Abstract
The study of natural products in biomedical research is not a modern concept. Many of the most successful medical therapeutics are derived from natural products, including those studied in the field of HIV/AIDS. Biomedical research has a rich history of discovery based on screens of medicinal herbs and traditional medicine practices. Compounds derived from natural products, which repress HIV and those that activate latent HIV, have been reported. It is important to remember the tradition in medical research to derive therapies based on these natural products and to overcome the negative perception of natural products as an “alternative medicine.”
Keywords: : natural products, HIV, AIDS, HIV suppression, HIV reactivation
Natural Products and Medical Discoveries
The study of natural products in drug discovery is not a modern concept. In fact many of the most successful medical therapeutics were originally found in nature. There are reports of ancient Egyptians using moldy bread to treat infections, which may have led to the discovery of Penicillin in 1928.1,2 Secreted products of the fungus Penicillium chrysogenum prevented bacterial colony growth and led to the development of Penicillin, which was eventually clinically available in the 1940's. As early as 400 BC the use of willow bark to alleviate pain was reported.3 The active compound was discovered in the 1800's to be salicin, which was later modified with an acetyl group to create aspirin. The bark of the cinchona tree had been used as far back as the 1600's to cure malaria, and was purified into the drug chloroquine in the 1940's.4 The active component of the anticancer drug Taxol is paclitaxel, derived from the bark of the Pacific Yew tree.5,6 Paclitaxel was discovered as part of a screen for anticancer natural plant products in the 1960's.
Many of these products are present as protective defense mechanisms for the plant. The caustic nature of these products is used to deter predators from ingesting the plant, and the medicinal properties are derived from the natural inflammatory and protective properties of these products.7 The origin of many of these drugs is based in traditional medicine practices and the use of the raw unpurified products in these practices was often effective without additional isolation, purification, synthesis, and patenting.
The 2015 Nobel Prize for medicine was shared for the discovery of two of these natural product-based therapeutics, artemisinin and ivermectin.8 Artemisinin was discovered in the late 1960's in a screen of antimalarial Chinese traditional medicinal herbs.9 A crude extract of the leaves of Artemisia annua, known in Traditional Chinese Medicine (TCM) as qinghaosu, was able to fully inhibit the rodent malarial parasite Plasmodium berghei and was later equally effective when given as a crude extract to humans. The isolation and large-scale synthesis of the active compound artemisinin followed in 1972. Avermectin was discovered in the early 1970's through a screen of soil-derived products which had medicinal potential.10 The fermentation product of Streptomyces avermitilis, a soil bacteria, avermectin was highly efficient at killing parasitic larvae. Avermectin was then further modified to create ivermectin, which had additional potency and was safe to use in humans and animals. In 1987 ivermectin was synthesized into an effective drug against river blindness. Altogether, these examples highlight the history of screening natural products for their medicinal properties, and the diverse targets which have benefited from such research.
Current HIV Therapy
While HIV treatments have progressed significantly in the past 20 years, current treatment strategies are far from perfect and most importantly none of the current treatment options results in HIV cure.11 With current antiretroviral therapy (ART), viral titers are maintained below the level of detection in most individuals. However, side effects and lifetime daily administration of these drugs, while the individual feels relatively normal, can result in nonadherence to treatment and treatment interruption. Even a brief interruption in ART results in a rapid rebound of viral titers, which increase the potential for ART escape mutants that require adjustments in maintenance of drug regimen.12–14
In spite of effective suppression of viral replication with suppressive ART, latently infected cells prevent complete clearance by the virus.15 Latently infected cells are not actively replicating or producing viral proteins, which are the targets for ART.16 Therefore, it is crucial to continue research to improve on this imperfect system by finding more effective suppressive agents and discovering a means to safely reactivate latent HIV, as a means to target and eliminate latently infected cells.
Natural Products and HIV
HIV suppression
The bulk of HIV research using natural product-based compounds is based on suppression of the virus. Several effective plant-based compounds were studied early in the history of HIV research and continue to be studied today. These novel inhibitory compounds may lead to new ART, which have increased suppressive function, are available in the case of escaped mutants, and are possibly more cost effective treatment options (Fig. 1).
