Abstract
Objective: To evaluate the short-term efficacy and safety of desvenlafaxine versus placebo in the treatment of children and adolescents with major depressive disorder (MDD).
Methods: Outpatient children (7–11 years) and adolescents (12–17 years) who met DSM-IV-TR criteria for MDD and had screening and baseline Children's Depression Rating Scale-Revised (CDRS-R) total scores >40 were randomly assigned to 8 weeks of treatment with placebo, low exposure desvenlafaxine (20, 30, or 35 mg/day based on baseline weight), or higher exposure desvenlafaxine (25, 35, or 50 mg/day based on baseline weight). The primary efficacy endpoint was change from baseline in CDRS-R total score at week 8, analyzed using a mixed-effects model for repeated measures. Secondary efficacy assessments included Clinical Global Impressions-Severity and Clinical Global Impressions-Improvement scales. Safety assessments included adverse events and the Columbia-Suicide Severity Rating Scale.
Results: The safety population included 363 patients (children, n = 109; adolescents, n = 254). No statistical separation from placebo was observed for either desvenlafaxine group for CDRS-R total score or for any secondary efficacy endpoint. At week 8, adjusted mean (standard error) changes from baseline in CDRS-R total score for the desvenlafaxine low exposure, desvenlafaxine high exposure, and placebo groups were −23.7 (1.1), −24.4 (1.1), and −22.9 (1.1), respectively. The incidence of adverse events was similar among groups.
Conclusion: Low and high exposure desvenlafaxine groups did not demonstrate efficacy for the treatment of MDD in children and adolescents in this double-blind, placebo-controlled trial. Desvenlafaxine (20–50 mg/day) was generally safe and well tolerated with no new safety signals identified in pediatric patients with MDD in this study.
Keywords: : desvenlafaxine, major depressive disorder, treatment efficacy, clinical trial, children, adolescents
Introduction
Major depressive disorder (MDD) is a primary cause of disability in the United States, with prevalence rates of up to ∼8% in adolescents and 2.5% in children (Birmaher et al. 1996). Depression in youth can substantially impair trajectories of development and overall quality of life (Siu 2016). Pediatric depression can negatively impact social and family relationships, and frequently disrupts academic performance and activities (Puig-Antich et al. 1993; Birmaher et al. 1996, 2007; Fergusson and Woodward 2002). Furthermore, pediatric patients with depression are at risk for substance abuse, suicidal behavior, medical and psychiatric hospitalization, and recurrence of depression (Weissman et al. 1999; Fergusson and Woodward 2002; Marmorstein 2009; Edwards et al. 2014). To address the significant morbidity and mortality associated with depression, the US Preventive Services Task Force recommends screening for MDD in all pediatric patients 12–18 years of age (Siu 2016).
Practice guidelines recommend antidepressant therapy, psychological intervention, or both, for the treatment of children and adolescents with moderate to severe depression (Birmaher et al. 2007; Cheung et al. 2007). The efficacy and safety of various selective serotonin reuptake inhibitors (SSRIs) and serotonin–norepinephrine reuptake inhibitors (SNRIs) have been studied in pediatric patients with MDD (Emslie et al. 1997, 2007, 2009, 2014; Wagner et al. 2003, 2004; Atkinson et al. 2014); however, only the SSRIs fluoxetine and escitalopram are approved in the United States for the treatment of MDD in children ≥8 years of age (fluoxetine) and adolescents 12–17 years of age (escitalopram) (Prozac Package Insert, 2014; Lexapro Package Insert, 2014). Currently available antidepressants may not be safe and effective for all children and adolescents with MDD; thus, there is an unmet need for additional treatment options.
Desvenlafaxine (administered as desvenlafaxine succinate) is an SNRI approved for the treatment of adults with MDD at the recommended therapeutic dose of 50 mg/day (Pristiq Package Insert, 2016). Treatment with desvenlafaxine doses lower than 50 mg has not demonstrated significant clinical benefit versus placebo in adults (Iwata et al. 2013; Liebowitz et al. 2013). The safety and tolerability of desvenlafaxine in children and adolescents with MDD were assessed in a small, 8-week, open-label, fixed-dose phase 2 study (Findling et al. 2014). Desvenlafaxine doses ranging from 10 to 200 mg/day were generally safe and well tolerated in that study. Although efficacy endpoints were exploratory, children and adolescents treated with desvenlafaxine demonstrated improvements from baseline in depressive symptoms assessed using the Children's Depression Rating Scale-Revised (CDRS-R) (Poznanski et al. 1985) total score (Findling et al. 2014). However, the study did not include a placebo control group for comparison. Improvements observed at the end of the 8-week open-label study appeared to be maintained among children and adolescents who participated in the 6-month, flexible-dose extension study (Findling et al. 2014). To further assess the efficacy and safety of desvenlafaxine in pediatric patients with MDD, the sponsor (Pfizer, Inc.) planned a total of four phase 3 studies, including two short-term and two corresponding 6-month extension studies. Pharmacokinetic data obtained using desvenlafaxine doses ranging from 10 to 200 mg in the phase 2 study (Findling et al. 2016) served as the basis for informing the desvenlafaxine exposure levels studied in the phase 3 studies.
This study (NCT01371734) is the second of two similar short-term, double-blind, placebo-controlled studies of desvenlafaxine for the treatment of pediatric patients with MDD. Findings from the first of these short-term studies (NCT01372150) have been published (Weihs et al. 2017), and results of the extension studies will be reported separately. The objectives of this study were to evaluate the efficacy, safety, and tolerability of desvenlafaxine in the treatment of children and adolescents with MDD. An additional objective was to evaluate the population pharmacokinetics of desvenlafaxine in children and adolescents with MDD; results of that analysis will be reported separately.
Methods
Patients were randomized at 33 sites in the United States and Chile. Principal investigators were child and adolescent, or general psychiatrists with experience in the diagnosis and treatment of pediatric depression and in conducting industry-sponsored studies. The study was conducted from August 2011 to September 2015 in accordance with the International Council for Harmonisation Guideline for Good Clinical Practice (International Council for Harmonisation 1998) and the ethical principles that have their origin in the Declaration of Helsinki. The study protocol and any amendments received institutional review board or independent ethics committee approval. Written informed consent and assent were obtained from legal guardians and study participants, respectively, before any protocol-required procedures were performed.
Study design
This was a phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study of desvenlafaxine in the treatment of children and adolescents with MDD. Patients who completed this acute-phase study were eligible to participate in a 6-month, open-label extension study of desvenlafaxine. Eligible patients were randomly assigned (1:1:1) to placebo, desvenlafaxine low exposure (based on body weight at baseline), or desvenlafaxine higher exposure (based on body weight at baseline) arms, and stratified by age group (child [7–11 years] or adolescent [12–17 years]) and country. (Country was not included as a factor in statistical analyses because only one subject was enrolled in Chile.) Eligible patients received 8 weeks of double-blind treatment. Patients not continuing into the extension study completed a 1-week blinded treatment taper.
Study treatment
The selection of doses was based on two factors: first, the highest dose used in the study was 50 mg/day because no efficacy benefit has been demonstrated at doses higher than 50 mg/day in adults and tolerability decreases at doses higher than 50 mg/day (Thase et al. 2009). Second, pharmacokinetic data from a phase 2a study in pediatric patients with MDD have shown that desvenlafaxine exposure may be predicted by body weight in this population (Findling et al. 2016). Therefore, dosing for low and high desvenlafaxine exposure groups in this study was based on each patient's body weight at baseline, starting at 50 mg/day for the highest body weight group in the “high exposure” desvenlafaxine arm. For the “high exposure” desvenlafaxine group, patients weighing 20 to <35 kg, 35 to <70 kg, and >70 kg at baseline received desvenlafaxine doses of 25, 35, and 50 mg, respectively. Patients in the “low exposure” desvenlafaxine group received desvenlafaxine doses of 20, 25, and 35 mg, respectively. Patients received a titration dose during the first week on-therapy. Desvenlafaxine dosing during titration, treatment, and taper (when applicable) are provided in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/cap).
