDR+: 0.6 (0.1–2.5) cells/105 PBMCs; and CD34+/CD133+/KDR+: 0.1 (0.0–0.9) cells/105 PBMCs. Importantly, undetectable CD34+/KDR+ and CD34+/CD133+/KDR+ EPC levels were found in 40% (23/57) and 44% (25/57) of participants (Fig. 1), respectively.
FIG. 1.
Distribution of CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs. Y axis: EPCs level/105 PBMCs. X axis: individual patient. EPC, endothelial progenitor cell; PBMCs: peripheral blood mononuclear cells.
Associations between EPCs and CVD risk factor burden
Participants with dyslipidemia (defined as diagnosis of lipid disturbance or on lipid-lowering therapy) tended to have fewer CD34+/KDR+ [0.04 (0.0–0.4) cells/105 PBMCs vs. 0.28 (0.0–1.6) cells/105 PBMCs, p = .06] and CD34+/CD133+/KDR+ [0.0 (0–0.42) cells/105 PBMCs vs. 0.28 (0.0–1.5) cells/105 PBMCs, p = .07] EPCs than those without dyslipidemia. These differences persisted irrespective of concomitant statin or angiotensin receptor blocker treatment (data not shown).
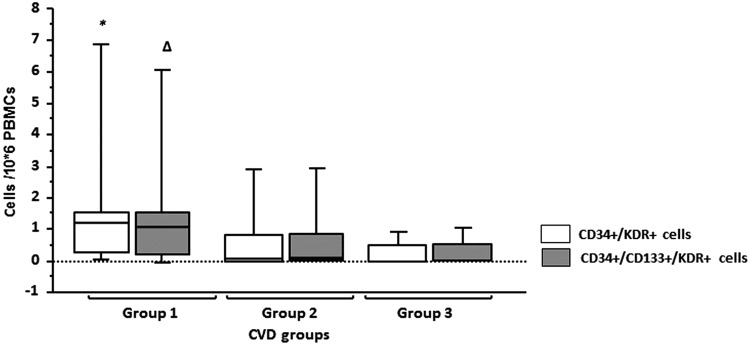
CD34+/KDR+ (tau −0.15, p = .04) and CD34+/CD133+/KDR+ (tau −0.14, p = .07) number of cells correlated with CVD risk factor burden: consistently, men with ≥2 CVD risk factors had significantly fewer CD34+/KDR+ [0.09 (0.0–0.6) cells/105 PBMCs vs. 1.4 (0.7–2.9) cells/105 PBMCs, p = .01] and CD34+/CD133+/KDR+ [0.08 (0.0–0.6) cells/105 PBMCs vs. 1.5 (0.7–3.0) cells/105 PBMCs, p = .01] EPCs than men with ≤1 CVD risk factor (Fig. 2). No correlation between number of EPCs subset and 10-year Framingham Risk Score was found.
FIG. 2.
EPCs by CVD risk profile. Group 1: 0–1 CVD risk factors; group 2: 2–3 CVD risk factors; group 3: ≥4 CVD risk factors. *p < .05, compared with group 2 and group 3. Δp < .05, compared with group 3. CVD, cardiovascular disease.
Men with undetectable CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs were more likely than those with detectable EPCs to have ≥2 CVD risk factors. Men with undetectable CD34+/KDR+ EPCs were also somewhat more likely to have diabetes than those with detectable EPCs (Table 2).
Table 2.
