Abstract
Background: Sexually transmitted infections (STIs) are an increasingly critical and costly health problem for American childbearing women. Pregnant women who misuse substances are more likely to engage in risky sexual behavior that leads to STIs. Substance use and risky sex during pregnancy are both associated with numerous negative consequences for the woman and the developing fetus.
Study Design: A two-group, randomized controlled trial.
Participants: Recruitment of 50 pregnant women (30% Latina; 24.4 years old [SD = 5.31]) with an average of 13 weeks gestation (SD = 4.5 weeks) was conducted at a prenatal clinic in a large inner-city hospital. Recruitment took place between 2015 and 2016, and data analysis took place in 2016.
Intervention: A computer-delivered, single-session brief motivational intervention plus booster session addressing both substance use and STI risk.
Objective: To assess participants' perceptions of the intervention and to examine the preliminary efficacy in reduction of substance use and risky sex at 4-month follow-up assessment.
Results: There were consistently very high ratings of acceptability of the intervention, ranging between 6.3 and 6.8 on a 1–7 scale. At the 4-month follow-up, participants in the intervention arm reported a significantly larger reduction (54%) in any marijuana or alcohol use compared with participants in the control group (16%) (p = 0.015) based on two-group clustered logistic regression using a generalized estimating equations approach. There was a higher reduction in condomless vaginal sex at follow-up in the health checkup for expectant moms (HCEM) arm than control (27% vs. 5%), although this was not significant (p = 0.127).
Conclusions: The results of this pilot study are encouraging with respect to the acceptability and preliminary efficacy of an intervention in reducing alcohol/marijuana use and condomless sex during pregnancy, supporting the next step of testing the intervention in a larger sample.
Keywords: : sexually transmitted infections, brief intervention, substance abuse, reproductive health, pregnancy
Introduction
Sexually transmitted infections (STIs) are on the rise in the United States,1 and STI risk is an increasingly critical and costly health problem for American women, especially for pregnant women who can pass these infections onto their babies. STIs among women are associated with significant morbidity and mortality, including premature death. The American Congress of Obstetricians and Gynecologists (ACOG) reported that over the past decade, childbearing women have comprised one of the most rapidly expanding groups infected by STIs, including HIV, in the United States.2 Among pregnant women who had been treated for an STI in the past 6 months, 30% tested positive for a current STI. Racial minority populations are at disproportionate risk of STIs—African American young women face nearly 5 times higher rates of chlamydia and 11 times higher rates of gonorrhea than among white females.5 Critical to this trend is the intersection of STIs and alcohol/drug use—both highly prevalent problems in women's lives.
Increasing abstinence from alcohol and illicit drug use among pregnant women is an objective of the Healthy People 2020.4 Recent national epidemiologic surveys have found that past year prevalence of illicit drug use was 5.4%5 for pregnant women. Regarding alcohol use, 19% of women in their first trimester of pregnancy report using alcohol and 3% report binge drinking (four or more drinks in a row).5 Since underreporting of alcohol use during pregnancy can be substantial, drinking during pregnancy is likely even higher.6,7
The co-occurrence of alcohol and substance use as well as sexual risk taking contribute significantly to STI acquisition, particularly in high-risk and vulnerable populations.8 Pregnant women who use alcohol or substances are more likely to engage in sexual risk behaviors than their nonpregnant counterparts.9 Illicit drug use is associated with major health consequences for pregnant women that can affect fetal development, rates of STI, depression, and partner violence, as well as contributing to significant prenatal and neonatal complications.10 Marijuana use during pregnancy is on the rise,11 and recent reviews suggest that infants whose mothers used marijuana during pregnancy, compared with those who did not, were more likely to have lower birth weight and require neonatal intensive care.12 It is well documented that prenatal alcohol exposure can lead to a wide range of adverse effects, known as fetal alcohol spectrum disorders, with an estimated 12,000 infants born with fetal alcohol syndrome (FAS) each year.