Study patients
Eligible study participants were male and female outpatients ≥7 to <18 years of age who weighed ≥20 kg at the screening and baseline visits. All enrolled patients met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Text Revision) criteria for MDD as the primary diagnosis, as assessed by the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (Kaufman et al. 1997) [K-SADS-PL] and clinical interview. A comprehensive diagnostic psychiatric evaluation, including collection of psychiatric history and treatments and confirmation of the MDD diagnosis, was performed by a psychiatrist at screening. Enrolled patients were required to have at least moderately severe depressive symptoms for ≥30 days before screening, and a CDRS-R score >40 and Clinical Global Impressions-Severity scale (CGI-S) (Guy 1976) score ≥4 at screening and baseline. Eligible patients were judged, in the investigator's opinion, to be likely to respond to antidepressant therapy without the need for concomitant psychotherapy.
Key exclusion criteria included history or presence of MDD with psychotic features or any psychotic disorder, bipolar disorder (or first-degree relative with bipolar disorder), manic episodes or other comorbid primary psychiatric conditions, or high risk of suicide (including first-degree relative who committed suicide). Detailed exclusion criteria and prohibited treatments are listed in Supplementary Data S1. Nonpsychopharmacologic drugs with psychotropic effects were permitted if the patient had been taking a stable dose for at least 90 days before study baseline. Previous formal psychotherapy for MDD was allowed only if it occurred more than 30 days before screening. Supportive nonbehavioral psychotherapy, family therapy, counseling, or play therapy with a focus other than on depressive symptoms were also permitted, provided that no changes in intensity or frequency were made within 90 days before study baseline and no change was anticipated for the duration of the study.
Study assessments
Efficacy
The primary efficacy outcome was change from baseline in CDRS-R total score at week 8. Total scores on the 17-item CDRS-R scale range from 17 to 113, with lower scores indicating lower symptom intensity. Other efficacy outcomes included change from baseline in CGI-S (key secondary efficacy outcome), distribution of Clinical Global Impressions-Improvement (CGI-I) scores, and CGI-I response (defined as a CGI-I score of 1 or 2). All efficacy assessments were administered at weeks 1, 2, 3, 4, 6, and 8, and/or at early termination in the double-blind phase. Individuals completing the CDRS-R, CGI-S, and CGI-I were qualified, trained by the sponsor, and approved as evaluators before conducting the assessments. Individuals completing the K-SADS-PL, CDRS-R, CGI-S, and CGI-I were qualified (with a minimum of 2 years of clinical experience with pediatric MDD), trained by the study sponsor, and approved as evaluators before conducting the assessments. Individuals completing the CDRS-R had at least 2 years' experience using the scale and were certified by the sponsor by a two-step process: raters had to (1) meet predefined inter-rater reliability criteria against the gold standard scores using video-taped assessments and (2) complete a one-on-one training on CDRS-R interview technique (applied training) by the rater training vendor, achieving acceptable technique and reliability in accordance with prespecified criteria using the Rater Applied Performance Scale (Kobak et al. 2005). The protocol recommended that, whenever possible, the same rater performed a given scale for a patient at each assessment.
Safety
Adverse events' (AEs, MedDRA v18), vital sign assessments', and Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner et al. 2011) evaluations were performed at each study visit. A physical examination with Tanner assessment and laboratory evaluations were conducted at screening and week 8, with serum lipids and liver function tests also assessed at week 4. Electrocardiogram (ECG) was performed at screening, baseline, and week 8. Potential clinically important (PCI) findings were identified based on categorical changes in vital signs, ECG results, and laboratory findings defined according to criteria prespecified by the sponsor (Supplementary Table S2).
Individuals completing the C-SSRS and Tanner assessments were qualified, trained by the study sponsor, and approved as evaluators before conducting those assessments. The protocol recommended that, whenever possible, the same rater administer the Tanner assessment for the patient at each study visit.
Statistical analysis
Sample size determination
The sample size estimate was conducted for the change from baseline in CDRS-R total score at week 8. It was estimated that 111 participants per treatment arm (N = 333) was sufficient to demonstrate a 5-point difference in the primary endpoint between the desvenlafaxine and placebo groups at a significance level of 5% and a power of 85%, assuming a pooled standard deviation (SD) of 12, and that no more than 5% of randomized subjects would fail to qualify for the primary analysis. Based on findings from a planned interim analysis (Supplementary Data S2), the sample size was increased from N = 333 to N = 360 (i.e., nine participants added to each treatment group).
Efficacy
Efficacy evaluations were conducted in the intent-to-treat (ITT) population, defined as all patients who were randomly assigned to treatment, received at least one dose of study drug, and had a baseline and at least one postbaseline primary efficacy assessment. Change from baseline in CDRS-R (primary analysis) was assessed using a mixed-effects model for repeated measures (MMRM) with terms for treatment, week, interaction of treatment and week, age group, gender, and baseline CDRS-R score. Change from baseline in CGI-S score was assessed using the same approach as used with the CDRS-R total score. A Hochberg step-up procedure was used to control for multiplicity associated with study-wise type I error across comparisons between each desvenlafaxine treatment group and placebo. If both desvenlafaxine treatment groups had p-values ≤0.05, both were considered statistically significant; if one had a p-value >0.05 and the other had a p-value ≤0.025, the latter desvenlafaxine treatment group alone was considered statistically significant. At each visit, the CGI-I was analyzed as a categorical variable using the Cochran-Mantel-Haenszel row-mean-score-difference test using ridit scores, controlling for age groups. Response rates at each visit, based on a CGI-I score of 1 or 2, were analyzed using a logistic regression model with treatment and age group as factors. Sensitivity analyses were conducted and are described in Supplementary Data S2.
Safety
The safety population included all patients who were randomly assigned to treatment and received at least one dose of study drug. The incidence of treatment-emergent adverse event (TEAE), discontinuations due to AEs, and serious AEs were summarized by treatment group. Vital signs, laboratory evaluations, ECG parameters, C-SSRS data, and Tanner assessments were summarized descriptively. The incidence rates of prespecified AEs of clinical interest for desvenlafaxine (tier-1 AEs) were compared between treatment groups using risk difference and exact 95% confidence intervals.
