Abstract
Background and Aim: The insulin to carbohydrate ratio (CR) is a parameter used by patients with type 1 diabetes (T1D) to calculate the premeal insulin bolus. Usually, it is estimated by the physician based on patient diary, but modern diabetes technologies, such as subcutaneous glucose sensing (continuous glucose monitoring, CGM) and insulin delivery (continuous subcutaneous insulin infusion, CSII) systems, can provide important information for its optimization. In this study, a method for CR optimization based on CGM and CSII data is presented and its efficacy and robustness tested in several in silico scenarios, with the primary aim of increasing protection against hypoglycemia.
Methods: The method is based on a validated index of insulin sensitivity calculated from sensor and pump data (SISP), area under CGM and CSII curves. The efficacy and robustness of the method are tested in silico using the University of Virginia/Padova T1D simulator, in several suboptimal therapy scenarios: with nominal CR variation, over/underestimation of meal size or suboptimal basal insulin infusion. Simulated CGM and CSII data were used to calculate the optimal CR. The same scenarios were then repeated using the estimated CR and glycemic control was compared.
Results: The optimized CR was efficacious in protecting against hypoglycemic events caused by suboptimal therapy. The method was also robust to possible error in carbohydrate count and suboptimal basal insulin infusion.
Conclusions: A novel method for CR optimization in T1D, implementable in daily life using CGM and CSII data, is proposed. The method can be used both in open- and closed-loop insulin therapy.
Keywords: : Diabetes therapy, Type 1 diabetes, Bolus calculator, Continuous glucose monitoring, Insulin bolus.
Introduction
Type 1 diabetes (T1D) therapy requires exogenous insulin administration, through a basal–bolus strategy with multiple daily injections (MDI) or insulin pump, tuned by the physician according to three to four self-monitoring blood glucose (SMBG) measurements per day. However, modern diabetes technologies, such as continuous glucose monitoring (CGM) sensors and continuous subcutaneous insulin infusion (CSII) systems, provide important information potentially allowing to improve diabetes management. In fact, CGM devices, at variance with the few SMBG measurements per day, provide a more comprehensive picture of postprandial and overnight glucose excursions,1–3 whereas CSII systems allow a more flexible tuning of insulin therapy, with respect to MDI, according to the patient's daily activities. Moreover, CSII technologies incorporate features, such as the “bolus calculator”,4–6 helping patients to accurately adjust premeal insulin bolus to avoid postprandial hypo/hyperglycemic episodes. However, this tool is based on patient-specific parameters, such as the insulin to carbohydrate ratio (CR) and the insulin correction factor, which need to be optimally estimated before being used in such decision support systems.6,7 In particular, CR represents the amount of meal-related carbohydrate cleared by 1 unit of insulin (g/U) and is used to compute the optimal premeal insulin bolus as the ratio between the amount of carbohydrate ingested in the meal (in g) and its value: a higher/lower CR value, given the same meal composition and amount of carbohydrate intake, corresponds to a lower/higher meal insulin bolus. Usually, CR is periodically updated by the physician according to some empirical guidelines,8–10 based on body weight (BW) and/or total daily insulin dose.
Recently, researchers have proposed more rigorous methods to calculate CR. For instance, in Bevier et al.,11 a systematic approach to estimate patient-specific CR has been developed, which consists of performing a meal challenge during a hyperinsulinemic-euglycemic clamp, but requires patient hospitalization and, thus, it is not applicable in practice. In Breton and Kovatchev,12 CR is calculated by using SMBG data collected in daily life conditions over a period of 2–6 weeks, but given the duration of the data collection period, it can only provide an average value of the optimal CR in that period, precluding the possibility to assess intra- and interday variability, likely to occur in real-life conditions.
In the last decades, with the increasing use of modern diabetes technology, CGM data were employed for CR optimization.13,14 In particular, in Herrero et al.,13 CR is adapted based on the minimum glucose value, measured by CGM, within a predefined postprandial time window (from 2 to 5 h postprandial and/or prior the following meal): if the minimum is higher/lower than the target, CR is decreased/increased so that more/less insulin is given. In Herrero et al.,14 CR adjustment is based on the area under CGM curve measured after the meal (e.g., 5 h); this method requires, for each subject, an optimal reference area under the CGM curve to compare with, which is easy to obtain in simulation, but more difficult to have it in real life.