The Centers for Disease Control and Prevention (CDC) and the ACOG have identified the prenatal period as an ideal opportunity for STI prevention and subsequent behavior change to reduce risk for acquiring infection and have recommended that information regarding STIs and behavior risk assessment should be provided to all pregnant women as part of routine healthcare.13 Despite the clear risks of STIs among pregnant adult women, very few STI-focused interventions have specifically targeted this group of women, and a review by the U.S. Preventive Services Task Force concluded that “methodologically rigorous trial evidence” is lacking for pregnant women.14 Only two RCTs have focused on pregnant women and included an STI/HIV prevention program, one of which was bundled into group prenatal care across two prenatal clinics in the United States and led to significantly increased condom use and decreased unprotected sex.15 The other study, specific to pregnant women at a methadone maintenance program, involved a motivational interviewing (MI)-based HIV risk behavior intervention and resulted in significantly less drug-related HIV risk behavior; however, the intervention did not include behavioral exercises to build skills to reduce condomless sex.16 Both of these interventions involved multiple sessions, with the latter including six sessions. A limitation of STI/HIV interventions with multiple sessions can be poor attendance (e.g., low follow-up and treatment completion rates for female participants with substance use).17 The use of technology to target stigmatized behaviors, including STI risk behaviors and substance use, is an empirically supported method of encouraging the disclosure of sexual risk-taking.18 Very few studies have assessed computer-based STI/HIV prevention interventions exclusively in pregnant women.19
The current study describes a pilot randomized controlled trial of an innovative computer-delivered intervention (the “HCEM”) that targets women at risk for STI/HIV and alcohol/drug use during pregnancy. The HCEM was designed and implemented using the Computerized Intervention Authoring System (CIAS20,21), an intervention development platform, which has been previously used in a number of studies, including those targeting risk behaviors during the prenatal and postpartum periods. The HCEM is a tailored, motivationally-focused STI/HIV and substance use risk reduction intervention that is consistent with key concepts from MI22 and provides training in several relevant skills, including male and female condom application, informed by the Information-Motivation-Behavior (IMB) model, which theorizes that information and motivation activate one's behavioral skills, which in turn lead to risk reduction.23 We previously reported on the iterative development and highly rated quantitative and qualitative feedback of users' experience of the HCEM in an open trial testing (G. Tzilos Wernette et al., under review).
The primary aims of the current study were to test the (1) feasibility of the HCEM, (2) acceptability via participant report of ease of use, helpfulness, and overall satisfaction, and (3) preliminary evidence for the hypothesized effects on outcomes: the proposed intervention, relative to a time-and-attention-matched control group, will produce reductions in self-reported condomless sex during the follow-up assessment at 4 months. Furthermore, we hypothesize that the intervention condition, relative to control, will produce reductions in self-reported alcohol/drug use (frequency, quantity, and heavy drinking/use frequency).
Methods
Participant inclusion criteria
We randomized 50 women who met study inclusion criteria. Inclusion criteria included pregnant women who endorsed (1) condomless vaginal (or anal) sex at least once in the past 30 days; (2) an unplanned pregnancy; and (3) current alcohol or drug use or are at-risk for prenatal alcohol/drug use, which was determined by a positive score on either the T-ACE, a screening tool for at-risk drinking developed for use in ob/gyn settings,24 or the Substance Use Risk Profile-Pregnancy scale (SURP-P), which has been used successfully to identify pregnant women at risk for substance use.25 Other inclusion criteria included an ability to understand study procedures in English and less than 5 months gestation, to increase likelihood of completing 4-month follow-up assessment during pregnancy. This study was approved by the Institutional Review Boards of Butler Hospital and Women and Infants Hospital in Providence, Rhode Island.
Participant characteristics and recruitment feasibility
Recruitment took place between December 2015 and April 2016. We approached 1,460 women and screened a total of 401 women over the course of 9 months during the randomized trial phase of the study (Fig. 1). Fifty eligible women (37%) were consented and enrolled, and the computer software randomized 31 (62%) into the HCEM intervention condition and 19 (38%) into the control condition. Of these 50 women, 15 (30%) identified themselves as Hispanic/Latina, and 35 (70%) identified as non-Hispanic. Thirteen women (26%) identified themselves as black or African American, 15 women (30%) identified as white, 7 (14%) identified as more than one race, and 13 women (26%) selected their race as “other.”
FIG. 1.
CONSORT flow diagram.
Study participants (24.4 years old [SD = 5.31]) were 50 pregnant women with an average of 13 weeks gestation. All 50 women (100%) completed a baseline assessment and intervention session. Of the 31 women randomized to the intervention condition, 30 (97%) completed a booster session. Of the 19 women randomized to the control condition, 19 (100%) completed a booster session. Forty-nine women (98%) completed the follow-up assessment 4 months later. One woman (2%) withdrew from the study after enrollment due to miscarriage. The majority of participants (48%) reported being single, 38% were living with a partner but not married, and 14% were married. Nearly half (44%) of the participants reported being unemployed, and 38% of the sample did not graduate high school. Characteristics between control and intervention group were not found to be significantly different (Table 1).
Table 1.