Results
Study patients
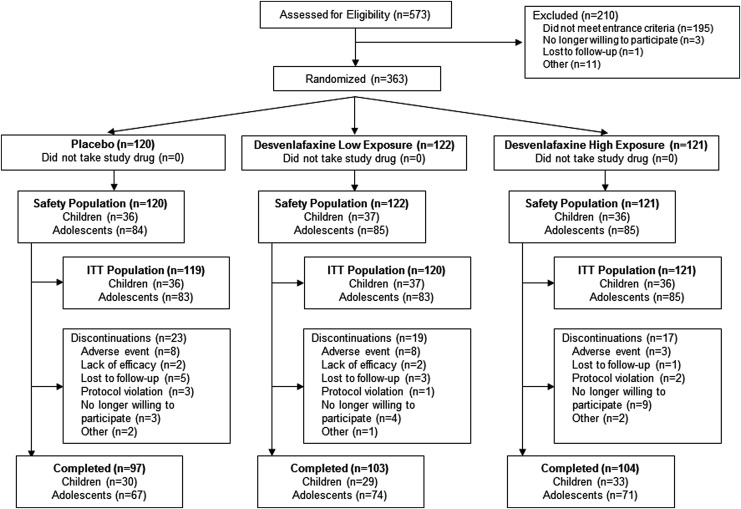
Of 573 individuals screened, 363 were randomly assigned to treatment, took at least one dose of study drug, and were included in the safety population (desvenlafaxine low exposure, n = 122; desvenlafaxine high exposure, n = 121; and placebo, n = 120) (Fig. 1). The safety population comprised 109 children and 254 adolescents; the ITT population included 109 children and 251 adolescents (total, 360 patients). A total of 59 patients discontinued treatment early (desvenlafaxine low exposure, 19 [16%]; desvenlafaxine high exposure, 17 [14.0%]; and placebo, 23 [19%]). Overall, the most common reasons for discontinuation were AEs (19 [5.2%]) and no longer willing to participate in the study (16 [4.4%]).
FIG. 1.
Study flow and patient disposition ITT. ITT, intent-to-treat.
The safety population was 56.5% female and median age was 14 years. Mean (SD) CDRS-R total score at baseline was 58.09 (9.19), reflecting moderately severe depression; baseline CDRS-R total scores ranged from 40 to 87 (one child with a baseline CDRS-R total score equal to 40 was enrolled; the child was excluded from the per-protocol population analysis due to the violation). Patient demographics and baseline clinical characteristics were similar among treatment groups (Table 1). Overall, 24.8% (90) of patients in the safety population had a psychiatric condition other than MDD in their medical history, and the percentages of patients across treatment groups were 27.3%, 23.0%, and 24.2% in the desvenlafaxine high exposure, desvenlafaxine low exposure, and placebo groups, respectively. The most common psychiatric conditions (reported in >5% of patients) included ADHD (high exposure, 9.9%; low exposure, 9.8%; and placebo, 7.5%), self-injurious behavior (high exposure, 9.1%; low exposure, 4.9%; and placebo, 6.7%), and insomnia (high exposure, 5.8%; low exposure, 7.4%; and placebo, 4.2%). A total of 13 (3.6%) patients in the safety population received some type of supportive therapy (e.g., psychotherapy, behavioral therapy, family therapy, or counseling) during study participation (desvenlafaxine high exposure, 5.0%; desvenlafaxine low exposure, 1.6%; and placebo, 4.2%). In 3 of these 13 subjects, the supportive therapy was prohibited per protocol, as the therapy was focused on the patient's depressive symptoms. Those patients were noted as having protocol deviation in the study report.
Table 1.
Demographic and Baseline Characteristics by Treatment and Age Group, Safety Population
| Treatment | Age group | ||||
|---|---|---|---|---|---|
| Placebo (n = 120) | Desvenlafaxine low exposure (n = 122) | Desvenlafaxine high exposure (n = 121) | Children (n = 109) | Adolescents (n = 254) | |
| Age, mean (SD), years | 13.15 (2.68) | 13.07 (2.80) | 12.87 (3.01) | 9.36 (1.32) | 14.61 (1.55) |
| Sex, n (%) | |||||
| Female | 60 (50.0) | 69 (56.56) | 76 (62.81) | 46 (42.20) | 159 (62.60) |
| Male | 60 (50.0) | 53 (43.44) | 45 (37.19) | 63 (57.80) | 95 (37.40) |
| Race, n (%) | |||||
| Asian | 1 (0.83) | 1 (0.82) | 0 | 0 | 2 (0.79) |
| Black | 25 (20.83) | 31 (25.41) | 33 (27.27) | 40 (36.70) | 49 (19.29) |
| White | 85 (70.83) | 86 (70.49) | 78 (64.46) | 59 (54.13) | 190 (74.80) |
| Other | 9 (7.50) | 4 (3.28) | 10 (8.26) | 10 (9.17) | 13 (5.12) |
| Ethnic origin, n (%) | |||||
| Hispanic/Latino | 18 (15.0) | 23 (18.85) | 18 (14.88) | 18 (16.51) | 41 (16.14) |
| Not Hispanic/Latino | 102 (85.0) | 99 (81.15) | 103 (85.12) | 91 (83.49) | 213 (83.86) |
| Height, mean (SD), cm | 159.43 (12.68) | 158.26 (13.18) | 155.80 (14.60) | 141.92 (9.61) | 164.65 (8.24) |
| Weight, mean (SD), kg | 61.39 (22.18) | 58.04 (19.96) | 59.82 (24.12) | 41.94 (14.18) | 67.38 (20.48) |
| BMI, mean (SD), kg/m2 | 23.64 (6.38) | 22.64 (5.45) | 23.96 (7.29) | 20.46 (5.38) | 24.68 (6.43) |
| Duration of current episode, mean (SD), months | 12.85 (12.10) | 11.23 (11.21) | 12.42 (13.24) | 11.39 (11.01) | 12.50 (12.68) |
| Baseline CDRS-R total score, mean (SD) | 57.28 (8.94) | 58.52 (9.18) | 58.45 (9.45) | 56.43 (8.73) | 58.80 (9.30) |
| Baseline CGI-S score, mean (SD) | 4.55 (0.58) | 4.61 (0.61) | 4.61 (0.58) | 4.59 (0.58) | 4.59 (0.59) |
BMI, body mass index; CDRS-R, Children's Depression Rating Scale-Revised; CGI-S, Clinical Global Impressions-Severity; SD, standard deviation.
Efficacy
Children's Depression Rating Scale-Revised
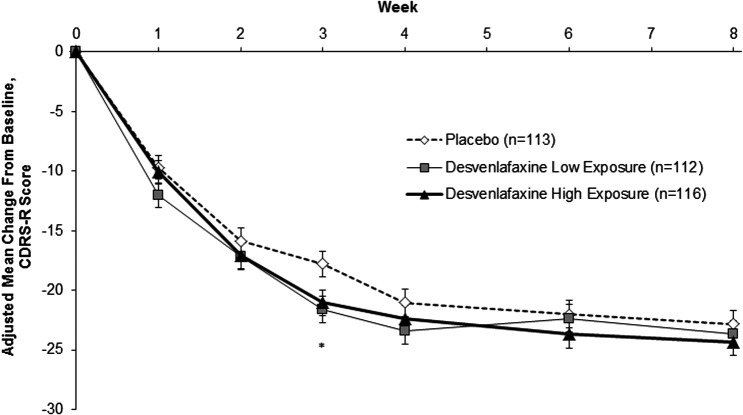
At week 8, the adjusted mean (standard error) changes from baseline in CDRS-R score were −23.7 (1.1), −24.4 (1.1), and −22.9 (1.1) for the desvenlafaxine low exposure, desvenlafaxine high exposure, and placebo groups, respectively. The adjusted mean difference versus placebo (95% CI) in change from baseline in CDRS-R score at week 8 did not differ statistically from placebo for either the desvenlafaxine low exposure (0.85 [−2.23, 3.94]) or desvenlafaxine high exposure (1.52 [−1.56, 4.61]) groups (Fig. 2). Overall, the profile of change from baseline in CDRS-R score during the course of the 8-week treatment phase was similar for the three treatment groups. Point differences were statistically significant between the desvenlafaxine low exposure group and placebo group at week 3 (p = 0.013), and between the desvenlafaxine high exposure group and placebo group at week 3 (p = 0.034). For all other time points (weeks 1, 2, 4, 6, and 8), no statistically significant differences were found between the active groups and placebo. Results were similar for children (7–11 years) and adolescents (12–17 years) across treatment groups (Supplementary Fig S1).
