Abstract
Objectives: An increasing number of abandoned clinical trials have forestalled efforts to advance the evidence base for the treatment of mood and anxiety disorders in children and adolescents. With this in mind, we sought to present and validate a Bayesian approach for the reanalysis of summary data in abandoned clinical trials and to review and re-evaluate available pharmacokinetic, tolerability, and efficacy data from two large, randomized controlled trials of buspirone in pediatric patients with generalized anxiety disorder (GAD).
Methods: Prospective, randomized, parallel-group controlled trials of buspirone in pediatric patients with GAD as well as associated pharmacokinetic studies were identified and data were extracted. In addition to descriptive statistics, marginal posterior densities for each variable of interest were determined and a Monte Carlo pseudosample was generated with random draws obtained from the Student's t-distribution to assess, with inferential statistics, differences in variables of interest.
Results: Buspirone was evaluated in one flexibly dosed (N = 227) and one fixed-dose (N = 341) trial in children and adolescents aged 6–17 years with a primary diagnosis of GAD. With regard to improvement in the sum of the Columbia Schedule for Affective Disorders and Schizophrenia GAD items, buspirone did not separate from placebo in the fixed-dose trial at low (95% CI: −0.78 to 2.39, p = 0.32) or high dose (95% CI: −0.87 to 1.87, p = 0.47) nor did it separate from placebo in the flexibly dosed study (95% CI: −0.3 to 1.9, p = 0.15). Drop out as a result of a treatment-emergent adverse event was significantly greater in buspirone-treated patients compared to placebo (p = 0.011). Side effects were consistent with the known profile of buspirone with lightheadedness occurring more frequently in buspirone-treated patients (p < 0.001).
Conclusions: Buspirone is well tolerated in pediatric patients with GAD, although two randomized controlled trials were underpowered to detect small effect sizes (Cohen's d < 0.15). Finally, Bayesian approaches may facilitate re-examination of data from abandoned clinical trials.
Keywords: : anxiety, clinical trial, generalized anxiety disorder (GAD), anxiolytic
Introduction
In medicine, randomized controlled trials are the primary machinery for providing reliable evidence for interventions. The last two decades have seen a number of efforts to increase the evidence base for psychopharmacologic treatment in children and adolescents. In 1997, the Food and Drug Administration Modernization Act (FDAMA) offered a 6-month extension of exclusivity rights to manufacturers of medications in exchange for submission of data from pediatric trials (United States Congress 1997). This was followed by additional Federal efforts to increase the study of pharmacologic treatments in pediatric populations, including the Best Pharmaceuticals for Children Act of 2002 (BCPA) (United States Congress 2002) and the Pediatric Research Equity Act of 2003 (amended in 2007 and renewed in 2012) (United States Congress 2003). However, despite an increased number of clinical trials in children and adolescents, relatively few are published. Furthermore, those sponsored by industry are more than three times less likely to be published than those with academic or Federal funding (Pica and Bourgeois 2016). The lack of publication of findings from these clinical trials has raised concerns “about relying on published research to reflect the truth.” As such, these invisible, unpublished trials contribute to publication bias violating the ethical mandates of the Declaration of Helsinki: “Researchers, authors, sponsors, editors and publishers all have ethical obligations with regard to the publication and dissemination of the results of research…Negative and inconclusive as well as positive results must be published or otherwise made publicly available” (World Medical Association 2013).
While the findings of many studies of psychopharmacologic interventions in youth with anxiety disorders have been published (for review see: Strawn et al. 2015a), these studies—with few exceptions (Strawn et al. 2017a)—focus on antidepressants and benzodiazepines. Importantly, nearly 40% of anxious youth do not respond adequately to antidepressant treatment (Walkup et al. 2008). As such, medications with alternate mechanisms of action are commonly utilized to treat antidepressant-resistant anxiety disorders (Strawn et al. 2012) despite limited or nonexistent safety, tolerability, and efficacy data in pediatric populations.
The non-benzodiazepine anxiolytic buspirone is approved for the treatment of generalized anxiety disorder (GAD) in adults (Bristol-Myers Squibb Company, Buspirone Package Insert Package Insert) and case reports and open-label case series suggest that it may be effective in youth with anxiety disorders (Kranzler 1988; Siméon 1993; Zwier and Rao 1994). However, to date, the only prospective published studies systematically evaluating the potential effectiveness of buspirone in any pediatric population have focused on irritability in youth with autism spectrum disorder (Ghanizadeh and Ayoobzadehshirazi 2015) and on inattention and impulsivity in adolescents with attention-deficit/hyperactivity disorder (Malhotra and Santosh 1998; Davari-Ashtiani et al. 2010; Mohammadi et al. 2012; Shahrbabaki et al. 2013).
With regard to anxious youth, buspirone was systematically evaluated in one pharmacokinetic study of children, adolescents, and adults (Salazar et al. 2001) and in two randomized controlled trials of pediatric patients with GAD nearly two decades ago (USFDA 1998, 2000). However, the results of these efficacy studies have not been published or disseminated beyond a brief addition to the medication “label” in 2001 (Bristol-Myers Squibb Company, Buspirone Package Insert Package Insert). With this in mind, we sought to (1) review and re-evaluate available pharmacokinetic, safety, tolerability, and efficacy data of buspirone in pediatric populations with GAD, or related anxiety disorders and (2) present and validate a Bayesian approach to reanalysis of clinical trial data in children and adolescents.
Methods
Background literature review
Two child and adolescent psychiatrists (J.R.S. and P.E.C.) independently examined the extant literature with the goal of identifying prospective, randomized, placebo-controlled trials of buspirone for GAD in children and adolescents (<18 years of age). A literature review of the National Library of Medicine (PubMed) from 1966 through March 2017 was completed using the following search terms: pediatric or child or adolescent or youth AND (GAD or overanxious disorder) AND buspirone. In addition, the Cochrane Library, the Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science were searched from inception through February 2017. Studies that were not published with English language were excluded. An additional search of clinicaltrials.gov was conducted and the package insert was reviewed to identify relevant studies. Thereafter, a Freedom of Information Act Request was made and following discussion with the U.S. Food and Drug Administration, FDA Supplement 43 was accessed (USFDA 2000).