Clinical Characteristics and Inflammatory Biomarkers Between HIV+ Men with Undetectable Versus Detectable CD34+/KDR+ Endothelial Progenitor Cells
| CD34+/KDR+ EPC | CD34+/CD133+/KDR+ EPC | |||||
|---|---|---|---|---|---|---|
| Undetectable (n = 23) | Detectable (n = 34) | p | Undetectable (n = 25) | Detectable (n = 32) | p | |
| Cells/105 PBMCs | 0 (0–0) | 0.6 (0.2–2.0) | 0 (0–0) | 0.7 (0.2–2.0) | ||
| ≥2 CVD risk factors | 100 | 85 | 0.06 | 100 | 84 | 0.04 |
| HTN | 35 | 24 | 0.36 | 32 | 25 | 0.56 |
| Tobacco | 26 | 38 | 0.34 | 28 | 38 | 0.45 |
| Dyslipidemia | 65 | 44 | 0.12 | 57 | 44 | 0.13 |
| Overweight | 70 | 53 | 0.21 | 68 | 53 | 0.26 |
| Diabetes | 26 | 9 | 0.08 | 24 | 9 | 0.14 |
| ABC use | 22 | 35 | 0.28 | 28 | 31 | 0.79 |
| PI use | 26 | 26 | 0.97 | 32 | 22 | 0.39 |
| Statin use | 43 | 50 | 0.63 | 48 | 47 | 0.93 |
| ARB use | 17 | 15 | 0.79 | 20 | 13 | 0.46 |
| AIDs | 35 | 38 | 0.79 | 36 | 38 | 0.91 |
| IL-6 (pg/ml) | 1.1 (0.8–1.5) | 1.7 (1.3–3.1) | 0.009 | 1.2 (0.8–1.8) | 1.7 (1.2–2.7) | 0.09 |
| sTNFR-I (pg/ml) | 1073.9 (1024.4–1175.0) | 1108.7 (1001.1–1320.9) | 0.56 | 1083.7 (1028.1–1177.3) | 1108.7 (999.0–1348.4) | 0.65 |
| sVCAM-1 (ng/ml) | 976.3 (775.7–1216.6) | 889.6 (755.4–1109.4) | 0.24 | 1021.4 (807.7–1205.8) | 874.3 (723.7–1093.9) | 0.11 |
| sCD14 (ng/ml) | 2050.8 (1676.2–2168.4) | 1950.7 (1772.4–2209.4) | 0.91 | 2050.8 (1678.9–2184.1) | 1950.6 (1776.4–2184;6) | 0.90 |
| sCD163 (ng/ml) | 771.0 (642.0–965.5) | 669.8 (550.0–725.3) | 0.06 | 771.0 (666.3–1000.8) | 666.7 (539.6–714.5) | 0.02 |
| ADMA (μmol/ml) | 0.46 (0.39–0.66) | 0.43 (0.37–0.59) | 0.36 | 0.48 (0.40–0.63) | 0.42 (0.37–0.57) | 0.23 |
Continuous values are expressed in median (IQR) and nominal values in absolute values (percentage).
p-values for comparison of undetectable versus detectable EPC groups. Data in bold for significant p-value <0.05.
ABC, abacavir; ADMA, asymmetric dimethylarginine; ARB, angiotensin receptor blocker; CV, cardiovascular; EPC, endothelial progenitor cell; HTN, hypertension; IL-6, interleukin-6; PBMCs, peripheral blood mononuclear cells; PI, protease inhibitor; sCD14, soluble CD14; sCD163, soluble CD163; sTNFR-I, soluble tumor necrosis factor-α receptor 1; SVCAM-1, soluble vascular cell adhesion molecule-1.
