Abstract
AGS-004 consists of matured autologous dendritic cells co-electroporated with in vitro transcribed RNA encoding autologous HIV antigens. In an open-label, single arm sub-study of AGS-004-003, AGS-004 was administered monthly to suppressed participants who started antiretroviral therapy (ART) during acute HIV infection. HIV-1 specific T cell responses were measured by multicolor flow cytometry after 3–4 doses. The frequency of resting CD4+ T-cell infection (RCI) was measured by quantitative viral outgrowth assay. Participants demonstrating increased immune response postvaccination were eligible for analytic treatment interruption (ATI). AGS-004 induced a positive immune response defined as ≥2-fold increase from baseline in the number of multifunctional HIV-1 specific CD28+/CD45RA− CD8+ effector/memory cytoxic T-lymphocytes (CTLs) in all six participants. All participants underwent ATI with rebound viremia at a median of 29 days. Immune correlates between time to viral rebound and the induction of effector CTLs were determined. Baseline RCI was low in most participants (0.043–0.767 IUPM). One participant had a >2-fold decrease (0.179–0.067 infectious units per million [IUPM]) in RCI at week 10. One participant with the lowest RCI had the longest ATI. AGS-004 dendritic cell administration increased multifunctional HIV-specific CD28+/CD45RA− CD8+ memory T cell responses in all participants, but did not permit sustained ART interruption. However, greater expansion of CD28−/CCR7−/CD45RA− CD8+ effector T cell responses correlated with a longer time to viral rebound. AGS-004 may be a useful tool to augment immune responses in the setting of latency reversal and eradication strategies.
Keywords: : dendritic cell vaccine, HIV, acute HIV infection, HIV eradication, analytic treatment interruption
Introduction
Given a rapidly expanding need for antiretroviral therapy (ART) for HIV-infected individuals worldwide, innovative therapies that control or cure HIV-1 infection are needed. One approach is to strengthen and broaden HIV-specific CD8+ T cell responses, based on the temporal association between the appearance of HIV-specific CD8+ responses and viral decline during acute HIV infection (AHI).1,2 One HIV therapeutic immunization strategy utilizes monocyte-derived dendritic cells (MD-DCs) loaded ex vivo with RNA encoding HIV antigens, as MD-DCs are potent antigen-presenting cells and induce primary and secondary immune responses in CD4+ and CD8+ T cells. This approach can induce and increase antigen-specific T cell responses in animal models and patients with chronic infections and cancer.3 Further, HIV-1 peptide-loaded DCs administered to HIV-infected individuals can induce some degree of HIV-specific immunogenicity.3–7
AGS-004-003 was a randomized controlled trial of AGS-004 on HIV RNA set point following analytic treatment interruption (ATI) in chronically HIV-infected (CHI) participants.4,8 AGS-004 is an autologous DC therapy consisting of matured patient-derived autologous DCs co-electroporated with in vitro transcribed RNA encoding HIV-1 Gag, Nef, Rev, and Vpr amplified from participants' pre-ART plasma and RNA encoding human CD40 ligand (CD40 L). Autologous DCs are matured ex vivo by sequential exposure to tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), and PGE2 before adding the CD40 L adaptive signal. CD40 L is employed to maximize DC antigen presentation9 and improve immunopotency, by inducing secretion of interleukin (IL)-12, a necessary cytokine for CD8+ T cell responses.10,11
In this sub-study, we evaluated AGS-004 in participants who started ART during AHI, as immune-enhancing strategies may be particularly suited to these individuals. ART initiation during AHI can preserve HIV-1-specific CD4+ and CD8+ T cell responses12 and limit the size of the latent reservoir.13–17 Further, most individuals are infected with a single or limited number of founder viruses18,19 and early treatment minimizes virus evolution, as few variants escape from CD8+ T cell pressure in the first weeks.18,20 The combination of preserved cellular responses and limited viral diversity in acutely treated individuals provides a unique population for vaccination studies.
Materials and Methods
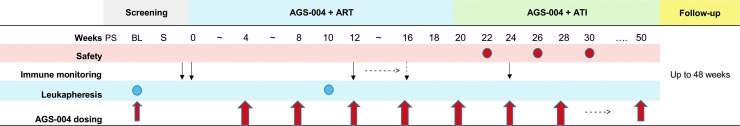
This was an open-label, two-site, single arm sub-study of AGS-004 DC therapy in participants ages 18–59 years who initiated ART within 45 days of AHI, defined as a negative or indeterminate enzyme immunoassay or negative HIV RNA test within 45 days of detectable plasma HIV RNA. Eligibility included HIV RNA levels <50 copies/ml (c/ml) on ART for at least 6 months, CD4+ nadir ≥200 cells/mm3, baseline CD4+ count >400 cells/mm3, and a pre-ART frozen plasma sample within 90 days of starting ART. AHI participants received five monthly doses of AGS-004 (weeks 0, 4, 8, 12, and 16) while on ART (Fig. 1), with each dose consisting of 1.2 × 107 DCs administered as three 0.2 ml intradermal injections. Participants began ATI at ∼week 20 and continued monthly AGS-004 dosing. In contrast to the parent study in CHI participants,4 only AHI participants with a positive CTL response postvaccination were eligible for voluntary ATI. CD4+ counts and HIV RNA levels were evaluated every 2 weeks following ATI. Restarting ART could be triggered by CD4+ count <350 cell/mm3, >20% decline in absolute CD4+ count or CD4+ count percentage, confirmed HIV RNA level ≥10,000 c/ml, or participant request. Participants were followed through week 48 after the last AGS-004 dose or ART reinitiation (Fig. 1). The University of North Carolina at Chapel Hill (UNC) and Duke University Institutional Review Boards approved the study. All participants provided written informed consent.
FIG. 1.
Study schema. ART, antiretroviral therapy; ATI, analytic treatment interruption; BL, baseline leukapheresis; PS, prescreen; S, screen.
Autologous DCs used to produce AGS-004 have been described.4 Monocytes are collected during leukapheresis and differentiated in culture. Autologous pre-ART HIV RNA encoding Gag, Nef, Vpr, and Rev (GNVR) is amplified and co-electroporated with synthetic CD40 L RNA into mature DCs.9 A reduced quantity of Nef RNA is used to preserve DC functionality by minimizing HLA downregulation.21 Vpr RNA is truncated due to its ability to inhibit production of IL-1222 and reduce activation of antigen-specific memory and recall CD8+ CTL responses.10,23,24
A second leukapheresis was collected before ATI for immune monitoring purposes. Autologous DCs were again co-electroporated with CD40 L RNA and GNVR, or with CD40 L RNA, and each individual antigen RNA or with CD40 L RNA and GFP RNA (DCs expressing green fluorescent protein (GFP) served as control DCs). This allows measurement of HIV-specific T cell responses in vitro to the combination RNA antigen payload (GNVR), to each individual HIV antigen (Gag, Nef, Vpr, or Rev) and to control DC for six separate DC targets. Peripheral blood mononuclear cells (PBMCs) were collected at baseline (immediately before the first AGS-004 dose), and 1 month after the third (week 12) or fourth dose (week 16), and 2 days after the fourth dose in one participant (51–102). Given a limited number of PBMCs collected, cultures were set up as individual wells and positive responses determined by subtracting two times the total number of functional CD28+/CD45RA− effector/memory CTL at baseline, from the total number of functional CD28+/CD45RA− effector/memory CTL measured in response to in vitro stimulation with DC encoding each antigen.