Data extraction
Summary data, including variance and significance findings, were extracted from the two efficacy studies for buspirone in pediatric patients with GAD (CN101-124 and CN101-125) contained within FDA Supplement 43 (USFDA 2000). In addition, selected pharmacokinetic data (Cmax and half-life [t½]) for both buspirone and the primary metabolite, 1-PP, were extracted from the supplement and, to validate the statistical approach (described in Bayesian modeling of response), these analyses were compared to pharmacokinetic data that were published separately from this study (Salazar et al. 2001).
Bayesian modeling of response
Given that the results presented in the study documents were primarily summary statistics, distributional assumptions were necessary. An assumption of normally distributed outcomes appears reasonable, given the results of large clinical trials of pediatric patients with anxiety disorders (Walkup et al. 2008). Given a normal likelihood, adopting an uninformative prior, 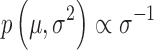 , results in a Student's t-marginal posterior for the mean,
, results in a Student's t-marginal posterior for the mean, 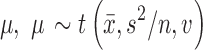 , where
, where 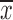 and s2 represent the sample mean and variance, respectively. The degrees of freedom are given by
and s2 represent the sample mean and variance, respectively. The degrees of freedom are given by 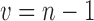 resulting in sufficient information to determine the distribution.
resulting in sufficient information to determine the distribution.
For comparison of buspirone versus placebo, the posteriors for the mean symptom severity ratings for each group were obtained as already described. Then, a Monte Carlo (MC) sample from each exact posterior distribution was obtained by random sampling from the distributions. These MC samples were combined to numerically obtain the posterior distribution of the difference in means for inference and hypothesis testing. Specifically, given sample means, 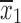 and
and 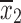 , sample standard deviations s1 and s2 and samples sizes n1 and n2 randomly draw R values,
, sample standard deviations s1 and s2 and samples sizes n1 and n2 randomly draw R values, 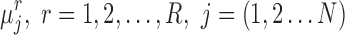 from each of the marginal posterior distributions
from each of the marginal posterior distributions 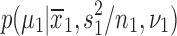 and
and 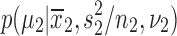 , which are Student's t-distributions. Then, R differences in means were computed and credible intervals, means, and so on were determined from the MC sample of R values from the posterior of differences in means (Lancaster 2004; Greenberg 2008). Finally, a post hoc power analysis was performed utilizing Bayesian updating (i.e., improving the prior probability estimate to produce a posterior probability estimate) to determine the necessary sample size for the demonstration of a statistically significant result for the primary outcome measure in study CN101-124. Additional details of this statistical approach are provided in Supplementary Data (Supplementary Data are available online at www.liebertpub.com/cap).
, which are Student's t-distributions. Then, R differences in means were computed and credible intervals, means, and so on were determined from the MC sample of R values from the posterior of differences in means (Lancaster 2004; Greenberg 2008). Finally, a post hoc power analysis was performed utilizing Bayesian updating (i.e., improving the prior probability estimate to produce a posterior probability estimate) to determine the necessary sample size for the demonstration of a statistically significant result for the primary outcome measure in study CN101-124. Additional details of this statistical approach are provided in Supplementary Data (Supplementary Data are available online at www.liebertpub.com/cap).
Statistical analyses
In addition to the models already described, descriptive statistics, t-tests, and χ2 tests were used to characterize the groups, frequencies of adverse events, and so on. p-Values ≤0.05 were considered statistically significant and no correction was made for multiple comparisons. Statistical analyses were performed using R (version 3.1.2).
Results
Background literature review
Two independent searches yielded concordant results. Four unstructured reviews discussed the use of buspirone for child and adolescent anxiety (Popper 1993; Velosa and Riddle 2000; Wagner 2001; Masi et al. 2002), one case report described the treatment course of an adolescent with anxiety who received buspirone (Kranzler et al. 1988), and an abstract from the 149th Annual Meeting of the American Meeting of the American Psychiatric Association reported the salutary effects of buspirone in adolescents with anxiety (Bouvard et al. 1996). However, the full abstract was not published or readily available. The authors of this abstract were contacted in an attempt to clarify the focus of the study and methodology. In addition, a case series of children and adolescents aged 6–20 years, of whom 4 had “anxiety and/or depressive disorders,” suggested improvement in anxiety symptoms at low doses (10–40 mg/day, mean dose 25 mg/day) over an average follow-up of 2.5 months, while a subsequent open-label trial (n = 13) in the same report suggested that patients aged 6–14 years (mean age 10 years), treated with open-label buspirone for 4 weeks at low doses (maximum dose 20 mg/day), had improvement in Clinical Global Impressions Scale scores, the Manifest Anxiety Scale, and the anxiety subscale of the Brief Psychiatric Rating Scale for Children (Siméon 1993). Otherwise, there were no published, randomized, placebo-controlled trials of buspirone for children and adolescents with anxiety. Hence, the subsequent results focus on FDA Supplement 43.
Pharmacokinetic studies of buspirone in pediatric patients with anxiety disorders
The confirmatory analyses of summary steady-state pharmacokinetic data for buspirone and the primary active metabolite, 1-PP, in children (n = 13) and adolescents (n = 12) with anxiety disorders and healthy adults (n = 14), contained within FDA Supplement 43, were consistent with the published data from this study (Salazar et al. 2001). In our independent analyses that were based on the summary FDA data, the t½buspirone was significantly higher in children compared to adults at the 7.5 mg BID dose (p = 0.002) but did not differ between adolescents and adults (p = 0.39) or between children and adolescents (p = 0.130). At the 15 mg BID dose, the t½buspirone did not differ between children and adolescents (p = 0.528) or between adolescents and adults (p = 0.898) nor between children and adults (p = 0.382). Finally, at the 30 mg BID dose, no statistically significant differences in t½buspirone were noted between children and adolescents (p = 0.162), between adolescents and adults (p = 0.966), or between children and adults (p = 0.148).