Demographic Characteristics of Sample, n = 50
| Control, n = 19 | Intervention, n = 31 | p | |
|---|---|---|---|
| Age (mean ± SD) | 23.2 ± 4.30 | 25.1 ± 5.79 | 0.22 |
| Pregnancy week (mean ± SD) | 13.9 ± 4.21 | 12.9 ± 4.76 | 0.46 |
| Ethnicity, n (%) | |||
| Latina | 8 (42) | 8 (26) | 0.23 |
| Non-Latina | 11 (58) | 23 (74) | |
| Race, n (%) | 0.20 | ||
| Caucasian | 8 (42) | 7 (23) | |
| African American | 2 (10) | 11 (35) | |
| More than one race | 3 (16) | 4 (12) | |
| Native American or Alaskan | 0 (0) | 2 (6) | |
| Other/unknown | 6 (32) | 7 (23) | |
| Education, n (%) | |||
| 0–8 grades | 0 (0) | 4 (12) | 0.22 |
| 9–11 grades | 8 (42) | 7 (23) | |
| High school grad | 6 (32) | 8 (26) | |
| Some college+ | 5 (26) | 12 (39) | |
| Employment, n (%) | |||
| Full time | 4 (22) | 7 (23) | 0.20 |
| Part time | 2 (10) | 6 (20) | |
| Student | 1 (5) | 2 (6) | |
| Homemaker | 5 (26) | 1 (3) | |
| Unemployed | 7 (37) | 15 (48) | |
| Marital status, n (%) | |||
| Single | 8 (42) | 16 (52) | 0.51 |
| First child, n (%) | 10 (53) | 12 (39) | 0.91 |
| Planned pregnancy, n (%) | 1 (5) | 2 (6) | 0.68 |
| Received public assistance in past year, n (%) | 13 (68) | 18 (58) | 0.46 |
| Food stamps assistance, n (%) | 12 (63) | 23 (74) | 0.41 |
p-values for differences between conditions were calculated using chi-square analyses for dichotomous data, and independent t-tests for continuous data.
Procedure
Participant recruitment and study procedures were conducted at a prenatal clinic in a large inner-city hospital that serves predominately low-income pregnant women. Research staff approached potential participants who were at the clinic for their prenatal visit and introduced the computer screener for the pilot study, which was described as a survey to help moms have healthier pregnancies. Women who expressed interest were screened for eligibility (∼5 minutes) using a program delivered on a tablet PC, given an information sheet (used to protect anonymity of those who did not qualify or chose not to participate in the full study), and received a $5 gift card. The computer program randomized participants to either the HCEM intervention condition or control condition. The control condition was found to be acceptable in our previous trials21,26 (S. Hill et al., under review) and included responding to questions related to television shows and providing subjective ratings.
The initial session included the computer-delivered assessment and either HCEM intervention or control condition (60 minutes). Within 1 month of the initial session, participants were asked to return to the study site, where they completed a 15-minute computer-delivered booster session or a control session. Whenever possible, the booster session was coordinated with a prenatal appointment and took place within 1 month following baseline. Participants returned for a computer-based 4-month follow-up assessment.
Measures
Acceptability of the computer software and the intervention was assessed after completing the baseline intervention session by the following computer-delivered measures: the satisfaction with CIAS software scale21 reported participant satisfaction with themes of likeability, ease of use, level of interest, and respectfulness using a 1–5 Likert scale (1 = low and 5 = high). Acceptability of HCEM intervention content (e.g., videos on condom use, personal testimonials) used a 1–7 Likert scale (1 = not helpful and 7 = very helpful). An in-person evaluation interview was conducted at the 1-month booster session. Interview questions were open-ended and designed specifically for this study, addressing various aspects, including comfort, ease of use, and likeability of the computer software, intervention content, and overall research procedures.
The timeline follow-back (TLFB), a well-validated and reliable method,27,28 was used to collect self-reported daily behaviors, using a calendar and multiple prompts to assist with the recall of alcohol use, drug use, and sexual behavior (e.g., condom use). The research assistant (RA) administered the TLFB during the baseline assessment to collect daily information over the past month, and the 4-month follow-up assessment included behaviors since the baseline assessment.
Biological testing
We collected hair sample testing (Psychemedics, Inc.) at baseline and at follow-up assessment to corroborate self-report of illicit drug use. The hair samples were obtained by a trained RA, collected from cosmetically undetectable areas on the scalp and sent off-site for analysis. We used the OSOM Trichomonas Rapid Test to test for an incident of vaginal trichomoniasis at baseline and at follow-up assessment. This self-administered vaginal swab specimen accurately diagnoses vaginal trichomoniasis and is a method that is feasible and acceptable to participants.29 Women who tested positive at any time during the study were linked to comprehensive STI care at the Women's Primary Care Center. We chose trichomoniasis, because it is the most common curable STI in young sexually active women in the United States.1 Participants could receive up to $70 for study completion.