In this work, a new algorithm for CR optimization from CGM sensor and CSII data is proposed and tested using the University of Virginia (UVA)/Padova T1D simulator.15,16 Several meal scenarios, that is, with errors on patient's nominal CR, over/underestimation of meal carbohydrate amount, and suboptimal basal insulin infusion, are performed to test efficacy and robustness of the method in preventing large glycemic excursions, especially in hypoglycemia, caused by suboptimal therapy. Each scenario consisted of two experiments: the simulated CGM and CSII data obtained in the first experiment are used to calculate the optimal CR with the proposed algorithm, which will be used in the second experiment, for each virtual subject, and glycemic control is compared. The method is also tested against an empirical rule, based on CGM sensor data only.
Methods
In this section the CR optimization algorithm is described. The performance of the algorithm is evaluated in silico by exploiting several single meal scenarios.
CR optimization algorithm
The method proposed for CR optimization is based on the fact that CR and insulin sensitivity (SI) of each patient are strictly related. In fact, the more sensitive is the subject to insulin, the higher is the corresponding CR, since the lower is the premeal insulin bolus to be administered to cover the meal. In this study, a validated index of SI estimated from sensor-augmented insulin pump data (SISP)17 is exploited to optimize CR.
The calculation of the optimal CR, for each meal, is basically a three-step procedure:
(1) computation of the insulin sensitivity index (SISP)17 from glucose sensor (S) and insulin pump (P) (SISP calculation);
(2) calculation of the new CR (CRnew) from SISP, glucose sensor, and insulin pump data (CR calculation);
(3) assessment of CRnew against the control performance obtained with the CR actually used (CR assessment).
SISP calculation
For each meal, the data needed for SISP calculation17 are: glucose excursion measured by CGM sensor from at least 3–6 h after the meal, basal and bolus insulin administered by insulin pump before (from at least 3 to 6 h) and during the meal, and the amount of carbohydrates ingested during the meal. Once these data are available, the index of insulin sensitivity (SISP) is estimated, for that meal, by using a simple algebraic formula.17
CR calculation
By rearranging the eqation of SISP in Schiavon et al.17 (see Appendix for details), and given that the premeal insulin bolus (Bolus), which should be administered to compensate the meal, can be calculated by dividing the amount of ingested carbohydrates (D) and CR
 |
together with the fact that the desired CR (CRnew) is the one that makes glucose to return to its premeal value at the end of the meal, one has CRnew (see Equation (2) below)
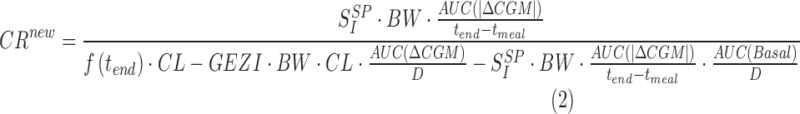 |
where AUC is the area under the CGM curve calculated from the start (tmeal) to the end (tend) of the meal, f(tend) is the fraction of the ingested dose which has reached plasma at the end of the meal, ΔCGM and |ΔCGM| are the above basal and the absolute value of above basal glucose excursion, and Basal is the basal insulin infusion rate during the meal. Other parameters needed in Equation 2 are body weight (BW), age, and height used for the estimation of plasma insulin clearance (CL).18 The glucose effectiveness at zero insulin (GEZI)19,20 and the volume of glucose distribution VG21 are fixed to population values.17
CR assessment
To guarantee patient safety, we propose to compare the estimated CRnew against the one actually used for the same meal (CRold) on the basis of the glycemic control obtained in the meal. The optimal CR proposed by the algorithm (CRSP) is thus determined according to the following rules in Equation (3) next page:
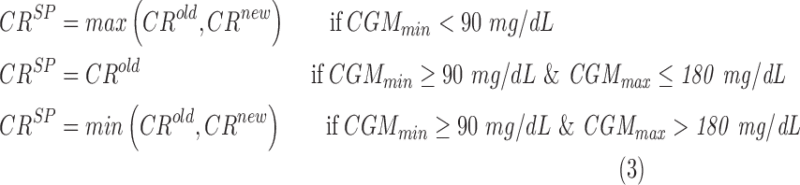 |
Hence, if CGM during the meal was too low (CGMmin <90 mg/dL), the proposed CR cannot be lower than CRold; whereas if CGM during the meal was maintained within 90 and 180 mg/dL, CRold should not be changed and if CGM during the meal was too high (CGMmax >180 mg/dL, with CGMmin ≥90 mg/dL), CR cannot be higher than CRold.