To determine immune responses by multicolor flow cytometry, PBMCs collected at baseline and after ≥3 immunizations were cultured in vitro with autologous DCs as described.10,25 PBMCs were labeled with BrdU (bromodeoxyuridine) to track CTL proliferation and cocultured with autologous DC targets prepared as above. Autologous DC targets for in vitro stimulation were either DCs co-electroporated with CD40 L RNA and all antigens (GNVR) or with DCs co-electroporated with CD40 L and each individual antigen. To determine background nonspecific activation of in vitro CTL cultures, PBMCs at each time point were stimulated with CD40 L DCs co-electroporated with GFP RNA. GFP RNA was not included in the administered product. Cocultures were incubated at 37°C for 6 days. On day 6, each culture was restimulated with the same autologous DCs used in the initial culture, anti-CD107a antibody was added and cultures were incubated at 37°C for 5 h. Viable cells were identified by staining for viability using Live/Dead Fixable Dye (Invitrogen) followed by surface staining with specific antibodies for CD28, CCR7, CD27, CD45RA, CD3, and CD8 expression. Cells were fixed, permeabilized, and DNase treated to detect BrdU (BD biosciences). Intracellular staining for IFN-γ, TNF-α, IL-2, GrB, and BrdU was performed. Cells were transferred to BD TruCount Tubes for analysis on a BD LSRII cytometer, with 400,000–600,000 events collected per sample.
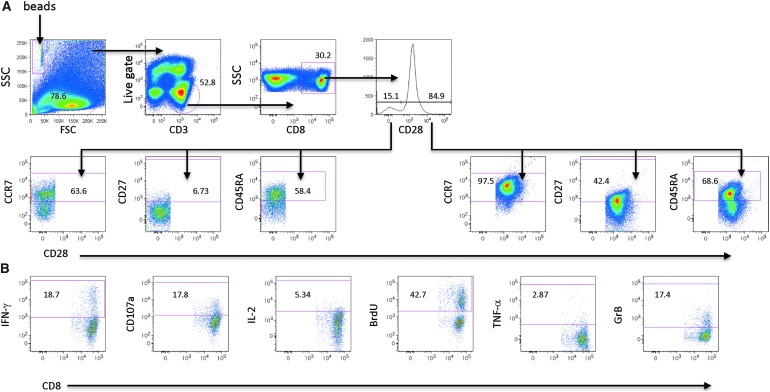
Viable CD3+CD8+ T cells were divided into either CD28 receptor-positive or CD28 receptor-negative subsets. Boolean gating was used to define the central memory (CM) effector memory (EM) and effector (E) CTL subsets defined by the expression of CD45RA, CCR7, and CD27 within the CD28 receptor-positive and CD28 receptor-negative CTL subsets (Fig. 2A). Within the CM, EM, and E CTL subsets, positive staining for cytokines (IFN-γ, TNF-α, and IL-2), cytolytic markers (Granzyme B [GrB], CD107a, and proliferation (BrdU) (Fig. 2B) identified functional marker expression. Increases in effector marker expression are reported as the absolute number of cells/ml determined using TruCount Tubes (BD biosciences) during sample acquisition by flow cytometry. The number of cells/ml within a given gate is calculated as follows: (number of cellular events collected/number of Trucount beads collected) × (Trucount bead concentration)/collected volume [μL]) × 1,000.
FIG. 2.
Determination of multifunctional T cell responses. PBMC collected pre and post-treatment were cultured in vitro with autologous AGS-004 DCs. The following gating strategy was used to identify central/memory, effector/memory, and effector CTLs. (A) After in vitro stimulation multicolor flow cytometry identified AGS-004-induced HIV Ag-reactive CTL subsets by first gating on live CD3-positive cells present in the lymphocyte gate forward scatter (FSC) vs. side scatter (SSC). CD8-positive cells were then gated through the CD28 histogram and the CD28-positive and CD28-negative subsets were further gated to identify the CCR7, CD27 CD45RA-positive and negative subsets. Boolean gating was used to identify CTL subsets based on the combinatorial expression of the surface markers CD28, CCR7, CD27, and CD45RA. (B) To identify functional cells/ml within each CTL subset the expression of functional markers was determined by gating on cells within the gates for cytokines (IFN-γ, TNF-α, and IL-2), cytolytic markers (Grb and CD107) and proliferation (BrdU). Trucount beads were identified in the FSC versus SSC gate. DC, dendritic cell; IL, interleukin; PBMC, peripheral blood mononuclear cells; FSC, forward scatter; SSC, side scatter.
As in the parent study (AGS-004-003),4 a positive response to AGS-004 was defined as >2-fold increase over baseline in the number of CD28+/CD45RA− effector/memory CTLs (cells/ml) exhibiting at least one effector function defined by the expression of IFN-γ, TNF-α, IL-2, GrB, CD107a, or proliferation to the total HIV antigen payload (GNVR) present. Statistically significant differences between CD28+/CD45RA− effector/memory CTL responses at baseline and after AGS-004 administration were determined using a two-tailed Student's t-TEST. Correlates between time to viral rebound and change in the number of effector/memory and effector multifunctional CTLs after AGS-004 administration were determined by nonparametric bivariate Spearman's Rho statistical analysis.
Plasma HIV RNA assays were performed by a single-copy assay (SCA), with two measurements at baseline and at weeks 10 and 16. An additional SCA was performed at week 28 if HIV RNA remained undetectable during ATI. The frequency of resting CD4+ T cell infection (RCI) was measured by quantitative viral outgrowth assay (QVOA) as described26,27 at baseline and pre-AGS-004 dosing and after three doses on ART (week 10). Correlation between time to viral rebound during ATI and RCI and CTL fold increase was assessed by Spearman correlation. In participant (51–102) with prolonged aviremia following rebound during the ATI period, antiretroviral drug testing was performed at McGill University and the UNC School of Pharmacy.
Results
Between June 2012 and January 2013, six male participants enrolled with a median age of 35 years (range 26–56; Table 1). At enrollment, the median CD4+ T cell count was 618 cells/mm3 (range 397–937), and the median time from AHI diagnosis to ART initiation was 15 days (range 9–20). The median time from participant ART start date to enrollment was 3.1 years (range 1.5–3.7). There were few treatment-related adverse events in the study; none >grade 1, with mild injection site reactions the most common. No participant discontinued or interrupted study treatment due to adverse events.
Table 1.