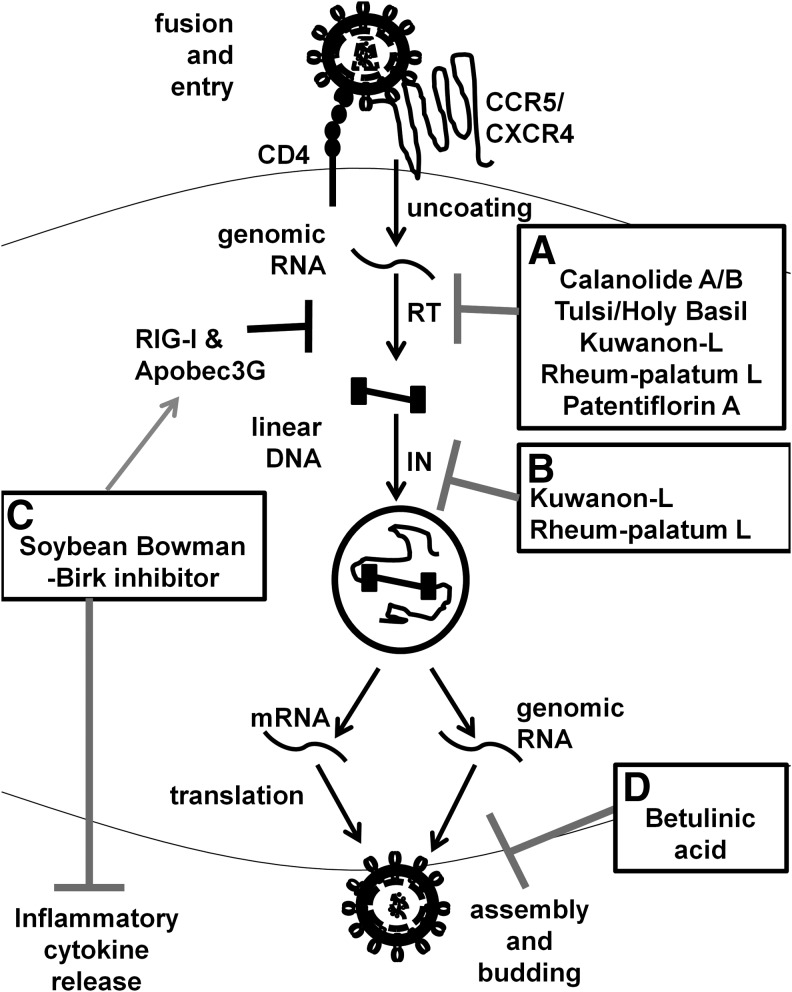
FIG. 1.
Natural products that suppress HIV replication. Natural product-derived compounds have been studied, which target multiple steps in HIV replication (A) Calanolide A/B, Tulsi/Holy Basil, Kuwanon-L, Rheum-palatum L, and Patentiflorin A inhibit HIV RT, blocking the reverse transcription of HIV genomic RNA into proviral DNA. (B) In addition to their anti-RT activity, Kuwanon-L and Rheum-palatum L have anti-IN activity, which prevents integration HIV proviral DNA into the host genome. (C) The soybean-derived Bowman-Birk inhibitor increases cellular expression of HIV restriction factors, RIG-I and Apobec3G, which also inhibit at the RT step. Additionally Bowman-Birk inhibitor also inhibits expression of inflammatory cytokines. (D) Betulinic acid inhibits HIV Gag processing and prevents release of infectious viral particles. IN, integrase; RT, reverse transcriptase.
Calanolides were among the first plant-based compounds described to have anti-HIV activity,17,18 derived from Calophyllum lanigerum, a mangosteen family tree found in the tropical rainforests of Malaysia. The medicinal properties were discovered after a 1992 National Institute of Health (NIH)-sponsored sample-collecting study. Calanolide A and B, isolated from fruit and twigs of the tree, were purified and determined to have nonnucleoside reverse transcriptase (RT) inhibitor activity17 (Fig. 1A). The scarcity of C. lanigerum, due to destruction of the natural rainforest habitat, made it necessary for Calanolide A and B to be synthesized.19 Both the drugs are well tolerated and have been in development.20
Promising research continues in a variety of natural plant-based therapeutics with anti-HIV activity. Tulsi or Holy Basil is traditionally used in Indian Ayurvedic medicine to treat a number of ailments and is generally regarded as promoting overall wellbeing. A combination of esters and amides isolated from tulsi have in vitro anti-RT activity21 (Fig. 1A). Kuwanon-L, isolated from the black mulberry tree Morus nigra, has anti-RT and integrase (IN) activity comparable to ART22,23 (Fig. 1A, B). Rheum palmatum L, a TCM plant, also showed potent anti-RT and IN activity comparable to ART24 (Fig. 1A, B). Patentiflorin A, an arylnaphthaline lignin glycoside isolated from the Vietnamese medicinal plant Justica gendarussa had RT-inhibitor activity and inhibited azidothymidine (AZT) and Nevirapine-resistant HIV isolates25 (Fig. 1A). A soybean-derived protease inhibitor, Bowman-Birk inhibitor, inhibits inflammatory cytokines and replication in macrophages in addition to increasing expression of antiviral factors, including RIG-I and Apobec 3G26 (Fig. 1C).
Betulinic acid and dihydro betulinic acid were isolated from the Chinese herb Syzygium claviflorum, or trumpet satinash, in 1996.27 Betulinic acid was effective at inhibiting late-stage processing Gag and resulted in the release of noninfectious viral particles28 (Fig. 1D). It has been synthesized as the drug, Bevirimat, is well tolerated, and continues in development following clinical trials in 2007.29 Possible candidates for ART may be discovered through further screens of medicinal plants as well as further development of these candidates currently under investigation.