In silico testing
The efficacy and robustness of the method against suboptimal therapy was assessed in silico with nine meal scenarios performed on the 100 virtual adults of the UVA/Padova T1D Simulator.15,16 Each scenario consisted in administering a meal with a carbohydrate content of 75 g in two experiments: Baseline (Bas) and Sensor&Pump (SP). The two experiments are identical apart from the fact that in SP, CR is set to CRSP calculated from CGM and CSII data obtained from Bas experiment. The nine-meal scenarios are performed with: (i) nominal (CR and basal insulin infusion) conditions; (ii) and (iii) CR variation, i.e., reduced and increased by 20%, respectively; (iv) and (v) error on meal carbohydrate amount, i.e., over/underestimated by 20%, respectively; (vi) and (vii) suboptimal basal insulin infusion, i.e., basal insulin increased/reduced by 20%, respectively, both in Bas and SP experiments; and (viii) and (ix) suboptimal (increased/reduced by 20%) basal insulin infusion in Bas, but restored to optimal in SP experiment. These last two scenarios, (viii) and (ix), resemble the case in which CR is optimized based on data gathered when basal insulin is maladapted and is then applied when optimal basal insulin infusion is restored.
To further strengthen the method, we simulated an Empirical (Emp) rule for CR optimization, based on CGM sensor data only, and assessed its performance in all the above meal scenarios. The Emp mimics a clinician adjusting CR up or down by a 10% by looking at CGM traces: CR is increased by 10% if CGMmin <90 mg/dL, whereas is reduced by 10% if CGMmin >90 mg/dL and CGMmax >180 mg/dL, and it is left unchanged otherwise.
Data analysis
Results are presented as mean ± SD and comparison is made by paired two-sample t-test for normally distributed indices, whereas for not normally distributed, comparison is done by Wilcoxon Signed-Rank test. Safety (reduction of hypoglycemia) and efficacy (attenuation of hyperglycemia) were quantitatively evaluated using the control variability grid analysis (CVGA).22 This tool provides a simultaneous visual and numerical assessment of the overall quality of glycemic control in a population by representing each profile as a point in the xy plane. In particular, the X-axis reports the minimum value associated with each profile in a linear reversed scale (from 110 mg/dL, left, to 50 mg/dL, right), whereas the Y-axis reports the maximum in a nonlinear scale (from 110 mg/dL, bottom to 300 mg/dL, top). The optimal control is located in the bottom left corner and the larger is the distance from the bottom left corner, the worse is the glucose control. The plane is divided in colored zones: A (very good control, light green), B (fairly good control, dark green), C (bad control, yellow), D (very bad control, red).
In addition, according to Maahs et al.,23 performances were assessed by calculating the following main outcome metrics based on CGM traces: % time below 70 mg/dL, % time in target (between 70 and 180 mg/dL), and % time above 180 mg/dL.
Results
For sake of clarity, below are reported only three of the nine tested scenarios, which show the efficacy and robustness of the method to protect from hypoglycemia caused by suboptimal therapy. The in silico results of the remaining six scenarios, which were essentially confirmatory, are reported in the Supplementary Data (available at http://online.liebertpub.com/doi/suppl/10.1089/dia.2017.0248).
CR underestimated by 20% (scenario ii)
In case of nominal CR reduced by 20%, 31% of the subjects experienced hypoglycemia. The simulated CGM and CSII data were used to calculate CRSP in each subject, which were significantly higher than CRBas (14.3 ± 5.2 g/U vs. 12.7 ± 4.3 g/U in SP and Bas, respectively, P < 0.001) resulting in a better glycemic control in all the subjects. In particular (Fig. 1), CRSP dramatically reduced hypo- (3% vs. 31% of the subjects in SP vs. Bas, respectively) at the cost of a modest increase in hyperglycemic events (74% vs. 64% of the subjects in SP vs. Bas, respectively). When comparing SP versus Emp, SP outperforms Emp since 3% versus 13% and 74% versus 66% of the subjects experienced hypo- and hyperglycemia with CRSP versus CREmp, respectively.
FIG. 1.