Analyses of the Cmax across the three age groups were generally consistent with the previously reported analyses and revealed no differences in Cmax for buspirone at the 7.5 mg BID dose between groups (children vs. adolescents, p = 0.938; adolescents vs. adults, p = 0.10), except when comparing children to adults (children vs. adults, p = 0.034). At the 15 mg BID dose, there was a trend toward a difference in Cmax between children and adults (p = 0.11), although no statistically significant differences in Cmax between children and adolescents (p = 0.986) or between adolescents and adults (p = 0.22). Finally, Cmax at the 30 mg BID dose did not differ (children vs. adolescents, p = 0.900; adolescents vs. adults, p = 0.352) and trended toward a difference for the children versus adults (p = 0.10).
Similarly, comparison of analyses performed from models utilizing the summary data for the t½1-PP across the three age groups was consistent with the previously published patient-level analyses (Salazar et al. 2001). These analyses revealed no differences in t½1-PP at the 7.5 mg BID dose (children vs. adolescents, p = 0.328; adolescents vs. adults, p = 0.386; children vs. adults, p = 0.716). Similarly, no differences among groups were detected for the 15 mg BID dose (children vs. adolescents, p = 0.746; adolescents vs. adults, p = 0.906; children vs. adults, p = 0.650) or the 30 mg BID dose (children vs. adolescents, p = 0.814; adolescents vs. adults, p = 0.124; children vs. adults, p = 0.124). Cmax for 1-PP was significantly higher in children compared to adults at all doses (7.5 mg BID: p = 0.012; 15 mg BID: p = 0.005; 30 mg BID: p = 0.006), consistent with the original report (Salazar et al. 2001). Similarly, the Cmax 1-PP was higher in adolescents compared to children, although this did not reach statistical significance at all doses (7.5 mg BID: p = 0.052; 15 mg BID: p = 0.034; 30 mg BID: p = 0.07). Finally, Cmax for 1-PP was similar at all buspirone doses between adolescents and adults (7.5 mg BID: p = 0.324; 15 mg BID: p = 0.358; 30 mg BID: p = 0.29).
Efficacy study designs
The safety and efficacy of buspirone in children and adolescents, aged 6–17 years, with GAD were evaluated in two 6-week trials of youth with a primary diagnosis of GAD (Study CN101-124 and Study CN101-125; Fig. 1). Patients were required to have a GAD score of at least 16 on the GAD module of the Schedule for Affective Disorders and Schizophrenia for School Age Children Columbia version (K-SADS) in both studies and exclusion criteria included a score of ≥45 on the Childhood Depression Rating Scale, pregnancy, lactation, major psychiatric illness other than GAD, or the concurrent use of psychotropic medications (NDA-18-731, SES-043, p. 5–6). The primary outcome measure was the sum of the four items in the K-SADS: (1) severity of anxiety or worry, (2) difficulty controlling worry, (3) severity of associated symptoms, and (4) global distress about symptoms. Secondary measures of efficacy included Clinical Global Impression-Severity (CGI-S) and Clinical Global Impression-Improvement (CGI-I), Children's Anxiety Ratings Scale (CARS), and the Screen for Child Anxiety-Related Emotional Disorders (SCARED) (Birmaher et al. 1997).
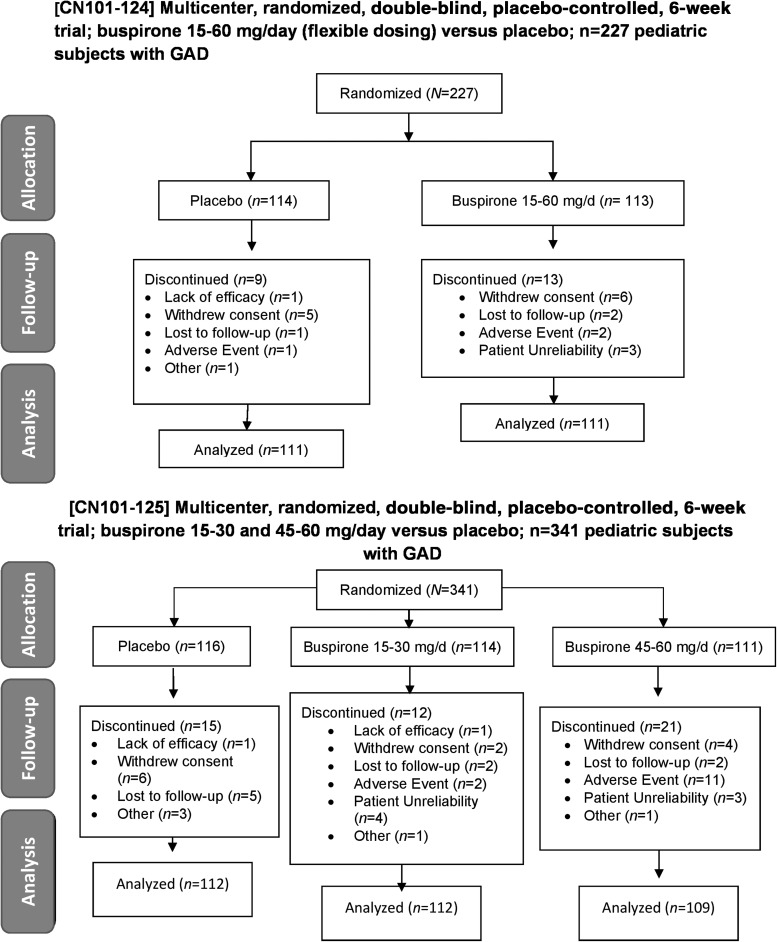
FIG. 1.
CONSORT diagrams for efficacy studies in children and adolescents with GAD. Study CN101-124 (top panel) was a 6-week, multicenter, randomized, double-blind, placebo-controlled trial of buspirone 15–60 mg per day versus placebo in children and adolescents with GAD. Study CN101-125 was a 6-week, multicenter, randomized, double-blind, placebo-controlled trial of buspirone 15–30 mg per day and 45–60 mg per day versus placebo in children and adolescents with GAD (bottom panel). GAD, generalized anxiety disorder.