HIV reactivation
An even more promising area of HIV research seeks to find natural products that activate HIV transcription with the goal toward achieving a functional cure30,31 (Fig. 2). Activation is required to target reservoirs of latently infected cells and enables the killing and clearance of latently infected cells through immune clearance and targeting by ART.16 A major hurdle toward reactivating latently infected cells is that these cells are quiescent and lack expression of important activating factors, which are required for host gene expression and efficient HIV transcription: nuclear factor kappa B (NF-κB), positive elongation factor b (P-TEFb), and cyclin-dependant kinase (CDK) 11.32,33 NF-κB regulates transcriptional initiation, and is induced by multiple signaling pathways.34 P-TEFb exists in the cell in equilibrium between an inactive complex (with 7SK-RNA and Hexim1) and an active complex of free P-TEFb.35,36 However, without sufficient expression of P-TEFb components, CDK9 and cyclin T1 (CycT1), reactivation therapeutics that function by releasing P-TEFb from this inactive complex are unable to function.32 CDK11 is important in proper 3′ end processing of HIV mRNA. Without appropriate cleavage and polyadenylation, HIV mRNA is degraded.33
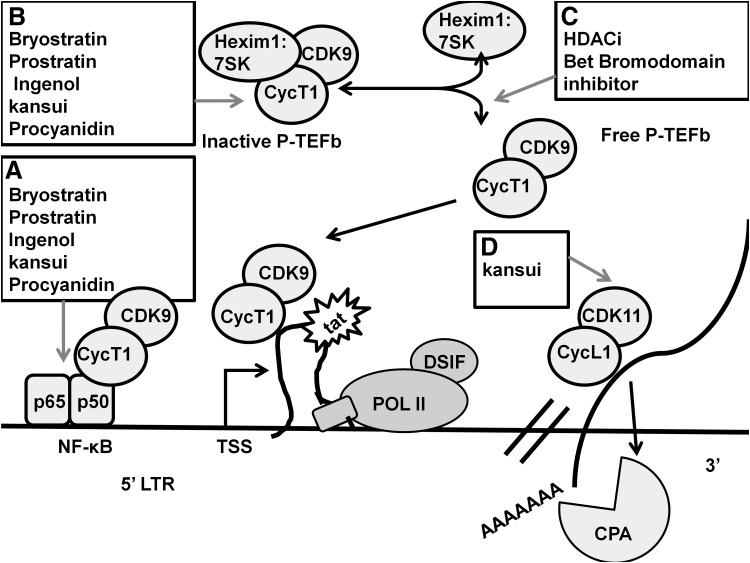
FIG. 2.
Natural products that activate HIV transcription. Natural product-derived compounds have been studied, which reactivate latent HIV by inducing HIV transcription. (A) PKC agonist (bryostatin, prostratin, and ingenol), a crude extract of kansui and procyanidin, activate cellular NF-κB, which contributes to transcription initiation. (B) PKC agonist (bryostatin, prostratin, and ingenol), a crude extract of kansui, and procyanidin induce cellular expression of CycT1, part of the P-TEFb complex. (C) Increase in total CycT1 permits HDACi and BET-bromodomain inhibitors to function by activating P-TEFb and releasing it from its inactive complex with Hexim1:7SK. Altogether, increase in expression and release of free P-TEFb allow recruitment of P-TEFb to NF-κB and POLII, resulting in transcription elongation. (D) A crude extract of kansui further reactivates latent HIV by increasing cellular expression of CDK11, which complexes with CycL to promote cleavage and polyadenylation (CPA) recruitment and proper mRNA end processing. NF-κB, nuclear factor kappa B; PKC, protein kinase C; P-TEFb, positive transcription elongation factor b.
To fully reactivate latent HIV, cellular expression of these factors must first be increased.16 The caveat to increasing cellular transcriptional factors is that activation is not HIV specific and care must be given to prevent global T cell activation and inflammatory cytokine storm, while still delivering a potent-enough activation signal to trigger transcription of latent HIV.37 Several groups are currently researching combination therapies, which will potentially reduce effective doses of each compound, so that nonspecific inflammatory activation is potentially reduced.37–39 Furthermore, reactivation while continuous ART administration is crucial to prevent uncontrolled viral rebound.
Activation through the protein kinase C (PKC) pathway has become the most well-studied method of latent HIV reactivation (Fig. 2). Several of the most prominent candidates are based on PKC agonists found in natural products. PKC agonists overcome the failing of the first studied reactivation compounds, primarily HDAC inhibitors (HDACi), which function through release of P-TEFb from its inactive complex40,41 (Fig. 2C). These drugs alone fail to reactive latent HIV in primary CD4+ latency models or in clinical trials, due to low levels of P-TEFb in resting cells.42,43 Because of the potent activation induced by PKC agonists, several groups are exploring combinatorial treatments which pair a low dose of a PKC agonist with a latency-reversing agent (LRA) (such as HDACi or a Bet bromodomain inhibitor).37–39,44 This treatment would allow for a nontoxic dose of the PKC agonist to prime the cell and allow for further activation by the LRA.
Prostratin, isolated from the bark of Homalanthus nutans, the Samoan mamala tree, was used by traditional medicine practitioners as a treatment for hepatitis.45 Samples of mamala were sent to NIH in 1990 to isolate the active compounds. A partnership was created between UC Berkeley and the Samoan islands for the rights to these trees and whatever medicinal compounds were isolated from them.46 Obtaining enough of the natural source of prostratin limits its wide-spread use as a therapeutic PKC agonist.