Simulated CGM profiles and CVGA22 (left and right, respectively) in the 100 T1D virtual subjects during Bas (average, blue line, and ± SD range, blue shaded area), SP (average, red line, and ± SD range, red shaded area), and Emp (average, green line, and ± SD range, green shaded area) experiments in case of underestimation of nominal CR by 20% in Bas. Bas, baseline; CGM, continuous glucose monitoring; CR, carbohydrate ratio; CVGA, control variability grid analysis; Emp, empirical; SP, sensor and pump; T1D, type 1 diabetes.
In terms of glycemic control in the whole population, SP outperforms Emp with fewer subjects in the C and D zones (14% and none vs. 27% and 1% of the subjects in C and D zone with SP vs. Emp, respectively) and a significant reduction of the % time spent in hypoglycemia, with SP almost completely avoiding hypoglycemia (from 3.4% in Bas to 0.1% vs. 0.7%, with P < 0.0001, in SP vs. Emp) at the cost of a minimal increase of the % time above 180 mg/dL (from 12.4% in Bas to 15.5% vs. 13.2%, with P < 0.0001 vs. P = 0.02, in SP vs. Emp) for both methods.
Meal size overestimated by 20% (scenario iv)
In case of overestimation of meal carbohydrate amount by 20% with nominal CR in Bas (Fig. 2), 23% and 64% of the subjects experienced hypo- and hyper-glycemia, while both SP and Emp reduced hypo- (4% and 6% of the subjects, respectively) with a minimal increase in hyperglycemic events (73% and 71% of the subjects, respectively).
FIG. 2.
Simulated CGM profiles and CVGA22 (left and right, respectively) in the 100 T1D virtual subjects during Bas (average, blue line, and ±SD range, blue shaded area), SP (average, red line, and ±SD range, red shaded area), and Emp (average, green line, and ±SD range, green shaded area) experiments in case of overestimation of meal size by 20% with nominal CR in Bas.
In terms of glycemic control, SP outperforms Emp with fewer subjects in the C zone (none in D zone) and a reduction of % time below 70 mg/dL (from 1.9% in Bas to 0.1% vs. 0.2%, with, P < 0.0001, in SP vs. Emp), at the cost of a modest increase of the % time above 180 mg/dL (from 13.2% in Bas to 15.6% vs. 14.2%, with P < 0.0001 vs. P = 0.02, in SP vs. Emp).
Basal insulin underestimated by 20%—restored (scenario ix)
In case of basal insulin infusion reduced by 20% in Bas, but restored to optimal when CRSP and CREmp are used, SP completely avoided hypoglycemic events at the cost of a minimal increase in hyperglycemia (0% vs. 3% hypo- and 77% vs. 71% hyperglycemic events with SP vs. Emp), with respect to Emp, together with a reduction of glycemic excursions (Fig. 3), i.e., 7% vs. 18% of the subjects in the C zone (none in D zone) for SP vs. Emp, respectively.
FIG. 3.
Simulated CGM profiles and CVGA22 (left and right, respectively) in the 100 T1D virtual subjects during Bas with nominal conditions (average, blue line, and ±SD range, blue shaded area), whereas SP (average, red line, and ±SD range, red shaded area) and Emp (average, green line, and ±SD range, green shaded area) experiments are performed with CR calculated in case of suboptimal basal insulin infusion reduced by 20% and basal insulin infusion restored to optimal.
Discussion
A crucial aspect in diabetes management is the optimization of insulin therapy, in particular the estimation of the CR and its daily variation, due for example, to patient variability, habits, and health conditions. In clinical practice, CR is periodically updated by the physician with a trial-and-error approach, based on some empirical guidelines8–10 and patient's diary, e.g., information about carbohydrate counting of the ingested meals, amount of insulin delivered, and pre/postprandial glucose levels.
CR is known to be related to insulin sensitivity. In this work, we exploited this knowledge and developed an algorithm for the optimization of patient-specific CR, based on a validated index of insulin sensitivity (SISP)17 estimated, for each meal, from glucose sensor (S) and insulin pump (P) data with the primary aim of preventing large glycemic excursions, especially in hypoglycemia, caused by suboptimal therapy.
The performance of the method was assessed in silico using the UVA/Padova T1D simulator15,16 with different scenarios. Two single-meal experiments were performed to test the feasibility and robustness of the method in simple experimental conditions: nominal, variation of CR, error in meal size estimation, and suboptimal basal insulin infusion. The simulated CGM and CSII data of the Baseline (Bas) experiment were used by the algorithm to calculate CRSP, which was then employed in the second experiment.