Associations between EPCs and inflammatory biomarker levels
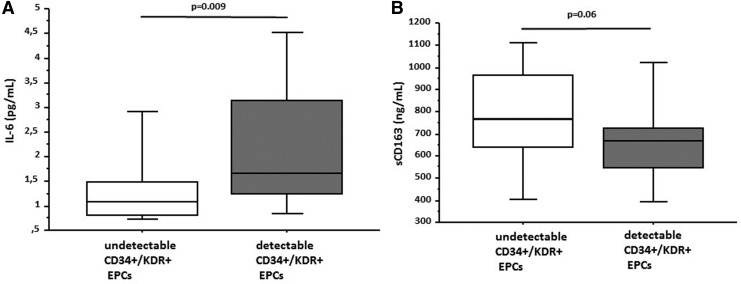
Overall, no significant (p < .05) correlations between number of EPCs subset and circulating inflammatory biomarker levels were observed. Interestingly, however, IL-6 levels were significantly lower in the subgroup of men with undetectable CD34+/KDR+ EPCs than those with detectable CD34+/KDR+ EPCs (1.1 vs. 1.7 pg/ml, p = .009), with a trend observed for undetectable CD34+/CD133+/KDR+ EPCs (undetectable 1.2 vs. 1.7 pg/ml, p = .09). In addition, sCD163 levels were higher in the subgroup of men with undetectable CD34+/CD133+/KDR+ EPCs than those with detectable CD34+/CD133+/KDR+ EPC levels (771.0 vs. 666.7 ng/ml, p = .02), with a trend observed for CD34+/KDR+ EPCs (undetectable 771.0 vs. 669.8 ng/ml, p = .06) (Fig. 3).
FIG. 3.
(A) Interleukin-6 levels in HIV+ men by CD34+/KDR+ EPC level detectability. (B) Soluble CD163 (sCD163) levels in HIV+ men by CD34+/KDR+ EPCs level detectability. IL-6, interleukin-6; sCD163, soluble CD163.
EPCs by ART class
Trends toward greater number of CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs were observed among men with prolonged exposure (>5 years) to NRTIs [CD34+/KDR+: >5 years 0.2 (0.0–0.1) cells/105 PBMCs, ≤5 years 0.0 (0.0–0.2) cells/105 PBMCs, between-group p = .07; CD34+/CD133+/KDR+: >5 years 0.2 (0.0–0.9) cells/105 PBMCs, ≤5 years 0.0 (0.0–0.2) cells/105 PBMCs, between-group p = .08]. No relationship was observed between number of EPCs subset and length of exposure to the PI, NNRTI, or INSTI classes of agents, nor were EPC differences observed among current PI- versus non-PI-treated or ABC- versus TDF-treated individuals.
EPCs, inflammatory markers, and HIV-related parameters
Number of EPCs subset did not vary by HIV-related parameters, including history of an AIDS diagnosis, CD4+: CD8+ T lymphocyte ratio, time since HIV diagnosis, or HCV coinfection status. Pertinent correlations were observed between HIV duration and sTNFR-I (r = 0.29, p = .03), sCD14 (r = 0.24, p = .07), sVCAM (r = 0.35, p = .008), and sCD163 (r = 0.31, p = .002) levels.
Discussion
In this pilot, cross-sectional study of older HIV+ men on suppressive ART, we observed (1) an association between increased CVD risk factor burden and fewer CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs; (2) that almost half of the participants had undetectable CD34+/KDR+ and CD34+/CD133+/KDR+ EPC levels, a striking finding; and (3) that IL-6 levels were significantly lower in HIV+ men with undetectable CD34+/KDR+ EPCs, whereas sCD163 levels were higher among men with undetectable CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs.
Consistent with some studies in the general population,13,14,19 we observed that men with higher CVD risk factor burden had significantly fewer CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs. Interestingly, overall number of CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs were extremely low, and 40% of our HIV+ population had undetectable CD34+/KDR+ EPCs. Although we did not have documented CVD prevalence by imaging, main CVD risk factors were frequent in our studied population, including dyslipidemia 53%, current smoking 33%, and hypertension 28%, suggesting a population with high-risk of atherosclerosis. Our findings raise the question of the significance of low number of EPCs in the context of a chronic inflammatory disease. Under the hypothesis that circulating EPCs reflect intact vascular regenerative capacity in persons at high risk of or with prevalent CVD, our data may reflect low or impaired vascular regenerative capacity among older HIV+ individuals with high CVD risk factor burden. However, although low number of EPCs may be associated with low vascular regenerative capacity, studies have shown that number of EPCs may increase during and immediately after acute CVD events,20,21 with an early return to baseline beginning 72 h postinjury21 and/or reaching levels similar to healthy controls at 2 months after acute myocardiac injury.22 Hence, in this setting, a low EPC level may reflect the absence of EPC mobilization rather than impaired vascular regenerative capacity. Of importance, our study excluded all patients with recent (<24 weeks) CVD events, and our data are believed to reflect an association between number of EPCs and preclinical atherosclerosis, as suggested by the high rate of traditional CVD risk factors in our cohort, but no causal relationship can further be presumed.