Bryostatin, from the marine bryozoans Bugula neritina, as discovered in 1968 after a marine biologist collected a sample of the seaweed-like Bugula and sent it to the National Cancer Institute to be analyzed for any possible anticancer properties.47 Bryostatin proved to be an effective in vitro anticancer agent, and more recently was reported to help prevent memory loss in Alzheimer's disease.47,48 However, synthesis of bryostatin is prohibitive to large-scale production and use as a therapeutic agent, as a ton of Bugula is required to make 1 g of bryostatin.49
Plants of the Euphorbia species are the natural plant source of a third PKC agonist, ingenol.50,51 Euphorbia are common plants found worldwide. Aveloz, or Euphorbia tirucalli, has been used in Brazil for the treatment of cancer.52 Ingenol mebutate, from the sap of Euphorbia peplums, is an approved topical skin treatment against precancerous actinic keratosis.53 Ingenol dibenzoate is a purified commercially available ingenol with very potent PKC agonist activity.54
Several groups have shown the efficacy of purified bryostatin55 (Fig. 2A, B), prostratin56 (Fig. 2A, B), and ingenol54,57 (Fig. 2A–C) as LRAs in cell lines, primary CD4+ T cells, and in HIV+ ART-suppressed patient cells in vitro. However, toxicity of these highly effective PKC agonists is an ongoing concern, as well as necessity to deliver the drugs through injection. Currently, synthetic analogs of bryostatin58,59 and prostratin60 are being explored, with reduced toxicity and cost of synthesis compared with their natural source counterparts. Ingenol B is a semisynthetic ingenol, which is less toxic and exhibits greater stability.32,61,62 Ingenol B has been safely tested in macaques.63
Euphorbia kansui (kansui), one of the plants of the Euphorbia species, has been used for thousands of years in TCM to treat fluid retention,64 cancer,52 and acities65 with minimal reported toxicity, primarily loose stool.66 Kansui contains 12 ingenols as well as sesquiterpenoids, triterpenoids, and euphols.50,51,67 Euphols have anti-inflammatory properties, which may dampen the inflammatory toxicity induced by the PKC agonist activity of ingenol.68 A crude extract of Euphorbia reactivates latent HIV by increasing cellular P-TEFb and CDK11 with similar efficacy to purified ingenol39 (Fig. 2A–C). Kansui is currently being studied in a small clinical trial with human and NHP subjects as a potential clinical LRA.69
Procyanidins are flavonoids found in a variety of plant sources, including grape, apple, cinnamon, and cacao.70–72 The antioxidant benefits of procyanidins have been reported, and plant source-derived procyanidin is available in supplement form. Procyanidin C1 isolated from Theobroma cacao, the plant source of cocoa reactivates latent HIV through the MAPK pathway, and shows synergistic activation when added in combination with the PKC agonist Phorbol 12-myristate 13-acetate (PMA)73 (Fig. 2A). Cocoa itself has a number of reported health benefits, including cardiovascular and metabolic health benefits as well as potential anti-inflammatory and anticancer properties.74,75 Procyanidin C-13,3′3″-tri-O-gallate was isolated from Polygonum cuspidatum Sieb. et Zucc, the Japanese knotweed plant.76 Knotweed, which been used in TCM to maintain cardiac health, also reactivates latent HIV in cell lines through activation of P-TEFb (Fig. 2B).
Functional Cure
A recent report from AIDS and Human Retroviruses suggests an intriguing cure strategy following treatment with combination of traditional Chinese herbs.77,78 A combination of 13 different plants, including Astragalus, Skullcap, and Ginseng, was administered to nine patients from 2001 to 2009.77 Eight patents displayed below detection limit viral titers in 2016; four of these patients were ART naive. Unfortunately, it is unclear of the underlying mechanism at work due to lack of follow through and collapse of the company conducting the study. However, the overall outcome is incredibly encouraging in spite of lack of consistent records and proper controls throughout the study. A follow-up on this study would provide insight into the mechanisms controlling suppression of HIV in the absence of sustained therapy, which were hinted at in this study.
Summary and Conclusions
It is clear from the historical evidence presented in this review that the scientific community has a long and rich history of screening plant compounds and mining traditional medical practices for the discovery of anti-HIV compounds (Table 1). Researchers need to apply same rigorous research standards to natural products as to purified compounds, to ensure that good science is the basis for these natural product-based discoveries. This includes well-controlled experiments, using the same gold standard experimentation used to test and validate the safety and efficacy of drugs. Extensive high-performance liquid chromatography will ensure a full understanding of the composition of the raw natural product, and whether other active components exist in the crude preparations, which have additional therapeutic effects. Pharmacokinetic studies in animal models are important to determine where in the body these products target and how stable they are following administration. Most importantly, clinical testing is essential, to ensure safe and consistent application of these products. Thorough safety and efficacy studies will provide nontoxic dosage guidelines and dosing strategies. Traditional medicine uses may provide a basis for safely tolerated doses and administration methods in humans. Often these traditional practices have safe doses and procedures, which can be used to implement these products into a conventional medical treatment. Additionally, most of these raw natural products are not considered drugs by the Food and Drug Administration, which may shorten the time required to approve their use. However, only after meticulously testing, should these natural product-based therapies be integrated into a physician-supervised treatment, including continued ART.