FIG. 2.
Adjusted mean (SE) change from baseline in CDRS-R score in children and adolescents; MMRM analysis, ITT population. *p = 0.013, desvenlafaxine low exposure versus placebo; p = 0.034, desvenlafaxine high exposure versus placebo. Adjusted mean difference vs placebo (95% CI), week 8: desvenlafaxine low exposure, 0.85 (−2.23, 3.94); desvenlafaxine high exposure, 1.52 (−1.56, 4.61). CDRS-R, Children's Depression Rating Scale-Revised; MMRM, mixed-effects model for repeated measures; SE, standard error.
Secondary efficacy endpoints
The results based on CGI-S and CGI-I scores for the desvenlafaxine groups versus placebo were generally consistent with those for the CDRS-R total score, with no statistically significant differences observed (Table 2).
Table 2.
Summary of Secondary Efficacy Outcomes at Week 8, Intent-to-Treat Population
| CGI-S | N | Adjusted mean change (SE) | Difference in adjusted means (placebo active) | 95% CI | p |
|---|---|---|---|---|---|
| Placebo | 102 | −1.49 (0.11) | |||
| Desvenlafaxine low exposure | 105 | −1.51 (0.11) | 0.015 | −0.29, 0.32 | 0.923 |
| Desvenlafaxine high exposure | 106 | −1.65 (0.11) | 0.161 | −0.14, 0.47 | 0.302 |
| CGI-Ia | N | Very much improved (%) | Much improved (%) | Minimally improved (%) | No change (%) | CMH test p-value |
|---|---|---|---|---|---|---|
| Placebo | 102 | 22 (21.6) | 35 (34.3) | 29 (28.4) | 16 (15.7) | |
| Desvenlafaxine low exposure | 105 | 20 (19.0) | 39 (37.1) | 26 (24.8) | 19 (18.1) | 0.696 |
| Desvenlafaxine high exposure | 106 | 27 (25.5) | 39 (36.8) | 23 (21.7) | 16 (15.1) | 0.462 |
| CGI-I responseb | Proportion responders | % | Adjusted odds ratio | Wald 95% CI | p |
|---|---|---|---|---|---|
| Placebo | 57/102 | 55.9 | |||
| Desvenlafaxine low exposure | 59/105 | 56.2 | 0.97 | 0.56, 1.69 | 0.925 |
| Desvenlafaxine high exposure | 66/106 | 62.3 | 0.76 | 0.44, 1.33 | 0.342 |
CGI-I scored as 1, very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; 7, very much worse; 1 patient in each desvenlafaxine group scored 5 at week 8 and no patient scored 6 or 7 at week 8.
CGI-I response was defined as CGI-I score of 1 (very much improved) or 2 (much improved).
CGI-I, Clinical Global Impressions-Improvement; CGI-S, Clinical Global Impressions-Severity; CI, confidence interval; CMH, Cochran-Mantel-Haenszel; SE, standard error.
Although the proportion of responders were numerically higher in the desvenlafaxine groups (desvenlafaxine low exposure, 56.2%, and desvenlafaxine high exposure, 62.3%) compared with placebo (placebo, 55.9%), there were no statistically significant differences between groups in the proportion of patients who were much improved or very much improved at week 8 LOCF.
Safety and tolerability
Adverse events
A total of 235/363 (64.7%) patients experienced 1 or more AE during the on-therapy period (desvenlafaxine low exposure, 81 [66.4%]; desvenlafaxine high exposure, 81 [66.9%]; and placebo, 73 [60.8%]). Most AEs were mild or moderate in severity. Moderate AEs considered by the investigator to be related to study medication were reported by 43 patients (desvenlafaxine low exposure, 17 [13.9%]; desvenlafaxine high exposure, 14 [11.6%]; and placebo, 12 [10.0%]). A total of two patients, both in the desvenlafaxine low exposure group, reported severe AEs that were considered by the investigator to be related to study treatment (tension headache and initial insomnia [adolescent]; and suicide attempt [adolescent]). The patient who made the suicide attempt (involving overdose of amitriptyline prescribed to a relative) was treated in the emergency department, admitted to the intensive care unit for observation, and then transferred to an in-patient psychiatric facility. Study drug was stopped after the event, and the patient was stabilized on treatment with sertraline.
Overall, 17 patients (4.7%) experienced AEs resulting in discontinuation (6 children and 11 adolescents), including 7 patients (5.7%) in the desvenlafaxine low exposure group (palpitations, liver function test abnormal, sedation, aggression, hypomania, irritability, and suicide attempt); 3 patients (2.5%) in the desvenlafaxine high exposure group (mydriasis, suicidal ideation, and dermatitis allergic); and 7 patients (5.8%) in the placebo group (anxiety, depression, oppositional defiant disorder, screaming, and dermatomyositis).
Treatment-emergent adverse events
TEAEs were reported by 81 patients (66.4%) in the desvenlafaxine low exposure group, 81 patients (66.9%) in the desvenlafaxine high exposure group, and 73 patients (60.8%) in the placebo group. The most common TEAEs reported by at least 5% in any treatment group are summarized by age group in Table 3. No statistically significant differences were observed between desvenlafaxine low exposure, desvenlafaxine high exposure, and placebo in the incidence of any tier-1 TEAE (Supplementary Table S3).
Table 3.