The first study, CN101-124, involved randomization (1:1) to flexibly dosed buspirone or placebo for 6 weeks. In this study, following screening, buspirone was initiated at 7.5 mg per day for days 1–4, increased to 15 mg per day on day 5, and continued at this dose until day 14. Then, during weeks 3–6, buspirone could remain at 15 mg per day or could be flexibly titrated to 30 mg per day, 45 mg per day, or 60 mg per day.
In the second study, CN101-125, buspirone was also initiated at 7.5 mg per day for the first 4 days of treatment and then was titrated to 15 mg per day and, during the second week, was increased to 30 mg per day, and finally increased to 45 mg per day during the third week of treatment. During the final 2 weeks—weeks 4–6—patients either continued at the 45 mg per day dose or the dose was increased to 60 mg per day.
Study CN101-124 was conducted at 34 sites within the United States, of which 25 actually enrolled subjects (NAcademic = 4; NCommunity-based = 21), while Study CN101-125 was conducted at 48 sites, of which 32 actually enrolled subjects (NAcademic = 9; NCommunity-based = 23). The average number of patients randomized per site in Study CN101-124 was 9 ± 1 patients per site, while in Study CN101-125, 11 ± 3 patients were randomized per site.
Analyses of efficacy studies in pediatric patients with GAD
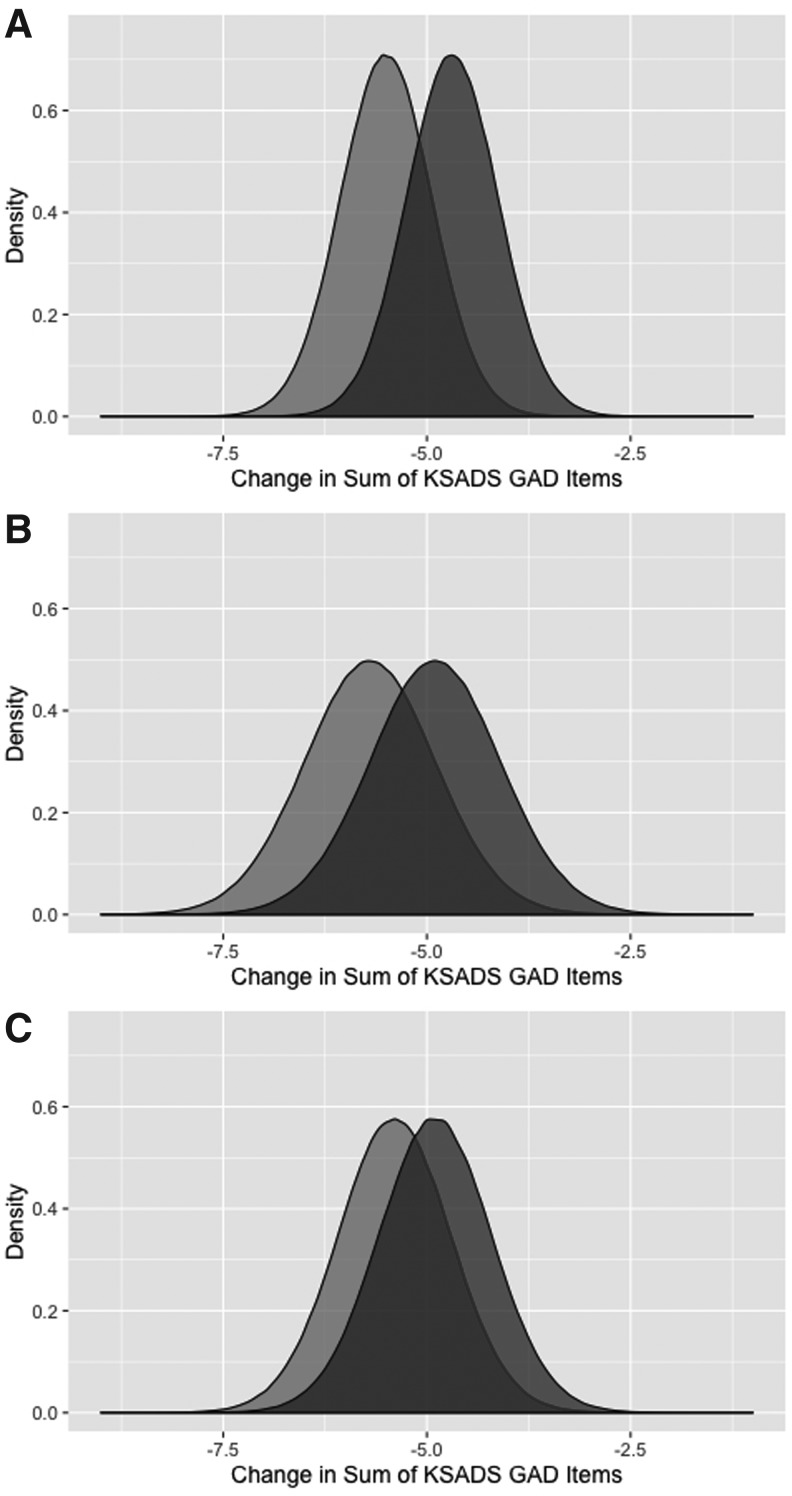
Reanalyses of summary data (last observation carried forward [LOCF]) from Study CN101-124 revealed a lack of a statistically significant difference between buspirone-treated and placebo-treated patients for the primary outcome measure, sum of K-SADS GAD items (95% credible interval [CI] for placebo/buspirone difference: −0.3 to 1.9, p = 0.152) (Fig. 2A). For Study CN101-124, the difference in improvement in GAD symptom burden between low-dose buspirone and placebo as well as between high-dose buspirone and placebo both failed to reach statistical significance (95% credible interval [CI] for placebo/buspirone difference: −0.79 to 2.38, p = 0.317; and 95% credible interval for placebo/buspirone difference: −0.87 to 1.9, p = 0.465, respectively; Fig. 2B, C). Finally, with regard to the standard effects (i.e., Cohen's d) for the studies, in CN101-124, the Cohen's d was 0.137, while in Study CN101-125, the Cohen's d for the low dose and high dose was 0.094 and 0.060, respectively.