Demographic and Clinical Characteristics of Acute Hiv Infection Participants Who Received AGS-004 Dendritic Cell Therapy
| Participant ID | Age (years) | Race/ethnicity | Gender | Baseline CD4 count (cells/mm3) | Baseline SCA (c/ml) | Week 12/pre-ATI SCA (c/ml) | Time to HIV RNA rebound (days) | ATI duration (days) | Reason for ART restart | Peak HIV RNA during ATI (c/ml) | Pretreatment frequency of RCI (IUPM) | Post-treatment frequency of RCI (IUPM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 51–100 | 37 | African American | Male | 662 | <0.6 | <0.5 | 28 | 36 | VL >10,000 c/ml | 267,000 | 0.266 | 0.140 |
| 51–102 | 33 | African American | Male | 397 | <0.4 | <0.4 | 28 | 280a | VL >10,000 c/ml | 11,900 | 0.767 | 0.572 |
| 54–100 | 56 | White, non-Hispanic | Male | 574 | <0.4 | 1.2 | 55 | 90 | VL >10,000 c/ml/>20% ↓ CD4% | 287,000 | 0.179 | 0.067 |
| 54–101 | 50 | White, non-Hispanic | Male | 482 | <0.5 | <0.5 | 49 | 151 | >20% ↓ CD4% | 3,290 | 0.043 | 0.049 |
| 54–102 | 26 | African American | Male | 937 | <0.5 | 0.48 | 29 | 42 | VL >10,000 c/ml | 24,000 | 0.088 | 0.195 |
| 54–104 | 26 | African American | Male | 714 | 0.8 | <0.4 | 13 | 33 | >20% ↓ CD4% | 19,300 | 0.525 | 0.691 |
Therapeutic drug testing detected ART medications during ATI.
ART, antiretroviral therapy; RCI, resting CD4+ T cell infection; SCA, single-copy assay; ATI, analytic treatment interruption; IUMP, infectious units per million
Three participants (51–100, 51–102, and 54–100) received AGS-004 DC therapy with all four RNAs (GNVR). Two participants (54–101 and 54–102) received AGS-004 with three RNAs, Gag, Nef, and Rev (GNR) as Vpr was not recovered in the pre-ART sample; one participant (54–104) received AGS-004 with three RNAs, Gag, Vpr, and Rev (GVR) as Nef was not recovered in the pre-ART sample. Previous work demonstrated that in vitro priming with postmatured DC co-electroporated with CD40 L RNA and HIV RNA encoding specific target antigens expanded multifunctional antigen-specific CTL, exhibiting a CD28+/CD45RA− effector/memory phenotype.4,11,28 Similar DC therapy administered to participants with metastatic renal cell carcinoma showed a significant correlation between the expansion of functional CD28+/CD45RA− effector/memory CTL and clinical outcomes.25 Therefore, our primary immune end point focused on the expansion of functional HIV-1-specific CD28+/CD45RA− effector/memory CTLs.
After in vitro stimulation, the number of CD8+ T cells expressing each functional marker was calculated at baseline (W0, pre-AGS-004) and after AGS-004 administration (W12 or W16) for each participant (Fig. 3). Statistically significant increases in the number of CD8+ T cells proliferating (BrdU) and expressing IFN-γ, CD107a and Grb were detected to the total GNVR total RNA payload after AGS-004 administration in all six participants. The number of CD8+ T cells expressing each functional marker (red dots) for a representative participant is shown (Fig. 4A). Post-AGS-004, the number of proliferating CTLs increased after in vitro restimulation from 14,011 to 32,875 cells/ml. Increases in the numbers of cells/ml were seen for the lytic markers CD107a (6,467–20,478) and GrB (15,971–27,793) and for the cytokines IFN-γ (6,718–20,557) and TNF-α (517–806). For this representative participant, IL-2 decreased from 7,307 cells/ml at baseline to 4,285 cells/ml post-AGS-004. Moreover, the majority of functional CTLs resided in the CD28+/CD5RA− effector/memory CTL subset (Fig. 4A red dots). Given increases in the number of functional CTL present in the CD28+/CD45RA− CTL population, we determined functional marker expression after AGS-004 administration as a quantitative value (cells/ml) for CTLs in the CD28+/CD45RA− effector/memory CTL subset. Determining changes in the number of cells/ml with functional activity in the CD28+/CD45RA− effector/memory CTL subset from baseline to post-treatment enables a direct comparison between measurements performed within a single participant and between participants. Six of six participants had greater than a two-fold increase in the total number of CD28+/CD45RA− effector/memory CTLs expressing any functional marker after post-AGS-004 (W12 or W16), and in five participants the increases over baseline (W0) were statistically significant (Fig. 4 B–F). While the increase in response for participant 054–104 (Fig. 4G) was not statistically significant, it was greater than two-fold over baseline (from 30,613 cells/ml at baseline to 95,709 cells/ml at week 12). This contrasted to responses measured to GFP where only two participants, 054–101 and 054–102 (Fig. 4E, F, respectively) had statistically significant increases in the total number of CD28+/CD45RA− effector/memory CTLs expressing any functional marker after AGS-004 administration.
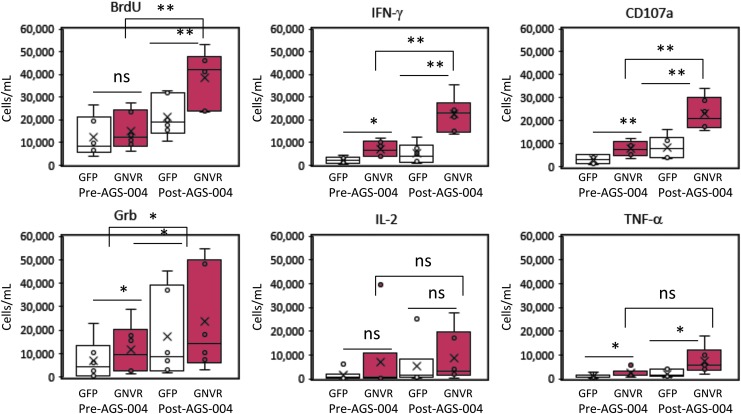
FIG. 3.
CD8+ T cell responses in participants receiving AGS-004. Box and whisker plots show the distribution of the number of CD8+ T cells (cells/ml) proliferating (BrdU), expressing IFN-γ, CD107a, Grb, IL-2, or TNF-α determined before or post AGS-004 administration for participants. Statistically significant differences between the GFP background response (open bar and whisker plots) and the total antigen payload (GNVR)-specific response (red bar and whisker plots) are shown. Statistically significant differences between the total antigen payload (GNVR) responses determined pre- and post-AGS-004 administration are shown. *p < .05; **p < .01. GNVR, Gag, Nef, Vpr, and Rev; NS, not statistically significant.