To date, no data related to EPCs level among HIV+ individuals virologically suppressed have been reported. HIV-related parameters have been associated with low EPC levels, as HIV may directly infect EPCs,6 and significantly fewer CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs have been reported in HIV+, ART-naive patients than in healthy controls.5 In addition, Gomez-Garre et al.4 observed that ART exposure (>5 years exposure to PI or NRTI) was the main predictor of low number of CD34+/KDR+ EPCs after controlling for CVD risk factors. However, in this study, 17% of ART-treated persons had detectable HIV-1 RNA, which may have confounded results.
In our study, no significant associations between EPC levels and ART classes or agents were found, although trends toward greater number of CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs among men with >5 years NRTI exposure were observed. We also cannot exclude other possible associations due to the high proportion of undetectable EPC levels in our sample, which could have prevented us from detecting these relationships. Given the limited and conflicting data, further studies to understand the relationship between ART use and EPCs are warranted.
From a clinical perspective, EPCs have been suggested as a predictive biomarker of CVD events.14,23 Schmidt-Lucke et al.23 reported an optimal threshold of 3.8 CD34+/KDR+ EPCs/105 PBMCs for the prediction of cardiovascular events in a study of HIV− individuals with CAD over a median duration of 10 months follow-up. Among HIV+ individuals, only one study10 has explored the relationship between EPCs change over time and atherosclerosis progression. This study was performed on frozen and not fresh PBMC samples, which the authors admitted their reduced ability to quantify EPCs. Despite this, the authors observed greater number of EPCs among HIV+ participants, and concluded a lack of association between changes in EPCs and change in carotid intima-media thickness for 1 year. Limitations of this study make it difficult to conclude whether EPCs can be used as a predictive marker of atherosclerotic disease in chronic HIV disease, especially in the absence of CVD event documentation. Further HIV+ cohort studies with measurements of CVD events as primary outcomes are needed.
In an exploratory analysis, we studied the interplay between EPCs and inflammatory biomarkers. Interestingly, our results showed significantly lower IL-6 levels in the subgroup of HIV+ men with undetectable CD34+/KDR+ EPCs. IL-6, as a marker of systemic inflammation, has been associated with CVD risk in HIV− and HIV+ populations.24,25 However, the relationship between IL-6 and atherogenesis during chronic HIV infection is less clear.26–28 IL-6 is a multifunctional, systemic cytokine involved in various inflammatory processes, including EPC proliferation, differentiation, and apoptosis.29,30 EPCs express the IL-6 receptor and IL-6 stimulates EPC migration and proliferation.31,32 This physiology is consistent with our finding that HIV+ men with undetectable CD34+/KDR+ EPCs had lower median IL-6 levels.
In contrast, sCD163 was higher in the subgroups of HIV+ men with undetectable CD34+/KDR+ and CD34+/CD133+/KDR+ EPCs than in those with detectable EPCs. Since number of EPCs is inversely correlated with the severity of atherosclerosis in the general population,9 our results may suggest significant subclinical atherosclerosis in these men. Furthermore, monocyte/macrophage infiltration is a hallmark of atherosclerosis,33 and higher circulating sCD163 levels have been associated with coronary plaque volume in HIV+ men.34 HIV+ studies with both EPCs and subclinical atherosclerosis assessment are needed to support our findings.