Table 1.
Summary of Natural Products and Their Activity Against HIV
| Natural product | Active compound(s) | Traditional use | Anti-HIV activity | In vivo application | |
|---|---|---|---|---|---|
| HIV suppression | Calophyllum lanigerum | Calanolide A and B | NNRT inhibitor | Safety and pharmacokinetics of calanolide A in HIV− patients.20 | |
| Syzygium claviflorum (trumpet satinash) | Betulinic acid and dihydro betulinic acid | Maturation inhibitor | Safety, pharmacokinetic, and antiviral effects of Bevirimat in HIV+ patients.29 | ||
| Tulsi or Holy Basil | Combination of esters and amides | Indian ayurvedic medicine | RT inhibitor | ||
| Morus nigra (black mulberry) | Kuwanon-L | RT and IN inhibitor | |||
| Rheum palmatum L | TCM | RT and IN inhibitor | |||
| Justica gendarussa | Patentiflorin A | Vietnamese medicinal plant | RT inhibitor | ||
| Soybean | Bowman-Birk inhibitor | Inhibits inflammatory cytokines, induces restriction factors | |||
| HIV reactivation | Theobroma cacao | Procyanidin C1 | MAPK agonist | ||
| Polygonum cuspidatum Sieb. et Zucc (Japanese knotweed) | Procyanidin C-13,3′3″-tri-O-gallate | TCM | P-TEFb and NF-κB | ||
| Bugula neritina | Bryostatin | PKC agonist | Safety and efficacy of bryostatin analogs in humanized mouse model.59 | ||
| Homalanthus nutans (Samoan mamala tree) | Prostratin | PKC agonist | |||
| Euphorbia kansui | Ingenol | TCM | PKC agonist | Safety and efficacy of Ingenol B in macaque CNS model of HIV infection.62 |
IN, integrase; NF-κB, nuclear factor kappa B; PKC, protein kinase C; P-TEFb, positive transcription elongation factor b; RT, reverse transcriptase; TCM, Traditional Chinese Medicine.
Many natural products contain combinations of active ingredients, which may mitigate the toxic effects of the primary active compounds. Many traditional medicinal plants are consumed as a crude extract or tea, which are proven to be effective in the unprocessed/unpurified form. This natural-occurring mixture may be more effective than single isolated compound. As is the case with kansui, the presence of anti-inflammatory compounds in the crude extract may diminish the toxic side effects of the potent active compound. The basis of ancient medical practices and use of crude plant extracts may even prove a better strategy than creating a drug based on a single isolated active ingredient. It may be beneficial to integrate certain traditional medicine dosing practices when using these natural products.
One major hurdle to overcome in the application of natural product-based strategies is the negative perception of therapeutics perceived as “alternative medicine.” Integration of these new treatments into western medical regimes, under regular physician observation is the key to prevent misuse, abandonment of suppressive ART, and self-diagnosis and treatment. Newly discovered suppressive agents could be used to enhance current ART, or provide more affordable options. Any latency reversal strategy would need to be conducted while on ART. Additionally, researchers should strive to publish in journals not solely devoted to natural product or alternative medicine research, and in turn reviewers should not balk at the mention of natural products or traditional medicinal plants. With vigorous and extensive research, these therapeutics are not just an alternative, but rather hypothesis-driven, science-based discoveries, which should be regarded the same as isolated, purified, and druggable compounds.
The extensive history and success of natural product-based therapeutics should inform the future of this line of research. Moving forward, embracing research into natural products can only benefit HIV/AIDS research. Solid science into purified and natural compounds should be regarded as equal. Above all, the welfare and needs of the HIV+ patient population are more important than procuring a patented drug. For these reasons, emerging research should continue to contribute to the rich history of discoveries and scientific progress based on products found in nature. In pursuing these leads, one could envision a collaborative effort between NIH-funded and pharmaceutical research, possibly guided by agricultural research. In this way, large screens of plants could identify novel natural product sources; the identification of purified active compounds from natural sources could be further pursued; and continued effort could be made to move forward with innovative therapeutic strategies.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Society AC: Discovery and development of penicillin. www.acs.org/content/acs/en/education/whatischemistry/landmarks/flemingpenicillin.html Accessed July9, 2017
- 2.Hare R: New light on the history of penicillin. Med Hist 1982;26:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desborough MJR, Keeling DM: The aspirin story—From willow to wonder drug. Br J Haematol 2017;177:674–683 [DOI] [PubMed] [Google Scholar]
- 4.Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, et al. : Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar J 2011;10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landmarks: ACSNHC. Discovery of Camptothecin and Taxol®. www.acs.org/content/acs/en/education/whatischemistry/landmarks/camptothecintaxol.html Accessed July9, 2017