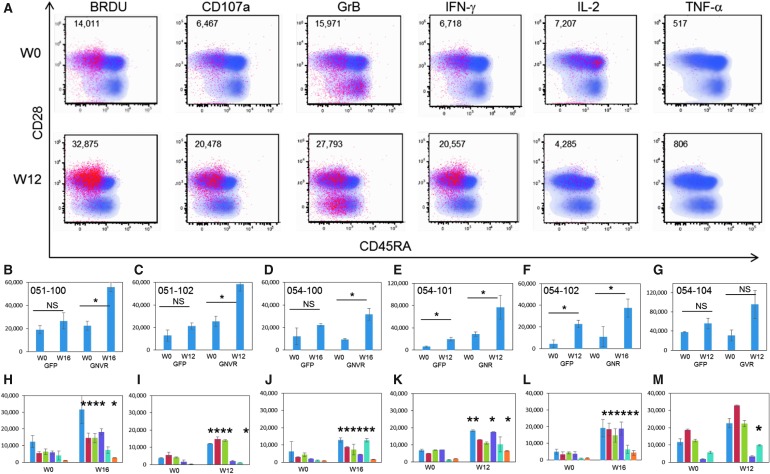
FIG. 4.
Multifunctional immune responses to the total antigen RNA payload in participants treated with AGS-004. CD8 T cell responses at baseline (W0) or after AGS-004 administration (W12 or W16) were measured after in vitro stimulation with AGS-004 autologous DCs and functional marker expression was detected by multicolor flow cytometry. (A)
Dot plot overlays from one representative participant before AGS-004 administration (W0) and after AGS-004 administration (W12) show the distribution of cellular events representing each functional marker (red dots) BrdU, CD107a, Grb, IFN-γ, IL-2, and TNF-α overlaid on the total CD8+ T cell population (blue dots) gated on the CD28 (y-axis) and CD45RA (x-axis) plots. The numbers are representative of cells/ml expressing each functional marker (red dots). (B–G) The total number of CD28+/CD45RA− CTL (cells/ml, Y-axis) expressing any functional marker after stimulation with DCs encoding GFP or the final product HIV payload (GNVR, GNR, or GVR) was calculated at baseline (W0) and post-AGS-004 (W12 or W16). Total numbers of cells/ml were determined by adding the numbers of cells/ml measured for each individual functional marker. (B) 51–100 to GNVR, (C) 51–102 to GNVR, (D) 54–100 to GNVR, (E) 54–101 to GNR, (F) 054–102 GNR, and (G) 054–104 to GVR. Statistically significant increases in the total number of cells/ml expressing functional markers over baseline are shown. NS, not significant. The contribution of each functional marker (cells/ml, y-axis) within the total response at baseline (W0) or post-AGS-004 (W12 or W16) for each participant is shown, (H) 51–100 to GNVR, (I) 51–102 to GNVR, (J) 54–100 to GNVR, (K) 54–101 to GNR, (L) 054–102 GNR, and (M) 054–104 to GVR. BrdU  , CD107a
, CD107a  , Grb
, Grb  , IFN-γ
, IFN-γ  , IL-2
, IL-2  , and TNF-α
, and TNF-α  . Starred (*) markers indicate statistically significant increased CTL responses from pre- to post-AGS-004 administration. GNR, Gag, Nef, and Rev; GVR, Gag, Vpr and Rev.
. Starred (*) markers indicate statistically significant increased CTL responses from pre- to post-AGS-004 administration. GNR, Gag, Nef, and Rev; GVR, Gag, Vpr and Rev.
We then examined the multifunctionality of the response, defined as an increase in at least two functional markers after post-AGS-004. Five of six participants had statistically significant increases in multiple functional markers with participants 54–100 (Fig. 4J) and 54–102 (Fig. 4L) having increases in all six functional markers. Participants 51–100 (Fig. 4H) and 51–102 (Fig. 4I) had statistically significant increases in all functional markers excluding IL-2, and participant 54–101 (Fig. 4K) had statistically significant increases in the number of cells/ml expressing markers for BrdU, CD107a, IFN-γ, and TNF-α. Participant 54–104 (Fig. 4M) had increases in functional markers over baseline; however, the only statistically significant change was detected for CTLs that produced IL-2.
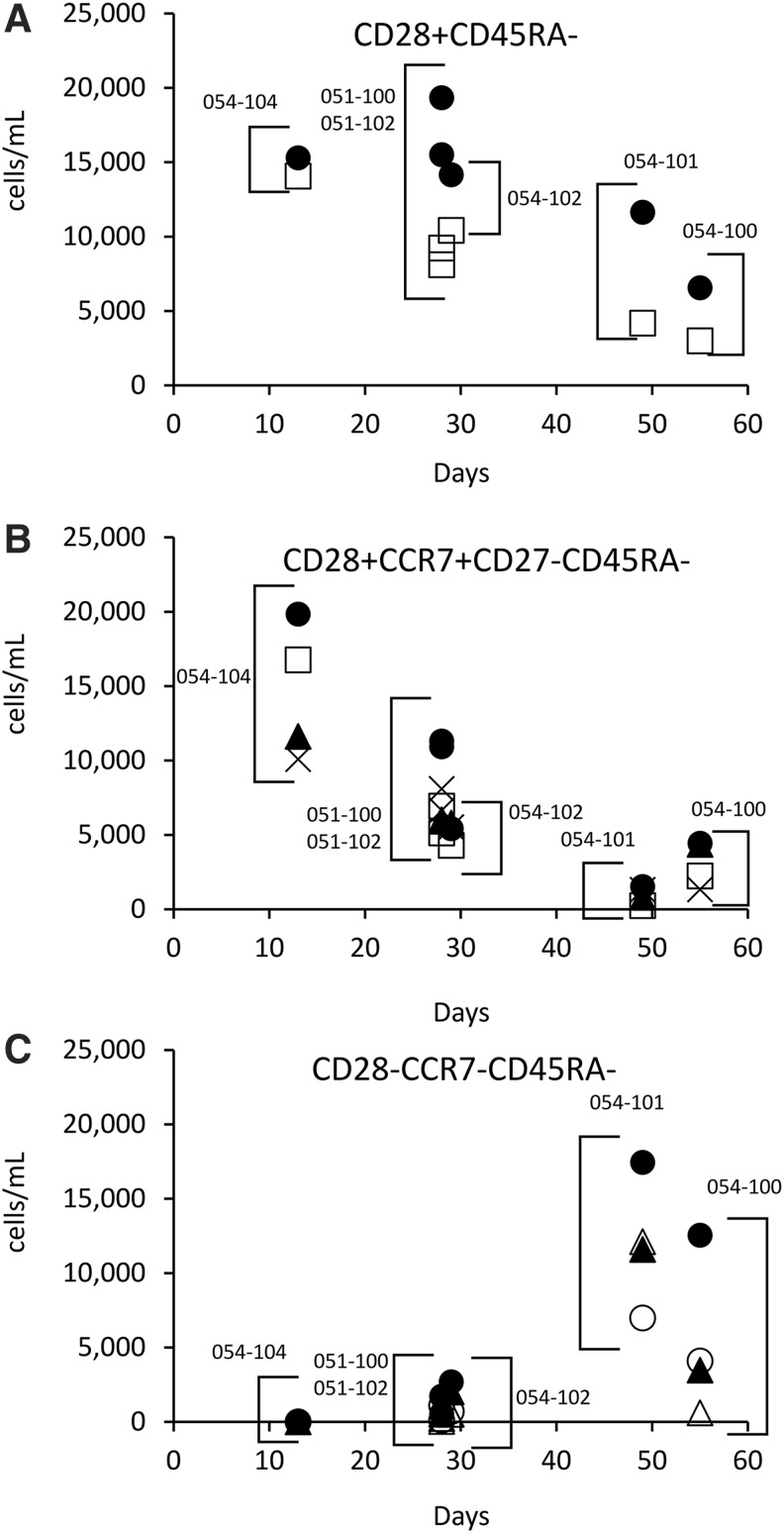