FIG. 2.
Distribution of anxiety symptom severity in children and adolescents with GAD in Buspirone efficacy studies. Posterior densities for the change in the summary K-SADS GAD items, at endpoint, for buspirone-treated patients (light gray) and those receiving placebo (dark gray). Panel (A) shows the buspirone/placebo differences in Study CN101-124 (95% CI: −0.3 to 1.9 [median 0.81], p = 0.15, odds against null hypothesis: 2.82), while the differences between buspirone- and placebo-treated patients for Study CN101-125 for the 15–30 mg per day dose (95% CI: −0.78 to 2.39 [median 0.79], p = 0.32, odds against null hypothesis: 1.64) are shown in (B) and for the 45–60 mg per day (95% CI: −0.87 to 1.87 [median 0.49], p = 0.47, odds against null hypothesis: 1.31) are shown in (C). CI; GAD, generalized anxiety disorder; K-SADS, Schedule for Affective Disorders and Schizophrenia for School Age Children Columbia version.
Adverse events
In the analysis of pooled adverse events for CN101-124 and CN101-125, discontinuation related to an adverse event occurred more commonly in patients treated with buspirone relative to those treated with placebo (p < 0.01). However, lightheadedness was the only adverse event that occurred more frequently, at a statistically significant level, in patients treated with buspirone relative to those receiving placebo (10% vs. 2%, p < 0.001). Finally, in the fixed-dose study, the number of patients who dropped out as a result of an adverse event only trended toward being statically significant between the high-dose and low-dose groups (χ2 = 2.53, p = 0.11) (Table 1).
Table 1.
Pooled Adverse Event Rates from Two Double-Blind, Placebo-Controlled Studies of Buspirone in Children and Adolescents with Generalized Anxiety Disorder
| Adverse events, frequency (%) | Buspirone n = 334 | Placebo n = 225 | Significance |
|---|---|---|---|
| Headache | 29 (9%) | 23 (10%) | χ2 = 0.22, p = 0.641 |
| Asthenia | 10 (3%) | 8 (4%) | χ2 = 0.02, p = 0.876 |
| Accidental injury | 7 (2%) | 7 (3%) | χ2 = 0.23, p = 0.633 |
| Nausea | 16 (5%) | 10 (4%) | χ2<0.01, p = 1 |
| Dyspepsia | 13 (4%) | 12 (5%) | χ2 = 0.36, p = 0.549 |
| Diarrhea | 5 (1.5%) | 7 (3%) | χ2 = 0.36, p = 0.546 |
| Vomiting | 6 (2%) | 7 (3%) | χ2 = 0.53, p = 0.468 |
| Lightheadedness | 34 (10%) | 5 (2%) | χ2 = 11.9,p< 0.001 |
| Somnolence | 14 (4%) | 4 (2%) | χ2 = 1.8, p = 0.180 |
| Insomnia | 5 (1.5%) | 7 (3%) | χ2 = 0.99, p = 0.320 |
| Nervousness | 5 (1.5%) | 3 (1%) | χ2<0.01, p = 1 |
| Upper respiratory tract infection | 5 (1.5%) | 5 (2%) | χ2 = 0.10, p = 0.757 |
| Rhinitis | 5 (1.5%) | 3 (1%) | χ2<0.01, p = 1 |
| Discontinuation secondary to an adverse event | 15 (5%) | 1 (<1%) | χ2 = 6.53,p = 0.011 |
Bold text reflects statistically significant differences.
Post hoc Bayesian power analyses for efficacy studies
Using Bayesian updating, the additional sample size required for the results of the flexibly dosed study of buspirone in adolescents with GAD (CN101-124) to be statistically significant was determined to be 100. As such, randomization of approximately 100 additional participants, with a preservation of the mean difference in K-SADS GAD score between patients randomized to buspirone and those randomized to placebo and variance (0.810 ± 0.409), could be combined with the prior sample to yield a Bayesian posterior p-value of 0.048 (95% credible interval: 0.007 to 1.612).
Discussion
The efficacy studies analyzed and presented herein represent the only randomized controlled trials of buspirone in pediatric patients with anxiety disorders. Moreover, these trials are rare; only one other study has evaluated a non-benzodiazepine, nonantidepressant medication in pediatric patients with anxiety disorders (Strawn et al. 2017a). This fact is particularly important given that as many as two in five pediatric patients with anxiety disorders may fail to respond to traditional first-line psychopharmacologic interventions (Walkup et al. 2008). In addition, the results of these efficacy studies are of interest with regard to a number of several scientific, economic, and clinical factors that have recently received substantial attention (Walkup 2017).
Recently, failed and negative trials in anxiety and depressive disorders have been attributed to high placebo response rates (Dobson and Strawn 2016). A recent meta-analysis of placebo response in pediatric anxiety disorders (n = 2230) identified a number of factors that were associated with increased placebo response and which are present in the two buspirone studies analyzed (Dobson and Strawn 2016). First, a large number of study sites with relatively few patients randomized per site have been associated with increased placebo response in pediatric patients with anxiety disorders (Dobson and Strawn 2016). Second, the majority of sites in both buspirone efficacy studies were nonacademic sites—a factor that is associated with a decreased likelihood of detecting an effect in both pediatric (Dobson and Strawn 2016) and adult studies of anxiety disorders (Rutherford et al. 2015). Finally, Study CN101-125, the fixed-dose study of buspirone, involved a 2:1 randomization to buspirone. This unbalanced randomization may increase both the patients' and clinicians' expectation of randomization to active drug and perhaps, eo ipso, treatment success. Furthermore, expectation of treatment outcome has been associated with placebo response in randomized controlled trials of pediatric patients with anxiety disorders (Strawn et al. 2017b).