In all simulations, the use of CRSP allowed to improve the overall glycemic control in a significant percentage of subjects, especially by dramatically reducing the number of hypo- at the cost of a slight increase in hyperglycemic events. This was particularly evident when CR was artificially reduced by 20% in Bas, causing hypoglycemia in 31% of subjects, whereas only 3% of them experienced hypoglycemia when CRSP was used (Fig. 1). Of note, depending upon the actual CR used at baseline, nominal, reduced, or increased, one may obtain similar but not identical CR values. This is certainly in part due to nonlinearities in the glucose–insulin system, but more likely to the fact that the algorithm may modify or not the suggested CR based on the measured CGM profile. However, this has no practical consequences since small differences in CR are generally absorbed by the quantization needed to set the value in a bolus calculator (which usually allows a resolution of 0.5 g/U).
Results also show the robustness of the method in case of over/underestimation of the carbohydrate content of the meal, usually leading glycemia to falling/rising toward hypo/hyperglycemia, respectively. In particular, again, the algorithm improved glucose control especially by preventing hypoglycemia. Moreover, the algorithm shows robustness in case of suboptimal basal insulin infusion. A systematic increase/decrease in basal insulin infusion of 20% is an important perturbation and one would expect that the algorithm is able to compensate this error. Actually, it does not since CRSP is designed to bring glucose to the premeal value: this is a good feature of the algorithm since the meal bolus has to compensate the carbohydrate intake and not to contrast other disturbances. In fact, when optimal basal insulin infusion is restored, CRSP, at variance with CREmp, avoided possible hypoglycemia. However, it is hard to segregate the effect of basal and bolus administrations during a meal and, when used in a real-life context, one should be aware that this algorithm does not interfere with possible algorithms optimizing basal insulin infusion.
The efficacy of CRSP was also tested against an empirical rule based on CGM sensor data only (CREmp), under the same experimental conditions. Results show that CRSP always outperforms CREmp in reducing hypoglycemic events with an equal/slightly higher number of hyperglycemic events. Moreover, thanks to the simultaneous use of glucose sensor and insulin pump data, CRSP is robust to errors in basal insulin infusion, outperforming CREmp especially when basal insulin infusion is restored to optimal.
A possible limitation of the proposed method is that it relies on data provided by a CGM device. Despite important improvements in CGM accuracy,24 occasionally, CGM traces can still suffer from inaccuracies. However, to improve the quality of CGM measurements, algorithms exist that can be used to recalibrate CGM traces25,26 (this was actually implemented in Dassau et al.27). Another drawback could be the fact that, in the formulas, some parameters are fixed to population values,19–21 whereas others are calculated from population models using anthropometric data.18 Nevertheless, the impact of fixing model parameters was already evaluated in Schiavon et al.,17 where it has been demonstrated that the estimate of SISP is modestly affected by the values of these parameters.
Furthermore, the algorithm was tested using standard mixed meal tests, which are generally assumed to be completely absorbed after 6 h from the beginning of the meal. This may be not always true when a meal has a high fat and/or protein content.28 In that case, SISP and CR can be accurately calculated by properly changing the model of glucose absorption determining the so-called carbohydrates on board concept.17 It must be pointed out that the algorithm is able to propose an optimization of CR, and thus of the total amount of insulin to be administered in correspondence to a meal, regardless form the modality of insulin administration (single bolus or dual wave), which depends on the patient's habits.
Finally, it is an accepted notion that simulation studies are not a substitute of clinical trials, but passing the in silico test is a prerequisite before going into in vivo trials. In view of this, the algorithm was also successfully tested in silico in virtual children and adolescent populations (results not shown) since a clinical trial is also planned in these populations.
Concerning the possible use of CRSP in real life, the algorithm can run, in principle, after each meal and provide the value of the optimal CR that should have been used to optimally cover that meal. However, it can also be used to optimize the CR daily pattern, i.e., the three CR values used by a subject to calculate the optimal premeal boluses related to the three main meals (breakfast, lunch, and dinner), provided that 7-day CGM and CSII data are available: 7-day is considered a good compromise between the need to reject possible outliers in the CR estimates (due, e.g., to noise in CGM data, calibration errors, sensor disconnections, pump malfunctions, and missing information on carbohydrate meal content) and the ability to detect variation in the optimal CR daily pattern. Basically, one has first to calculate the optimal CR for each available meal, then to label each value as breakfast, lunch, or dinner, according to the time of the day of each meal, and, finally, to calculate the average estimate of CR daily pattern and its variability. Moreover, in real life, one can also set a maximum allowable deviation, with respect to the original CR pattern (e.g., 20%), to increase patient safety and make him more confident on the algorithm suggestions.