The increased numbers of CD28+/CD45RA− effector/memory CTL (cells/ml) determined from pre- to post-AGS-004 treatment for each functional marker was plotted versus time to viral rebound and showed an inverse correlate between time to rebound and the magnitude of proliferating CTL (ρ = −0.811 p = < .05 or CTL expressing Grb (ρ = −0.811 p = < .05) (Fig. 5A). If the CD28+/CD45RA− subset was further sub-gated to include only CTL expressing CCR7, to define CM CTL, additional inverse correlates were seen with CTL expressing IFN-γ (ρ = −0.927 p = < .007), and CD107a (ρ = −0.927 p = < .007), BrdU (ρ = −0.927 p = < .007), and GrB (ρ = −0.927 p = < .007) (Fig. 5B). This prompted analysis of other CTL subsets defined by the absence of the CD28 receptor and CCR7, to see whether other functional subsets correlated with time to viral rebound. Interestingly, the increase in the number of functional CTL representative as effector CTL defined as CD28−/CCR7−/CD45RA− positively correlated with time to viral rebound (Table 1) for numbers of CTL that are BrdU positive (ρ = 0.811 p = < .05) express CD107a, (ρ = 0.927 p = < .007), IL-2 (ρ = 0.840 p = < .036), or TNF-α (ρ = 0.927 p = < .007) (Fig. 5C). These data suggest participants with the longest time to viral rebound accumulated increased numbers of effector CTL in peripheral blood with a concurrent decrease in numbers of CM CTL in the periphery.
FIG. 5.
Increased numbers of effector CTL within the CD28−/CCR7−/CD45RA− CTL subset correlates with longer time to viral rebound. CD8+ T cell responses at baseline (W0) or after AGS-004 administration (W12 or W16) were measured after in vitro stimulation with AGS-004 autologous DCs and functional marker expression was detected by multicolor flow cytometry. (A) Absolute numbers of CTL (cells/mL) for each effector marker (BrdU •, CD107a ▲, Grb □, IFN-γ X, IL-2 ◯, and TNF-α △) were calculated by subtracting the number of CTL determined at baseline (W0) from the number of CTL determined after AGS-004 administration (W12 or W16). Absolute numbers of CTL with effector marker expression for the CD28+/CD45RA− CTL subset (A), the CD28+CCR7+CD27−CD45RA− CTL subset (B), and the CD28−CCR7−CD45RA− CTL subset (C) were plotted versus time to viral rebound (days) for each participant. For each subset only those effector markers that had either a positive correlate with time to viral rebound or an inverse correlate with time to viral rebound are shown. Correlates were determined by nonparametric bivariate Spearman's Rho statistical analysis.
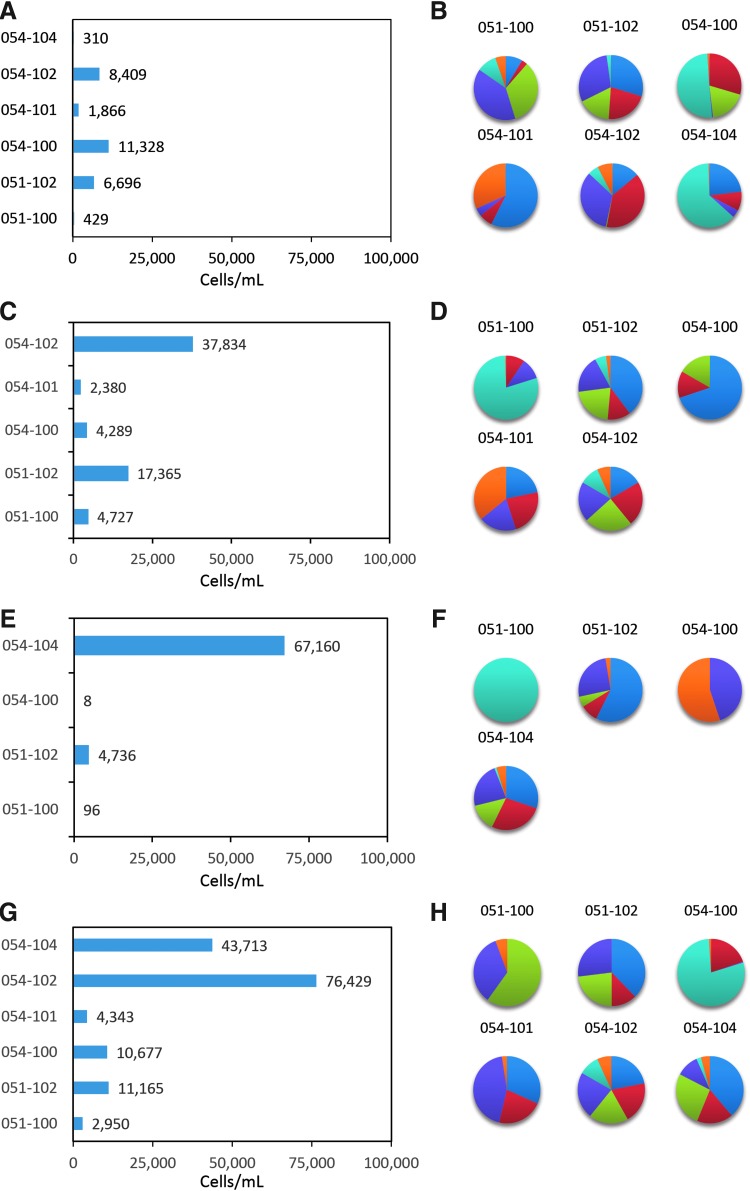
Evaluation of response to individual RNA antigens after in vitro stimulation revealed an increase in total numbers of CD28+/CD45RA− effector/memory CTLs expressing any functional marker for each participant to each individual HIV antigen (Fig. 6). All six participants had ≥2-fold increased responses over baseline to Gag (range 310–11,323 cells/ml) (Fig. 6A), and responses were multifunctional. The percentage of each functional marker within the total response to Gag is represented for each participant (Fig. 6B). While all participants contained multifunctional CTLs, there was no clear pattern of functional marker expression across participants. In some cases the CTL population response to Gag contained CTLs that proliferated (shown in blue), or produced cytokines IFN-γ (purple) or TNF-α (orange), or IL-2 (light blue). Lytic activity was detected with GrB expression in three participants (green) and CD107a (red) for all participants.