Number (%) of Patients Reporting Treatment-Emergent Adverse Events with Incidence ≥5% in Any Treatment Group, On-Therapy Period, Safety Population
| Children | Adolescents | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 36) | Desvenlafaxine low exposure (n = 37) | Desvenlafaxine high exposure (n = 36) | Placebo (n = 84) | Desvenlafaxine low exposure (n = 85) | Desvenlafaxine high exposure (n = 85) | Placebo (n = 120) | Desvenlafaxine low exposure (n = 122) | Desvenlafaxine high exposure (n = 121) | |
| Any TEAE | 21 (58.3) | 24 (64.9) | 24 (66.7) | 52 (61.9) | 57 (67.1) | 57 (67.1) | 73 (60.8) | 81 (66.4) | 81 (66.9) |
| Abdominal pain, upper | 4 (11.1) | 3 (8.1) | 5 (13.9) | 5 (6.0) | 4 (4.7) | 6 (7.1) | 9 (7.5) | 7 (5.7) | 11 (9.1) |
| Accidental overdose | 1 (2.8) | 0 | 2 (5.6) | 0 | 0 | 1 (1.2) | 1 (0.8) | 0 | 3 (2.5) |
| Aggression | 0 | 2 (5.4) | 0 | 0 | 0 | 0 | 0 | 2 (1.6) | 0 |
| Blood triglycerides increased | 0 | 2 (5.4) | 0 | 0 | 0 | 0 | 0 | 2 (1.6) | 0 |
| Cough | 2 (5.6) | 1 (2.7) | 1 (2.8) | 3 (3.6) | 1 (1.2) | 2 (2.4) | 5 (4.2) | 2 (1.6) | 3 (2.5) |
| Decreased appetite | 1 (2.8) | 0 | 3 (8.3) | 5 (6.0) | 6 (7.1) | 3 (3.5) | 6 (5.0) | 6 (4.9) | 6 (5.0) |
| Diarrhea | 0 | 2 (5.4) | 2 (5.6) | 2 (2.4) | 1 (1.2) | 1 (1.2) | 2 (1.7) | 3 (2.5) | 3 (2.5) |
| Dizziness | 0 | 1 (2.7) | 0 | 3 (3.6) | 4 (4.7) | 6 (7.1) | 3 (2.5) | 5 (4.1) | 6 (5.0) |
| Dysmenorrheaa | 0 | 0 | 0 | 1 (2.1) | 1 (1.8) | 4 (7.1) | 1 (1.7) | 1 (1.4) | 4 (5.3) |
| Fatigue | 1 (2.8) | 2 (5.4) | 2 (5.6) | 1 (1.2) | 2 (2.4) | 7 (8.2) | 2 (1.7) | 4 (3.3) | 9 (7.4) |
| Feeling jittery | 0 | 0 | 2 (5.6) | 0 | 1 (1.2) | 2 (2.4) | 0 | 1 (0.8) | 4 (3.3) |
| Viral gastroenteritis | 2 (5.6) | 0 | 1 (2.8) | 0 | 2 (2.4) | 2 (2.4) | 2 (1.7) | 2 (1.6) | 3 (2.5) |
| Headache | 2 (5.6) | 8 (21.6) | 5 (13.9) | 13 (15.5) | 14 (16.5) | 20 (23.5) | 15 (12.5) | 22 (18.0) | 25 (20.7) |
| Insomnia | 0 (0) | 3 (8.1) | 3 (8.3) | 1 (1.2) | 4 (4.7) | 1 (1.2) | 1 (0.8) | 7 (5.7) | 4 (3.3) |
| Nausea | 2 (5.6) | 2 (5.4) | 4 (11.1) | 5 (6.0) | 10 (11.8) | 10 (11.8) | 7 (5.8) | 12 (9.8) | 14 (11.6) |
| Nasopharyngitis | 1 (2.8) | 3 (8.1) | 1 (2.8) | 1 (1.2) | 5 (5.9) | 6 (7.1) | 2 (1.7) | 8 (6.6) | 7 (5.8) |
| Psychomotor hyperactivity | 0 | 1 (2.7) | 3 (8.3) | 0 | 0 | 1 (1.2) | 0 | 1 (0.8) | 4 (3.3) |
| Pyrexia | 0 | 3 (8.1) | 1 (2.8) | 0 | 0 | 1 (1.2) | 0 | 3 (2.5) | 2 (1.7) |
| Skin abrasion | 0 | 0 | 2 (5.6) | 0 | 0 | 0 | 0 | 0 | 2 (1.7) |
| Upper respiratory tract infection | 1 (2.8) | 0 | 0 | 2 (2.4) | 4 (4.7) | 6 (7.1) | 3 (2.5) | 4 (3.3) | 6 (5.0) |
| Vomiting | 0 | 0 | 3 (8.3) | 4 (4.8) | 1 (1.2) | 6 (7.1) | 4 (3.3) | 1 (0.8) | 9 (7.4) |
Percentage calculated using number of females as denominator.
TEAE, treatment-emergent adverse event.
Deaths and SAEs
No deaths occurred during the study. Seven randomized patients (1.9%) experienced SAEs: three assigned to the desvenlafaxine low exposure group (aggression [child; discontinued]; homicidal ideation and suicidal ideation [adolescent; events occurred after last on-therapy dose, discontinued]; and suicide attempt [adolescent; discontinued, as described above]), one assigned to the desvenlafaxine high exposure group (appendicitis and abscess [adolescent; temporarily discontinued for treatment]), and three assigned to placebo (suicidal ideation [adolescent; event occurred after last on-therapy dose, discontinued], dermatomyositis [adolescent; discontinued], and suicide attempt and suicidal ideation [adolescent]; suicide attempt involving ibuprofen overdose with vomiting, no change to study drug during the event, recovered from suicide attempt, and study drug stopped ∼1 week later due to insufficient response; suicidal ideation occurred after the patient stopped taking study drug, hospitalized]).
Suicidality
Treatment-emergent suicidal ideation or suicidal behavior, which included both new onset and worsening suicidal ideation or behavior, was reported for 39 (10.8%) of 360 patients who had a C-SSRS assessment at baseline and at 1 or more postbaseline time points (Table 4 [treatment-emergent events; full data presented in Supplementary Table S4]). A total of 39/360 (10.8%) patients had treatment-emergent suicidal ideation, and two patients (0.6%) also had treatment-emergent suicidal behavior. Both suicidal behavior events were categorized as actual attempt, one reported in a placebo-treated adolescent and the other in an adolescent treated with low exposure desvenlafaxine (Supplementary Table S4). The desvenlafaxine low exposure-treated patient was discontinued due to a serious AE of suicide attempt, and the placebo-treated patient remained in the study as described above (“Deaths and SAEs” section).
Table 4.
Summary of Treatment-Emergent Suicidal Ideation and Behavior Reported on the Columbia-Suicide Severity Rating Scale at Any Postbaseline Assessment, Safety Population
| Placebo (n = 119) | Desvenlafaxine low exposure (n = 120) | Desvenlafaxine high exposure (n = 121) | Total (N = 360) | |
|---|---|---|---|---|
| Treatment-emergent SIBa, n/N (%) | 16/119 (13.4) | 9/120 (7.5) | 14/121 (11.6) | 39/360 (10.8) |
| New-onset SIBb | 14/107 (13.1) | 9/113 (8.0) | 13/108 (12.0) | 36/328 (11.0) |
| Worsening SIBc | 2/12 (16.7) | 0/113 (0) | 1/13 (7.7) | 3/32 (9.4) |
| Treatment-emergent SId, n/N (%) | 16/119 (13.4) | 9/120 (7.5) | 14/121 (11.6) | 39/360 (10.8) |
| New-onset SIe | 14/107 (13.1) | 9/113 (8.0) | 13/108 (12.0) | 36/328 (11.0) |
| Wish to be dead | 5 | 5 | 5 | 15 |
| Nonspecific active suicidal thoughts | 4 | 3 | 4 | 11 |
| Active suicidal ideation with any methods (no plan) without intent to act | 4 | 0 | 4 | 8 |
| Active suicidal ideation with specific plan and intent | 1 | 1 | 0 | 2 |
| Worsening SIf | 2/12 (16.7) | 0 | 1/13 (7.7) | 3/32 (9.4) |
| Shift to nonspecific active suicidal thoughts | 1 | 0 | 0 | 1 |
| Shift to active suicidal ideation with any methods (no plan) without intent to act | 0 | 0 | 1 | 1 |
| Shift to active suicidal ideation with specific plan and intent | 1 | 0 | 0 | 1 |
| Treatment-emergent SBg, n/N (%) | 1/119 (0.8) | 1/120 (0.8) | 0/121 (0) | 2/360 (0.6) |
| New-onset suicidal behaviorh | 1/119 (0.8) | 1/120 (0.8) | 0/121 (0) | 2/360 (0.6) |
| Suicide attempt | 1 | 1 | 0 | 2 |
| Worsening SBi | 0 | 0 | 0 | 0 |
There was one poststudy suicide attempt reported as a serious adverse event that was not captured on the C-SSRS; C-SSRS was not performed following that event. N represents the number of subjects in this analysis, that is, subjects who had a baseline and a postbaseline C-SSRS assessment.
Treatment-emergent SIB is defined as (1) new-onset SI or SB, (2) worsening SI or SB, or (3) postbaseline SB on subjects reporting SI at baseline.
New-onset SIB is defined as any SI or SB reported postbaseline on subjects who reported no SI and no SB at baseline.
Worsening SIB is defined as (1) shift from SI at baseline to a more severe SI postbaseline, (2) shift from SI at baseline (and no SB at baseline) to any SB postbaseline, or (3) shift from SB at baseline to a more severe SB postbaseline
Treatment-emergent SI is defined as new-onset SI or worsening SI.