Treatment conditions
Health checkup for expectant moms
Participants randomized to HCEM interacted with the computer and were guided by an animated narrator, which engages in a MI-consistent style, has the ability to use emotionally expressive statements and empathic reflection, and has been found in previous research to be well liked and understood by low-income, pregnant, and postpartum women.21 HCEM was self-administered with the assistance of the RA and included a behavioral skills component, in which the RA facilitated the setup of models for male and female condom use application. This portion of HCEM included video instruction that was guided by the computer. HCEM presented information and education regarding health risks and included testimonial videos of women who were HIV positive and pictures of STIs. All participants had the option to create a personalized safety plan that was tailored and designed to increase awareness of the interconnected risk factors for STI/HIV and alcohol/drug use in the woman's life. At the booster session, the narrator reviewed the components of the intervention session (e.g., goal-setting), and participants reviewed their personalized plan and identified any barriers to increasing safety behaviors.
Control condition
Participants randomized to the control group also interacted with the computer and were guided by the same narrator. The content of the control condition at both the baseline and booster session included watching brief segments of popular television shows with subsequent questions for ratings of their subjective preference. This control condition controlled for time effects and has been used successfully in previous behavioral trials21,26 (S. Hill et al., under review). Participants in both conditions received brochures specifically designed to facilitate health risk behaviors during pregnancy.
Statistical analysis
Key study variables were summarized through descriptive statistics. Demographic characteristics were compared between control and intervention groups by means of two-sample t-test (continuous) and chi-square tests. The primary risk behaviors of interest were condomless sex, alcohol, and drug use, as assessed by the TLFB. Although the TLFB assessed the use of several drugs (marijuana, cocaine, opiates, prescription, and “other” drugs), we report and focus here only on marijuana use as it was the only illicit drug reported among participants with the exception of two individuals who endorsed both cocaine and marijuana use at baseline assessment. There was no self-reported use of drugs other than marijuana at follow-up. Endorsement of any marijuana or alcohol use and condomless sex was compared between study arms at both baseline and follow-up using Fisher's exact test.
For each individual, a binary indicator of any substance use, representing the use of marijuana or alcohol at any time over the 90-day TLFB period at both pre and post, was created. The proportion of reduction in any substance use between the control and intervention groups was compared using a clustered logistic regression with time (pre vs. post), group and time-by-group interaction as the primary factors. The clustering within subject was accounted for using a generalized estimating equations (GEE) approach. Models were further adjusted for age and marital status. Because all but one participant engaged in condomless vaginal sex at baseline, a cross-sectional logistic regression was run with this outcome only at follow-up with factors and controlling covariates identical to the other models.
Results of trichomoniasis tests at baseline and 4-month follow-up assessments and hair sample drug screening at follow-up are descriptively presented. Formal statistical tests were not performed due to limited amount of data.
Results
Acceptability of HCEM
The overall acceptability of HCEM was supported by quantitative feedback. Participant self-reported ratings of both the software as well as aspects of the HCEM intervention were consistently high. With regard to the overall utility, 100% of the women reported that the intervention was useful in helping them to have a healthy pregnancy. Mean ratings of individual questions tapping satisfaction with the computer software ranged from a low of 4.6 (out of 5), “How interesting was it,” to a high of 5.0, “Was it respectful of you?” Mean ratings of the specific components of HCEM content ranged from a low of 6.3 (out of 7), “How helpful do you think the narrator was?” to a high of 6.8, “How helpful do you think the videos of women talking about HIV were?” and “How helpful do you think the information on how women can have safer sex was?”
Preliminary efficacy of HCEM
Timeline follow-back
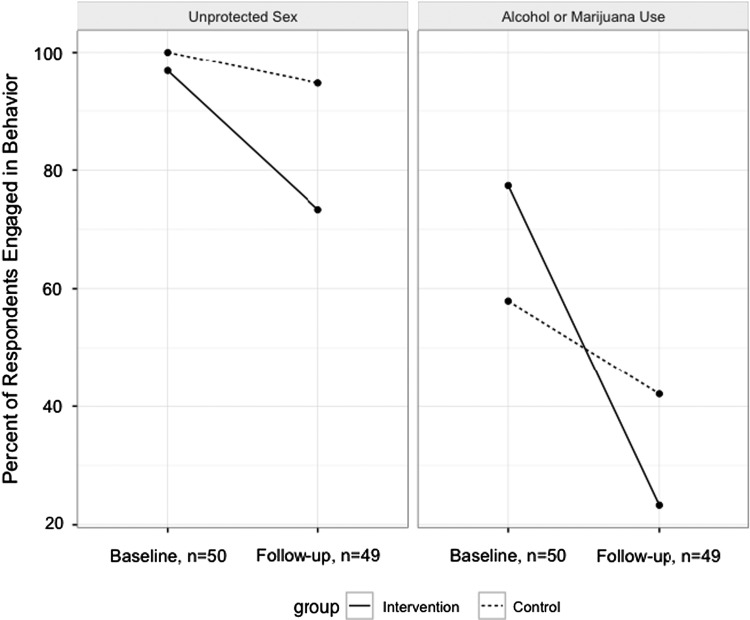
At the baseline assessment, 70% of participants endorsed substance use (alcohol or marijuana) (58% control vs. 77% intervention, Fisher's p-value = 0.20), decreasing to 31% at 4-month follow-up assessment (42% control vs. 23% intervention, Fisher's p-value = 0.21). All but one intervention participant engaged in condomless vaginal sex at baseline (98%) compared with 82% at 4-month assessment (95% control vs. 73% intervention, Fisher's p-value = 0.13) (Fig. 2).