We simulated a 7-day open-loop scenario, with randomness in meal timing and amount of carbohydrate content in case of nominal CR and nominal CR reduced/increased by 20%, to assess the robustness of the method to challenges which may occur in real life. The algorithm used the 7-day CGM and CSII to calculate the CR daily pattern and showed the ability to improve glucose control by properly increasing/reducing CR when CR was artificially reduced/increased, respectively (see results in Multiple Meal Scenario section in Supplementary Data). In this in silico scenario well-spaced meals were performed while, in real life, meals can be closer one to each other. However, as shown in Schiavon et al.17 for SISP, the method is also able to provide a robust estimation of CR for each meal whenever the distance between consecutive meals is at least 3 h, otherwise the method provides a single CR value accounting for the overall contribution of all meal amounts and administered insulin. Neither intra- nor interday variability of insulin sensitivity was included in this scenario and the problem of tracking changes in CR daily pattern is currently under development.
Finally, we note that the SP method was already successfully employed in a clinical trial for the optimization of CR daily pattern before starting27 and during a closed-loop session.29 In fact, the CR algorithm can be employed both in open- (e.g., decision-support systems,6,7 like “bolus calculator”) and in closed-loop setting, whenever the closed-loop algorithm works on the top of the preprogrammed open-loop insulin therapy.30,31
Conclusions
The insulin to carbohydrate ratio is a key parameter in everyday life in subjects with T1D for the optimization of insulin therapy. In this work, a method for the optimization of CR, for each meal, based on CGM and CSII data is proposed. In all simulations, the method improves glycemic control, in particular by protecting against hypoglycemic events caused by suboptimal therapy. The method is also robust to over/underestimation of the carbohydrate content of the meal and suboptimal basal insulin infusion.
The method has great potential for optimizing both standard open- and closed-loop insulin therapy and the next step will be to test it in vivo.
Supplementary Material
Appendix
In this study, a detailed description of CR calculation is reported.
By rearranging the equation of SISP in Schiavon et al.,A1 one can obtain carbohydrate ratio (CR) as the ratio between the amount of carbohydrates ingested (D) and the premeal insulin bolus (Bolus) as
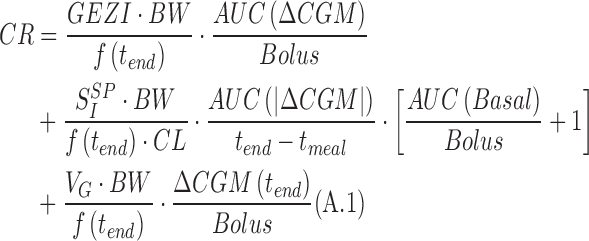 |
where AUC is the area under the CGM curve calculated from the start (tmeal) to the end (tend) of the meal, f(tend) is the fraction of the ingested dose which has reached plasma at the end of the meal, ΔCGM and |ΔCGM| are the above basal and the absolute value of above basal glucose excursion, and Basal is the basal insulin infusion rate during the meal. Other parameters needed in Equation A.1 are body weight (BW), age, and height used for the estimation of plasma insulin clearance (CL).A2 The glucose effectiveness at zero insulin (GEZI)A3,A4 and the volume of glucose distribution VGA5 are fixed to population values.A1
Finally, since the premeal insulin bolus (Bolus) is obtained by dividing the amount of carbohydrates ingested (D) and CR
 |
by substituting Equation A.2 into Equation A.1, one obtains
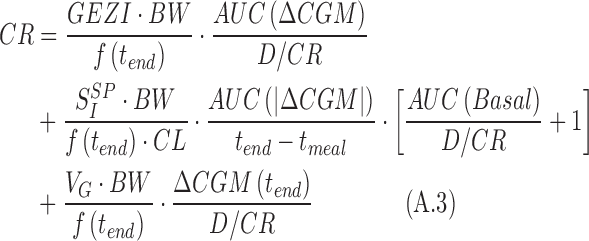 |
The desired CR (CRnew) is the one that allows glucose to return to its premeal value at the end of the meal. Hence, one can assume 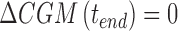 leading Equation A.3 to be simplified as
leading Equation A.3 to be simplified as
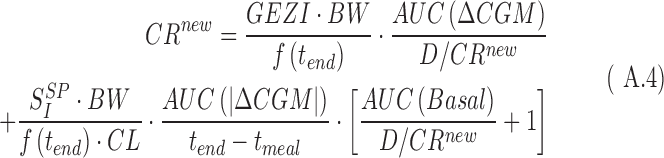 |
and thus one has CRnew (see A.5 below).