New-onset SI is defined as any SI reported postbaseline on subjects who reported no SI at baseline.
Worsening SI is defined as shift to a more severe SI postbaseline on subjects reporting SI at baseline.
Treatment-emergent SB is defined as new-onset SB or worsening SB.
New-onset SB is defined as any SB reported postbaseline on subjects who reported no SB at baseline.
Worsening SB is defined as shift to a more severe SB postbaseline on subjects reporting SB at baseline.
C-SSRS, Columbia-Suicide Severity Rating Scale; SI, suicidal ideation; SB, suicidal behavior; SIB, suicidal ideation or behavior.
New-onset self-injurious behavior without suicidal intent was reported for one patient (0.8%) in the desvenlafaxine low exposure group, one patient (0.8%) in the desvenlafaxine high exposure group, and three patients (2.5%) in the placebo group.
Other safety measures
A total of 202 patients had on-therapy PCI vital sign values (low exposure desvenlafaxine, 76/120 [63.3%]; high exposure desvenlafaxine, 66/121 [54.5%]; and placebo, 60/119 [50.4%]). Upon data review by the medical monitor, PCI vital sign findings in 43 patients were considered to be clinically important. The most common clinically important findings were postural hypotension (desvenlafaxine low and high exposure, n = 14 each; and placebo, n = 9), weight gain (desvenlafaxine low exposure, n = 1; desvenlafaxine high exposure, n = 3; and placebo, n = 1), weight loss (desvenlafaxine low exposure, n = 2; desvenlafaxine high exposure, n = 1; and placebo, n = 1), and abnormal blood pressure (desvenlafaxine low and high exposure, n = 1 each). Mean changes from baseline in vital signs and weight are reported by age group in Table 5.
Table 5.
Physical and Vital Sign Results, Mean Change from Baseline at Final On-Therapy Evaluation, Safety Population
| Children | Adolescents | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 36) | Desvenlafaxine low exposure (n = 37) | Desvenlafaxine high exposure (n = 36) | Placebo (n = 84) | Desvenlafaxine low exposure (n = 85) | Desvenlafaxine high exposure (n = 85) | |
| Systolic BP, supine (mm Hg) | 0.6 | 0.1 | 0.5 | 0.3 | −0.1 | −0.4 |
| Diastolic BP, supine (mm Hg) | −0.6 | −0.4 | 1.9 | 0.2 | 0.9 | 0.1 |
| Pulse, supine (beats/min) | 1.5 | 3.7 | −1.4 | 2.1 | 1.7 | 1.4 |
| Systolic BP, orthostatic (mm Hg) | 0.9 | 0.4 | −0.3 | −1.3 | −0.6 | −0.2 |
| Diastolic BP, orthostatic (mm Hg) | −0.1 | 2.3 | −0.3 | −0.1 | 0 | 0 |
| Pulse, orthostatic (beats/min) | 1.4 | 1.1 | 4.3 | −2.0 | 1.1 | 2.5 |
| Weight (kg) | 1.0 | 0.1 | 0.5 | 0.4 | −0.2 | 0.2 |
| BMI (kg/m2) | 0.3 | −0.1 | 0.1 | 0 | −0.2 | −0.1 |
| Height (cm) | 0.6 | 0.5 | 0.5 | 0.4 | 1.7 | 0.5 |
BMI, body mass index; BP, blood pressure.
Expected shifts associated with development assessed by Tanner staging were observed during the study. No clinically important ECG findings were reported, and no clinically meaningful differences between treatment groups were observed.
A total of 253 patients had at least 1 on-therapy PCI laboratory test result (low exposure desvenlafaxine, 86/117 [73.5%]; high exposure desvenlafaxine, 87/116 [75.0%]; and placebo, 80/115 [69.6%]). Of these, clinically important changes in laboratory findings were observed in 18 patients, including 11 patients in the desvenlafaxine low exposure group (urine protein [n = 4], triglycerides [n = 4], prolactin [n = 2], abnormal liver function tests [n = 1], and fasting glucose [n = 1]), 5 patients in the desvenlafaxine high exposure group (urine protein [n = 4] and triglycerides [n = 1], and 2 patients in the placebo group (prolactin [n = 1] and triglycerides [n = 1]). Mean changes from baseline for selected laboratory values are reported by age group Supplementary Table S5.
Discussion
In this phase 3 multicenter, randomized, double-blind, placebo-controlled, 8-week, parallel-group study of children and adolescents with MDD, there was no statistically significant difference for either desvenlafaxine exposure group compared with placebo on the primary efficacy measure, or other efficacy measures, at week 8. Efficacy of desvenlafaxine for treating MDD in pediatric patients therefore was not demonstrated in this study. A large placebo response was observed across all measures of efficacy; this likely reduced the ability to detect a statistically significant difference between the desvenlafaxine low exposure, desvenlafaxine high exposure, and placebo groups on the primary or secondary efficacy endpoints. Patients in all treatment groups demonstrated substantial improvement from baseline in depressive symptoms based on the CDRS-R total score at week 8. Findings for the secondary efficacy endpoints (CGI-S; CGI-I) were similar to and consistent with the primary (CDRS-R total score) findings. Desvenlafaxine was generally well tolerated in children and adolescents, with no new safety signals identified. Safety results were consistent with previous adult and pediatric desvenlafaxine MDD trials.
The findings in this study are consistent with the previously published small, open-label study in pediatric patients in which desvenlafaxine doses of 10–100 mg/day in children (n = 27) and 25–200 mg/day in adolescents (n = 24) were shown to be generally safe and well tolerated (Findling et al. 2014). Exploratory efficacy findings from that study demonstrated that improvements from baseline in CDRS-R at the end of the fixed-dose study were maintained during a 6-month flexible dosing extension (Findling et al. 2014). Results of this study are also comparable with those of a companion study (NCT01372150) that assessed the efficacy and tolerability of desvenlafaxine (n = 115), fluoxetine (n = 112), and placebo (n = 112) in pediatric patients with MDD (Weihs et al. 2017). In that study, all treatment groups (including placebo) showed improved CDRS-R total scores from baseline (primary endpoint), with no differences observed between groups for any of the primary or secondary efficacy endpoints due to the large placebo effect. The study was deemed inconclusive with regard to desvenlafaxine efficacy, given that even the active comparator, fluoxetine, which has well-established efficacy in the pediatric MDD population, also did not separate from placebo.
The mean changes in CDRS-R scores observed with desvenlafaxine in this study (−23.7 and–24.4 for the low and high exposure groups, respectively) are comparable with antidepressant drug response observed in positive clinical trials of pediatric patients with MDD (Emslie et al. 1997, 2002, 2009; Wagner et al. 2003, 2004). However, the large placebo response observed in this study (−22.9) was similar to that reported in negative or inconclusive trials of paroxetine (Emslie et al. 2006), duloxetine (Atkinson et al. 2014; Emslie et al. 2014), and venlafaxine (Emslie et al. 2007) for pediatric MDD, and may have influenced study outcomes. Factors associated with placebo response and potential strategies for reducing placebo response have been identified and discussed in the published literature (Bridge et al. 2009; Enck et al. 2013; Rief et al. 2016). Several of those strategies, including limiting number of treatment arms, requiring rater certification for the CDRS-R, and using the same rater for a given patient whenever possible, were used in this trial, but it is not clear that any of these was effective. The placebo response rate for CGI-I in this trial (55.9%) fell in the range of placebo response rates reported in a review of other industry-sponsored trials for pediatric depression (50%–60%) (Walkup 2017).