This pilot study has several limitations, including a small sample size that limited our ability to perform multivariate analysis and decreased our power to detect relationships between EPCs and inflammatory biomarkers. In addition, the lack of a control group (HIV− and/or HIV+, ART-naive individuals) prevents complete interpretation of EPCs values and comparison of EPC levels by HIV serostatus or ART treatment status; however, given the accelerated or earlier onset of CVD in HIV-infected patients, the length of duration of HIV infection and long ART treatment history in this cohort, and the high CVD risk factor burden, acquisition of well-matched controls would be difficult and was beyond the scope of this pilot study. Lastly, our results cannot be generalized to all patients living with HIV, as only men who were predominantly white were enrolled in this convenience sample. Despite this limitation, the dramatically low EPC levels in our participants compared with previously reported levels in HIV− and younger HIV+ individuals suggest that a carefully matched control group will be critical for future studies in this population, which is our next step. Despite these limitations, our findings support an association between circulating number of EPCs and CVD risk in treated HIV infection.
In conclusion, our study reports lower and frequently undetectable EPC levels among older, HIV+, ART-treated men with high CVD risk factor burden, and potential relationships between CD34+/KDR+ and CD34+/CD133+/KDR+ EPC subset frequency and circulating levels of IL-6 and monocyte activation. Importantly, our data specifically reflect the physiology of an older group of men with long-standing HIV infection on suppressive ART, who are at higher CVD risk than previously studied, younger HIV+ populations. Therefore, weight must be given to the potential value of our findings, particularly the frequency of extreme EPC suppression, in this high-risk population. Additional clinical studies are needed to further define relationships between EPCs, chronic inflammation and immune activation, and CVD outcomes among older HIV+ adults.
Acknowledgments
We gratefully thank M. Carlson and E. Chang for their contribution in the enrollment of participants, and S. Hirata for her assistance with data collection. This work was supported by National Institutes of Health (grants P30 AG028748, K23 AI110532 to JEL, and 5P30 AI028697).
Author Disclosure Statement
S.S., T.K., D.H., S.P., A.A.M., and J.S.C. have no conflict of interest. J.E.L. has served as a consultant for Gilead Sciences and Merck.
References
- 1.Scarpino M, Pinzone MR, Di Rosa M, et al. : Kidney disease in HIV-infected patients. Eur Rev Med Pharmacol Sci 2013;17:2660–2667 [PubMed] [Google Scholar]
- 2.Currier JS: Update on cardiovascular complications in HIV infection. Top HIV Med 2009;17:98–103 [PubMed] [Google Scholar]
- 3.Post WS, Budoff M, Kingsley L, et al. : Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014;160:458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Garre D, Estrada V, Ortega-Hernandez A, et al. : Association of HIV-Infection and antiretroviral therapy with levels of endothelial progenitor cells and subclinical atherosclerosis. J Acquir Immune Defic Syndr 2012;61:545–551 [DOI] [PubMed] [Google Scholar]
- 5.Lopez M, Vispo E, San Roman J, et al. : Short communication high risk of endothelial dysfunction in HIV individuals may result from deregulation of circulating endothelial cells and endothelial progenitor cells. AIDS Res Hum Retroviruses 2012;28:656–659 [DOI] [PubMed] [Google Scholar]
- 6.Teofili L, Iachininoto MG, Capodimonti S, et al. : Endothelial progenitor cell trafficking in human immunodeficiency virus-infected persons. AIDS 2010;24:2443–2450 [DOI] [PubMed] [Google Scholar]
- 7.Gehling UM, Ergun S, Schumacher U, et al. : In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 2000;95:3106–3112 [PubMed] [Google Scholar]
- 8.Khan SS, Solomon MA, McCoy JP, Jr.: Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom 2005;64:1–8 [DOI] [PubMed] [Google Scholar]
- 9.Chironi G, Walch L, Pernollet MG, et al. : Decreased number of circulating CD34+KDR+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis 2007;191:115–120 [DOI] [PubMed] [Google Scholar]