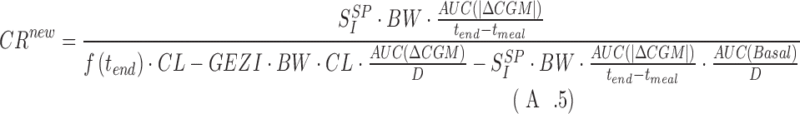 |
Appendix References
- A1.Schiavon M, Dalla Man C, Kudva YC, et al. : Quantitative estimation of insulin sensitivity in type 1 diabetic subjects wearing a sensor-augmented insulin pump. Diabetes Care 2014;37:1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A2.Campioni M, Toffolo GM, Basu R, et al. : Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetics parameters. Am J Physiol Endocrinol Metab 2009;297:E941–E948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A3.Basu A, Dalla Man C, Basu R, et al. : Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 2009;32:866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A4.Hinshaw L, Dalla Man C, Nandy DK, et al.: Diurnal pattern of insulin action in type 1 diabetes: implications for a closed-loop system. Diabetes 2013;62:2223–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A5.Dalla Man C, Caumo A, Basu R, et al. : Minimal model estimation of glucose absoprtion and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–E643 [DOI] [PubMed] [Google Scholar]
Acknowledgments
The work was partially supported by the National Institutes of Health (DP3DK094331) and by the Ministero dell'Università e della Ricerca Scientifica (Progetto di Ateneo dell'Università di Padova 2014).
Authors Disclosure Statement
No competing financial interests exist.
Authorship Contribution
M.S., C.D.M., and C.C. developed the method, analyzed the results, and drafted and revised the article.
Editor's Note:
The Carbohydrate-to-Insulin Ratio (CR) is also designated as the Carbohydrate Ratio (CR), Carbohydrate Factor (Carb-F), and Carb:Insulin ratio (C:I), among other options. The ratio is also referred to as Insulin-to-Carbohydrate Ratio (I:C) ratio.
The Correction Factor (Corr-F or CF) is also commonly referred to as the Insulin Sensitivity Factor.
References
- 1.Boland E, Monsod T, Delucia M, et al. : Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care 2001;24:1858–1862 [DOI] [PubMed] [Google Scholar]
- 2.Kaufman FR, Gibson LC, Halvorson M, et al. : A pilot study of the continuous glucose monitoring system: clinical decisions and glycemic control after its use in pediatric type 1 diabetic subjects. Diabetes Care 2001;24:2030–2034 [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson J, Hanas R: Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics 2003;111:933–938 [DOI] [PubMed] [Google Scholar]
- 4.Gross TM, Kayne D, King A, et al. : A bolus calculator is an effective means of controlling postprandial glycemia in patients on insulin therapy. Diabetes Technol Ther 2003;5:365–369 [DOI] [PubMed] [Google Scholar]
- 5.Zisser H, Robinson L, Bevier W, et al. : Bolus calculator: a review of four “smart” insulin pumps. Diabetes Technol Ther 2008;10:441–444 [DOI] [PubMed] [Google Scholar]
- 6.Walsh J, Roberts R, Bailey T: Guidelines for optimal bolus calculator settings in adults. J Diabetes Sci Technol 2011;5:129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klonoff DC: The current status of bolus calculator decision-support software. J Diabetes Sci Technol 2012;6:990–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson PC, Hebblewhite HR, Steed RD, Bode BW: Analysis of guidelines for basal-bolus insulin dosing: basal insulin, correction factor, and carbohydrate-to-insulin ratio. Endocr Pract 2008;14:1095–1101 [DOI] [PubMed] [Google Scholar]
- 9.Walsh J, Roberts R, Bailey T: Guidelines for insulin dosing in continuous subcutaneous insulin infusion using new formulas from a retrospective study on individuals with optimal glucose levels. J Diabetes Sci Technol 2010;4:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King AB, Armstrong DU: A prospective evaluation of insulin dosing recommendations in patients with type 1 diabetes at near normal glucose control: bolus dosing. J Diabetes Sci Technol 2007;1:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bevier WC, Zisser H, Parlerm CC, et al. : Calculating the insulin to carbohydrate ratio using the hyperinsulinaemic-euglycaemic clamp–a novel use for a proven technique. Diabetes Metab Res Rev 2007;23:472–478 [DOI] [PubMed] [Google Scholar]