In this study, a possible contributor to the large placebo effect was the actual process of clinician-administration of the CDRS-R. This process alone may have provided a therapeutic impact for patients comparable with a cognitive behavior therapy session. The potential for psychological assessment procedures to emerge as a therapeutic tool was described in a recent evaluation of children with mood disorders (including MDD), who were participating in clinical trials (Young et al. 2016). The authors reported significant, clinically meaningful improvements in mood severity following psychodiagnostic screening assessments (such as CDRS-R), but before randomization or administration of study treatment (Young et al. 2016). However, while a potential psychotherapeutic effect of the CDRS-R assessments may have contributed to the placebo response in this study, it cannot fully explain the failure of desvenlafaxine to separate from placebo. Several positive pediatric studies with lower placebo response rates have also used the CDRS-R assessment (Emslie et al. 1997, 2002, 2009; Wagner et al. 2003, 2004).
Nonpharmacological interventions (i.e., psychotherapy) are recognized as an effective treatment option for pediatric patients with MDD (Clark et al. 2012). Thus, for pediatric patients, the potential therapeutic effect of simply participating in a clinical trial may partly explain why no separation was observed for active treatment (desvenlafaxine) from placebo. In addition, the study protocol allowed randomization of patients who participated in previous psychotherapy sessions (greater than 30 days before screening), and also allowed other types of therapy in which the focus was not on depressive symptoms (e.g., supportive nonbehavioral psychotherapy, family therapy, counseling, or play therapy) as long as the intensity and frequency of sessions were stable for 90 days before treatment with no anticipated change throughout the duration of the study. However, only 13 patients (3.6%) received such supportive therapy.
This study had several limitations. The use of inclusion and exclusion criteria to enroll individuals without comorbid psychiatric conditions, other unstable medical illnesses, or a risk for suicide limited the study population to one that did not reflect the larger pediatric MDD population. In addition, the inclusion of two desvenlafaxine arms in the study design may have increased expectation of treatment success, enhancing the placebo response (Rutherford and Roose 2013). This study did not include an active control to provide assay sensitivity, which may have increased understanding of factors contributing to the efficacy outcome (Temple and Ellenberg 2000). Finally, this study did not use independent raters for efficacy evaluations. It may be that the use of centralized raters could reduce placebo response (Kobak et al. 2010), but data suggest that rater competence and reliability may be more critical than whether raters are site based or centralized (Targum et al. 2013).
Conclusions
In this phase 3, double-blind, placebo-controlled study, low doses (20–35 mg/day based on baseline weight) and higher doses (25–50 mg/day based on baseline weight) of desvenlafaxine did not demonstrate efficacy for the treatment of MDD in children and adolescents. Statistically significant differences between desvenlafaxine and placebo were not observed for any efficacy measure assessed. Throughout the study, similar improvements in depressive symptoms were observed in all three treatment groups, with no meaningful differences observed between patients who received desvenlafaxine versus placebo. These results, and those from a similarly designed sister study (Weihs et al. 2017), provide no evidence for the use of desvenlafaxine as a first-line treatment for MDD in pediatric patients. Treatment with desvenlafaxine 20–50 mg/day was generally safe and well tolerated in this study with no new safety signals observed. The safety and tolerability profile of desvenlafaxine was consistent with that observed in previous clinical trials of children, adolescents, and adults with MDD (Findling et al. 2014; Carrasco et al. 2016).
Clinical Significance
Efficacy of desvenlafaxine at two weight-based dosing levels was not demonstrated in this 8-week, double-blind, placebo-controlled study. No statistically significant difference from placebo on the primary efficacy endpoint (CDRS-R) or any secondary efficacy endpoint was observed for either of the two desvenlafaxine treatment arms. Treatment with desvenlafaxine 20–50 mg/day was generally safe and well tolerated in this study with no new safety signals observed.
Supplementary Material
Acknowledgments
Medical writing support was provided by Kathleen M. Dorries, PhD, at Peloton Advantage, LLC, and funded by Pfizer Inc. The authors would like to thank the patients and family members who contributed their time to this study. Site investigators, coordinators, as well as study monitors, data managers, and data analysts were essential to the accomplishment of this trial and appreciated by the authors. Trial registration number NCT01371734.
Authors' Contributions
Study design: S.R., S.L., R.D.E., R.L.F., and D.W. (SAP amendment). Study investigator: S.A. and R.L.F. Enrolled patients: S.A. and R.L.F. Collection and assembly of data: S.R. and S.L. Data analysis: D.B.W. (statistical analysis). Data review: R.D.E. (medical monitoring), S.L., S.R., and R.A. Data interpretation: All authors. Article preparation: All authors. Article review and revisions: All authors. Final approval of article: All authors.
Disclosures
S.A.: Receives or has received research support from Pfizer, Acadia, Allergan [Forest, Naurex, Actavis], Blackthorn, Roche, Janseen, NIMH, SYNRX, AssureRX, Axsom, Boehringer Ingelheim, Otsuka, and Sunovion. S.L. and S.R.: Employee of Pfizer, Inc., and has Pfizer stock and Pfizer stock options. R.D.E., D.B.W., and R.A.: Employee of Pfizer, Inc., owns Pfizer stock, and has Pfizer stock options. R.L.F.: Receives or has received research support, acted as a consultant and/or served on a speaker's bureau for Actavis, Akili, Alcobra, American Academy of Child & Adolescent Psychiatry, American Psychiatric Press, Bracket, CogCubed, Cognition Group, Coronado Biosciences, Elsevier, Epharma Solutions, Forest, Genentech, GlaxoSmithKline, Guilford Press, Ironshore, Johns Hopkins University Press, KemPharm, Lundbeck, Medgenics, Merck, NIH, Neurim, Novartis, Otsuka, PCORI, Pfizer, Physicians Postgraduate Press, Purdue, Rhodes Pharmaceuticals, Roche, Sage, Shire, Sunovion, Supernus Pharmaceuticals, Syneurx, Takeda, Teva, Tris, Validus, and WebMD.