FIG. 2.
Reductions in main outcomes of alcohol or drug use and unprotected sex.
Results of the GEE analyses revealed that those in HCEM, compared with those in control, had a significantly larger reduction in the odds of any self-reported marijuana or alcohol use from baseline to follow-up (time-by-group interaction p = 0.015). Specifically, odds of alcohol or marijuana use at baseline were 11.7 times higher at baseline compared with follow-up in those assigned to HCEM (adjusted odds ratio, AOR [95% confidence interval, CI] = 11.7 [4.2, 33.0], p < 0.001; Table 2). For participants in the control arm, odds of alcohol or marijuana use were 1.9 times higher at follow-up than at baseline, but that change was not statistically significant (AOR [95% CI] = 1.90 [0.63, 5.74], p = 0.255).
Table 2.
Regression Results for Risk Behaviors
| Any drug or alcohol usea | Condomless vaginal sexb | |||
|---|---|---|---|---|
| AOR (95% CI) | p | AOR (95% CI) | p | |
| Age | 0.94 (0.84, 1.05) | 0.27 | 0.91 (0.79, 1.04) | 0.17 |
| Marital status | ||||
| Married/living married | Reference | Reference | ||
| Single | 0.75 (0.24, 2.38) | 0.63 | 1.09 (0.21, 5.55) | 0.92 |
| Group | ||||
| HCEM | Reference | Reference | ||
| Control | 2.09 (0.60, 7.34) | 0.25 | 5.50 (0.59, 51.21) | 0.13 |
| Time | ||||
| Baselinec | 11.7 (4.16, 32.97) | <0.001 | ||
| Follow-up | Reference | |||
| Time × group | 0.16 (0.04, 0.74) | 0.02 | ||
Boldface indicates statistical significance (p < 0.05).
Results of clustered logistic regression using data from both time points.
Results of logistic regression on follow-up engagement only, time and time × group terms not applicable.
Effect shown is baseline versus follow-up for HCEM group, same effect for control group was not significant: AOR (95% CI) = 1.9 (0.6, 5.7), p = 0.26.
CI, confidence interval; HCEM, health checkup for expectant moms; AOR, adjusted odds ratio.
Logistic regression analyses indicated that there was not a statistically significant difference between groups in the odds of condomless sex engagement at follow-up (AOR [95% CI] = 5.5 [0.6, 51.2], p = 0.13; Table 2). However, examination of the raw change in engagement from baseline to follow-up shows a substantially higher reduction in the intervention arm than in the control (27% vs. 5%) (Fig. 2).
Biological testing
Trichomoniasis test results were available for 47 individuals at baseline (17 control and 30 intervention) and 37 at follow-up (14 control and 23 intervention). Two intervention participants had positive test results at baseline, one of which became negative at follow-up and the other refused follow-up testing. At follow-up, there were three positive results (two intervention and one control), all of whom had negative results at baseline. The participants who had positive test results at both baseline and follow-up also reported having condomless sex at the corresponding time points and were included in the analysis. Hair samples were collected for 35 participants (14 control and 21 intervention) at the follow-up assessment. However, eight were deemed insufficient samples for detecting Cocaine, Opiates, PCP, and Amphetamines and 12 insufficient for marijuana. Of the valid 27 samples, 5 were positive for cocaine (all were in the intervention condition), 1 of whom was also positive for opiates. An additional three were positive for marijuana (one control and two intervention). The two intervention participants who tested positive for marijuana also endorsed marijuana use on the TLFB at follow-up, and one of the two also tested positive for cocaine. Of the 23 valid hair samples for marijuana use, the findings for 20 (87%) conformed to the self-report TLFB data. Of the nonconcordant, one positive hair sample did not self-report and two individuals self-reported who had negative hair samples. Hair test data were used to corroborate self-report and were not an outcome measure.
Discussion
The current findings demonstrate the feasibility, acceptability, and the preliminary efficacy of the HCEMs in a sample of high risk, low-income pregnant women. With regard to study procedures, 96% of women who were approached for the study were interested in completing the screener, yielding a very low refusal rate. Follow-up rates were high with 49 out of 50 participants completing the 4-month follow-up assessment. The findings of this pilot trial were encouraging and consistent in the overall high ratings of acceptability of the software and HCEM across several domains, including content, appearance, and utility. The high ratings mirror similar results, using this software in other studies with low-income, at-risk women20 (S. Hill et al., under review). Moreover, high acceptability ratings of the intervention content, including the video demonstration of female condom use and the testimonial videos of women who were HIV positive are particularly encouraging and support a larger trial to test the efficacy of HCEM in reducing alcohol/drug use and HIV risk in a more diverse study sample, both with respect to demographic diversity and regarding diversity of substance use.