FIG. 6.
Multifunctional immune responses to the individual HIV antigens present in the administered AGS-004 payload. The total number of CD28+/CD45RA− CTL (cells/ml, X-axis) expressing the any functional marker after in vitro stimulation with DCs encoding the single HIV antigens are expressed as the values (cells/ml) greater than twofold over baseline. (A) Participant responses to GAG, (C) participant responses to Nef, (E) participant responses to Vpr, and (G) participant responses to Rev. The percent contribution of each individual functional marker to the total response is represented by pie charts for participant responses to (B) GAG, (D) Nef, (F) Vpr, and (H) Rev. Pie charts of representative responses to BrdU  , CD107a
, CD107a  , Grb
, Grb  , IFN-γ
, IFN-γ  , IL-2
, IL-2  , TNF-α
, TNF-α  are shown clockwise starting at 12 o'clock.
are shown clockwise starting at 12 o'clock.
The multifunctional responses to Nef (Fig. 6C) ranged from 2,380 to 37,834 cells/ml. Proliferating CTLs were observed in three participants and two of these participants contained all six functional markers. Similarly, the composition of responses were multifunctional with 051–100 containing CTL expressing CD107a, IFN-γ, and IL-2, participant 051–102 contained proliferating CTL and all six functional markers, 054–100 contained proliferating CTL and CD107a and GrB. 054–101 contained proliferating CTL and CD107a, IFN-γ, and TNF-α. 054–102 contained CTL expressing all six functional markers (Fig. 6D).
Four participants tested for response to Vpr revealed responses over baseline ranging from 8 to 67,160 cells/ml and were multifunctional for two participants (051–102 and 054–104), expressing all six functional markers. Responses for participants 051–100 and 054–100 were below 100 cells/ml and more mono-functional with CTL expressing only IL-2, (051–100) or IFN-γ and TNF-α (054–100) (Fig. 6F).
Responses to Rev over baseline ranged from 2,950 to 76,429 cells/ml (Fig. 6G); all participants had CTLs that expressed two functional markers. For participant 51–100, the response was dominated by CTL expressing IFN-γ, GrB, and TNF-α, and for 051–102, the response contained proliferating CTL and CD107a, GrB, and IFN-γ. 051–100 contained CTL expressing IL-2 and CD107a, 054–101 contained proliferating CTL and IFN-γ, CD017a, and TNF-α. Both 054–102 and 054–104 contained CTL expressing all six functional markers (Fig. 6H).
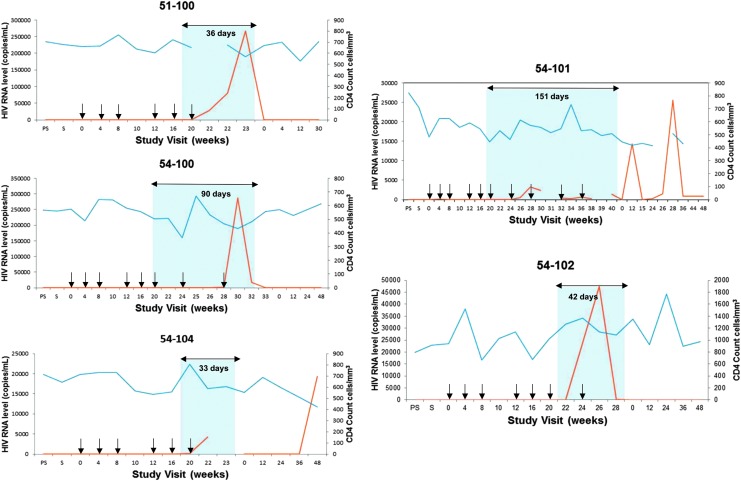
After confirming increases in the number of functional CD28+/CD45RA− memory CTL responses post-treatment, all participants underwent ATI. Figure 7 shows the time course of CD4+ T cell counts and changes in viral load for participants. Participants rebounded to ≥40 copies/ml at a median of 29 days (range 13–55), with a median ATI of 66 days (range 33–280; Table 1). Three participants restarted ART due to confirmed HIV RNA >10,000 c/ml, two restarted due to a >20% decline in CD4+ count, and one restarted after meeting both CD4+ and viral load criteria. The median observed peak HIV RNA level following ATI was 21,650 c/ml (range 3,290–287,000), and no symptoms consistent with acute retroviral syndrome were observed. All participants rapidly suppressed viremia after restarting pre-entry ART regimens (Fig. 7); however, two had confirmed viremia during follow-up with self-reported nonadherence to resumed ART (54–101, 54–104).
FIG. 7.
Measurement of viral load and CD4 T cell counts in participants receiving AGS-004. Plasma HIV RNA levels (red lines) and CD4 cell counts (blue lines) are shown for participants treated with AGS-004. The blue shaded areas indicate the period of analytic treatment interruption (ATI) across study visits and overlying arrows indicate the duration of ATI (days). AGS-004 administrations are denoted by black arrows. Participant 51–102 is not shown given drug monitoring revealed ongoing ART administration during the ATI period. ART, antiretroviral therapy; PS, prescreen; S, screen.
In one participant (051–102), initial viral rebound to >10,000 c/ml was followed by repeat viral load of 3,579 c/ml and continued ATI. Due to prolonged aviremia (280 days) following initial viral rebound in this participant, therapeutic drug testing was performed for efavirenz, emtricitabine, and tenofovir on stored samples obtained during ATI. Efavirenz was not detected eight days following ATI and before viral rebound, but detected from three subsequent stored samples from separate time points over 3 months during the ATI; tenofovir and emtricitabine were detected in the one sample tested. This participant denied restarting ART, and had a second viral rebound of 12,200 c/ml at day 280.
The frequency of RCI at pre-entry and pre-AGS-004 dosing was low in most participants, ranging 0.043–0.767 infected resting CD4+ cells per million (IUPM; Table 1), and in comparison with participants treated during CHI.29 Time to viral rebound was inversely correlated with RCI (ρ = −0.84; p = .04). The only participant (054–100) with a statistically significant decrease in RCI (2.5-fold from 0.179 IUPM at baseline to 0.067 IUPM at week 10)30 also had increases in the total number of functional CD28+/CD45RA− CTLs (Fig. 4D), and increases in CD28−/CCR7−/CD45RA− CTLs after AGS-004 dosing, but did not have the longest ATI. Increases were multifunctional in nature with CTLs positive for all six functional markers (Fig. 4J). Participant 054–100 had increases in the number of CTL responding to Gag (Fig. 6A, B), Nef (Fig. 6C, D), and Rev (Fig. 6G, H) and the longest time to rebound (55 days, Table 1) with an ATI duration of 90 days (Fig. 7C) in the absence of an HLA allele associated with HIV control.31 Despite an increase in both effector/memory and effector CTL responses, this participant had the highest observed peak HIV RNA level off ART (287,000 c/ml), but suppressed to <50 c/ml 4 weeks after reinitiating ART.