References
- Atkinson SD, Prakash A, Zhang Q, Pangallo BA, Bangs ME, Emslie GJ, March JS: A double-blind efficacy and safety study of duloxetine flexible dosing in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:180–189, 2014 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent D, Bernet W, Bukstein O, Walter H, Benson RS, Chrisman A, Farchione T, Greenhill L, Hamilton J, Keable H, Kinlan J, Schoettle U, Stock S, Ptakowski KK, Medicus J: Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 46:1503–1526, 2007 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, Perel J, Nelson B: Childhood and adolescent depression: A review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry 35:1427–1439, 1996 [DOI] [PubMed] [Google Scholar]
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA: Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166:42–49, 2009 [DOI] [PubMed] [Google Scholar]
- Carrasco JL, Kornstein SG, McIntyre RS, Fayyad R, Prieto R, Salas M, Mackell J, Boucher M: An integrated analysis of the efficacy and safety of desvenlafaxine in the treatment of major depressive disorder. Int Clin Psychopharmacol 31:134–146, 2016 [DOI] [PubMed] [Google Scholar]
- Cheung AH, Zuckerbrot RA, Jensen PS, Ghalib K, Laraque D, Stein RE: Guidelines for Adolescent Depression in Primary Care (GLAD-PC): II. Treatment and ongoing management. Pediatrics 120:e1313–e1326, 2007 [DOI] [PubMed] [Google Scholar]
- Clark MS, Jansen KL, Cloy JA: Treatment of childhood and adolescent depression. Am Fam Physician 86:442–448, 2012 [PubMed] [Google Scholar]
- Edwards AC, Joinson C, Dick DM, Kendler KS, Macleod J, Munafo M, Hickman M, Lewis G, Heron J: The association between depressive symptoms from early to late adolescence and later use and harmful use of alcohol. Eur Child Adolesc Psychiatry 23:1219–1230, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Findling RL, Yeung PP, Kunz NR, Li Y: Venlafaxine ER for the treatment of pediatric subjects with depression: Results of two placebo-controlled trials. J Am Acad Child Adolesc Psychiatry 46:479–488, 2007 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Nilsson M, Jacobson JG: Fluoxetine for acute treatment of depression in children and adolescents: A placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry 41:1205–1215, 2002 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Prakash A, Zhang Q, Pangallo BA, Bangs ME, March JS: A double-blind efficacy and safety study of duloxetine fixed doses in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:170–179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J: A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 54:1031–1037, 1997 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S: Escitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry 48:721–729, 2009 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Wagner KD, Kutcher S, Krulewicz S, Fong R, Carpenter DJ, Lipschitz A, Machin A, Wilkinson C: Paroxetine treatment in children and adolescents with major depressive disorder: A randomized, multicenter, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 45:709–719, 2006 [DOI] [PubMed] [Google Scholar]
- Enck P, Bingel U, Schedlowski M, Rief W: The placebo response in medicine: Minimize, maximize or personalize? Nat Rev Drug Discov 12:191–204, 2013 [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ: Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry 59:225–231, 2002 [DOI] [PubMed] [Google Scholar]
- Findling RL, Groark J, Chiles D, Ramaker S, Yang L, Tourian KA: Safety and tolerability of desvenlafaxine in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:201–209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Groark J, Tourian KA, Ramaker SA, Chiles D, Yang L, Nichols AI: Pharmacokinetics and tolerability of single-ascending doses of desvenlafaxine administered to children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 26:909–921, 2016 [DOI] [PubMed] [Google Scholar]
- Guy W: Clinical Global Impressions. In: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD, US Department of Health, Education, and Welfare, 1976, pp. 217–222 [Google Scholar]
- International Council for Harmonisation: ICH harmonised tripartite guideline: Statistical principles for clinical trials E9. 1998. www.ich.org/products/guidelines/efficacy/efficacy-single/article/statistical-principles-for-clinical-trials.html Accessed May15, 2017
- Iwata N, Tourian KA, Hwang E, Mele L, Vialet C, For the Study 3359 Investigators: Efficacy and safety of desvenlafaxine 25 and 50 mg/day in a randomized, placebo-controlled study of depressed outpatients. J Psychiatr Pract 19:5–14, 2013 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kobak KA, Leuchter A, Debrota D, Engelhardt N, Williams JB, Cook IA, Leon AC, Alpert J: Site versus centralized raters in a clinical depression trial: Impact on patient selection and placebo response. J Clin Psychopharmacol 30:193–197, 2010 [DOI] [PubMed] [Google Scholar]
- Kobak KA, Lipsitz JD, Williams JB, Engelhardt N, Bellew KM: A new approach to rater training and certification in a multicenter clinical trial. J Clin Psychopharmacol 25:407–412, 2005 [DOI] [PubMed] [Google Scholar]
- Lexapro [package insert]. St. Louis, MO, Forest Pharmaceuticals, Inc., 2014 [Google Scholar]
- Liebowitz MR, Tourian KA, Hwang E, Mele L, For the Study 3362 Investigators: A double-blind, randomized, placebo-controlled study assessing the efficacy and tolerability of desvenlafaxine 10 and 50 mg/d in adult outpatients with major depressive disorder. BMC Psychiatry 13:94, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein NR: Longitudinal associations between alcohol problems and depressive symptoms: Early adolescence through early adulthood. Alcohol Clin Exp Res 33:49–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ: The Columbia-suicide severity rating scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Freman LN, Mokros HB: Children's depression rating scale-revised (September 1984). Psychopharmacol Bull 21:979–989, 1985 [Google Scholar]
- Pristiq [package insert]. Philadelphia, PA, Wyeth Pharmaceuticals, Inc, a subsidiary of Pfizer, Inc., 2016 [Google Scholar]
- Prozac [package insert]. Indianapolis, IN, Eli Lilly and Company, 2014 [Google Scholar]
- Puig-Antich J, Kaufman J, Ryan ND, Williamson DE, Dahl RE, Lukens E, Todak G, Ambrosini P, Rabinovich H, Nelson B: The psychosocial functioning and family environment of depressed adolescents. J Am Acad Child Adolesc Psychiatry 32:244–253, 1993 [DOI] [PubMed] [Google Scholar]
- Rief W, Barsky AJ, Bingel U, Doering BK, Schwarting R, Wohr M, Schweiger U: Rethinking psychopharmacotherapy: The role of treatment context and brain plasticity in antidepressant and antipsychotic interventions. Neurosci Biobehav Rev 60:51–64, 2016 [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Roose SP: A model of placebo response in antidepressant clinical trials. Am J Psychiatry 170:723–733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu AL: Screening for depression in children and adolescents: U.S. preventive services task force recommendation statement. Ann Intern Med 164:360–366, 2016 [DOI] [PubMed] [Google Scholar]
- Targum SD, Wedel PC, Robinson J, Daniel DG, Busner J, Bleicher LS, Rauh P, Barlow C: A comparative analysis between site-based and centralized ratings and patient self-ratings in a clinical trial of Major Depressive Disorder. J Psychiatr Res 47:944–954, 2013 [DOI] [PubMed] [Google Scholar]
- Temple R, Ellenberg SS: Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: Ethical and scientific issues. Ann Intern Med 133:455–463, 2000 [DOI] [PubMed] [Google Scholar]
- Thase ME, Kornstein SG, Germain JM, Jiang Q, Guico-Pabia C, Ninan PT: An integrated analysis of the efficacy of desvenlafaxine compared with placebo in patients with major depressive disorder. CNS Spectrums 14:144–154, 2009 [DOI] [PubMed] [Google Scholar]
- Wagner KD, Ambrosini P, Rynn M, Wohlberg C, Yang R, Greenbaum MS, Childress A, Donnelly C, Deas D: Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: Two randomized controlled trials. JAMA 290:1033–1041, 2003 [DOI] [PubMed] [Google Scholar]
- Wagner KD, Robb AS, Findling RL, Jin J, Gutierrez MM, Heydorn WE: A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents. Am J Psychiatry 161:1079–1083, 2004 [DOI] [PubMed] [Google Scholar]
- Walkup JT: Antidepressant efficacy for depression in children and adolescents: Industry- and NIMH-Funded studies. Am J Psychiatry 174: 430–437, 2017 [DOI] [PubMed] [Google Scholar]
- Weihs K, Murphy W, Abbas R, Chiles D, England R, Ramaker S, Wajsbrot DB: Desvenlafaxine vs placebo in a fluoxetine-referenced study of children and adolescents with MDD. J Child Adolesc Psychopharmacol 2017;28:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, Klier CM, Ryan ND, Dahl RE, Wickramaratne P: Depressed adolescents grown up. JAMA 281:1707–1713, 1999 [DOI] [PubMed] [Google Scholar]
- Young AS, Meers MR, Vesco AT, Seidenfeld AM, Arnold LE, Fristad MA: Predicting therapeutic effects of psychodiagnostic assessment among children and adolescents participating in randomized controlled trials. J Clin Child Adolesc Psychol, 2016. [Epub ahead of print]. DOI: 10.1080/15374416.2016.1146992 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.