The findings support the preliminary efficacy of HCEM to reduce the risky behaviors of alcohol/marijuana use during pregnancy. There is evidence that computer-based brief interventions can reduce substance use in adults.30,31 The majority of existing interventions have had a single focus either on reducing alcohol/drug use or improving sexual health, and very few of these have targeted pregnant women exclusively.20,32–34 A single session brief intervention targeting sexual risk reduction in a sample of men and women patients at a STI clinic found that the intervention indirectly affected sexual risk behavior through alcohol-related factors, suggesting the importance of targeting risk-related alcohol use.35 Recently, the NIH issued a strong recommendation36 for physicians to advise pregnant women to avoid marijuana use, with growing concerns of its use by women to treat nausea during pregnancy.
With regard to reduction in condomless sex, the direction and magnitude of the effect was very promising and would be of clinical significance, however, it was not significant with this very small sample size. It may be that the lack of significance regarding unprotected sex reduction may indicate that additional contact may be needed for our high-risk group of pregnant women. The literature supports brief (10–15 minute), multicontact interventions for high-risk groups.37 The advantage of fewer sessions, if efficacious, increases the potential feasibility of implementation in a wide variety of settings—and the overall impact is greater when accessible and disseminable to larger populations.
HCEM is innovative in targeting STI/HIV risk behaviors, including substance use during pregnancy with a brief intervention format. Very few studies have rigorously examined brief interventions for pregnant women who may be at risk for STI, particularly among women who may also be at risk for substance use during pregnancy, and no such studies have evaluated a single-session, brief intervention plus booster intervention. A limitation of HIV interventions with multiple sessions can be poor attendance,17 which could be especially problematic for low-income pregnant women who have obstetrician appointments in addition to other multiple demands. Furthermore, a Cochrane review38 of psychosocial interventions for reducing injection and sexual HIV risk behavior in drug users concluded that while multisession psychosocial interventions have been effective, there are minimal differences on outcomes between these interventions and brief standard educational interventions as a cost effective option. Screening and brief intervention approaches, especially delivered in a technology-based format, have great potential to reach pregnant women who otherwise would not be identified and would not receive an intervention of any kind.
The current study included a number of strengths. We had a high rate of completion at the 4-month follow-up assessment for participants in both conditions (98%). Our control condition was matched for time and included no overlapping content with HCEM, providing a reasonable test of the effect of HCEM. Furthermore, this control condition using the CIAS platform has been found acceptable and engaging in at least 4 behavioral studies that we know of, including our own previous trials26,33 (S. Hill et al., under review). A limitation of prior MI interventions is that long-lasting effects are a challenge. In HCEM, we included a booster session to bolster and maintain the effects of the intervention. Our current sample endorsed marijuana use as their primary substance and, therefore, we could not examine the impact of HCEM on other drug use due to the small number of women reporting other drug use. We obtained biological samples from participants, including hair testing and trichomoniasis testing, for the purpose of sensitivity analysis.
The limitations of the current study included the use of self-report in the disclosure of risk behaviors, the inclusion of only English-speaking participants, an imbalance in the computerized randomization, the inability to generalize study results, particularly given the low enrollment rate as the study sample, may not be representative of the population of pregnant women, and including an unblinded research assistant who instructed participants who were randomized to HCEM condition on behavioral skills (e.g., condom use application). While the RA did not have a role as a study interventionist, the RA contact in facilitating the setup of the behavioral skills component (e.g., setup of the models for male and female condom application) may have contributed to the intervention effect. With regard to enrollment rate, we screened a large number of women for inclusion; however, the enrollment rate (13%) is comparable to other prevention trials, including studies for postpartum depression.39,40
Regarding feasibility of study procedures, we improved upon our collection rates of biological samples (e.g., women allowing hair sample) over the course of the study based on modifications that we made to our approach (e.g., completing additional training for RAs on the procedures for the collection of biological samples, improving upon our script to the participant, and increasing incentive). Refusal rates for both the trichomoniasis testing and hair sampling decreased over the course of the study. The refusal rate for the hair sampling of the first 25 participants at the 4-month follow-up was 36%; the refusal rate for the last 25 participants was 16%. The refusal rate for the trichomoniasis test at follow-up for the first 25 participants was 24%; the refusal rate for the last 25 participants was 16%. At the 4-month follow-up assessment, there were 12 missing biological samples (2 were phone assessments and 10 refused to provide hair samples).