The participant (54–101, Fig. 7D) with the lowest baseline RCI (0.043 IUPM) rebounded at 49 days, but underwent ATI for 151 days, reinitiating ART due to decline in CD4+ count percentage (44.1%–33.8%). The peak observed HIV RNA level during this participant's ATI was 3,290 c/ml followed by rapid suppression after restarting ART, but developed subsequent viremia with reported nonadherence to ART. This participant showed an increase in the total number of functional CTL after AGS-004 administration (Fig. 4E) and in the CD28−CCR7−CD45RA− effector CTL subset with a multifunctional response to the total GNR HIV antigen payload and to the individual antigens Gag (Fig. 6A), Nef (Fig. 6C) and Rev (Fig. 6G).
HIV RNA measurement by SCA was detectable above 1 c/ml in only one participant at one time point after dosing, preventing assessment of the impact of AGS-004 on low-level viremia. No association of virologic or T cell response was observed with HLA type (data not shown).
Discussion
AGS-004 DC therapy administered to individuals who started ART during AHI led to statistically significant increases in HIV-specific CD28+/CD45RA− effector/memory CTL responses to the total antigen payload in five of six participants. Further, increased responses could be detected to single HIV antigens present in the payload. All six participants had an increased response to Gag and Rev, five participants had increased responses to Nef, and two participants to Vpr. The induced responses to the total antigen payload or the individual antigens displayed a multifunctional phenotype characterized by CD28+/CD45RA− effector/memory CTL that proliferated, produced cytokines such as IFN-γ, IL-2, and TNF-α and exhibited markers of lytic activity, a critical function necessary to kill virally infected cells. Further, the expansion of multifunctional CD28−/CCR7−/CD45RA− effector CTLs correlated with a longer time to viral rebound during ATI.
We observed a significant association between reservoir size measured by QVOA and time to rebound, supporting the hypothesis that a replication competent reservoir in resting CD4 cells fuels HIV rebound. Of note, the one participant with a significant decline in RCI had the longest time to rebound. Given concerns that even brief periods of viral rebound would be unacceptable among individuals treated during AHI, conservative criteria for reinitiating ART resulted in variability in the reason for reinitiation, and limited our ability to determine correlations. Due to the small sample size, comparison of immune responses and time to viremia with CHI participants who received AGS-004 or other acutely treated patients is limited.
We hypothesized that time to viral rebound in acutely treated participants might be substantially delayed as reported in other cohorts treated early following acquisition.12,32,33 However, despite expansion of multifunctional CD28+/CD45RA− effector/memory CTL responses, extended viral control was not observed. A lack of delay in viral rebound has been recently reported in a large cohort of participants treated during AHI.34 In fact, an inverse correlate with time to viral rebound was seen with expansion of both effector/memory and central/memory CTLs. Unexpectedly, a positive correlation between longer time to viral rebound and expansion of multifunctional effector CTL defined by the lack of CD28, CCR7, and CD45RA was observed in our study. Taken together, these data suggest that a conversion of AGS-004 induced CD28+/CCR7+/CD45RA− central/memory CTL toward multifunctional effector CTL may contribute to immunological control of viral rebound. This hypothesis is supported by the observation that participants with the longest time to viral rebound during ATI shifted from central/memory multifunctional CTLs toward a more differentiated effector CTL phenotype that maintained multifunctionality. The ability to convert expanded CM CTL to effector CTL may be essential to an effective antiviral immune response.
These correlations must be confirmed prospectively in future clinical trials. Lack of a control group prevents determining whether HIV-specific CTL responses induced by AGS-004 contributed to viremic control. The lack of prolonged viral control despite enhanced HIV-specific CD28+/CD45RA− effector/memory CTL responses may be due to insufficient frequencies of induced responses, lack of differentiation of central/memory CTL to effector CTL, lack of responses to critical HIV-1 antigen targets not incorporated in AGS-004, or the presence of preexisting escape variants. It is possible that early after AGS-004 treatment, CD8+ T cell activation induces CTLs, identified as effector/memory by the expression of CD28 and CCR7. Expansion of this pool of CTLs leads to a differentiation of effector CTL, which lack CD28 and CCR7. Therefore, while AGS-004 administration induces expansion of effector/memory and central/memory CTL, it may require further differentiation of these CTL subsets into a more effector like phenotype shown to correlate with control of viral rebound.
Our findings highlight challenges of studies in patients treated during AHI. The preservation of immune function in AHI participants suggests they are the population most likely to respond to immune enhancing interventions in proof-of-concept eradication studies, supported by the observation that multifunctional CTL responses could be recalled in vitro after administration of AGS-004 in all participants. Consistent with other findings,17 AHI participants in our study had <1 c/ml measured by SCA, precluding our ability to assess whether immunization impacted low level viremia. Further, all participants demonstrated low frequency of RCI at baseline compared to CHI patients17 on suppressive therapy,35 limiting the ability to assess depletion of RCI. We were also precluded from comparing viral set point pre- and postvaccination as participants initiated ART before establishing a set point during AHI. Therefore, the virologic impact of AGS-004 in acutely treated participants was limited to evaluating time-to-viral-rebound during ATI.
Our findings may inform the use of ATI in HIV eradication studies. In a study of six participants with durably suppressed HIV, one participant surreptitiously resumed ART during the ATI, highlighting a need to incorporate the measurement of ART levels in any ATI study. As two previously adherent participants became nonadherent after restarting ART, our findings suggest nonadherence following ATI should be addressed as a risk for participants.36
Although immune responses induced by AGS-004 did not prevent rebound viremia off ART as an approach to functional cure, the finding that AGS-004 induced responses in all participants suggests AGS-004 might enhance clearance of virus-expressing cells in the setting of latency reversal in ART-suppressed individuals. Given ex vivo data suggest augmentation of HIV-specific immunity will be critical for HIV eradication, including one which reactivates latent HIV,37 our findings indicate AGS-004 should be considered in future combined eradication strategies.
Acknowledgments
We earnestly thank Esther Villiard, Dain Melendez, Ken Wood, Amanda Crooks, Deborah McMullen, and Nilu Goonetilleke for their invaluable work on this study. We are indebted to all of the participants who participated in this study.
Disclosure Statement
C.G. has received research support from Bristol Myers Squibb, Gilead Sciences, Abbott, and Janssen (formerly Tibotec Therapeutics). C.H. has received grant support and/or consulting/honoraria from BMS, GSK, Merck, Tibotec Therapeutics, Gilead, Myriad Pharmaceuticals, and Pfizer. D.M. has received research support from Bristol Myers Squibb, Gilead Sciences, and Janssen, has consulted for Merck, and holds common stock in Gilead Sciences. J.E. receives research support from ViiV Healthcare and is a consultant to Bristol Myers Squibb, Merck, Gilead, Janssen, and ViiV Healthcare. M.D., I.T., E.V., A.G., W.L., and C.N. are employees of Argos Therapeutics, Inc., and M.D., E.V., I.T., and C.N have been granted stock options in the company. A.C., J.K., K.M., and M.M. have no competing financial interests.