- 10.Papasavvas E, Hsue P, Reynolds G, et al. : Increased CD34+/KDR+ cells are not associated with carotid artery intima-media thickness progression in chronic HIV-positive subjects. Antivir Ther 2012;17:557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vecchiet J, Iachininoto MG, Capodimonti S, et al. : Effect of antiviral therapy on pro-angiogenic hematopoietic and endothelial progenitor cells in HIV-infected people. Thromb Res 2013;131:238–243 [DOI] [PubMed] [Google Scholar]
- 12.Costiniuk CT, Hibbert BM, Filion LG, et al. : Circulating endothelial progenitor cell levels are not reduced in HIV-infected men. J Infect Dis 2012;205:713–717 [DOI] [PubMed] [Google Scholar]
- 13.Ballard VL, Edelberg JM: Stem cells and the regeneration of the aging cardiovascular system. Circ Res 2007;100:1116–1127 [DOI] [PubMed] [Google Scholar]
- 14.Hill JM, Zalos G, Halcox JP, et al. : Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600 [DOI] [PubMed] [Google Scholar]
- 15.Redondo S, Hristov M, Gordillo-Moscoso AA, Ruiz E, Weber C, Tejerina T: High-reproducible flow cytometric endothelial progenitor cell determination in human peripheral blood as CD34+/CD144+/CD3− lymphocyte sub-population. J Immunol Methods 2008;335:21–27 [DOI] [PubMed] [Google Scholar]
- 16.Sibal L, Aldibbiat A, Agarwal SC, et al. : Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia 2009;52:1464–1473 [DOI] [PubMed] [Google Scholar]
- 17.Costiniuk CT, Hibbert BM, Simard T, Ghazawi FM, Angel JB, O'Brien ER: Circulating endothelial progenitor cells in HIV infection: A systematic review. Trends Cardiovasc Med 2013;23:192–200 [DOI] [PubMed] [Google Scholar]
- 18.Lake JE, Seang S, Kelesidis T, Currier JS, Yang OO: Telmisartan increases vascular reparative capacity in older HIV-infected adults: A pilot study. HIV Clin Trials 2016;17:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimmeler S, Zeiher AM: Vascular repair by circulating endothelial progenitor cells: The missing link in atherosclerosis? J Mol Med (Berl) 2004;82:671–677 [DOI] [PubMed] [Google Scholar]
- 20.George J, Goldstein E, Abashidze S, et al. : Circulating endothelial progenitor cells in patients with unstable angina: Association with systemic inflammation. Eur Heart J 2004;25:1003–1008 [DOI] [PubMed] [Google Scholar]
- 21.Adams V, Lenk K, Linke A, et al. : Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol 2004;24:684–690 [DOI] [PubMed] [Google Scholar]
- 22.Massa M, Rosti V, Ferrario M, et al. : Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 2005;105:199–206 [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. : Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005;111:2981–2987 [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH: Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000;101:1767–1772 [DOI] [PubMed] [Google Scholar]
- 25.Nordell AD, McKenna M, Borges AH, et al. : Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014;3:e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross AC, Rizk N, O'Riordan MA, et al. : Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009;49:1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parra S, Coll B, Aragones G, et al. : Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: Relationships with serum markers of oxidation and inflammation. HIV Med 2010;11:225–231 [DOI] [PubMed] [Google Scholar]
- 28.Merlini E, Luzi K, Suardi E, et al. : T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One 2012;7:e46073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitani H, Katayama N, Araki H, et al. : Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. Br J Haematol 2000;109:288–295 [DOI] [PubMed] [Google Scholar]
- 30.Gabay C: Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006;8 Suppl 2:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Y, Ye J, Shen F, et al. : Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab 2008;28:90–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon YW, Heo SC, Jeong GO, et al. : Tumor necrosis factor-alpha-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim Biophys Acta 2013;1832:2136–2144 [DOI] [PubMed] [Google Scholar]
- 33.Hansson GK, Libby P: The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol 2006;6:508–519 [DOI] [PubMed] [Google Scholar]
- 34.Burdo TH, Lo J, Abbara S, et al. : Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011;204:1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]