- 6.Cragg GM: Paclitaxel (Taxol): A success story with valuable lessons for natural product drug discovery and development. Med Res Rev 1998;18:315–331 [DOI] [PubMed] [Google Scholar]
- 7.Dias DA, Urban S, Roessner U: A historical overview of natural products in drug discovery. Metabolites 2012;2:303–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su X-Z, Miller LH: The discovery of artemisinin and Nobel Prize in Physiology or Medicine. Sci China Life Sci 2015;58:1175–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu Y: The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med 2011;17:1217–1220 [DOI] [PubMed] [Google Scholar]
- 10.Landmarks: ACSNHC. Discovery of ivermectin. www.acs.org/content/acs/en/education/whatischemistry/landmarks/ivermectin-mectizan.html Accessed July9, 2017
- 11.UNAIDS: AIDS by the numbers 2015. Joint United Nations Programme on HIV/AIDS (UNAIDS) 2015
- 12.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, et al. : Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 1997;94:13193–13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. : Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science (New York, NY) 1997;278:1295–1300 [DOI] [PubMed] [Google Scholar]
- 14.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. : Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science (New York, NY) 1997;278:1291–1295 [DOI] [PubMed] [Google Scholar]
- 15.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, et al. : Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013;155:540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cary DC, Fujinaga K, Peterlin BM: Molecular mechanisms of HIV latency. J Clin Invest 2016;126:448–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashman Y, Gustafson KR, Fuller RW, Cardellina JH, 2nd, McMahon JB, Currens MJ, et al. : The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J Med Chem 1992;35:2735–2743 [DOI] [PubMed] [Google Scholar]
- 18.McKee TC, Covington CD, Fuller RW, Bokesch HR, Young S, Cardellina IJ, et al. : Pyranocoumarins from tropical species of the genus Calophyllum: A chemotaxonomic study of extracts in the National Cancer Institute collection. J Nat Prod 1998;61:1252–1256 [DOI] [PubMed] [Google Scholar]
- 19.Newman RA, Chen W, Madden TL: Pharmaceutical properties of related calanolide compounds with activity against human immunodeficiency virus. J Pharm Sci 1998;87:1077–1080 [DOI] [PubMed] [Google Scholar]
- 20.Creagh T, Ruckle JL, Tolbert DT, Giltner J, Eiznhamer DA, Dutta B, et al. : Safety and pharmacokinetics of single doses of (+)-calanolide a, a novel, naturally occurring nonnucleoside reverse transcriptase inhibitor, in healthy, human immunodeficiency virus-negative human subjects. Antimicrob Agents Chemother 2001;45:1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonar VP, Corona A, Distinto S, Maccioni E, Meleddu R, Fois B, et al. : Natural product-inspired esters and amides of ferulic and caffeic acid as dual inhibitors of HIV-1 reverse transcriptase. Eur J Med Chem 2017;130:248–260 [DOI] [PubMed] [Google Scholar]
- 22.Esposito F, Tintori C, Martini R, Christ F, Debyser Z, Ferrarese R, et al. : Kuwanon-L as a new allosteric HIV-1 integrase inhibitor: Molecular modeling and biological evaluation. Chembiochem 2015;16:2507–2512 [DOI] [PubMed] [Google Scholar]
- 23.Martini R, Esposito F, Corona A, Ferrarese R, Ceresola ER, Visconti L, et al. : Natural product Kuwanon-L inhibits HIV-1 replication through multiple target binding. Chembiochem 2017;18:374–377 [DOI] [PubMed] [Google Scholar]
- 24.Esposito F, Carli I, Del Vecchio C, Xu L, Corona A, Grandi N, et al. : Sennoside A, derived from the traditional chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine 2016;23:1383–1391 [DOI] [PubMed] [Google Scholar]
- 25.Zhang HJ, Rumschlag-Booms E, Guan YF, Wang DY, Liu KL, Li WF, et al. : Potent inhibitor of drug-resistant HIV-1 strains identified from the medicinal plant Justicia gendarussa. J Nat Prod 2017;80:1798–1807 [DOI] [PubMed] [Google Scholar]
- 26.Ma TC, Zhou RH, Wang X, Li JL, Sang M, Zhou L, et al. : Soybean-derived Bowman-Birk Inhibitor (BBI) inhibits HIV replication in macrophages. Sci Rep 2016;6:34752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto F, Kashiwada Y, Cosentino LM, Chen CH, Garrett PE, Lee KH: Anti-AIDS agents—XXVII. Synthesis and anti-HIV activity of betulinic acid and dihydrobetulinic acid derivatives. Bioorg Med Chem 1997;5:2133–2143 [DOI] [PubMed] [Google Scholar]
- 28.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH: Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem 1996;39:1016–1017 [DOI] [PubMed] [Google Scholar]
- 29.Smith PF, Ogundele A, Forrest A, Wilton J, Salzwedel K, Doto J, et al. : Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-o-(3′,3′-dimethylsuccinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob Agents Chemother 2007;51:3574–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Archin NM, Margolis DM: Emerging strategies to deplete the HIV reservoir. Curr Opin Infect Dis 2014;27:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deeks SG: HIV: Shock and kill. Nature 2012;487:439–440 [DOI] [PubMed] [Google Scholar]