It is noteworthy that these two buspirone studies were completed in the early years following the passing of the FDAMA (United States Congress 1997). Because of financial incentives to complete the pediatric study before patent expiration and to benefit from the 6-month patient extension (i.e., pediatric exclusivity), these early trials faced scientific and implementation difficulties. The sponsors of these pediatric studies needed to quickly identify large groups of investigators who could recruit significant populations of participants and conduct the study quickly (Walkup 2017). Not unexpectedly, the assembly of such a study team often required study investigators who were not child and adolescent psychiatrists, but rather were general adult psychiatrists, pediatricians, and family practitioners in addition to research-dedicated clinics (Walkup 2017). These characteristics are reflected in the efficacy studies described herein. In essence, the regulatory and economic factors in this era resulted in a “confluence of pressure to recruit a large number of participants in a tight time frame, large numbers of sites with small Ns per site, site investigators with unknown pediatric [anxiety] or clinical trial experience [and]…financial incentives to retain participants in the trial and implicit pressures for participants to get better (i.e., observer bias, or enhanced expectancy effects)” (Walkup 2017).
The outcome measure for these clinical trials also warrants further discussion. For studies of pediatric patients with major depressive disorder, the Children's Depression Rating Scale (Poznanski et al. 1984) is frequently utilized as a primary outcome measure. However, for clinical trials involving anxious youth, there is considerable heterogeneity in the outcome measures. Many studies (Pine et al. 2001; Rynn et al. 2007; Walkup et al. 2008; Strawn et al. 2015b, 2017a) utilized the Pediatric Anxiety Rating Scale (PARS) (RUPP 2002), although some others used other measures, including the Hamilton Anxiety Rating Scale (HAM-A) (Rynn et al. 2001) or social anxiety-specific measures (Beidel et al. 2007; March et al. 2007). These instruments assess different symptoms. For example, some heavily weight somatic symptoms (HAM-A), while others heavily weight cognitive symptoms of anxiety (SCARED) and do not necessarily measure impairment or frequency of symptoms. Furthermore, some scales that assess the frequency, severity, and impairment (e.g., PARS) do not reflect the type of anxiety symptoms. The use of K-SADS symptom burden as the outcome measure may have resulted in both the measurement of side effects of treatment (e.g., fatigue) and somatic symptoms of anxiety and may not have actually assessed impairment. In addition, the Clinical Global Impressions scores that were presented to the FDA (not included in this analysis) suggest that CGI was treated as a continuous variable rather than as a categorical variable, with dichotomized definitions of “response” or “nonresponse” as is standard in nearly all recent clinical trials of psychopharmacologic interventions in anxious youth (Pine et al. 2001; March et al. 2007; Rynn et al. 2007; Walkup et al. 2008; Strawn et al. 2015b, 2017a). These concerns, with regard to the measurement of anxiety symptoms and impairment, may reflect that this was one of the early psychopharmacologic treatment trials of anxious youth. Another consideration is the short time frame over which the trial was implemented and conducted in an effort to satisfy regulatory requirements. Nonetheless, while it remains to be determined how a different primary outcome measure might have affected separation between buspirone and placebo, the high placebo response rate likely degraded the ability to detect drug/placebo differences.
Bayesian modeling represents an approach that has been utilized in analyses of clinical trial data over the last decade (Greco et al. 2013; Monden et al. 2016). These models has been leveraged to elucidate relationships between antidepressant dose and response, evaluate pharmacotherapy in a number of psychiatric disorders, evaluate psychotropic medication tolerability in youth (Cohen et al. 2012), evaluate the impact of clinical characteristics on antidepressant treatment response (Fountoulakis et al. 2013), and facilitate efficacy and adverse event comparisons in network meta-analyses of antidepressants in youth with major depressive disorder (Cipriani et al. 2016). However, while we have utilized similar Bayesian models to evaluate placebo response in pediatric patients with anxiety disorders (Strawn et al. 2017b), to the best of our knowledge, Bayesian methods have never been used to analyze abandoned clinical trial data, in which only summary-level data are available. Several strengths of this approach include the ability to provide credible intervals for the difference between buspirone and placebo that provide clinically significant insight into the potential magnitude of the effect of buspirone in reducing anxiety symptoms in youth with GAD. This, coupled with the posterior probability, provides further insight into whether a larger study would be informative. Importantly, the potential magnitude of the effect suggests that a subsequent study would likely not be worth pursuing—a conclusion that cannot be ascertained from a p-value. Additional strengths of this approach include the need for fewer restrictive assumptions regarding the relative variance of treatment groups (e.g., placebo vs. buspirone), and other restrictions inherent to the data can be easily incorporated (e.g., bounds above zero). Further, the ability to examine and visualize the exact, small-sample posterior densities for each group and for the difference in symptomatic improvement between groups, with no reliance on large-sample assumptions, provides a wealth of information beyond that obtainable from a p-value. Finally, posterior odds against the null hypothesis of no difference in effect can be determined and can minimize the linear combination of type I and II errors, rather than requiring a sole reliance on p-values. Importantly, p-values may fix the nominal size of the type I error—a significant problem in the clinical trials' statistics.
Limitations
While this is the first report of randomized trials evaluating buspirone in anxious youth and, to our knowledge, is the first use of a Bayesian modeling approach to examine summary data from abandoned clinical trials in a pediatric population, there are several important limitations. First, assumptions regarding the variance and symmetry of the distribution of change in anxiety symptoms were made; however, similar variance distributions were observed in our recent analyses of the Child/Adolescent Anxiety Multimodal Study (CAMS), which has a similar unbalanced randomization and in which we used a similar probabilistic Bayesian analysis (Strawn et al. 2017b). Second, with only summary statistics available, these analyses rely on the assumption that the placebo and medication effects are normally distributed. While this assumption is standard and can be justified by the central limit theorem and the maximum entropy principle, availability of the raw data would allow for testing of this assumption. Third, the high placebo response rate in these studies may have been attributed to the early post-FDMA climate of the early 2000s (Walkup 2017), the outcome measure used or to other intrinsic aspects of the population (Strawn et al. 2017b) or the design of the clinical trial (Dobson and Strawn 2016).