HCEM is a brief intervention (one session plus a booster session) that is theory-driven and adapted from a previously tested and acceptable brief intervention for at-risk women. MI and the IMB model are well-established models and found to be effective for HIV/STI risk reduction, both generalizable to different populations, including low-income urban women.41 The results from this pilot trial are very encouraging with respect to the acceptability and feasibility of HCEM, as well as the preliminary efficacy of HCEM in reducing alcohol and marijuana use and condomless sex during pregnancy. Future research to test HCEM with a larger and more diverse sample is warranted.
Acknowledgments
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) R21HD075658 (PIs: G.T.W., C.Z.; Co-I: C.W.K). The authors gratefully acknowledge the women who participated in this study, as well as the research staff at WIH (Ms. Cheryl Santos, Ms. Kristen DeLayo, and Ms. Michelle Scully) for their assistance with data collection and computer programming, and the continued support of Dr. Steven Ondersma. This trial is registered with ClinicalTrials.gov (NCT02120716).
Author Disclosure Statement
No competing financial interests exist for authors Golfo Tzilos Wernette, Melissa Plegue, Christopher Kahler, and Ananda Sen. Caron Zlotnick's husband is a consultant for Soberlink.
References
- 1.Centers for Disease Control and Prevention (CDC). CDC Fact Sheet. Reported STDs in the United States. 2014 National Data for Chlamydia, Gonorrhea, and Syphilis. Available at: https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/std-trends-508.pdf Accessed September20, 2017
- 2.Hader SL, Smith DK, Moore JS, Holmberg SD. HIV infection in women in the United States: Status at the Millenium. JAMA 2001;285:1186–1192 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). 2015. Sexually Transmitted Diseases Surveillance. STDs in Racial and Ethnic Minorities. Available at: https://www.cdc.gov/std/stats15/minorities.htm Accessed September20, 2017
- 4.Healthy People 2020. Pregnancy Health and Behaviors. MICH-11.4. 2016. Available at: www.healthypeople.gov Accessed September20, 2017
- 5.Substance Abuse and Mental Health Services Administration, Results from the 2013 National Survey on Drug Use and Health. Summary of National Findings, NSDUH Series H-48, HHS Publication No (SMA) 14-4863 Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014 [Google Scholar]
- 6.Keough V, Jennrick J. Including a screening and brief alcohol intervention program in the care of the obstetric patient. J Obstet Gynecol Neonatal Nurs 2009;38:715–722 [DOI] [PubMed] [Google Scholar]
- 7.Sommers M, Dyehouse J, Howe S, Wekselman K, Felming M. “Nurse, I only had a couple of beers”: Validity of self-reported drinking before serious vehicular injury. Am J Crit Care 2002;11:106–114 [PubMed] [Google Scholar]
- 8.Rehm J, Shield KD, Joharchi N, Shuper PA. Alcohol consumption and the intention to engage in unprotected sex: Systematic review and meta-analysis of experimental studies. Addiction 2012;107:51–59 [DOI] [PubMed] [Google Scholar]
- 9.Ramsey SE, Engler PA, Stein MD. Addressing HIV risk behavior among pregnant drug abusers: An overview. Prof Psychol Res Pr 2007;38:518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gouin K, Murphy K, Shah P. Effects of cocaine use during pregnancy on low birthweight and preterm birth: A systematic review and meta-analyses. Am J Obstet Gynecol 2011;204:340. [DOI] [PubMed] [Google Scholar]
- 11.Brown QL, Shmulewitz D, Martins SS, Wall MM, Sarvet AL, Hasin DS. Trends in Marijuana use among pregnant and non-pregnant reproductive-aged women. JAMA 2017;317:207–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunn JK, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-anaysis. BMJ Open 2016;6:e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). Morbidity and Mortality Weekly Report: Revised Recommendations for HIV Screening of Pregnant Women. Washington, DC: CDC; September, 2006. 55 (RR14); 1–17 [PubMed] [Google Scholar]
- 14.Lin J, Whitlock E, O'Connor E, Bauer V. Behavioral counseling to prevent sexually transmitted infections: A systematic review for the U.S. preventive services task force. Ann Intern Med 2008;149:497–508, W96–W99 [DOI] [PubMed] [Google Scholar]
- 15.Kersshaw TS, Magriples U, Westdahl C, Rising SS, Ickovics JR. Pregnancy as a window of opportunity for HIV prevention: Effects of an HIV intervention delivered within prenatal care. Am J Public Health 2009;99:2079–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Neill K, Baker A, Cooke M, Collins E, Heather N, Wodak A. Evaluation of a cognitive-behavioural intervention for pregnant injecting drug users at risk of HIV infection. Addiction 1996;91:1115–1125 [DOI] [PubMed] [Google Scholar]