Funding Sources: This project has been funded in whole or in part by Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. N01-AI-60019, AI50410 to the UNC CFAR, and in part by NIH U19-AI096113 to D.M.M. Single-copy assays were funded by intramural NIH funding.
Prior presentation of interim data by: Gay C, Archin N, Tcherepanova I, Villiard E, Hicks C, Kearney M, Coffin J, DeBenedette, M, Eron J, Nicolette C, Margolis DM. Immunogenicity of AGS-004 Dendritic Cell Therapy in Patients Treated during Acute HIV Infection. 21st Conference on Retroviruses and Opportunistic Infections 2014, Boston, MA. March 2–6, Abstract 344.
References
- 1.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB: Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 1994;68:6103–6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koup RA, Safrit JT, Cao Y, et al. : Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 1994;68:4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia F, Routy JP: Challenges in dendritic cells-based therapeutic vaccination in HIV-1 infection Workshop in dendritic cell-based vaccine clinical trials in HIV-1. Vaccine 2011;29:6454–6463 [DOI] [PubMed] [Google Scholar]
- 4.Jacobson JM, Routy JP, Welles S, et al. : Dendritic cell immunotherapy for HIV-1 infection using autologous HIV-1 RNA: A randomized, double-blind, placebo-controlled clinical trial. J Acquir Immune Defic Syndr 2016;72:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu W, Arraes LC, Ferreira WT, Andrieu JM: Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med 2004;10:1359–1365 [DOI] [PubMed] [Google Scholar]
- 6.Garcia F, Lejeune M, Climent N, et al. : Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J Infect Dis 2005;191:1680–1685 [DOI] [PubMed] [Google Scholar]
- 7.Garcia F, Climent N, Guardo AC, et al. : A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med 2013;5:166ra2. [DOI] [PubMed] [Google Scholar]
- 8.Debenedette M, Tcherepanova I, Gamble AH, et al. : Immune Function and Viral Load Post AGS-004 Administration to Chronic HIV Subjects Undergoing STI. 2014 Conference on Retroviruses and Opportunistic Infections. The CROI Foundation, Boston, MA, 2014 [Google Scholar]
- 9.Routy JP, Boulassel MR, Yassine-Diab B, et al. : Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol 2010;134:140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBenedette MA, Calderhead DM, Ketteringham H, et al. : Priming of a novel subset of CD28+ rapidly expanding high-avidity effector memory CTL by post maturation electroporation-CD40 L dendritic cells is IL-12 dependent. J Immunol 2008;181:5296–5305 [DOI] [PubMed] [Google Scholar]
- 11.Calderhead DM, DeBenedette MA, Ketteringham H, et al. : Cytokine maturation followed by CD40 L mRNA electroporation results in a clinically relevant dendritic cell product capable of inducing a potent proinflammatory CTL response. J Immunother 2008;31:731–741 [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg ES, Altfeld M, Poon SH, et al. : Immune control of HIV-1 after early treatment of acute infection. Nature 2000;407:523–526 [DOI] [PubMed] [Google Scholar]
- 13.Gianella S, von Wyl V, Fischer M, et al. : Impact of Early ART on Proviral HIV-1 DNA and Plasma Viremia in Acutely Infected patients. 17th Conference on Retroviruses and Opportunistic Infections. The CROI Foundation, San Francisco, CA, 2010 [Google Scholar]
- 14.Schmid A, Gianella S, von Wyl V, et al. : Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection. PLoS One 2010;5:e13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archin NM, Cheema M, Sackman R, et al. : Correlation of peak and duration of viremia and resting CD4+ T-Cell infection in acute HIV infection. 17th Conference on Retroviruses and Opportunistic Infections, Abstract 464; 2010; San Francisco, CA [Google Scholar]
- 16.Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. : Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS 2010;24:1598–1601 [DOI] [PubMed] [Google Scholar]
- 17.Archin NM, Vaidya NK, Kuruc JD, et al. : Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A 2012;109:9523–9528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. : The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med 2009;206:1253–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. : Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008;105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF: The immune response during acute HIV-1 infection: Clues for vaccine development. Nat Rev Immunol 2010;10:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quaranta MG, Mattioli B, Giordani L, Viora M: The immunoregulatory effects of HIV-1 Nef on dendritic cells and the pathogenesis of AIDS. FASEB J 2006;20:2198–2208 [DOI] [PubMed] [Google Scholar]
- 22.Tcherepanova I, Starr A, Lackford B, et al. : The immunosuppressive properties of the HIV Vpr protein are linked to a single highly conserved residue, R90. PLoS One 2009;4:e5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumder B, Janket ML, Schafer EA, et al. : Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: Implications for viral immune escape. J Virol 2005;79:7990–8003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muthumani K, Desai BM, Hwang DS, et al. : HIV-1 Vpr and anti-inflammatory activity. DNA Cell Biol 2004;23:239–247 [DOI] [PubMed] [Google Scholar]
- 25.Amin A, Dudek AZ, Logan TF, et al. : Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results. J Immunother Cancer 2015;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archin NM, Eron JJ, Palmer S, et al. : Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS 2008;22:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archin NM, Cheema M, Parker D, et al. : Antiretroviral intensification and valproic acid lack sustained effect on residual HIV-1 viremia or resting CD4+ cell infection. PLoS One 2010;5:e9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeBenedette MA, Calderhead DM, Tcherepanova IY, Nicolette CA, Healey DG. Potency of mature CD40 L RNA electroporated dendritic cells correlates with IL-12 secretion by tracking multifunctional CD8(+)/CD28(+) cytotoxic T-cell responses in vitro. J Immunother 2011;34:45–57 [DOI] [PubMed] [Google Scholar]
- 29.Eriksson S, Graf EH, Dahl V, et al. : Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 2013;9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crooks AM, Bateson R, Cope AB, et al. : Precise quantitation of the latent HIV-1 reservoir: Implications for eradication strategies. J Infect Dis 2015;212:1361–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goulder PJ, Walker BD: HIV and HLA class I: an evolving relationship. Immunity 2012;37:426–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufmann DE, Lichterfeld M, Altfeld M, et al. : Limited durability of viral control following treated acute HIV infection. PLoS Med 2004;1:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. : Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013;9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colby D, Chomont N, Kroon E, et al. : HIV RNA rebound postinterruption in persons suppressed in Fiebig I Acute HIV, Abstract #124. Conference on Retroviruses and Opportunistic Infections; February 13–16, 2017; Seattle, WA [Google Scholar]
- 35.Ananworanich J, Schuetz A, Vandergeeten C, et al. : Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012;7:e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson GE. The Ethics of HIV “Cure” research: What can we learn from consent forms? AIDS Res Hum Retroviruses 2014;31:56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shan L, Deng K, Shroff NS, et al. : Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012;36:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]