- 32.Pandelo Jose D, Bartholomeeusen K, da Cunha RD, Abreu CM, Glinski J, da Costa TB, et al. : Reactivation of latent HIV-1 by new semi-synthetic ingenol esters. Virology 2014;462–463:328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pak V, Eifler TT, Jager S, Krogan NJ, Fujinaga K, Peterlin BM. CDK11 in TREX/THOC Regulates HIV mRNA 3′ End Processing. Cell host & microbe. 2015;18:560–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nabel G, Baltimore D: An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 1987;326:711–713 [DOI] [PubMed] [Google Scholar]
- 35.Sedore SC, Byers SA, Biglione S, Price JP, Maury WJ, Price DH: Manipulation of P-TEFb control machinery by HIV: Recruitment of P-TEFb from the large form by Tat and binding of HEXIM1 to TAR. Nucleic Acids Res 2007;35:4347–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He N, Pezda AC, Zhou Q: Modulation of a P-TEFb functional equilibrium for the global control of cell growth and differentiation. Mol Cell Biol 2006;26:7068–7076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laird GM, Bullen CK, Rosenbloom DI, Martin AR, Hill AL, Durand CM, et al. : Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest 2015;125:1901–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF: New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014;20:425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cary DC, Fujinaga K, Peterlin BM: Euphorbia kansui reactivates latent HIV. PLoS One 2016;11:e0168027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM: Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem 2012;287:36609–36616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartholomeeusen K, Fujinaga K, Xiang Y, Peterlin BM: Histone deacetylase inhibitors (HDACis) that release the positive transcription elongation factor b (P-TEFb) from its inhibitory complex also activate HIV transcription. J Biol Chem 2013;288:14400–14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, et al. : An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 2013;9:e1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blazkova J, Chun TW, Belay BW, Murray D, Justement JS, Funk EK, et al. : Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4(+) T cells from infected individuals receiving effective antiretroviral therapy. J Infect Dis 2012;206:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang G, Mendes EA, Kaiser P, Wong DP, Tang Y, Cai I, et al. : Synergistic reactivation of latent HIV expression by ingenol-3-angelate, PEP005, targeted NF-kB signaling in combination with JQ1 induced p-TEFb activation. PLoS Pathog 2015;11:e1005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox PA, Johnson HE, Tavana G: Giving Samoan healers credit for prostratin. Science (New York, NY) 2008;320:1589. [DOI] [PubMed] [Google Scholar]
- 46.Cox PA: Ensuring equitable benefits: The falealupo covenant and the isolation of anti-viral drug prostratin from a samoan medicinal plant. Pharm Biol 2001;39 Suppl 1:33–40 [DOI] [PubMed] [Google Scholar]
- 47.Pettit GR, Kamano Y, Herald CL: Antineoplastic agents, 118. Isolation and structure of bryostatin 9. J Nat Prod 1986;49:661–664 [DOI] [PubMed] [Google Scholar]
- 48.Schrott LM, Jackson K, Yi P, Dietz F, Johnson GS, Basting TF, et al. : Acute oral Bryostatin-1 administration improves learning deficits in the APP/PS1 transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res 2015;12:22–31 [DOI] [PubMed] [Google Scholar]
- 49.Schaufelberger DE, Koleck MP, Beutler JA, Vatakis AM, Alvarado AB, Andrews P, et al. : The large-scale isolation of bryostatin 1 from Bugula neritina following current good manufacturing practices. J Nat Prod 1991;54:1265–1270 [DOI] [PubMed] [Google Scholar]
- 50.Wang HY, Wang JS, Wei DD, Wang XB, Luo J, Yang MH, et al. : Bioactivity-guided isolation of antiproliferative diterpenoids from Euphorbia kansui. Phytotherapy research: PTR 2012;26:853–859 [DOI] [PubMed] [Google Scholar]
- 51.Shi QW, Su XH, Kiyota H: Chemical and pharmacological research of the plants in genus Euphorbia. Chem Rev 2008;108:4295–4327 [DOI] [PubMed] [Google Scholar]
- 52.Kupchan SM, Uchida I, Branfman AR, Dailey RG, Jr., Fei BY: Antileukemic principles isolated from euphorbiaceae plants. Science (New York, NY) 1976;191:571–572 [DOI] [PubMed] [Google Scholar]
- 53.Anderson L, Schmieder GJ, Werschler WP, Tschen EH, Ling MR, Stough DB, et al. : Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol 2009;60:934–943 [DOI] [PubMed] [Google Scholar]
- 54.Spivak AM, Bosque A, Balch AH, Smyth D, Martins L, Planelles V: Ex vivo bioactivity and HIV-1 latency reversal by ingenol dibenzoate and panobinostat in resting CD4+ T cells from aviremic patients. Antimicrob Agents Chemother 2015;59:5984–5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez M, de Vinuesa AG, Sanchez-Duffhues G, Marquez N, Bellido ML, Munoz-Fernandez MA, et al. : Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Curr HIV Res 2010;8:418–429 [DOI] [PubMed] [Google Scholar]