- 17.Tross S, Campbell A, Cohen L, et al. Effectiveness of HIV/STD sexual risk reduction groups for women in substance abuse treatment programs: Results of a NIDA Clinical Trials Network Trial. J Acquir Immune Defic Syndr 2008;48:581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richens J, Copas A, Sadiq S, et al. A randomized controlled trial of computer-assisted interviewing in sexual health clinics. Sex Transm Dis 2010;86:310–314 [DOI] [PubMed] [Google Scholar]
- 19.Noar S, Black H, Pierce L. Efficacy of computer technology-based HIV prevention interventions: A meta-analysis. AIDS 2009;23:107–115 [DOI] [PubMed] [Google Scholar]
- 20.Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women. Am J Prev Med 2007;32:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ondersma S, Chase S, Svikis D, Schuster C. Computer-based brief motivational intervention for perinatal drug use. J Subst Abuse Treat 2005;28:305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller W, Rollnick S. Motivational interviewing: helping people change. Third Edition. New York: Guilford Press, 2013. [Google Scholar]
- 23.Fisher J, Fisher W. Changing AIDS-risk behavior. Psychol Bull 1992;111:455–474 [DOI] [PubMed] [Google Scholar]
- 24.Sokol R, Martier S, Ager J. The T-ACE questions: Practical prenatal detection of risk-drinking. Am J Obstet Gynecol 1989;160:863–870 [DOI] [PubMed] [Google Scholar]
- 25.Yonkers KA, Gotman N, Kershaw T, Forray A, Howell HB, Rounsaville BJ. Screening for prenatal substance use: Development of the substance use risk profile-pregnancy scale. Obstet Gynecol 2010;116:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzilos GK, Solol R, Ondersma S. A randomized phase I trial of a brief computer-delivered intervention for alcohol use during pregnancy. J Womens Health 2011;20:1517–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobell LC, Sobell MB. Timeline follow-back: a calendar method for assessing alcohol and drug use. Toronto, Ontario, Canada: Addiction Research Foundation, 1996 [Google Scholar]
- 28.Wray T, Braciszewski JM, Zywiak W, Stout R. Examining the reliability of alcohol/drug use and HIV-risk behaviors using Timeline Follow-Back in a pilot sample. J Subst Use 2016;21:294–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiesenfeld H, Lowry D, Heine R, et al. Self-collection of vaginal swabs for the detection of Chlamydia, Gonorrhea, and Trichomoniasis: Opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis 2001;28:321–325 [DOI] [PubMed] [Google Scholar]
- 30.Portnoy DB, Scott-Sheldon LA, Johnson BT, Carey MP. Computer-delivered interventions for health promotion and behavioral risk reduction: A meta-analysis of 75 randomized controlled trials, 1988–2007. Prev Med 2008;47:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore BA, Fazzino T, Garnet B, Cutter CJ, Barry DT. Computer-based interventions for drug disorders: A systematic review. J Subst Abuse Treat 2011;40:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farr SL, Hutchings YL, Ondersma SJ, Creanga AA. Brief intervention for illicit drug use among peripartum women. Am J Obstet Gynecol 2014;211:336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ondersma SJ, Svikis D, Thacker LR, Beatty JR, Lockhart N. Computer-delivered screening and brief interention (e-SBI) for postpartum drug use: A randomized trial. J Subst Abuse Treat 2014;46:52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnet B, Liu J, DeVoe M, Duggan A, Gold MA, Pecukonis E. Motivational intervention to reduce rapid subsequent births to adolescent mothers: A community-based randomized trial. Ann Fam Med 2009;7:436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins PR, Jenkins RA, Nannis ED, McKee KT, Jr, Temoshok LR. Reducing risk of sexually transmitted disease (STD) and human immunodeficiency virus infection in a military STD clinic: Evaluation of a randomized preventive intervention trial. Clin Infect Dis 2000;30:730–735 [DOI] [PubMed] [Google Scholar]
- 36.Volkow N, Compton W, Wargo E. The risks of Marijuana use during pregnancy. JAMA 2016;317:129–130 [DOI] [PubMed] [Google Scholar]
- 37.Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: A systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2012;157:645–654 [DOI] [PubMed] [Google Scholar]
- 38.Meader N, Li R, Jarlais D, Pilling S. Psychosocial interventions for reducing injection and sexual risk behavior for preventing HIV in drug users. Cochrane Database Syst Rev 2010:CD007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dennis CL, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev 2013:CD001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zlotnick C, Tzilos GK, Miller I, Seifer R, Stout R. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affect Disord 2015;189:263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carey M, Maisto S, Kalichman S, Forsyth A, Wright E, Johnson B. Enhancing motivation to reduce the risk of HIV infection for economically disadvantaged urban women. J Consult Clin Psychol 1997;65:531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]