Conclusions
The results from these studies suggest that buspirone is generally well tolerated in pediatric patients with anxiety disorders, has a side effect profile that is consistent with the known tolerability profile of buspirone in adults (Goldberg and Finnerty 1979; Lader and Scotto 1998), and is associated with discontinuation rates similar to selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors (SSNRIs) in this population. However, these trials suffered from a high placebo response rate and were underpowered to detect small treatment effects for buspirone in youth with GAD. Thus, if the true effect size for buspirone in pediatric GAD is ∼0.1 to 0.15, a larger and adequately powered trial would be required to conclusively evaluate the potential efficacy of buspirone in this population. Nonetheless, the efficacy studies analyzed and presented herein represent the only randomized controlled trials of buspirone in pediatric patients with anxiety disorders and, collectively, represent half of the non-benzodiazepine, nonantidepressant studies in pediatric patients with anxiety disorders.
Clinical Significance
While these studies do not conclusively support or refute the potential efficacy of buspirone in pediatric patients with GAD given their significant limitations, they suggest a tolerability profile that is similar to the tolerability profile of buspirone in adults (Goldberg and Finnerty 1979; Lader and Scotto 1998). Moreover, the results of these studies underscore the scientific and clinical factors that may have coalesced in the post-FDAMA regulatory environment and resulted in a number of “failed trials” in children and adolescents.
Supplementary Material
Acknowledgments
The authors appreciate the assistance of the Food and Drug Administration in obtaining the summary data presented herein, and thank the original investigators and the patients and families who participated in these clinical trials.
Disclosures
Dr. Strawn has received research support from Edgemont, Eli Lilly, Shire, Forest Research Institute, Lundbeck, and the National Institute of Health (NIMH and NIEHS). He receives royalties from Springer Publishing for two texts and has received material support from Assurex. Dr. Keeshin receives research support from the State of Utah and from the Substance Abuse and Mental Health Administration (SAMHSA). Dr. Croarkin receives research support from the National Institute of Mental Health, Neuronetics, and GeneSight/Assurex. The other study investigators have no potential financial conflicts of interest.
References
- Beidel DC, Turner SM, Sallee FR, Ammerman RT, Crosby LA, Pathak S: SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry 46:1622–1632, 2007 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM: The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry 36:545–553, 1997 [DOI] [PubMed] [Google Scholar]
- Bouvard M, Braconnier A, Dissoubray C: Buspirone in adolescents with Anxiety Disorders. New York (NY), 49th Annual Meeting of the American Psychiatric Association, 1996 [Google Scholar]
- Buspirone (package insert). Bristol-Myers Squibb Company, Princeton, NJ, 2010. www.accessdata.fda.gov/drugsatfda_docs/label/2001/18731s39s45lbl.pdf Accessed August18, 2017 [Google Scholar]
- Cipriani A, Zhou X, Giovane CD, Hetrick SE, Qin B, Whittington C, Coghill D, Zhang Y, Hazell P, Leucht S, Cuijpers P, Pu J, Cohen D, Ravindran AV, Liu Y, Michael KD, Yang L, Liu L, Xie P: Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet 388:881–890, 2016 [DOI] [PubMed] [Google Scholar]
- Cohen D, Bonnot O, Bodeau N, Consoli A, Laurent C: Adverse effects of second-generation antipsychotics in children and adolescents: A Bayesian meta-analysis. J Clin Psychopharmacol 32:309–316, 2012 [DOI] [PubMed] [Google Scholar]
- Davari-Ashtiani R, Shahrbabaki ME, Razjouyan K, Amini H, Mazhabdar H: Buspirone versus methylphenidate in the treatment of attention deficit hyperactivity disorder: A double-blind and randomized trial. Child Psychiatry Hum Dev 41:641–648, 2010 [DOI] [PubMed] [Google Scholar]
- Dobson ET, Strawn JR: Placebo response in pediatric anxiety disorders: Implications for clinical trial design and interpretation. J Child Adolesc Psychopharmacol 26:686–693, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Veroniki AA, Siamouli M, Möller H-J: No role for initial severity on the efficacy of antidepressants: Results of a multi-meta-analysis. Ann Gen Psychiatry 12:26, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh A, Ayoobzadehshirazi A: A randomized double-blind placebo-controlled clinical trial of adjuvant buspirone for irritability in autism. Pediatr Neurol 52:77–81, 2015 [DOI] [PubMed] [Google Scholar]
- Goldberg HL, Finnerty RJ: The comparative efficacy of buspirone and diazepam in the treatment of anxiety. Am J Psychiatry 136:1184–1187, 1979 [DOI] [PubMed] [Google Scholar]
- Greco T, Landoni G, Biondi-Zoccai G, D'Ascenzo F, Zangrillo A: A Bayesian network meta-analysis for binary outcome: How to do it. Stat Methods Med Res 25:1757–1773, 2016 [DOI] [PubMed] [Google Scholar]
- Greenberg E: Introduction to Bayesian Econometrics. New York, Cambridge University Press, 2008 [Google Scholar]
- Kranzler HR: Use of buspirone in an adolescent with overanxious disorder. J Am Acad Child Adolesc Psychiatry 27:789–790, 1988 [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Murphy S, Owen RT, Hart JT, Pearch P, Sykora K: Use of buspirone in an adolescent with overanxious disorder. J Am Acad Child Adolesc Psychiatry 27:789–790, 1988 [DOI] [PubMed] [Google Scholar]
- Lader M, Scotto JC: A multicentre double-blind comparison of hydroxyzine, buspirone and placebo in patients with generalized anxiety disorder. Psychopharmacol (Berl) 139:402–406, 1998 [DOI] [PubMed] [Google Scholar]
- Lancaster T: Introduction to Modern Bayesian Econometrics. Oxford, Blackwell, 2004 [Google Scholar]
- Malhotra S, Santosh PJ: An open clinical trial of buspirone in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 37:364–371, 1998 [DOI] [PubMed] [Google Scholar]