- 56.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA: Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol 2002;76:8118–8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang P, Lu P, Qu X, Shen Y, Zeng H, Zhu X, et al. : Reactivation of HIV-1 from latency by an ingenol derivative from Euphorbia kansui. Sci Rep 2017;7:9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeChristopher BA, Loy BA, Marsden MD, Schrier AJ, Zack JA, Wender PA: Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat Chem 2012;4:705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marsden MD, Loy BA, Wu X, Ramirez CM, Schrier AJ, Murray D, et al. : In vivo activation of latent HIV with a synthetic bryostatin analog effects both latent cell “kick” and “kill” in strategy for virus eradication. PLoS Pathog 2017;13:e1006575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beans EJ, Fournogerakis D, Gauntlett C, Heumann LV, Kramer R, Marsden MD, et al. : Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci U S A 2013;110:11698–11703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abreu CM, Price SL, Shirk EN, Cunha RD, Pianowski LF, Clements JE, et al. : Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: Inhibition of de novo infection and activation of viral LTR. PLoS One 2014;9:e97257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang G, Mendes EA, Kaiser P, Sankaran-Walters S, Tang Y, Weber MG, et al. : Reactivation of HIV latency by a newly modified Ingenol derivative via protein kinase Cdelta-NF-kappaB signaling. AIDS (London, England) 2014;28:1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gama L, Abreu CM, Shirk EN, Price SL, Li M, Laird GM, et al. : Reactivation of SIV reservoirs in the brain of virally suppressed macaques. AIDS (London, England) 2017;31:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gu XD, Zhang Q: Clinical progress in the treatment of severe acute pancreatitis with integrative Chinese and Western medicine. Chin J Integr Med 2007;13:235–240 [DOI] [PubMed] [Google Scholar]
- 65.Xing F, Tan Y, Yan GJ, Zhang JJ, Shi ZH, Tan SZ, et al. : Effects of Chinese herbal cataplasm Xiaozhang Tie on cirrhotic ascites. J Ethnopharmacol 2012;139:343–349 [DOI] [PubMed] [Google Scholar]
- 66.Bensky D, Clavey S, Stoger E: Chinese Herbal Medicine: Materia Medica, 3rd ed. Eastland Press, Vista, CA, 2004 [Google Scholar]
- 67.Hou JJ, Wu WY, Liang J, Yang Z, Long HL, Cai LY, et al. : A single, multi-faceted, enhanced strategy to quantify the chromatographically diverse constituents in the roots of Euphorbia kansui. J Pharm Biomed Anal 2014;88:321–330 [DOI] [PubMed] [Google Scholar]
- 68.Dutra RC, Claudino RF, Bento AF, Marcon R, Schmidt EC, Bouzon ZL, et al. : Preventive and therapeutic euphol treatment attenuates experimental colitis in mice. PLoS One 2011;6:e27122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.ClinicalTrials.gov: Immunologic Response to Kansui in Treated HIV+ Individuals: A Dose Escalation Study. (2015). Retreived from https://clinicaltrials.gov/ct2/show/NCT02531295 (Id No: NCT02531295)
- 70.De Rosso M, Panighel A, Vedova AD, Gardiman M, Flamini R: Characterization of non-anthocyanic flavonoids in some hybrid red grape extracts potentially interesting for industrial uses. Molecules (Basel, Switzerland) 2015;20:18095–18106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byun EB, Sung NY, Byun EH, Song DS, Kim JK, Park JH, et al. : The procyanidin trimer C1 inhibits LPS-induced MAPK and NF-kappaB signaling through TLR4 in macrophages. Int Immunopharmacol 2013;15:450–456 [DOI] [PubMed] [Google Scholar]
- 72.Sung NY, Yang MS, Song DS, Byun EB, Kim JK, Park JH, et al. : The procyanidin trimer C1 induces macrophage activation via NF-kappaB and MAPK pathways, leading to Th1 polarization in murine splenocytes. Eur J Pharmacol 2013;714:218–228 [DOI] [PubMed] [Google Scholar]
- 73.Hori T, Barnor J, Huu TN, Morinaga O, Hamano A, Ndzinu J, et al. : Procyanidin trimer C1 derived from Theobroma cacao reactivates latent human immunodeficiency virus type 1 provirus. Biochem Biophys Res Commun 2015;459:288–293 [DOI] [PubMed] [Google Scholar]
- 74.Khan N, Khymenets O, Urpi-Sarda M, Tulipani S, Garcia-Aloy M, Monagas M, et al. : Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014;6:844–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andujar I, Recio MC, Giner RM, Rios JL: Cocoa polyphenols and their potential benefits for human health. Oxid Med Cell Longev 2012;2012:906252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang C, Yang S, Lu H, You H, Ni M, Shan W, et al. : A natural product from Polygonum cuspidatum Sieb. Et Zucc. promotes Tat-dependent HIV latency reversal through triggering P-TEFb's release from 7SK snRNP. PLoS One 2015;10:e0142739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Jin F, Wang Q, Suo Z: Long-term survival of AIDS patients treated with only Traditional Chinese Medicine. AIDS Res Hum Retroviruses 2017;33:90–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hope TJ: Can a Traditional Chinese Medicine contribute to a cure for HIV? AIDS Res Hum Retroviruses 2017;33:89. [DOI] [PubMed] [Google Scholar]