- 12.Breton MD, Kovatchev BP: Method, system and computer program product for evaluation of insulin sensitivity, insulin/carbohydrate ratio, and insulin correction factors in diabetes from self-monitoring data. PCT international patent application PCT/US2008/069416, filed on July 8, 2008
- 13.Herrero P, Pesl P, Bondia J, et al. : Method for automatic adjustment of an insulin bolus calculator: in silico robustness evaluation under intra-day variability. Comput Methods Programs Biomed 2015;119:1–8 [DOI] [PubMed] [Google Scholar]
- 14.Herrero P, Pesl P, Reddy M, et al. : Advanced insulin bolus advisor based on run-to-run control and case-based reasoning. IEEE J Biomed Health Inform 2015;19:1087–1096 [DOI] [PubMed] [Google Scholar]
- 15.Kovatchev BP, Breton M, Dalla Man C, Cobelli C: In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol 2009;3:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalla Man C, Micheletto F, Lv D, et al. : The UVA/Padova type 1 diabetes simulator: new features. J Diabetes Sci Technol 2014;8:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiavon M, Dalla Man C, Kudva YC, et al. : Quantitative estimation of insulin sensitivity in type 1 diabetic subjects wearing a sensor-augmented insulin pump. Diabetes Care 2014;37:1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campioni M, Toffolo GM, Basu R, et al. : Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetics parameters. Am J Physiol Endocrinol Metab 2009;297:E941–E948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu A, Dalla Man C, Basu R, et al. : Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 2009;32:866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinshaw L, Dalla Man C, Nandy DK, et al. : Diurnal pattern of insulin action in type 1 diabetes: implications for a closed-loop system. Diabetes 2013;62:2223–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalla Man C, Caumo A, Basu R, et al. : Minimal model estimation of glucose absoprtion and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–E643 [DOI] [PubMed] [Google Scholar]
- 22.Soru P, De Nicolao G, Toffanin C, et al. : MPC based Artificial Pancreas: strategies for individualization and meal composition. Annu Rev Control 2012;36:118–128 [Google Scholar]
- 23.Maahs DM, Buckingham BA, Castle JR, et al. : Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care 2016;39:1175–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey TS, Chang A, Christiansen M: Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol 2015;9:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Favero S, Facchinetti A, Sparacino G, Cobelli C: Improving accuracy and precision of glucose sensor profiles: retrospective fitting by constrained deconvolution. IEEE Trans Biomed Eng 2014;61:1044–1053 [DOI] [PubMed] [Google Scholar]
- 26.Del Favero S, Facchinetti A, Sparacino G, Cobelli C; AP@home consortium: Retrofitting of continuous glucose monitoring traces allows more accurate assessment of glucose control in outpatient studies. Diabetes Technol Ther 2015;17:355–363 [DOI] [PubMed] [Google Scholar]
- 27.Dassau E, Brown SA, Basu A, et al. : Adjustment of open-loop settings to improve closed-loop results in type 1 diabetes: a multicenter randomized trial. J Clin Endocrinol Metab 2015;100:3878–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell KJ, Smart CE, Steil GM, et al. : Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015;38:1008–1015 [DOI] [PubMed] [Google Scholar]
- 29.Dassau E, Pinsker JE, Kudva YC, et al. : 12 week 24/7 ambulatory artificial pancreas with weekly adaptation of insulin delivery setting: effect on hemoglobin A1C and hypoglycemia. Diabetes Care 2017;40:1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magni L, Forgione M, Toffanin C, et al. : Run-to-run tuning of model predictive control for type 1 diabetes subjects: in silico trials. J Diabetes Sci Technol 2009;3:1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toffanin C, Messori M, Cobelli C, Magni L: Automatic adaptation of basal therapy for type 1 diabetic patients: a run-to-run approach. Biomed Signal Process Control 2017;31:539–549 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