- March JS, Entusah AR, Rynn M, Albano AM, Tourian KA: A Randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry 62:1149–1154, 2007 [DOI] [PubMed] [Google Scholar]
- Masi G, Millepiedi S, Mucci M: Efficacy of newer antidepressants for childhood anxiety disorders. Expert Rev Neurother 2:523–531, 2002 [DOI] [PubMed] [Google Scholar]
- Mohammadi M-R, Hafezi P, Galeiha A, Hajiaghaee R, Akhondzadeh S: Buspirone versus methylphenidate in the treatment of children with attention- deficit/hyperactivity disorder: Randomized double-blind study. Acta Med Iran 50:723–728, 2012 [PubMed] [Google Scholar]
- Monden R, de Vos S, Morey R, Wagenmakers E-J, de Jonge P, Roest AM: Toward evidence-based medical statistics: A Bayesian analysis of double-blind placebo-controlled antidepressant trials in the treatment of anxiety disorders. Int J Methods Psychiatr Res 25:299–308, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica N, Bourgeois F: Discontinuation and nonpublication of randomized clinical trials conducted in children. Pediatrics 138:e20160223–e20160223, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Walkup JT, Labellarte MJ, Riddle MA, Greenhill L, Klein R, Davies M, Sweeney M, Abikoff H, Hack S, Klee B, McCracken J, Bergman L, Piacentini J, March J, Compton S, Robinson J, O'Hara T, Baker S, Vitiello B, Ritz L, Roper M: Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med 344:1279–1285, 2001. 11323729 [Google Scholar]
- Popper CW: Psychopharmacologic treatment of anxiety disorders in adolescents and children. J Clin Psychiatry 54:52–63, 1993 [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R: Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Psychiatry 23:191–197, 1984 [DOI] [PubMed] [Google Scholar]
- RUPP (Research Units on Pediatric Psychopharmacology Anxiety Study Group): The Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J Am Acad Child Adolesc Psychiatry 41:1061–1069, 2002 [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Bailey V, Schneier FR, Pott E, Brown PJ, Roose SP: Influence of study design on treatment response in anxiety disorder clinical trials. Depress Anxiety 32:944–957, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynn MA, Riddle MA, Yeung PP, Kunz NR: Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: Two placebo-controlled trials. Am J Psychiatry 164:290–300, 2007 [DOI] [PubMed] [Google Scholar]
- Rynn MA, Siqueland L, Rickels K: Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am J Psychiatry 158:2008–2014, 2001 [DOI] [PubMed] [Google Scholar]
- Salazar DE, Frackiewicz EJ, Dockens R, Kollia G, Fulmor IE, Tigel PD, Uderman HD, Shiovitz TM, Sramek JJ, Cutler NR: Pharmacokinetics and tolerability of buspirone during oral administration to children and adolescents with anxiety disorder and normal healthy adults. J Clin Pharmacol 41:1351–1358, 2001 [DOI] [PubMed] [Google Scholar]
- Shahrbabaki ME, Sabzevari L, Haghdoost A, Ashtiani RD: A randomized double blind crossover study on the effectiveness of buspirone and methylphenidate in treatment of attention deficit/hyperactivity disorder in children and adolescents. Iran J Psychiatry Clin Psychol 18:292–297, 2013 [Google Scholar]
- Siméon JG: Use of anxiolytics in children. Encephale 19:71–74, 1993 [PubMed] [Google Scholar]
- Strawn JR, Compton SN, Robertson B, Albano AM, Hamdani M, Rynn MA: Extended release guanfacine in pediatric anxiety disorders: A pilot, randomized, placebo-controlled trial. J Child Adolesc Psychopharmacol 27:29–37, 2017a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Dobson ET, Mills JA, Cornwall GJ, Sakolsky D, Birmaher B, Compton SN, Piacentini J, McCracken JT, Ginsburg GS, Kendall PC, Walkup JT, Albano AM, Rynn MA: Placebo response in pediatric anxiety disorders: Results from the child/adolescent anxiety multimodal study. J Child Adolesc Psychopharmacol 2017b. [Epub ahead of print]; DOI: 10.1089/cap.2016.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Prakash A, Zhang Q, Pangallo BA, Stroud CE, Cai N, Findling RL: A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 54:283–293, 2015b [DOI] [PubMed] [Google Scholar]
- Strawn JR, Sakolsky DJ, Rynn MA: Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am 21:527–539, 2012 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA: Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depress Anxiety 32:149–157, 2015a [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Buspirone HCl (BuSpar) Pediatric Supplement NDA 18-731/Supplement 43. Washington, DC: Bristol-Myers Squibb Company, 2000 [Google Scholar]
- U.S. Food and Drug Administration. Proposed Pediatric Study Request for BuSpar (buspirone HCl). Washington, DC: Bristol-Myers Squibb Company, 1998 [Google Scholar]
- United States Congress: Food and Drug Administration Modernization Act of 1997. United States Congress, 1997 [Google Scholar]
- United States Congress (Christopher Dodd): Best Pharmaceuticals for Children Act of 2002. United States Congress, 2002 [Google Scholar]
- United States Congress (Michael “Mike” DeWine): Pediatric Research Equity Act of 2003. United States Congress, 2003 [Google Scholar]
- Velosa JF, Riddle MA: Pharmacologic treatment of anxiety disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am 9:119–133, 2000 [PubMed] [Google Scholar]
- Wagner KD: Generalized anxiety disorder in children and adolescents. Psychiatr Clin North Am 24:139–153, 2001 [DOI] [PubMed] [Google Scholar]
- Walkup JT: Antidepressant efficacy for depression in children and adolescents: Industry- and NIMH-funded studies. Am J Psychiatry 174:430–437, 2017 [DOI] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC: Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 359:2753–2766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. Available at https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ Accessed August172017
- Zwier KJ, Rao U: Buspirone use in an adolescent with social phobia and mixed personality disorder (cluster A type). J Am Acad Child Adolesc Psychiatry 33:1007–1011, 1994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.